Abstract
Background
Patients with KRAS mutations do not respond to epidermal growth factor receptor (EGFR) inhibitors and fail to benefit from adjuvant chemotherapy. Mutation analysis of KRAS is needed before starting treatment with monoclonal anti-EGFR antibodies in patients with metastatic colorectal cancer (mCRC). The objective of this study is to develop a multiplex allele-specific PCR (MAS-PCR) assay to detect KRAS mutations.
Methods
We developed a single-tube MAS-PCR assay for the detection of seven KRAS mutations (G12D, G12A, G12R, G12C, G12S, G12V, and G13D). We performed MAS-PCR assay analysis for KRAS on DNA isolated from 270 formalin-fixed paraffin-embedded (FFPE) colorectal cancer tissues. Sequences of all 270 samples were determined by pyrosequencing. Seven known point-mutation DNA samples diluted with wild-type DNA were assayed to determine the limitation of detection and reproducibility of the MAS-PCR assay.
Results
Overall, the results of MAS-PCR assay were in good concordance with pyrosequencing, and only seven discordant samples were found. The MAS-PCR assay reproducibly detected 1 to 2% mutant alleles. The most common mutations were G13D in codon 13 (49.17%), G12D (25.83%) and G12V (12.50%) in codon 12.
Conclusion
The MAS-PCR assay provides a rapid, cost-effective, and reliable diagnostic tool for accurate detection of KRAS mutations in routine FFPE colorectal cancer tissues.
Introduction
Colorectal cancer (CRC) is the most common cancer and the third leading cause of cancer death in the world [1]. In Thailand, CRC is the third most common cancer among men and the fifth most common one among women [2, 3]. One of the major molecular pathways in CRC development is the induction of an activating mutation in the proto-oncogene KRAS (Kirsten rat sarcoma viral oncogene) [4, 5]. The KRAS gene is a member of the RAS gene family and encodes a 21-kDa RAS protein, which is a downstream GTP-binding protein in the epidermal growth factor receptor (EGFR) signal transduction pathway. The oncogenic forms of KRAS mutations constitutively express the active RAS protein leading to increased cell division, cell proliferation, prevention of apoptosis process, induction of angiogenesis and increased metastasis [6]. Recently, cancer therapies have been developed using monoclonal antibodies, including cetuximab and panitumumab, to target the EGFR [7, 8]. These agents are designed to block ligand-induced EGFR tyrosine kinase activation and, thus, inhibit downstream signaling [9]. However, only the CRC with wild-type KRAS proto-oncogene responds to anti-EGFR antibodies treatment, whereas no therapeutic response occurs in CRC with KRAS mutations [9–11]. The European Society for Medical Oncology and the American Society of Clinical Oncology have established major oncology guidelines that these antibodies be restricted to patients with KRAS wild-type colorectal cancers [12, 13]. Therefore, detection of KRAS gene mutations has critical clinical relevance for developing individualized patient therapeutic strategies [7].
Several molecular methods have been developed for detecting KRAS mutations. These methods include direct sequencing [10], real-time PCR [11], high resolution melting (HRM) [14], amplification refractory mutation system polymerase chain reaction [15, 16], pyrosequencing [17, 18], co-amplification at lower denaturation temperature PCR [19], mutant-enriched PCR [20], and digital PCR [21]. In addition, several commercial molecular kits are commonly available for KRAS mutation detection, including the cobas® KRAS Mutation Test [22], 3D-Gene® KRAS mutation assay kit, therascreen® KRAS RGQ PCR Kit [23], EntroGen’s KRAS Mutation Analysis Kit for Real-Time PCR [23], and KRAS PyroMark Q96 V2.0 Kit [24]. However, all of these methods require technical expertise and specialized equipment and instruments, and are too expensive as prognostic and diagnostic tools for cancer patients in developing countries. By contrast, Multiplex allele-specific Polymerase Chain Reaction (MAS-PCR) is a simple, reliable and inexpensive method for detection of known mutations and single-nucleotide polymorphism [25, 26]. MAS-PCR is characterized by primers with an allele-specific 3’ terminus that anneals specifically to mutated or wild-type DNA template only [26, 27]. Wild-type and allele-specific primers generate different sized PCR products permitting easy detection of a known gene mutation.
In this study, we developed a MAS-PCR assay for analysis of the mutational status of KRAS codons 12 and 13. Single nucleotide point mutations in the KRAS gene occur most frequently in codons 12 and 13 accounting for 80 to 82% and 15 to 17% of the mutations, respectively [28–31]. Mutations in other positions, such as codons 61, 117, 146 and 154, are much less frequent amounting to approximately 1% of all KRAS gene mutations [17, 32]. In this study, presence of the most common point mutations in codons 12 and 13, which are G12D, G12A, G12R, G12C, G12S, G12V, and G13D [18, 30, 32, 33], was assessed in formalin-fixed, paraffin-embedded tissue samples from 270 CRC patients. Pyrosequencing, a robust and sensitive method, was used as a reference method to compare the sensitivity of MAS-PCR assay for detection of the KRAS mutant alleles.
Materials and Methods
Preparation of Clinical Samples
Formalin-fixed, paraffin-embedded colorectal adenocarcinomas from 270 patients with CRC were collected from the Institute of Pathology, Ministry of Public Health, Bangkok, Thailand. The study was approved by the Ethics Committee of the Institute of Pathology (IOP-KM-R57-007). The ethics committee waived the need for consent because the data of tissue samples were analyzed anonymously and reported. An experienced pathologist reviewed and marked the adenocarcinoma areas of the hematoxylin and eosin stained slides. Tumors were manually micro-dissected from paraffin-embedded blocks, and 10 μm thick sections were collected in a 1.5 ml tube. Paraffin was removed from the tissue blocks with xylene, and samples were air-dried. DNA was extracted from the samples and purified using a QIAamp DNA FFPE Tissue Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. DNA quantity was determined by NanoDrop spectrophotometry (NanoDrop Technologies, Wilmington, DE).
