Abstract
The diverse host range, high transmissibility, and rapid evolution of influenza A viruses justify the importance of containing pathogenic viruses studied in the laboratory. Other than physically or mechanically changing influenza A virus containment procedures, modifying the virus to only replicate for a single round of infection similarly ensures safety and consequently decreases the level of biosafety containment required to study highly pathogenic members in the virus family. This biological containment is more ideal because it is less apt to computer, machine, or human error. With many necessary proteins that can be deleted, generation of single-cycle infectious influenza A viruses (sciIAV) can be achieved using a variety of approaches. Here, we review the recent burst in sciIAV generation and summarize the applications and findings on this important human pathogen using biocontained viral mimics.
Keywords: Reporter virus, viral vectors, single-cycle virus, influenza vaccine, influenza HA-expressing MDCK cells (MDCK-HA), reverse genetics
1. Introduction
1.1 Influenza viruses as important human pathogens
Influenza virus infections cause both seasonal epidemics and occasional pandemics and remain an enormous clinical and public health problem worldwide. The financial burden in the United States (US) averages more than 87 billion dollars annually, due to prophylactic and therapeutic costs, hospital costs, and missed school or work days (Gasparini et al., 2012; Keech and Beardsworth, 2008; Molinari et al., 2007; Paul Glezen et al., 2013). Influenza viruses belong to the family Orthomyxoviridae of which there are six genera, or types: Influenza virus A, Influenza virus B, Influenza virus C, Isavirus, Quaranjavirus and Thogotovirus (International Committee on Taxonomy of Viruses. and King, 2012; Shaw and Palese, 2013). Type A and B influenza viruses infect humans regularly, however, only type A is believed to possess pandemic potential. The ability to cause pandemics is due to the natural host reservoir of influenza A virus (IAV), wild aquatic waterfowl, and the host’s ability to reassort divergent influenza A strains into novel pandemic viruses (Glezen, 1996; Taubenberger and Morens, 2009; Webster et al., 1992). IAV is further classified into different subtypes based on the antigenic major surface glycoproteins: hemagglutinin (HA; 18 subtypes) and neuraminidase (NA; 11 subtypes) (Shaw and Palese, 2013; Tong et al., 2012; Tong et al., 2013). Influenza virus infections are primarily spread by person-to-person transmission via aerosolized droplets. Additionally, zoonoses can occur, as evidenced by the current outbreaks of H7N9 and H5N1, which may contain pandemic potential if acquiring the ability to readily transmit between humans (Uyeki and Cox, 2013; Yen and Webster, 2009). In the 20th century, three documented IAV pandemics have occurred: the Spanish flu (H1N1) of 1918, the Asian flu (H2N2) of 1957, and the Hong Kong flu (H3N2) of 1968 (Kilbourne, 2006; Knipe and Howley, 2013). Of these three, the Spanish flu (1918) was especially fatal and was associated with approximately 50 million deaths worldwide (Taubenberger and Morens, 2006). The first influenza pandemic of the 21st century was declared in 2009 after the emergence of a quadruple-reassortant swine-origin H1N1 virus (Smith et al., 2009) that in less than one year infected more than 600,000 individuals worldwide, causing nearly 16,000 deaths in over 200 countries (CDC, 2009; WHO, 2010).
Current available options to counter the respiratory system attack by influenza viruses include both vaccines and antivirals. Vaccines, due to the induction of sterilizing immunity, are the primary means to prevent infection. However, the three types of vaccines available (inactivated influenza vaccine, IIV; live attenuated influenza vaccine, LAIV; and recombinant influenza vaccine, RIV) have moderate efficacy that changes seasonally due to constant viral evolution (Carrat and Flahault, 2007). Recently, the Advisory Committee on Immunization Practices (ACIP) recommended that each vaccine should be available in quadrivalent formulations, containing two strains of type A (H1N1 and H3N2) and two lineages of type B (Victoria and Yamagata) influenza viruses (Grohskopf et al., 2014). Options to control influenza after infection are limited to two classes of US Food and Drug Administration (FDA)-approved antivirals, targeting either the viral matrix 2 (M2) ion channel (amantadine, rimantidine) (Hay et al., 1985) and subsequently inhibiting viral entry, or the sialidase active site of NA (oseltamivir, zanamivir) (Gubareva et al., 2000) and inhibiting virus release. Similar to the challenges of vaccination, viral mutations can arise that render influenza antivirals ineffective, like in the case of M2 blockers, which are no longer recommended against circulating strains (Pizzorno et al., 2011). Despite our best efforts to prevent (vaccines) and treat (antivirals) infections, influenza annually accounts for upwards of approximately 49,000 deaths in the US and 500,000 worldwide (CDC, 2010; WHO, 2014b). Improved strategies preventing the morbidity and mortality associated with influenza virus infections are warranted, and studies aimed to better understand the biology of and develop new countermeasures against this important human pathogen are desperately needed.
1.2 Influenza virus biology
Influenza viruses contain a segmented single-stranded RNA genome of negative polarity that is packed within spherical or filamentous, lipid-enveloped particles (Shaw and Palese, 2013). The eight RNA segments of IAV encode at least 12 proteins (Table 1), using alternative splicing (nuclear export protein, NEP; and M2), alternative open reading frames (PB1-F2) (Chen et al., 2001), or ribosomal frame-shifting (PA-X) (Jagger et al., 2012) mechanisms.
Table 1.
Influenza A virus proteins and their functions
| Segment | Primary transcript | Secondary transcript | ||||
|---|---|---|---|---|---|---|
| N0 | Segment length | 3′/5′ ψ | Protein | Function | Protein | Function |
| 1 | 2,341 | 30/120 | PB2 | Component of viral polymerase complex. Host cell capped mRNA recognition and binding. | ||
| 2 | 2,341 | 60/120 | PB1 | Component of viral polymerase complex. RNA-dependent RNA polymerase. Endonuclease activity. | PB1-F2 | Induces cell death. |
| 3 | 2,233 | 12/21 | PA | Component of viral polymerase complex. Involved in cap-snatching. | PA-X | Repression of cellular host gene expression. |
| 4 | 1,775 | 45/80 | HA | Glycosylated surface protein. Binding to cellular receptor. Major antigenic determinant. | ||
| 5 | 1,565 | 60/120 | NP | Nucleoprotein. Encapsidation of the RNA segments to form the viral Ribonucleoprotein complexes. | ||
| 6 | 1,409 | 183/157 | NA | Glycosylated surface protein. Neuraminidase activity to release newly made virus from infected cells. | ||
| 7 | 1,027 | 222/220 | M1 | Matrix protein 1. Mediate viral encapsidation. Involved in export of vRNPs from the nucleus. | M2 | Matrix protein 2. Ion channel. |
| 8 | 890 | 35/35 | NS1 | Non-structural protein 1. Inhibits the host interferon response. | NEP | Mediates nuclear export of vRNPs. |
N0, influenza virus segment number.
Ψ, packaging signals: numbers represent nucleotide positions in the negative sense (obtained from www.fludb.org).
Infection with IAV begins when HA binds to its cellular receptor, sialic acid (SA)-linked glycoproteins containing SA in alpha 2,3 or alpha 2,6 linkages (Skehel and Wiley, 2000). Following endocytosis, the viral HA undergoes a conformational change, exposing a fusion peptide that inserts into the endosomal membrane and facilitating virion-endosome fusion (Harrison, 2015; Skehel and Wiley, 2000). Next, the proton-conducting M2 ion channel promotes the cation-mediated release of the viral ribonucleoprotein (vRNP) complexes from the virion core to the cell cytoplasm (Pinto et al., 1992; Wise et al., 2012). The vRNPs then translocate to the nucleus, where viral replication and transcription takes place, a unique mechanism compared to other negative-sense, single-stranded, RNA viruses (Lamb and Choppin, 1983).
Influenza negative-sense viral RNA (vRNA) is not recognized by host cell polymerases, thus viral genome replication and transcription requires the virus-encoded RNA-dependent RNA polymerase (RdRp) (Neumann et al., 2004). The influenza RdRp is a trimeric complex consisting of polymerase acidic (PA) and basic 1 and 2 (PB1, PB2) proteins that together with nucleoprotein (NP) are the minimal components for viral replication and transcription (Huang et al., 1990). The IAV vRNA segments are flanked at both termini by non-coding regions (NCRs) that serve as promoters to initiate transcription by the viral polymerase (Flick et al., 1996; Fodor et al., 1995). To coordinate movement of newly synthesized vRNPs from the nucleus to the site of viral release at the plasma membrane of infected cells, NEP and M1 are required, though the mechanism by which eight unique vRNAs are packaged into each budding virion is not completely understood, and is discussed further below (Hutchinson et al., 2010; Rossman and Lamb, 2011). Once assembled at the cellular membrane, nascent viral particles are released from infected cells by the receptor-destroying enzymatic activity of NA (Varghese et al., 1992).
The segmented genome of IAV provides an evolutionary advantage granting the ability to reassort or exchange homologous gene segments between different influenza strains of the same type. Reassortment can allow for co-circulating H1N1 and H3N2 strains to explore fitness landscapes, which can additionally result in antigenic shift (Laver and Webster, 1979; Rambaut et al., 2008). In fact, the emergence of pandemic IAV strains often is the result of reassortment events, as occurred with the quadruple- reassortant swine-origin H1N1 virus in 2009 (Garten et al., 2009). In addition to antigenic shift, influenza viruses can accumulate mutations within antigenic sites of the viral glycoproteins (antigenic drift) (Carrat and Flahault, 2007; Laver et al., 1979; Sandbulte et al., 2011), leading to resistance against existing antivirals or neutralizing antibodies (NAb) that were generated against the original strain (de Jong et al., 2005; Hurt et al., 2009; Krammer and Palese, 2015; Monto et al., 2006).
1.3 Decreasing the biosafety level of containment is beneficial for influenza virus research
Highly virulent IAV have the potential to pose a greater threat to human health than many other pathogens classified as biosafety level (BSL)-3 and BSL-4 agents, due to efficient transmission and limited antiviral therapeutic options (Sandbulte et al., 2011). While efficient human infection of highly pathogenic avian IAV H5N1 is rare, the case fatality rate is high (Fiebig et al., 2011; Horimoto and Kawaoka, 2001; WHO, 2014a). Recent laboratory studies suggest that although transmission of wild isolates is limited, adaptation of avian influenza (H5N1) to acquire aerosol transmissibility can be achieved in ferrets and that just a few mutations in HA are required (Herfst et al., 2012; Imai et al., 2012). The 2013 outbreak of H7N9 influenza in China, responsible for at least 571 human infections and 212 deaths, has reaffirmed the importance of surveillance against these threats (WHO, 2015). Therefore, when novel IAV are introduced into humans, rapid analysis of their virulence would be beneficial to better determine public health impact and the immediacy of implementing countermeasures. Concomitantly, screening current antiviral options against newly emerged viruses, while evaluating novel antivirals for prophylaxis against circulating influenza viruses, is of great importance (Hayden and de Jong, 2011).
Repeat infection with antigenically identical influenza viruses is rare in immunocompetent humans because prior exposure can result in the generation of NAbs. Most NAbs target IAV HA and prevent receptor binding or fusion. Common serologic assays to detect IAV Abs or NAbs targeting the viral HA are hemagglutination inhibition (HI) or virus microneutralization (MN) assays (2003; de Jong et al., 2003; Rowe et al., 1999; WHO, 2011). These assays have traditionally necessitated the manipulation of live-replicating viruses, though inactivated viruses can be used for HI (WHO, 2011). These assays can be used to evaluate potential antivirals that target HA receptor binding, such as HI, or steps in the viral life cycle, such as MN. The HI assay is problematic for detecting NAbs against avian influenza HA due to difficulty with interpreting results, as was found with avian H5 (Beare and Webster, 1991; Rowe et al., 1999; Stephenson et al., 2003). Some improvement in interpreting HI results can be achieved by using horse erythrocytes (Stephenson et al., 2003; WHO, 2013). New methods that overcome the safety concerns that accompany using live forms of IAV are therefore warranted. New approaches should be at least as sensitive as current available techniques while decreasing biosafety concerns associated with highly pathogenic IAV.
1.4 Single-cycle infectious viruses
Single-cycle viruses are defective for one or more essential functions that can be involved in viral genome synthesis, assembly and release of viral particles, or re- infection of new host cells (Dudek and Knipe, 2006). These defective viruses are typically generated in complementing cell lines that provide the missing gene product or its function in trans. While permissive infection of normal cells is possible, no infectious viral progeny will be produced. Genetically abrogating one or more steps in the virus life cycle makes these defective viruses very safe, highlighting their potential to serve as effective laboratory tools. Single-cycle virus technology has been broadly used with IAV and also with other viral pathogens (Evans et al., 2005; Gao and Palese, 2009; Hai et al., 2011; Jia et al., 2009; Rodrigo et al., 2011; Suzuki et al., 2009), which we will not discuss in this review. The continued use and improvement of single-cycle viruses is evidence of their utility in laboratory settings and as potential vaccine candidates.
High-throughput screening (HTS) has been performed using single-cycle viruses by including a reporter gene as a valid surrogate of viral infection (Beyleveld et al., 2013; Hertzberg and Pope, 2000; Rodrigo et al., 2011). For example, screening drugs to identify antivirals, and investigating cellular host protein interactions important for viral replication have also been accomplished utilizing single-cycle reporter-expressing viruses (Beyleveld et al., 2013). Single-cycle viruses have also proven useful to evaluate virus-host interactions and host immune responses during a single round of infection (Hirsch, 2010; Konig et al., 2010). Moreover, since blocking one or more steps in the viral life cycle makes these viruses replication defective, they have the potential to serve as safe vaccine candidates or vaccine vectors, which may prove to be advantageous over classical viral immunization approaches (Dudek and Knipe, 2006). Lastly, single-cycle infectious viruses can also serve as novel delivery vectors modified to express cellular modulators like interleukins or small non-coding RNAs (Chua et al., 2013; Dudek and Knipe, 2006).
2. Single-cycle infectious influenza A viruses (sciIAV)
2.1 Influenza plasmid-based reverse genetics techniques
Plasmid-based reverse genetics allows for the simultaneous expression of the IAV RdRp and negative-stranded genome in transiently transfected mammalian cells, which together generate de novo, or rescue, recombinant IAVs (Shaw and Palese, 2013). Luytjes et al, described a system that allowed the use of recombinant DNA technology to modify the genome of IAV and to engineer vectors for the expression of foreign genes (Luytjes et al., 1989). Recombinant RNA was expressed from a plasmid, and transfected together with purified IAV polymerase proteins in the presence of a helper virus. Then the recombinant RNA was expressed and packaged into virus particles.
Originally established in 1999, IAV reverse genetics that do not rely on “helper viruses” have revolutionized the influenza research field (Fodor et al., 1999; Neumann et al., 1999), leading to advances in virus biology, vaccines, and therapeutics (Neumann and Kawaoka, 2002; Neumann et al., 2002; Ye et al., 2014). The initial description of IAV reverse genetics required the use of 12 plasmids to rescue recombinant influenza viruses: four protein expression plasmids for vRNP reconstitution plus eight vRNA expression plasmids for the eight genomic vRNA segments. However, it was later described that only eight plasmids were needed, where an ambisense coding strategy generates both polymerase I-driven vRNA transcription and polymerase II-driven mRNA transcription from the same plasmid (Hoffmann et al., 2000a; Hoffmann et al., 2000b).