PCR Amplification and Pyrosequencing
Pyrosequencing for analysis of a KRAS gene fragment spanning codons 12 and 13 was performed as previously described with some modifications [18]. Sequences of the primers were previously described [18]. Reaction conditions with 1 μM forward primer (5’-GGCCTGCTGAAAATGACTGAA-3’), biotinylated reverse primer (5’-biotin-TTAGCTGTATCGTCAAGGCACTCT-3’), and 50 to 100 ng/μl DNA template yielded a 82-bp product. Reactions were performed in 1x PCR buffer containing 2.5 mM MgCl2, 0.2 mM dNTP (BioLabs, England), and 0.625 U Amplitag Gold DNA Polymerase (Applied Biosystems, USA) in a 30 μl total volume. PCR conditions were a denaturing step at 95°C for 10 min, then 1 min at 95°C, 1 min at 55°C, 1 min at 72°C for 35 cycles, and followed by 7 min at 72°C. PCR products were confirmed by 8% polyacrylamide gel electrophoresis at 140 V for 40 min, and gels were stained with SYBR Green I Nucleic Acid Gel Stain (1:400, Lonza, USA) for 30 min. The PCR products were sequenced by pyrosequencing using the PyroMark Gold Q96 reagent (Qiagen, Germany). PCR products in 30 μl were mixed with 3 μl streptavidin-conjugated Sepharose beads (Streptavidin Sepharose HP, Amersham Biosciences AB, Sweden), 40 μl binding buffer and 17 μl distilled water, followed by shaking at 1400 rpm for 10 min. The immobilized biotinylated PCR products: streptavidin-conjugated Sepharose beads complexes were captured using a vacuum prep tool. Single-stranded DNA purification was achieved by 1x washing the vacuum prep tool sequentially with 70% ethanol for 5 s, denaturation solution for 5 s, and washing buffer for 10 s. Biotinylated single-stranded DNA was added to a 96-well microtiter plate that contained 40 μl of 0.4 μM sequencing PF1-primer (5’-TGTGGTAGTTGG AGCTG-3’) for analysis at nucleotides 35 and 38 positions, and PF2-primer (5’-TGTGGTAGTTGGAGCT-3’) for analysis at nucleotide 34 position [18]. The plate was incubated at 80°C for 2 min, followed by cooling to room temperature for 5 min, and loaded onto the PyroMark Q96 ID system (Qiagen, Germany).
DNA Cloning
Genomic DNA of eight clinical samples harboring KRAS wild-type DNA and seven point mutations in KRAS codons 12 and 13 (G12D, G12A, G12R, G12C, G12S, G12V, and G13D) were subjected to PCR amplification using universal KRAS primers (K-ras-codon 12/13-F 5’-CTG GTG GAG TAT TTG ATA GTG TAT T-3’ and K-ras-codon 12/13-R 5’-ATC TGT ATC AAA GAA TGG TCC TG-3’). All 259-bp PCR products were cloned into psc-A-amp/kan vector and transformed into competent Escherichia coli cells using a Strataclone PCR cloning kit (Agilent Technologies; United States). The transformed bacteria were spread onto selective LB-agar plates (Oxoid, USA) with ampicillin and X-Gal (Promega, USA). After an overnight incubation at 37°C, white colonies were selected randomly and cultured in LB medium, overnight. Plasmids were extracted using Wizard® genomic DNA purification kit (Geneaid, Taipei, Taiwan) and subsequently screened for the insert fragment by PCR. Positive PCR products were sequenced by the Bioneer Corporation, Daejeon, Republic of Korea.
Primer Design
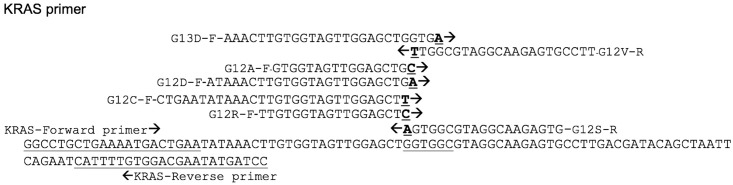
Allele-specific (AS) primers were designed for each of seven mutations, and a mutation-unspecific region was used as a reference amplicon. The 3′ terminal base of each AS primer was adopted according to its corresponding mutation. Amplification reactions were performed with the main KRAS forward primer and five AS primers (G12R-F, G12C-F, G12D-F, G12A-F, and G13D-F) sharing with one common antisense KRAS reverse primer, and reactions with two AS-primers (G12S-R and G12V-R) sharing with one common sense KRAS forward primer (Fig 1). Sequences of the primers used in this study are listed in Table 1. All primers were synthesized and supplied by BioDesign Co., Ltd. (BioDesign, Pathumthani, Thailand).
Fig 1. Target region of KRAS gene at codons 12 and 13 for amplification and the design of primers.
The positions of primers are illustrated. AS primers share the same forward primer or reverse primer. Solid arrows indicate the forward and reverse primers. The 3′ terminal base of each AS primers was adapted according to its corresponding mutation, and is bold and underlined. Codons and mutated bases are underlined.