2.2 Importance of packaging signals for the development of sciIAV
The vRNA segments of IAV share a common organization: a long central coding region, sometimes encoding more than one major transcript (Table 1), flanked by relatively short NCRs (Shaw and Palese, 2013). This genome segmentation confers evolutionary advantages, but also poses a problem for the virus. Genetic evidence indicates that IAV virions normally incorporate exactly eight vRNAs, and at least one copy of each of the eight viral vRNAs must be packaged within a single nascent virion to create productively infectious viral progeny (Chou et al., 2012; Gavazzi et al., 2013b; Hutchinson et al., 2010). If a packaging network exists, selective incorporation of the eight vRNAs requires a minimum of seven inter-segment interactions that could, in principle, be mediated by RNA-binding proteins that interconnect two vRNA segments. However, to date, no such cellular or viral protein has been identified, leaving direct vRNA-vRNA interactions within defined vRNA packaging sequences as an alternative hypothesis supported by in vitro observations (Essere et al., 2013; Fournier et al., 2012a; Fournier et al., 2012b; Gavazzi et al., 2013a). Electron microscopy and electron cryotomography of various IAV strains revealed that seven vRNAs are organized around a central segment forming a star-like structure (Essere et al., 2013; Fournier et al., 2012a; Fournier et al., 2012b; Gavazzi et al., 2013a). Surprisingly, evidence suggests that this interaction network, and more specifically, the regions and domains of the vRNAs involved in specific intermolecular interactions is not conserved between H3N2 and H5N2 IAV, suggesting that different vRNA interactions may drive selective packaging across IAV subtypes (Fournier et al., 2012a; Gavazzi et al., 2013a). These studies support previous evidence that conserved codons are important for vRNA packaging in H1N1 IAVs A/WSN/33 (WSN) but not in A/Puerto Rico/8/34 (PR8), indicating that some packaging signals are not conserved even among IAVs of the same subtype (Gog et al., 2007; Marsh et al., 2008).
To generate single-cycle infectious influenza A virus (sciIAV), an essential viral gene segment is removed, which then needs to be provided in trans to support viral propagation. However, early attempts to generate seven-segmented IAVs yielded phenotypes with reduced replicative fitness, even when the protein encoded by the deleted gene was provided in trans (Marsh et al., 2007). These findings suggested that an intact vRNA packaging network may be important for optimal virus fitness. Engineered IAV segments containing the 3′ and 5′ NCRs were replicated and transcribed by the viral RdRp but were not incorporated during budding to reconstitute an eight-segmented virus (Luytjes et al., 1989; Noda and Kawaoka, 2010). Incorporation of foreign gene segments into influenza virions would therefore need to hijack the proposed packaging signals.
Reverse genetics helped to identify packaging signals by flanking reporter transcripts with varying lengths of IAV vRNA segments. Packaging signals were first determined for NA (Fujii et al., 2003) by engineering constructs encoding green fluorescent protein (GFP) flanked by the 3′ NCR and 183 nucleotides (nt) of the 3′ NA; and the 5′ NCR and 157 nt of the 5′ NA, which were successfully incorporated into budding virions. Similar approaches corroborated the existence of packaging signals for the 7 remaining IAV vRNA segments (Dos Santos Afonso et al., 2005; Fujii et al., 2005; Fujii et al., 2009; Fujii et al., 2003; Gog et al., 2007; Hutchinson et al., 2010; Liang et al., 2005; Marsh et al., 2007; Marsh et al., 2008; Muramoto et al., 2006; Watanabe et al., 2003) (Table 1). Defective interfering (DI) particles generated from vRNA segments during IAV replication gave further evidence of the presence of segment-specific packaging signals within approximately 100 to 300 nt from each end of the vRNAs (Hutchinson et al., 2010). Sequence analysis further supported this hypothesis by determining that the most conserved codons are found to accumulate at the termini of each viral segment (Gog et al., 2007; Hutchinson et al., 2008; Marsh et al., 2008). Using the incorporation of GFP flanked by varying lengths of vRNA termini, it was found that not all vRNA segments have equivalent proportions of their length dedicated to virion packaging (Table 1). In these studies, the packaging signals were mapped within either PR8 or WSN by providing the deleted gene, or function thereof, in trans (Fujii et al., 2009; Fujii et al., 2003; Gao et al., 2012; Hutchinson et al., 2010; Liang et al., 2005; Marsh et al., 2007; Muramoto et al., 2006; Ozawa et al., 2009). However, the packaging signal domains were not completely conserved between these two H1N1 strains, and were even more disparate between different IAV subtypes.
Nucleotide sequences at IAV segment termini have been recently suggested to have two functions, one to control an individual segment’s incorporation (packaging signal), and another to orchestrate an entire eight-segmented packaging event (bundling signal) (Goto et al., 2013). Specific vRNA-vRNA interactions have been mapped to these same terminal locations, via in vitro transcribed vRNA plus native agarose gel electrophoresis, suggesting a role for these interactions in packaging (Fournier et al., 2012a; Fournier et al., 2012b; Gavazzi et al., 2013a) and potentially serving as reassortment barriers between IAV subtypes (Essere et al., 2013). Furthermore, incompatible packaging signals between IAV and influenza B virus (IBV) are likely to be the bottleneck that prevents intertypic reassortment (Baker et al., 2014). Whether or not similar packaging signals in IBV are present in each vRNA segment, and whether the organization of the eight vRNA segments is similar to that found in IAV, remains to be evaluated.
2.3 Generation of protein-expressing cell lines
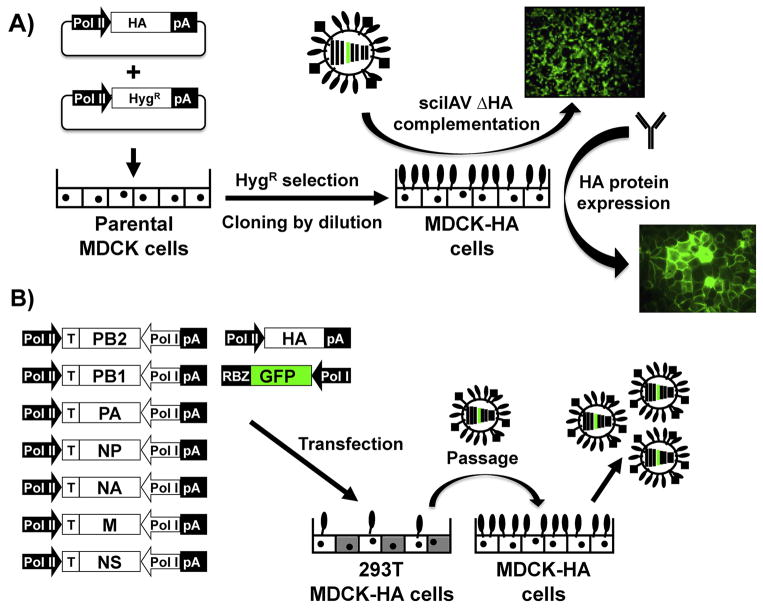
Several methods have been described for transfection-based generation of viral protein-expressing cell lines (e.g., calcium phosphate, cationic lipids, and electroporation), allowing for the transient or stable expression of proteins in diverse cell lines. In this review, we will focus on a brief overview of the generation of Madin-Darby canine kidney (MDCK) cell lines that express IAV HA (MDCK-HA) (Fig. 1A). Membrane-bound HA readily complements sciIAV lacking HA (ΔHA) (described in more detail below), and the antigenicity of the HA carried by the cell transfers to the pseudotyped particles produced during budding (Martinez-Sobrido et al., 2010). MDCK-HA cells were generated by transfection with either an HA protein expression plasmid, modified to also encode for a hygromycin resistance gene inserted downstream of an additional IRES element (Marsh et al., 2007), or co-transfected with HA and a hygromycin B resistance vector (Martinez-Sobrido et al., 2010). After transfection, cells are seeded at a low density so that hygromycin-resistant cells can be clonally isolated. To screen for homogenous and functional HA expression between cell clones, the ability to complement sciIAV ΔHA expressing GFP is evaluated at a low multiplicity of infection (MOI), where there are approximately 1,000 cells per infectious particle at the start of the experiment (Fig. 1A). The observable spread of GFP by fluorescence microscopy and concomitant titer increase of infectious or hemagglutinating units (HAU) in tissue culture supernatants qualitatively and quantitatively indicates the functionality of the MDCK-HA cells. To observe the relative quantity and uniformity of HA expression in MDCK-HA clones, immunofluorescence (IFA) or flow cytometry assays that incorporate anti-HA specific Abs are used (Fig. 1A). Analyzing both the percentage and functionality of HA expression in MDCK-HA clones ensures sciIAV ΔHA growth phenotypes that recapitulate wild type (WT) IAV replication kinetics, provided that the heterotypic HA is compatible with the sciIAV backbone. We have described here one method to generate MDCK stable cell lines, but additional methods that are more suitable for various species or cell types may also be used (Bussow, 2015; Lanza et al., 2013). Moreover, transgenic animals expressing complementing IAV proteins in tissues could also be used, similar to those expressing fluorescent proteins in specific cell types (Abe and Fujimori, 2013). For instance, Shih et al. described the generation of transgenic mice expressing the HA from IAV PR8 in different tissues, including lungs (Shih et al., 1997). It is thus feasible to infect these mice with a sciIAV ΔHA, which should be complemented and subsequently propagate in vivo but not be transmissible except to identical transgenic mice.
Figure 1. Generation of stable MDCK-HA cell lines and rescue of single-cycle infectious influenza A virus (sciIAV).
A) Schematic representation for the generation of stable MDCK-HA cells: Polymerase II (Pol II) driven plasmids encoding IAV HA (top) and hygromycin-B resistance (bottom) are co-transfected (ratio 3:1) into parental MDCK cells. After transfection, cells are seeded at low density (cloning dilution) and hygromycin-resistant clones are individually selected. HA-expressing MDCK clones are screened by HA-deficient sciIAV complementation using microscopic analysis of reporter gene (GFP) expression (top), and by HA protein expression using specific Abs by immunofluorescence (right). B) Plasmid-based reverse genetics to generate HA-deficient sciIAV: Ambisense plasmids encoding PB2, PB1, PA, NP, NA, M, NS as well as a Polymerase I (Pol I) driven plasmid encoding the reporter gene (GFP) flanked by the IAV HA NCR and packaging signals and a Pol II driven HA protein expression plasmid are co-transfected into co-cultures of 293T/MDCK-HA cells. Virus containing tissue culture supernatants are subsequently passaged onto MDCK-HA cells for the amplification of the sciIAV ΔHA. pA: polyadenylation signal. RBZ: Hepatitis Delta Virus ribozyme.
2.4 Generation of sciIAV
IAV requires the presence of eight vRNA segments for efficient virus fitness. Therefore, to reconstitute the full genome when generating sciIAV, the modified gene segment should encode for a trans gene flanked by the packaging signals and NCRs of the native segment. For example, sciIAV ΔHA was generated by using GFP flanked by the 3′ NCR and 45 nt of 3′ HA, and the 5′ NCR and 80 nt of 5′ HA (Fig. 1B). Compared with other non-infectious or single-cycle infectious virus vectors, sciIAV allows for the expression of foreign genes with nt lengths that are, at least, similar to those of the segments replaced (Dudek and Knipe, 2006), although the size limit for the foreign sequence has not been empirically defined for each gene segment. This represents an advantage over the generation of replication competent reporter-expressing influenza viruses that are limited by nt length insertion (Nogales et al., 2015; Tran et al., 2013). There are numerous sciIAV that differ in the gene deleted, the foreign sequence introduced, as well as the viral backbone used, which to date has been limited to H1N1 viruses WSN, PR8, A/California/04/2009 (pH1N1), and A/Swine/Saskatchewan/18789/02 (SW02) and H3N2 X31 (Table 2). Whether or not different packaging signals need to be used to generate non-H1N1 sciIAVs remain to be evaluated. And this could be a limitation to generate sciIAV using other backbones or genes deleted. In the last decade, this viral system has experienced a notable increase in use, exemplifying their versatility, ease of production, and utility for multiple in vitro and in vivo applications.
Table 2.
Single-cycle infectious influenza A viruses (sciIAV)
| Gene deleted | IAV backbone(1) | Transgene(2) | Complementation | Application | References |
|---|---|---|---|---|---|
| PB2 | PR8 | GFP or dsRed | Stable cell line | Segment incorporation | Inagaki et al. (2012) |
| PB2 | PR8* | GFP, Rluc, Fluc | Stable cell line | Neutralization assays | Ozawa et al (2011) |
| PB2 | PR8 | GFP | Stable cell line | Influenza vaccine | Victor et al. (2012) |
| PB2 | PR8 | HA from pH1N1 or VN1203 | Stable cell line | Bivalent influenza vaccine | Uraki et al (2013) |
| PB2 | PR8 | HPIV3 HN | Stable cell line | Bivalent vaccine | Kobayashi et al. (2013) |
| PB2 | PR8 | RSV F | Stable cell line | Bivalent vaccine | Fonseca et al. (2014) |
| PB1 | WSN | GFP, mCherry | Stable cell line | Oseltamivir resistance | Bloom et al (2010) |
| PB1 | pH1N1 | GFP | Stable cell line | Oseltamivir resistance | Bloom et al. (2011) |
| PB1 | WSN | GFP | Stable cell line | NA virus attachment | Hooper et al. (2015) |
| PB1 | WSN | GFP | Stable cell line | Oseltamivir resistance | Hooper and Bloom (2013) |
| HA | WSN | GFP, mRFP | Stable cell line | Packaging signals | Marsh et al. (2007) |
| HA | WSN | GFP | Stable cell line | NAb screening | Martinez-Sobrido et al. (2010) |
| HA | pH1N1 | GFP | Stable cell line | Influenza vaccine | Baker et al. (2013) |
| HA | X31 | GFP | Stable cell line | Influenza vaccine | Guo et al. (2014) |
| HA | PR8 | HA | Stable cell line | Influenza vaccine | Powell et al. (2012) |
| HA | pH1N1 | HA | Stable cell line | Influenza vaccine | Katsura et al. (2012) |
| HA | PR8 | PspA | Stable cell line | Bivalent vaccine | Katsura et al. (2014) |
| HA | PR8 | GFP | Stable cell line | HA complementation | Baker et al. (2014) |
| HA | pH1N1 | GFP | Stable cell line | NAb screening | Ramon et al. (2014) |
| HA | WSN | GFP | Stable cell line | Virus entry into bat cells | Poole et al. (2014) |
| HA | WSN | Rluc | Single infection | Genome-wide RNAi screen | Konig et al. (2010) |
| HA | WSN | Rluc | Stable cell line | Antiviral screen | Bottini et al. (2012) |
| HA | PR8 | GFP, miR-124 | Single infection | siRNA delivery | Schmid et al. (2014) |
| HA | PR8 | NEP, scramble | Single infection | Viral vector | Chua et al. (2013) |
| NA | PR8 | GFP | Exogenous NA | Virus attachment | Rimmelzwaan et al. (2007) |
| NA | PR8** | GFP | Exogenous NA | NAb screening | Rimmelzwaan et al. (2011) |
| NA | PR8 | GFP, mCherry | Single infection | Co-infection | Bodewes et al. (2012) |
| NA | PR8 | GFP or dsRed | None | Segment incorporation | Inagaki et al. (2012) |
| NA | WSN | GFP | None | Packaging signals | Fujii et al. (2003) |
| NA | WSN | GFP | None | Influenza vaccine | Shinya et al. (2004) |
| NA | SW02 | HA from SW98 | Exogenous NA | Bivalent influenza vaccine | Masic et al. (2013), Pyo et al. (2014) |
PR8, A/Puerto Rico/8/34 (H1N1); WSN, A/WSN/33 (H1N1); pH1N1, A/California/04/2009 (pH1N1); X31, PR8 internal segments with HA and NA from A/Hong Kong/2/1968 (H3N2); SW02, A/Swine/Saskatchewan/18789/02 (H1N1).