Table 1. Primers used in MAS-PCR for detecting the most common mutations in codons 12 and 13 of KRAS gene.
| Primer | Sequence | Conc. (μM) | Product length (bp) |
|---|---|---|---|
| KRAS-F | 5’-GGCCTGCTGAAAATGACTGAA-3’ | 0.05 | 113 bp |
| KRAS-R | 5’-GGATCATATTCGTCCACAAAATG-3’ | 0.075 | 113 bp |
| G12S-R | 5’-CACTCTTGCCTACGCCACT-3’ | 0.05 | 64 bp |
| G12R-F | 5’-TTGTGGTAGTTGGAGCTC-3’ | 0.025 | 85 bp |
| G12C-F | 5’-CTGAATATAAACTTGTGGTAGTTGGAGCTT-3’ | 0.01 | 97 bp |
| G12D-F | 5’- ATAAACTTGTGGTAGTTGGAGCTGA-3’ | 0.02 | 91 bp |
| G12A-F | 5’- GTGGTAGTTGGAGCTGC-3’ | 0.02 | 83 bp |
| G12V-R | 5’- AAGGCACTCTTGCCTACGCCAA-3’ | 0.02 | 68 bp |
| G13D-F | 5’- AAACTTGTGGTAGTTGGAGCTGGTGA-3’ | 0.0125 | 89 bp |
Multiplex Allele-Specific PCR (MAS-PCR) Assay
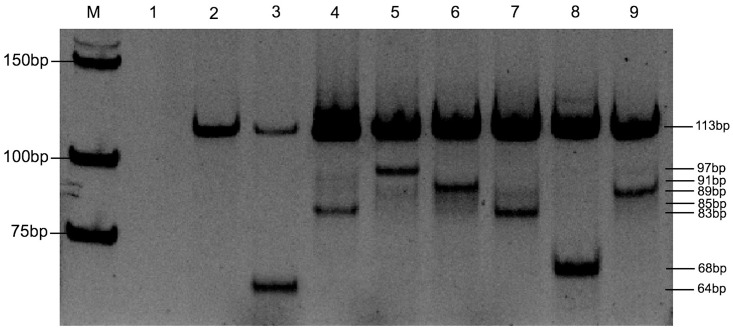
A single reaction had two main KRAS primers and seven AS primers targeting on seven mutated nucleotides within the KRAS gene. The MAS-PCR reaction in 50 μl total volume contained 1x PCR buffer, 2.5 mM MgCl2, 0.2 mM dNTP (BioLabs, England), 0.625 U Amplitag Gold DNA Polymerase (Applied Biosystems, USA), 50 to 100 ng/μl DNA template. The optimized concentrations of each primer are shown in Table 1. The reaction was amplified under the following conditions: an initial denaturation step at 95°C for 10 min, followed by 10 cycles at 95°C for 30 s, 60°C for 45 s, 72°C for 60 s, a second step of 20 cycles at 95°C for 30 s, 64°C for 45 s, 72°C for 60 s, and a third step of 20 cycles at 95°C for 30 s, 55°C for 45 s, 72°C for 60 s, and finally 10 min at 72°C. After amplification, the amplicons with a loading volume of 20 μl were analyzed by 8% polyacrylamide gel electrophoresis at 140 V for 60 min, and gels were stained with SYBR Green I Nucleic Acid Gel Stain (1:400, Lonza, USA) for 30 min. Because of the 3′ end of each AS primer pairs with the respective nucleotide base in the mutant sequence of KRAS gene, the allele-specific fragment was amplified yielding one of 97-bp, 91-bp, 89-bp, 85-bp, 83-bp, 68-bp and 64-bp products (represented the G12C, G12D, G13D, G12R, G12A, G12V, and G12S mutants, respectively) along with the positive 113-bp band as an internal control. While wild-type DNA at any of the seven positions prevents allele-specific amplification resulting in a corresponding missing band (Fig 2).
Fig 2. MAS-PCR assay of KRAS gene.
Assay distinguished wild-type KRAS gene codons 12 and 13 from different mutants using AS primers. Lane M: Low molecular weight DNA ladder; Lane 1: negative control; Lane 2: wild-type; Lane 3: G12S mutant; Lane 4: G12R mutant; Lane 5: G12C mutant; Lane 6: G12D mutant; Lane 7: G12A mutant; Lane 8: G12V mutant; Lane 9: G13D mutant.
Sensitivity of MAS-PCR Assay
Eight plasmid clones of KRAS wild-type and seven KRAS mutants (G12D, G12A, G12R, G12C, G12S, G12V, or G13D) were extracted. Each mutated plasmid DNA was mixed with a wild-type plasmid DNA in total of 100 ng. The proportion of mutant plasmid DNA was gradually reduced to obtain decreasing ratios of mutant to wild-type DNA at 100%, 50%, 25%, 10%, 5%, 2%, 1% and 0.1%. The precision and reproducibility were determined in four repeated run by analyzing the mixtures at the range of lowest detection limit which were demonstrated by MAS-PCR.
Data analysis
Results obtained from MAS-PCR and pyrosequencing were compared for the 270 formalin-fixed, paraffin-embedded specimens and were evaluated for significance using the Kappa statistics. A value of k > 0.81 was considered to be significant and to indicate that both methods provide almost perfect results. Positive and negative agreement confident intervals were determined for the MAS-PCR results. Differences in the categorical variables such as age, gender, histologic grade and site of tumor between patients and with KRAS mutations were evaluated for significance with chi-square test. Statistical tests were two-sided, and p < 0.05 was considered significant. Statistics were carried out using SPSS software (version 11.5).