PR8*: HA/NA from PR8, pH1N1 or VN1203: A/Vietnam/1203/04 (H5N1).
PR8**: HA from PR8, Neth178: A/Netherlands/178/95 (H3N2), VN1194: A/Vietnam/1194/04 (H5N1), VN1203, Tur05: A/Turkey/Turkey/1/05 (H5N1), HK97: A/Hong Kong/156/97 (H5N1).
GFP, Green fluorescent protein; DsRed, Discosoma red fluorescent protein; Rluc, Renilla luciferase; Fluc.\, Firefly luciferase; mRFP, monomeric red fluorescent protein; HIPV3 HN, Human parainfluenza virus type 3 hemagglutinin-neuraminidase; RSV F, Respiratory syncytial virus F glycoprotein; PspA, Streptococcus pneumoniae surface protein A; SW98, A/Swine/Texas/4199-2/98 (H3N2).
2.4.1 sciIAV ΔHA
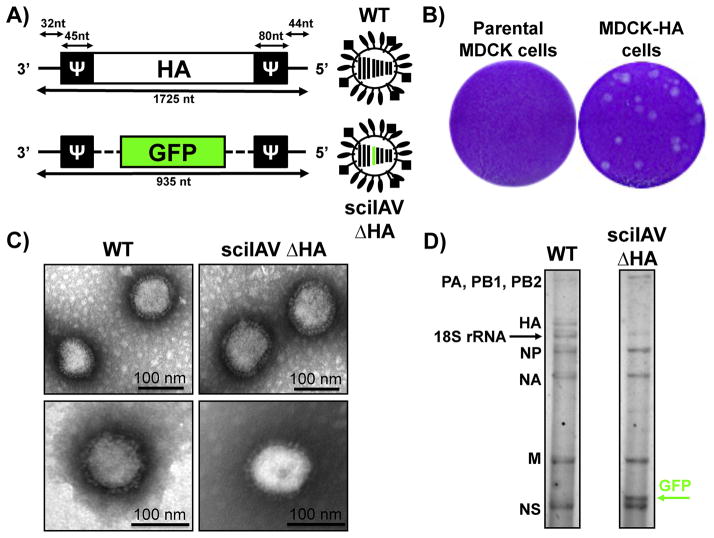
To study IAV assembly and the packaging of the HA segment into infectious virions, Marsh et al. rescued a sciIAV with the WSN or PR8 backbones and without the WT HA vRNA (sciIAV ΔHA) (Marsh et al., 2007). The modified IAV HA vRNA segment incorporated reporter genes, GFP or RFP, flanked by 45 and 80 nt in the 3′ and 5′ end of the vRNA, respectively (Fig. 2A) (Marsh et al., 2007). The packaging signals in the 3′ end differed from the 9 nt required as previously described for WSN (Watanabe et al., 2003). This sciIAV ΔHA could be efficiently passaged in cells constitutively expressing WSN-HA protein; however, and as expected, it could not spread in parental MDCK cells (Fig. 2B). Upon further investigation, it was found that although an IAV containing seven segments could be rescued, the replication was reduced compared to a virus whose missing segment was replaced with a recombinant reporter segment (containing HA packaging signals) that restored the eight-segmented IAV (Marsh et al., 2007). The seven-segmented ΔHA virus resulted in a 40 to 60% reduction in the packaging of the PA, NP, NA, M, and NS vRNAs, as measured by quantitative PCR, and the packaging of these vRNAs was partially restored in the presence of GFP/RFP packaging constructs.
Figure 2. Characterization of sciIAV ΔHA.
A) Schematic representation of the ΔHA/GFP vRNA: Top, wild-type IAV HA vRNA segment. Bottom, HA(45)GFP(80) vRNA. NCR (thin black lines) at each vRNA termini and the IAV HA packaging signals (Ψ) necessary for the incorporation of vRNA into virions. Nucleotide lengths of NCR, packaging signals and vRNAs are shown. B) Plaque morphology of sciIAV ΔHA in parental and MDCK-HA cells: Confluent parental and MDCK-HA cells were infected with ~25 PFU of the sciIAV ΔHA and, at 3 days post-infection, monolayers were fixed and stained with crystal violet. C) Electron micrographs of WT IAV (left) and sciIAV ΔHA (right). Scale bar, 100 nm. D) sciIAV ΔHA RNA composition: vRNAs of purified WT (left) and sciIAV ΔHA (right) virions are indicated. RNAs were separated by electrophoresis on polyacrylamide gels containing 7 M urea and silver stained. The positions of the IAV vRNA segments are indicated. The black arrow indicates 18S ribosomal RNA (18S rRNA). The green arrow indicates the GFP vRNA segment in the sciIAV ΔHA.
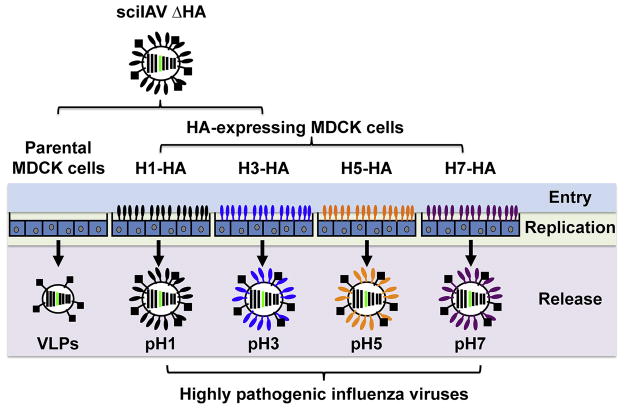
Martínez-Sobrido et al. demonstrated the application of sciIAV ΔHA for the screening of NAbs against H1 or H5 influenza virus, including the highly pathogenic Spanish flu (A/Brevig Mission/18 [H1N1]) and Bird Flu (A/Vietnam/1203/04 [H5N1]) (Martinez-Sobrido et al., 2010). Because the plasma membrane-bound HA from MDCK-HA cells is incorporated into budding sciIAV, progeny virions from infected MDCK-HA (A/Brevig Mission/18 or A/Vietnam/1203/04) bear the antigenicity of the cellular-expressed virus protein. Therefore, sciIAV ΔHA could be HA-pseudotyped with different IAV HAs by propagation in different MDCK-HA cells (Martinez-Sobrido et al., 2010) (Fig. 3). This antigenicity was demonstrated using monoclonal NAbs, and importantly, these neutralization assays could be conducted at BSL-2 conditions, even when evaluating NAbs against highly pathogenic IAVs (Martinez-Sobrido et al., 2010).