Results
Pyrosequencing analysis of KRAS gene mutations in CRC specimens
Two hundred and seventy clinical samples were first analyzed by pyrosequencing for 6 different point mutations in codon 12 (G12S, G12R, G12C, G12D, G12A, and G12V) and one point mutation in codon 13 (G13D) of the KRAS gene. The mutations selected for this study are among the most frequently found in colorectal cancer patients. Of the 270 tissue specimens, the sequencing results revealed that 120 cases (44.44%) had a mutation in either codon 12 or 13 in the KRAS gene, while 150 cases (55.55%) were defined as KRAS wild type (Table 2). Of the 120 cases with a KRAS mutation, 61 (50.83%) patients had a codon 12 mutation. The most frequent codon 12 mutation was G12D at 25.83%, followed by G12V at 12.50%. The incidence of G12A, G12S and G12C mutations ranged from 6 to 3%, while that of G12R was the lowest at < 1%. The codon 13 mutation G13D found at 49.17% was more prevalent than any of the codon 12 mutations. Patients had either one or no detectable mutation.
Table 2. KRAS mutational status in 270 CRC patients.
| KRAS status | Type of mutations Amino acid change | Pyrosequencing Number of mutations (%) | MAS-PCR Number of mutations (%) |
|---|---|---|---|
| Mutant | All Codon 12 and 13 | 120/270 | 113/270 |
| (44.44%) | (41.85%) | ||
| Mutated Codon 12 | All Codon 12 | 61/120 | 54/113 |
| (50.83%) | (47.79%) | ||
| G12D | GGT>GAT | 31/120 | 28/113 |
| Gly→Arg | (25.83%) | (24.78%) | |
| G12V | GGT>GTA | 15/120 | 15/113 |
| Gly→Val | (12.50%) | (13.27%) | |
| G12A | GGT>GCT | 7/120 | 4/113 |
| Gly→Ala | (5.83%) | (3.54%) | |
| G12S | GGT>AGT | 3/120 | 3/113 |
| Gly→Ser | (2.50%) | (2.65%) | |
| G12C | GGT>TGT | 4/120 | 4/113 |
| Gly→Cys | (3.33%) | (3.54%) | |
| G12R | GGT>CGT | 1/120 | 0/113 |
| Gly→Arg | (0.83%) | ND | |
| G13D | GGC>GAC | 59/120 | 59/113 |
| Gly→Asp | (49.17%) | (52.21%) |
Underlined bases represent the substitutions in the respective codon.
ND denotes not detected.
MAS-PCR analysis of KRAS gene mutations in CRC clinical samples
The MAS-PCR assay, performed on the 270 CRC tissue specimens to detect the KRAS codon 12 and codon 13 mutations, revealed results that closely agreed with those of pyrosequencing. As shown on Table 2, 113 (41.85%) cases of KRAS mutation in codons 12 and 13 and 157 cases (58.15%) of KRAS wild type were identified by MAS-PCR assay. Among the 113 mutated cases, 47.79% had a mutation at codon 12. Moreover in reference to pyrosequencing results, seven mutated cases (three G12D, one G12R, and three G12A) were not detected by MAS-PCR assay. However all codon 13 mutations were correctly identified, as with pyrosequencing results.
Correlation of patients’ characteristics with KRAS codon 12 and 13 mutations
The median age of patients was 62 years ranging from 27 to 90 years-old. Majority of the patients were between 60 and 79 years of age (55.19%). Male to female ratio was 1.55:1. The dominant histological type at 64.81% (175/270) was moderately differentiated, and 88.52% of the cases (239/270) were colorectal primary tumors (Table 3). A possible correlation between patients’ demographic characteristics and detected KRAS mutations was examined as shown in Table 3. Of the studied patients presenting KRAS mutated carcinoma, no significant differences were found with regard to age, gender, histologic grade and tumor site. Mutations in codons 12 and 13 were fairly and evenly distributed within the groups being close to 1:1 ratio regardless of the characteristic.
Table 3. Correlation between KRAS mutation and Patients’ characteristics of 270 colorectal carcinomas.
| Characteristics | N (%) | WT KRAS N (%) | MT KRAS N (%) | P Value | Codon 12 mutated N (%) | Codon 13 mutated N (%) | P Value |
|---|---|---|---|---|---|---|---|
| Total patients | 270 | ||||||
| Median age (range) | 62 | ||||||
| 20–39 years | 11 (4.07%) | 7 (63.64%) | 4 (36.36%) | 0.117 | 1 (25.00%) | 3 (75.00%) | 0.268 |
| 40–59 years | 98 (36.30%) | 48 (48.98%) | 50 (51.02%) | 28 (56.00%) | 22 (44.00%) | ||
| 60–79 years | 149 (55.19%) | 85 (57.05%) | 64 (42.95%) | 30 (46.88%) | 34 (53.12%) | ||
| 80–90 years | 12 (4.44%) | 10 (83.33%) | 2 (16.67%) | 2 (100%) | 0 | ||
| Gender: | |||||||
| Male | 164 (60.74%) | 95 (57.93%) | 69 (42.07%) | 0.329 | 36 (52.17%) | 33 (47.83%) | 0.733 |
| Female | 106 (39.26%) | 55 (51.89%) | 51 (48.11%) | 25 (49.02%) | 26 (50.98%) | ||
| Histologic Grade: | |||||||
| Well differentiated | 77 (28.52%) | 35 (45.45%) | 42 (54.55%) | 0.057 | 19 (45.24%) | 23 (54.76%) | 0.641 |
| Moderate differentiated | 175 (64.81%) | 102 (58.29%) | 73 (41.71%) | 39 (53.42%) | 34 (46.58%) | ||
| Poorly differentiated | 18 (6.67%) | 13 (72.22%) | 5 (27.78%) | 3 (60.00%) | 2 (40.00%) | ||
| Site: | |||||||
| Colorectal primary | 239 (88.52%) | 128 (53.56%) | 111 (46.44%) | 0.066 | 55 (49.55%) | 56 (50.45%) | 0.323 |
| Metastasis | 31 (11.48%) | 22 (70.97%) | 9 (29.03%) | 6 (66.67%) | 3 (33.33%) |
Comparison of MAS-PCR and pyrosequencing
Results for the 270 FFPE samples were subjected to agreement analyses, and the MAS-PCR findings were compared to those of the pyrosequencing method (Table 4). Concordant results of 263 out of 270 samples showed that both methods provided perfect results (p < 0.05) with no statistically significant difference between the assays (k = 0.947, 95% CI = 0.909 to 0.986). For 7 cases, discordant results were obtained, mainly regarding codon 12 mutations which were detectable by pyrosequencing and sanger direct sequencing but not with MAS-PCR assay. The positive agreement and negative agreement were 94% and 100%, respectively.
Table 4. Pairwise comparison and agreement analyses between MAS-PCR and pyrosequencing.
| Pyroseqencing | |||
|---|---|---|---|
| Number Mutant | Number Wild-type | Totals | |
| MAS-PCR | |||
| Number Mutant | 113 | 0 | 113 |
| Number Wild-type | 7 | 150 | 157 |
| Totals | 120 | 150 | 270 |
Positive agreement: 94% Cl 95% [88%; 97%]
Negative agreement: 100% Cl 95% [98%; 100%]
Sensitivity, precision and reproducibility of the MAS-PCR for KRAS mutation
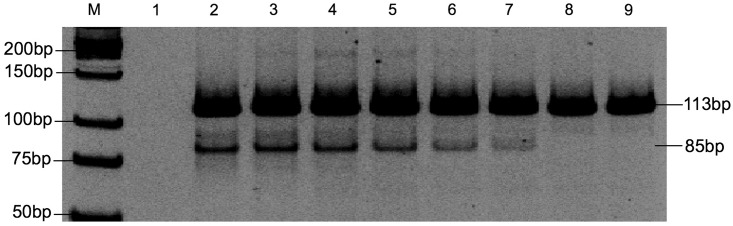
To evaluate the sensitivity of the MAS-PCR assay, plasmid DNA of each seven KRAS mutant clones was diluted in separate amplification reactions with plasmid DNA of a wild-type KRAS clone. The proportion of mutant DNA was gradually reduced to obtain decreasing ratios of mutant to wild-type DNA. The MAS-PCR assay detected mutant alleles down to 1% for the G12S mutant, and 2% for the G12R, G12C, G12D, G12A, G12V, and G13D mutants. The representative example of MAS-PCR assay for lowest limit of detection is shown in Fig 3. To test for precision and reproducibility of our MAS-PCR assay, we quantified KRAS mutations in the DNA mixtures containing each of the seven mutant KRAS DNA and wild-type DNA at varying ratios (10%, 5%, 2%, 1%, 0.1% and 0.01%) in four repeated runs. The results were precise and reproducible, of which the same lowest amount of KRAS mutant alleles were detected in all repeated runs.
Fig 3. Sensitivity of MAS-PCR assay for identifying KRAS gene mutations.
A representative gel is shown. Dilutions of G12R mutant plasmid and wild-type plasmid DNA (from 100%, 50%, 25%, 10%, 5%, 2%, 1% and 0.1% mutated alleles) Lane M: Low molecular weight DNA Ladder; Lane 1: negative control; Lane 2–9: corresponded to PCR products from 100% to 0.1%.
Discussion
Many studies have examined the association of KRAS mutations with CRC [8, 33–37]. KRAS mutations in CRC patients correlate with resistance to anti-EGFR treatment, such as cetuximab or panitumumab [7]. Hence, accurate prediction of therapeutic responses will spare patients from unnecessary treatment while focusing on more individualized effective therapy. A wide variety of methods have been developed, and several commercial molecular kits are commonly available for detecting KRAS mutations [10, 11, 14, 17, 20, 23]. Each technique has its own set of problems and issues. For example, direct sequencing is the most commonly used approach to screen for KRAS mutations, however sensitivity for detecting mutant DNA is low and requires at least 10%-30% of mutated alleles in a wild-type background [14, 18, 38]. HRM is a rapid methodology that enables high-throughput screening of KRAS mutations with a moderate analytical sensitivity of 5% to 6% [14], however, its main limitation is the inability to identify which codon is mutant. HRM results should be confirmed, and identification of specific mutations requires another technique, such as direct sequencing [39]. Pyrosequencing is accurate, feasible and has superior analytical sensitivity of approximately 5% mutant allele [14, 18]. Nevertheless, this assay requires a costly instrument, and expensive reagents and consumables, making it cost prohibitive for use in developing countries. Commercial molecular kits have various advantages, including high sensitivity (i.e., detection limit around 1% to <5%), speed, easy data interpretation, and numerous detectable positions of KRAS mutations, however this assay also requires a costly instrument, expensive reagents, and has a relatively high cost per sample [40]. Therefore, there is a need to develop an accurate, simple, and cost-effective method to detect KRAS mutations known to be associated with CRC that can be utilized in developing countries.