Figure 3. Generation of HA-pseudotyped sciIAV.
sciIAV ΔHA can be pseudotyped with various IAV HA proteins by infection of various MDCK-HA cell lines. Infection of parental MDCK cells will result in VLPs released that do not contain HA on the surface. Infection of MDCK-HA cells will produce sciIAV complemented with the cell-derived IAV HA, allowing the generation of HA-pseudotyped sciIAV.
To compare the replication kinetics of sciIAV ΔHA with WT virus, it was first demonstrated that WT virus grew to similar titers in parental and MDCK-HA cells, whereas sciIAV replicated to high titers only in MDCK-HA and failed to replicate in parental MDCK cells (Marsh et al., 2007; Martinez-Sobrido et al., 2010) (Fig. 2B). At the protein level, sciIAV has a similar composition as WT virus, with the exception of the levels of HA that appear to be slightly reduced (Martinez-Sobrido et al., 2010). Electron microscopy analysis of sciIAV and WT virus revealed that the sciIAVs ΔHA have morphology and particle size similar to those of the WT WSN virus (Martinez-Sobrido et al., 2010) (Fig. 2C). Importantly, the vRNA composition of the HA-deficient sciIAV was different than that of WT IAV because the GFP vRNA-like segment was incorporated into viral particles instead of the IAV HA vRNA (Fig. 2D).
Recently, our group has generated GFP-expressing sciIAV ΔHA on the backbone of, pH1N1, X31, and PR8 (Baker et al., 2013; Baker et al., 2014; Guo et al., 2014). The pH1N1 sciIAV ΔHA was used as a proof of concept for the development of safe alternative vaccines to prevent IAV infection. Powell et al. generated pH1N1 and PR8-backbone sciIAV ΔHA by suppressing the HA signal sequence and replacing the original ATG start codon with TAG to suppress translation (Powell et al., 2012). A single base at the end of the signal sequence at nucleotide position 83 was removed to ensure that if the original ATG was reconstituted, it would read out of frame. Additionally, this sciIAV ΔHA contained an inactivated HA cleavage site, and the resulting sciIAV was shown to only propagate in MDCK-HA cells. This recombinant virus lost virulence in mice but could still induce heterotypic protection, which lasted for at least four months post-vaccination. Kawaoka’s group also demonstrated the ability of sciIAV ΔHA to serve as a safe vaccine by mutating the HA cleavage site, so that the HA1 C-terminal arginine residue was replaced with threonine, rendering host cell proteases incapable of cleaving HA1-HA2 and eliminating infectivity of progeny virus (Katsura et al., 2012).
2.4.2 sciIAV ΔNA
IAV NA glycoprotein is required for virus release during budding, and deleting the gene would be expected to limit IAV to a single cycle of infection. Reporter-expressing sciIAVs ΔNA were developed as vaccine candidates (Shinya et al., 2004), to evaluate viral attachment to the host cell (Rimmelzwaan et al., 2007), rate of co-infections (Bodewes et al., 2012), as a tool for NAb screening (Rimmelzwaan et al., 2011), or for inhibition during intertypic infection (Wanitchang et al., 2012). Although recombinant sciIAV lacking NA enzymatic activity has similar growth kinetics as WT virus in the presence of exogenous Vibrio cholerae sialidase, sciIAV ΔNA grows with reduced replication levels compared with WT virus lacking enzyme supplementation, thus it can be considered a pseudo-sciIAV (our unpublished results) (Fujii et al., 2003; Inagaki et al., 2012). An alternative to complement sciIAV ΔNA viruses has been reported, which is based on the use of modified MDCK cells that express reduced levels of sialic acid on the cell surface (Shinya et al., 2004).
2.4.3 sciIAV ΔPB2
SciIAVs can also be generated by deleting the PB2 gene (sciIAV ΔPB2) and providing the missing protein in trans by generating PB2-expressing stable cell lines (Ozawa et al., 2011). Foreign reporter genes (GFP, DsRed, and renilla or firefly luciferases) were introduced between the NCR and packaging signals of the PR8 PB2 segment. The sciIAV ΔPB2 grows in PB2-expressing cells with similar kinetics compared to WT PR8 (Inagaki et al., 2012; Ozawa et al., 2011). Importantly, the stability of the foreign reporter gene is maintained over 5 serial passages. Reporter-expressing sciIAV ΔPB2 that contained heterologous HA and NA genes from pH1N1 were used to screen for NAb reactivity in convalescent ferret sera (Ozawa et al., 2011). The potential of sciIAV ΔPB2 as a vaccine candidate against influenza was also assessed in mice (Victor et al., 2012). Interestingly, Abs against GFP (expressed in place of PB2) were also detected in vaccinated mouse sera, suggesting the possibility of using PB2-deficient sciIAV as vectors to deliver antigens from disparate pathogens. Indeed, this hypothesis was proven correct when three separate reports detailed bivalent protection conferred by sciIAV ΔPB2 viruses that harbored either heterologous IAV HA, human parainfluenza virus type 3 (HPIV-3) hemagglutinin-neuraminidase (HN), or respiratory syncytial virus (RSV) F glycoprotein sequences instead of the PB2 vRNA segment (Fonseca et al., 2014; Kobayashi et al., 2013; Uraki et al., 2013). Besides inducing Abs against the heterologous immunogens, both PB2-deficient sciIAVs retained their protection efficacy against lethal IAV challenge.
2.4.4 sciIAV ΔPB1
To study the fitness of oseltamivir-resistant IAV mutants, Bloom et al. generated a recombinant WSN virus possessing PB1 segments in which most of the coding sequence was replaced by a gene encoding either GFP or mCherry flanked by the PB1 NCRs and packaging signals (Bloom et al., 2010). The PB1 segment was also modified to mutate the ATG start codon of PB1 and destroy the alternative start codon normally leading to PB1-F2 transcription. The WSN sciIAV ΔPB1 grew to high titers in cells constitutively expressing the PB1 protein. The authors used this virus to show that viruses containing the NA H274Y mutation, which alone reduced cell surface expression of NA, evolved compensatory mutations that increased NA surface expression (Bloom et al., 2011). More recently, by utilizing the same sciIAV ΔPB1 platform, the discovery of a new mutation in NA (G147R) was reported that provided a gain-of–function phenotype to the N1-NA protein. The protein acquired the receptor-binding function normally performed by HA (Hooper and Bloom, 2013; Hooper et al., 2015). Interestingly, even though this mutation was generated in the laboratory, it has also been found in several recent H1N1 and H5N1 natural isolates, suggesting that IAV could potentially switch the receptor-binding function between its two viral glycoproteins (Hooper and Bloom, 2013).
3. SciIAV applications
3.1 Identification of neutralizing antibodies (NAbs)
Abs that neutralize IAV typically bind to HA, and NAb induction serves as a surrogate for seroconversion following infection or vaccination in humans (Belshe et al., 2000; He et al., 2015). New interest has arisen in the identification of monoclonal NAbs due to their therapeutic benefit, with either specific or broad (predominantly HA stalk- reactive) spectrum activity against IAV (see (Laursen and Wilson, 2013) for review). Traditional HI and MN or plaque reduction neutralization test (PRNT) assays used to identify NAbs have various advantages and limitations. The HI assay provides a rapid read-out (same day), but sensitivity is limited because of the large amount of virus required to agglutinate erythrocytes (105–106 egg infectious dose50 (Killian, 2008) and the Abs inhibiting the HI does not necessarily neutralize the virus infection. In addition, HI assays are hampered by inconsistencies between interpretation of results across research personnel (Stephenson et al., 2007), an inability to discriminate between non- infectious and infectious viruses in viral preparations, improper sera preparation to deactivate non-specific virus inhibitors, and restriction of only being able to identify Abs that directly interfere with the binding of HA to its SA receptor on red blood cells (Hirst, 1942; Salk, 1944). Comparatively, MN or PRNT is advantageous because of its increased sensitivity over HI (only 100 – 200 egg infectious doses 50% are required; (Rimmelzwaan et al., 1998). However, the assays are disadvantageous with long protocol times, with results taking several days (2–4 days) when evaluating direct cytopathic effect (CPE). However, new MN methods that combine tissue culture cells and antigen detection by enzyme-linked immunosorbent assay (ELISA) can yield results within 2 days and are more sensitive. However, inter-laboratory discrepancies can arise due to variable assay endpoints such as cell staining by IFA, ELISA or HI assays (Stephenson et al., 2007; WHO, 2011).