In this study, we successfully developed highly sensitive and specific MAS-PCR assay, targeting the seven (i.e., G12S, G12R, G12C, G12D, G12A, G12V, and G13D) most common mutations in codons 12 and 13 of KRAS gene. We designed specific primers for each mutation, and a mutation-nonspecific region was used as a reference amplicon. The 3′-terminal base of each AS primer was adopted according to its corresponding mutation. The MAS-PCR assay was optimized for each primer in terms of the primer concentration and amplification parameters. The MAS-PCR assay we reported herein is the first development of multiplex allele-specific PCR employing the standard PCR method that could detect the seven most common KRAS gene mutations. The method could simply be performed in low resource laboratories. Further investigation is on the way to develop a simple, rapid and user-friendly Nucleic Acid Lateral Flow (NALF) immunoassay [41] using biotinylated primers based on primers developed in this study. Moreover probe-based real-time PCR could be established using our MAS-PCR primer set to detect KRAS mutations. Both methods could be advantageous in terms of preventing crosstalk between samples and/or environmental contaminations during conducting experimentations.
In the present study, we observed that the frequency of KRAS oncogene mutations in codons 12 and 13 in 270 samples of colorectal cancer Thai patients was 44.44%. Similar frequencies, ranging from 20 to 50%, have been previously described [28, 30, 33]. We found the rates of mutations in codon 12 and codons 13 were at 50.83% and 49.17%, respectively, which were higher than those reported in other studies (codon 12: 70–90%, codon 13: 10–30%) [28, 30, 31, 33]. However, our results gave similar frequencies (codon 12: 52.62% (10/19), codon 13: 42.12% (8/19)) as reported by Poehlmann et al [17]. The frequently found G13D, G12D, and G12V mutations identified in this study were among the commonest KRAS mutations, in agreement with other studies [17, 28, 33]. Our data showed that no significant association between KRAS mutations and age, gender, histologic grade or tumor site. However, some previous reports found that the rate of KRAS mutations were higher in females than males [10, 42] which was opposite to the study of Poehlmann et al [17].
Our data showed a superior consistency between MAS-PCR assay and pyrosequencing (k = 0.947). Seven discordances were found according to pyrosequencing results. Direct sequencing and pyrosequencing revealed the same mutant alleles, which comprised of 3 cases of G12D, 3 cases of G12A, and 1 case of G12R. One possible outcome is the formation of cross-dimers between primers leading to biased amplification, which may reduce sensitivity of the MAS-PCR assay [43]. In addition, FFPE tissue samples may have degraded nucleic acids due to the fixation process, and the effect of cross-linking fixatives on the nucleic acids is detrimental causing PCR inhibition [44, 45]. Interestingly, our MAS-PCR assay showed high analytical sensitivity of detection, with approximately 1–2% mutant allele detected based on DNA mixing experiments using genomic DNA isolated from plasmid cloned DNA. However, such sensitivity might be affected considering the use of clinical samples that are usually complicated with the presence of PCR interfering components in the samples. Nevertheless, most interfering components could be eliminated by DNA purification using FFPE tissue kits as we employed in this study and as previously described [46, 47]. Moreover, the MAS-PCR assay that we developed has a higher sensitivity than that reported for direct sequencing and HRM (i.e., 5% to 20%), and is equivalent to that reported for pyrosequencing and commercial molecular kits (i.e., 1% to 5%) [14, 18, 38]. Furthermore, we found a good precision and reproducibility of the developed assay, as four repeated run yielded the same results. In addition, MAS-PCR is a rapid assay requiring only < 4 h to complete the assay (excluding DNA isolation); it is inexpensive, costing approximately $8 per test. Moreover, the assay employs only a PCR instrument, and does not require a high level of technical expertise and specialized equipment.
Conclusions
In conclusion, we developed a MAS-PCR assay for detection of the seven most common mutations in codons 12 and 13 of the KRAS gene. MAS-PCR assay is a DNA-based protocol that was easy to perform, being rapid, cost-effective, highly sensitive and highly specific. An assay with these characteristics is important for analysis of clinical samples, such as FFPE tissues, in particular to assist clinicians in predicting the clinical course of monoclonal anti-EGFR antibody treatment of mCRC patients.
Acknowledgments
The authors would like to thank Dr. Chupong Ittiwut and Dr. Rungnapa Ittiwut (Center of excellence in medical genetics, Chulalongkorn University) for their advice about pyrosequencing techniques. We would like to thank Department of Medical Services, Institute of Pathology, Ministry of Public Health for providing CRC tissue specimens and the pyrosequencing instrument. We would like to express our gratitude to Prof. Dr. Kathleen L. McCoy (Department of Microbiology and Immunology, School of Medicine, Virginia Commonwealth University), who was financially supported by the grant for foreign academics and researchers to increase published articles in international journals through the Chulalongkorn University Ratchadaphiseksomphot Endowment Fund 2015 for her critical reading of this manuscript. We also would like to acknowledge Assoc. Prof. Dr. Pornpimol Rongnoparut for proofreading assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
SS was supported by the 90th anniversary of Chulalongkorn University fund (Ratchadaphiseksomphot Endowment Fund, GCUGR1125572134), and NC was supported by the Faculty of Allied Health Sciences Fund 2014 (AHS-CU 58004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2): 104–117. 10.3322/caac.21220 [DOI] [PubMed] [Google Scholar]
- 2.Sriamporn S, Wiangnon S, Suwanrungruang K, Rungsrikaji D, Sukprasert A, Thipsuntornsak N, et al. Risk factors for colorectal cancer in northeast Thailand: lifestyle related. Asian Pac J Cancer Prev. 2007;8(4): 573–577. [PubMed] [Google Scholar]
- 3.Sangrajrang S, Chokvanitphong V, Sumetchotimaytha W, Khuhaprema T. Evaluation of health status of a population underwent routine medical check up at the high risk screening clinic in National Cancer Institute. Asian Pac J Cancer Prev. 2012;13(11): 5759–5762. [DOI] [PubMed] [Google Scholar]
- 4.Dolatkhah R, Somi MH, Bonyadi MJ, Asvadi Kermani I, Farassati F, Dastgiri S. Colorectal cancer in iran: molecular epidemiology and screening strategies. J Cancer Epidemiol. 2015;2015: 643020 10.1155/2015/643020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosman F, Yan P. Molecular pathology of colorectal cancer. Pol J Pathol. 2014;65(4): 257–266. [DOI] [PubMed] [Google Scholar]
- 6.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11): 1160–1174. 10.1056/NEJMra0707704 [DOI] [PubMed] [Google Scholar]
- 7.Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8): 3992–3995. [DOI] [PubMed] [Google Scholar]
- 8.Bos JL. p21ras: An oncoprotein functioning in growth factor-induced signal transduction. Eur J Cancer. 1995;31A(7–8): 1051–1054. [DOI] [PubMed] [Google Scholar]
- 9.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377(9783): 2103–2114. 10.1016/S0140-6736(11)60613-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Yang H, Shen Y, Wang S, Lin D, Ma L, et al. Direct sequencing is a reliable assay with good clinical applicability for KRAS mutation testing in colorectal cancer. Cancer Biomark. 2013;13(2): 89–97. 10.3233/CBM-130334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amicarelli G, Shehi E, Makrigiorgos GM, Adlerstein D. FLAG assay as a novel method for real-time signal generation during PCR: application to detection and genotyping of KRAS codon 12 mutations. Nucleic Acids Res. 2007;35(19): e131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27(12): 2091–2096. 10.1200/JCO.2009.21.9170 [DOI] [PubMed] [Google Scholar]
- 13.Van Cutsem E, Oliveira J, Group EGW. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4: 61–63. 10.1093/annonc/mdp130 [DOI] [PubMed] [Google Scholar]
- 14.Tsiatis AC, Norris-Kirby A, Rich RG, Hafez MJ, Gocke CD, Eshleman JR, et al. Comparison of Sanger Sequencing, pyrosequencing, and melting curve analysis for the Detection of KRAS Mutations: diagnostic and clinical implications. J Mol Diagn. 2010;12(4): 425–432. 10.2353/jmoldx.2010.090188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox JC, England J, White P, Ellison G, Callaghan K, Charlesworth NR, et al. The detection of K-ras mutations in colorectal cancer using the amplification-refractory mutation system. Br J Cancer. 1998;77(8): 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogasawara N, Bando H, Kawamoto Y, Yoshino T, Tsuchihara K, Ohtsu A, et al. Feasibility and robustness of amplification refractory mutation system (ARMS)-based KRAS testing using clinically available formalin-fixed, paraffin-embedded samples of colorectal cancers. Jpn J Clinical Oncol. 2011;41(1): 52–56. [DOI] [PubMed] [Google Scholar]
- 17.Poehlmann A, Kuester D, Meyer F, Lippert H, Roessner A, Schneider-Stock R. K-ras mutation detection in colorectal cancer using the Pyrosequencing technique. Pathol Res Pract. 2007;203(7): 489–497. [DOI] [PubMed] [Google Scholar]
- 18.Ogino S, Kawasaki T, Brahmandam M, Yan L, Cantor M, Namgyal C, et al. Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diagn. 2005;7(3): 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu P, Liang H, Xue L, Yang C, Liu Y, Zhou K, et al. Potential clinical significance of plasma-based KRAS mutation analysis using the COLD-PCR/TaqMan(®) -MGB probe genotyping method. Exp Ther Med. 2012;4(1): 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang YS, Er TK, Lu HC, Yeh KT, Chang JG. Detection of KRAS codon 12 and 13 mutations by mutant-enriched PCR assay. Clin Chim Acta. 2014;436: 169–175. 10.1016/j.cca.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 21.Taly V, Pekin D, Benhaim L, Kotsopoulos SK, Le Corre D, Li X, et al. Multiplex picodroplet digital PCR to detect KRAS mutations in circulating DNA from the plasma of colorectal cancer patients. Clin Chem. 2013;59(12): 1722–1731. 10.1373/clinchem.2013.206359 [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Brophy VH, Cao J, Velez M, Hoeppner C, Soviero S, et al. Analytical performance of a PCR assay for the detection of KRAS mutations (codons 12/13 and 61) in formalin-fixed paraffin-embedded tissue samples of colorectal carcinoma. Virchows Arch. 2012;460(2): 141–149. 10.1007/s00428-011-1180-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakai K, Yoneshige A, Ito A, Ueda Y, Kondo S, Nobumasa H, et al. Performance of a novel KRAS mutation assay for formalin-fixed paraffin embedded tissues of colorectal cancer. Springerplus. 2015;4: 7 10.1186/2193-1801-4-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Macedo MP, de Melo FM, Lisboa BC, Andrade LD, de Souza Begnami MD, Junior SA, et al. KRAS gene mutation in a series of unselected colorectal carcinoma patients with prognostic morphological correlations: a pyrosequencing method improved by nested PCR. Exp Mol Pathol. 2015;98(3): 563–567. 10.1016/j.yexmp.2015.03.038 [DOI] [PubMed] [Google Scholar]