To overcome limitations, such as biosafety and the requirement of secondary assays, posed by traditional NAb identification approaches, self-limiting, reporter- expressing sciIAVs are a feasible alternative (Fig. 4A). HA-pseudotyped lentivirus particles expressing reporter genes have been experimentally used to evaluate influenza NAb generation (Nefkens et al., 2007). However, HA-pseudotyped lentivirus particles lack IAV internal proteins, and the altered stoichiometry of HA protein on the surface of the lentiviral particles can produce variability in defining NAbs, including stalk- reactive NAbs (Sui et al., 2009; Temperton et al., 2007). As an alternative to the use of WT IAV or HA-pseudotyped lentivirus particles, we and others have used sciIAV in reporter-based MN assays, which (i) can be performed with BSL-2 containment, (ii) are highly sensitive (100 – 200 FFU required), (iii) use a reporter protein to identify infected cells and eliminate the need for secondary assays while providing quantitative results that limit the error of interpretation, and (iv) can be completed within 24 hours (Hooper and Bloom, 2013; Martinez-Sobrido et al., 2010; Ozawa et al., 2011; Ramon et al., 2014; Rimmelzwaan et al., 2011). In addition, sciIAV represents a bonafide IAV and therefore can be inhibited at any step of the viral life cycle, including the use of non-HA NAbs (e.g. NA NAbs).
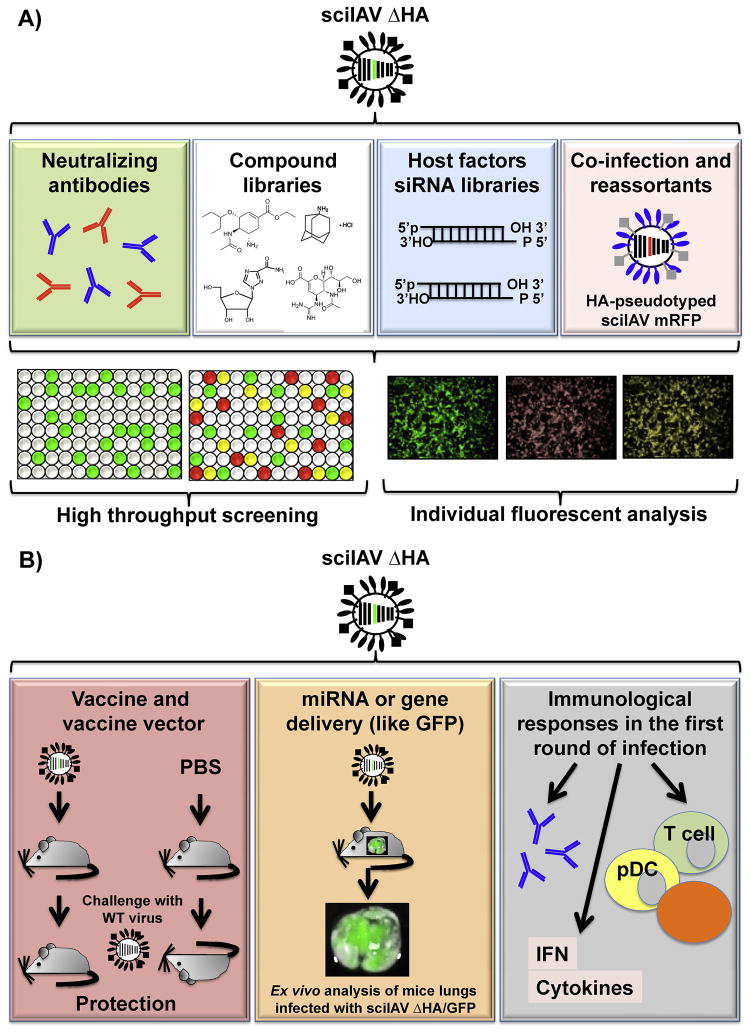
Figure 4. In vitro and in vivo applications of sciIAV.
A) In vitro applications: sciIAV can be used for the screening of influenza neutralizing antibodies (NAbs) and the identification of antiviral compounds. Fluorescent/luminescent assays can also be conducted to investigate host-virus factors important for IAV replication as well as to investigate co-infection and reassortment mechanisms of IAV infection. Color code in 96-well plates indicate single (green and red) or double (yellow) infections with sciIAV expressing green or red fluorescent proteins. B) In vivo applications: sciIAV can be used as vaccines or vaccine vectors to provide protection against subsequent viral lethal challenges, as viral vectors (left) for in vivo delivery of miRNA or genes like GFP (center), and to study the immunological consequences of an IAV single round of infection (right). For more details, see text.
Martínez-Sobrido et al. first described the use of a GFP-expressing sciIAV ΔHA as a viable surrogate to evaluate NAbs against different IAVs, including highly pathogenic IAVs (Martinez-Sobrido et al., 2010). The study demonstrated that both monoclonal NAbs and polyclonal human sera could be evaluated, and showed comparable, and possibly more sensitive, results than traditional HI assays. In further support, Rimmelzwaan et al. used sciIAV ΔNA to evaluate human, rabbit, ferret, sheep and swan sera, and demonstrated that sciIAV ΔNA produces results comparable to the classic MN and HI assay (Rimmelzwaan et al., 2011). Likewise, Ozawa et al. showed that both GFP fluorescence and renilla or firefly luciferase luminescence reporter proteins can be used to quantify sciIAV ΔPB2 infection, and that luminescence signals can be used to identify NAbs from ferret sera with high sensitivity (Ozawa et al., 2011). Additionally, Hooper and Bloom explored the effect of oseltamivir on neutralization of GFP-expressing sciIAV ΔPB1, when HA had reduced affinity for sialic acid linkages and NA could bind receptor (Hooper and Bloom, 2013). Through these four gene-deficient examples, sciIAV use in immunoassays was demonstrated to have distinct advantages for the evaluation of NAbs as compared to the use of replication-competent influenza viruses or pseudotyped lentivirus.