- 25.Shi X, Zhang C, Shi M, Yang M, Zhang Y, Wang J, et al. Development of a single multiplex amplification refractory mutation system PCR for the detection of rifampin-resistant Mycobacterium tuberculosis. Gene. 2013;530(1): 95–99. 10.1016/j.gene.2013.07.060 [DOI] [PubMed] [Google Scholar]
- 26.Gaudet M, Fara AG, Beritognolo I, Sabatti M. Allele-specific PCR in SNP genotyping. Methods Mol Biol. 2009;578: 415–424. 10.1007/978-1-60327-411-1_26 [DOI] [PubMed] [Google Scholar]
- 27.Little S. Amplification-refractory mutation system (ARMS) analysis of point mutations. Curr Protoc Hum Genet. 2001; Chapter 9: Unit 9.8. [DOI] [PubMed] [Google Scholar]
- 28.Bader T, Ismail A. Higher prevalence of KRAS mutations in colorectal cancer in Saudi Arabia: Propensity for lung metastasis. Alexandria J Med. 2014;50(3): 203–209. [Google Scholar]
- 29.Brink M, de Goeij AF, Weijenberg MP, Roemen GM, Lentjes MH, Pachen MM, et al. K-ras oncogene mutations in sporadic colorectal cancer in The Netherlands Cohort Study. Carcinogenesis. 2003;24(4): 703–710. [DOI] [PubMed] [Google Scholar]
- 30.Miglio U, Mezzapelle R, Paganotti A, Allegrini S, Veggiani C, Antona J, et al. Mutation analysis of KRAS in primary colorectal cancer and matched metastases by means of highly sensitivity molecular assay. Pathol Res Pract. 2013;209(4): 233–236. 10.1016/j.prp.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 31.Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72(10): 2457–2467. 10.1158/0008-5472.CAN-11-2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6(9): 519–527. 10.1038/nrclinonc.2009.111 [DOI] [PubMed] [Google Scholar]
- 33.Licar A, Cerkovnik P, Ocvirk J, Novakovic S. KRAS mutations in Slovene patients with colorectal cancer: frequency, distribution and correlation with the response to treatment. Int J Oncol. 2010;36(5):1137–1144. [DOI] [PubMed] [Google Scholar]
- 34.Barbacid M. ras genes. Annu Rev Biochem. 1987;56: 779–827. [DOI] [PubMed] [Google Scholar]
- 35.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49(17): 4682–4689. [PubMed] [Google Scholar]
- 36.Span M, Moerkerk PT, De Goeij AF, Arends JW. A detailed analysis of K-ras point mutations in relation to tumor progression and survival in colorectal cancer patients. Int J Cancer. 1996;69(3): 241–245. [DOI] [PubMed] [Google Scholar]
- 37.Villa E, Dugani A, Rebecchi AM, Vignoli A, Grottola A, Buttafoco P, et al. Identification of subjects at risk for colorectal carcinoma through a test based on K-ras determination in the stool. Gastroenterology. 1996;110(5): 1346–1353. [DOI] [PubMed] [Google Scholar]
- 38.Gao J, Li YY, Sun PN, Shen L. Comparative analysis of dideoxy sequencing, the KRAS StripAssay and pyrosequencing for detection of KRAS mutation. World J Gastroenterol. 2010;16(38): 4858–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karim B, Florence C, Kamel R, Nadia K, Ines O, Raja M, et al. KRAS mutation detection in Tunisian sporadic coloractal cancer patients with direct sequencing, high resolution melting and denaturating high performance liquid chromatography. Cancer Biomark. 2010;8(6): 331–340. 10.3233/CBM-2011-0222 [DOI] [PubMed] [Google Scholar]
- 40.Herreros-Villanueva M, Chen CC, Yuan SS, Liu TC, Er TK. KRAS mutations: analytical considerations. Clin Chim Acta. 2014; 431:211–220. 10.1016/j.cca.2014.01.049 [DOI] [PubMed] [Google Scholar]
- 41.Kamphee H, Chaiprasert A, Prammananan T, Wiriyachaiporn N, Kanchanatavee A, Dharakul T. Rapid Molecular Detection of Multidrug-Resistant Tuberculosis by PCR-Nucleic Acid Lateral Flow Immunoassay. PloS One. 2015;10(9): e0137791 10.1371/journal.pone.0137791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liou JM, Wu MS, Shun CT, Chiu HM, Chen MJ, Chen CC, et al. Mutations in BRAF correlate with poor survival of colorectal cancers in Chinese population. Int J Colorectal Dis. 2011;26(11): 1387–1395. 10.1007/s00384-011-1229-1 [DOI] [PubMed] [Google Scholar]
- 43.Zhang C, Liu Y, Ring BZ, Nie K, Yang M, Wang M, et al. A novel multiplex tetra-primer ARMS-PCR for the simultaneous genotyping of six single nucleotide polymorphisms associated with female cancers. PloS One. 2013;8(4): e62126 10.1371/journal.pone.0062126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dietrich D, Uhl B, Sailer V, Holmes EE, Jung M, Meller S, et al. Improved PCR performance using template DNA from formalin-fixed and paraffin-embedded tissues by overcoming PCR inhibition. PloS One. 2013;8(10): e77771 10.1371/journal.pone.0077771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludyga N, Grunwald B, Azimzadeh O, Englert S, Hofler H, Tapio S, et al. Nucleic acids from long-term preserved FFPE tissues are suitable for downstream analyses. Virchows Arch. 2012;460(2): 131–140. 10.1007/s00428-011-1184-9 [DOI] [PubMed] [Google Scholar]
- 46.Potluri K, Mahas A, Kent MN, Naik S, Markey M. Genomic DNA extraction methods using formalin-fixed paraffin-embedded tissue. Anal Biochem. 2015; 486: 17–23. 10.1016/j.ab.2015.06.029 [DOI] [PubMed] [Google Scholar]
- 47.Janecka A, Adamczyk A, Gasinska A. Comparison of eight commercially available kits for DNA extraction from formalin-fixed paraffin-embedded tissues. Anal Biochem. 2015; 476: 8–10. 10.1016/j.ab.2015.01.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.