When comparing various sciIAVs for use in NAb screening, sciIAV ΔHA is advantageous because the backbone of the virus only requires one rescue, and the virus can then be pseudotyped with a variety of IAV or IBV HAs by propagation on HA-expressing MDCK cells (Baker et al., 2014; Martinez-Sobrido et al., 2010). The sciIAV ΔHA is also advantageous in that two identical sciIAV varying only in their reporter gene could be quickly pseudotyped with HA to develop flexible multivalent screening assays (Baker et al., 2015). Additionally, the HA cell lines generated could theoretically be used in a virus-free ELISA to determine HA-binding Abs with monoclonal or polyclonal samples (our unpublished results). Drawbacks of using sciIAV ΔHA include nonspecific NAb reactivity to the cell line (MDCK-HA) on which the assay is performed. The Ab can bind to HA expressed on MDCK-HA cells, instead of the viral particles. Since pseudotyped sciIAV is incubated with putative NAbs, and the mixture is then added to MDCK-HA cells to allow for multicycle infection and reporter gene expression, there is the possibility of the binding of the Ab to the HA expressed from the cell line. This can be controlled by using irrelevant MDCK-HA cell substrates for infection or parental MDCK cells, although this would decrease the sensitivity of the assay because a higher MOI is needed to enhance signal intensity (Ozawa et al., 2011). Also, in terms of evaluating NAbs against newly emerging viruses, one must either quickly generate an MDCK-HA cell line or transiently transfect large batches of cells to generate stocks of novel pseudotyped sciIAV ΔHA. Transfection efficiencies may, however, be a limiting factor for pseudotype virus production and increase the variability between the viral stocks produced. Therefore, although the generation of stable cell lines take more time, it is highly recommendable.
Other gene-deficient sciIAVs are also ideal for NAb identification. However, sciIAV ΔNA are somewhat replication competent (Inagaki et al., 2012; Shinya et al., 2004) (our unpublished results), and the stoichiometry of HA on the virion may be inaccurate in the absence of NA. Conversely, sciIAV ΔPB2 or ΔPB1 are limited to a single cycle of replication and have appropriate HA and NA gene expression in infected cells, which should lead to accurate surface glycoprotein stoichiometry. All ΔPB2, ΔPB1, and ΔNA sciIAVs would require de novo rescue to change the HA antigenicity, which is disadvantageous to screen against diverse isolates. Reporter-based NAb screening using sciIAV can also be utilized to identify stalk-reactive broad spectrum NAbs (Baker et al., 2015), which would be advantageous over plaque reduction neutralization assays (Lee et al., 2012). Although sciIAV has been used only to evaluate the presence of NAbs, showing a comparable sensitivity to traditional assays, we cannot discard their limitations to detect other inhibitory Abs, such as those that prevent viral egress. However, given that sciIAV mirrored the viral replication of WT viruses when infecting trans-complementing cell lines, other kinds of inhibitory Abs could also be detected using sciIAV, although this hypothesis needs to be experimentally evaluated. In the future, the sciIAV platform could be adapted for high-throughput screening (HTS) identification of influenza NAbs.
3.2 HTS to identify host factors or compounds affecting IAV replication
The development of new antiviral strategies against IAV would be beneficial to combat the uniform viral resistance in circulating strains to adamantanes (M2 blockers) and previous widespread resistance to NA inhibitors (Bright et al., 2006; de Jong et al., 2005; Garten et al., 2009; Lackenby et al., 2008; Shiraishi et al., 2003). Identifying new viral or host targets for antiviral development would be ideal, since no virus has been described yet to gain resistance against such targets, like the viral RdRp and/or NP (Kao et al., 2010; Su et al., 2010). Current and traditional technologies to detect antivirals against IAV have been extensively reviewed elsewhere (Beyleveld et al., 2013). The following section will focus on the application and limitations of sciIAVs for the screening of new antivirals.
Due to the extremely large size of compound libraries, HTS approaches are the most ideal way to test for viral inhibition, and these approaches commonly employ a reporter protein that is quantifiable with optimal z-score (Hertzberg and Pope, 2000). IAV HTS assays have been reported to identify compounds that inhibit viral NA activity, NS1 function, and host factors that inhibit influenza viral replication and transcription using siRNA libraries (Beyleveld et al., 2013). A major drawback of using single-cycle or WT IAV for drug screens is that specialized cells need to be used that either complement the single-cycle deficiency or that encode for a reporter protein activated by the IAV RdRp, respectively. This consideration is important if host-specific pathways are inadequately recapitulated in the specialized cell lines. Replication-competent, reporter-expressing IAV may be more desirable in this regard, but special safety requirements may need to be considered when using IAV strains that contain genes from highly pathogenic isolates.
The major advantages of using sciIAV to screen for influenza antivirals is their versatility to support multiple reporter genes, which are compatible with HTS (Hertzberg and Pope, 2000) (Fig. 4A). Moreover the use of BSL-2 facilities for screening bypasses the limitations associated with containment when working with highly pathogenic IAV strains. Bottini et al. recently used renilla luciferase-expressing sciIAV ΔHA to screen for antivirals in MDCK-HA cells (Bottini et al., 2012). The authors used an HTS approach to evaluate a library consisting of approximately 14,000 chemical compounds, resulting in the identification of one antiviral (compound 7). The hit was validated by characterizing NP mRNA expression by quantitative real-time PCR in MDCK-HA or A549 cells infected with the sciIAV or WT viruses, respectively. Interestingly, the compound identified by this approach showed promise when it also protected mice against WT IAV challenge. However, the specific function or target of this class of antiviral compounds has yet to be determined. These results provide evidence that application of sciIAV in HTS assays for the detection of novel antiviral compounds is a promising approach capable of identifying antivirals that are active in in vitro and in vivo models of IAV infection (Fig. 4A).
An HTS approach to identify cellular proteins involved in the IAV life cycle was taken by using a library of small interfering RNA that targets the host cell transcriptome prior to reporter-expressing sciIAV infection (Hirsch, 2010; Konig et al., 2010) (Fig. 4A). Here, Konig et al. used sciIAV ΔHA expressing renilla luciferase and a genome-wide RNA interference (RNAi) library to identify key cellular factors required for successful replication of IAV. Using this approach, 295 cellular host factors were found that are required for IAV replication in human lung epithelial (A549) cells, a non-complementing cell line. Reporter-expressing sciIAV was beneficial to initially screen the library consisting of small interfering RNAs that together target 19,000 human genes. The rate of false-positives was acceptable, as 219 of the 295 genes targeted also affected WT virus replication over multiple cycles. It is important to consider that sciIAV in HTS screens can be used to identify targets that affect a single round of replication, which may not be identifiable during multiple rounds of infection due to signal saturation. Perhaps targeting genes that redundantly affect a single-cycle infection may prove advantageous in inhibiting multi-round infection.
3.3 Influenza vaccines and vaccine vectors
Currently available non-replicating virus vaccines (IIV and RIV) induce moderate humoral immunity that depends on the immunologic fitness of the recipient. Elderly and immunocompromised individuals typically have reduced responses compared to young healthy individuals (DiazGranados et al., 2014). IIV and RIV also afford limited protection during years where the vaccine formulation does not match circulating strains, although the vaccines may still offer some level of cross-reactivity (Ohmit et al., 2006; Osterholm et al., 2012; Treanor et al., 2007). On the other hand, LAIV, which replicates slowly due to its temperature-sensitive (ts), cold-adapted (ca) and attenuated (att) phenotype, generates more robust humoral and cellular immunity (Maassab and Bryant, 1999). Due to safety concerns in the young and asthmatics, and limited efficacy in the elderly, LAIV is only recommended for immunocompetent, non-asthmatic patients between the ages of 2–49 (Ambrose et al., 2012; Belshe et al., 2008; Powers et al., 1991). One study has found that combining inactivated and LAIVs is well-tolerated and efficacious in elderly individuals (Tang, 2012; Treanor and Betts, 1998). Therefore, there is great interest and need to improve the immunogenicity of IIV and RIV or the safety of LAIV, and implement new vaccination strategies that prevent infection in susceptible populations.
The safety of sciIAV was shown in a mouse model of influenza infection by delivering virus intranasally that did not produce overt signs of illness, including weight loss, ruffling of the fur, and hunching (Baker et al., 2013; Katsura et al., 2012; Powell et al., 2012; Shinya et al., 2004; Uraki et al., 2013; Victor et al., 2012). Recent characterization of an LAIV mouse model (Cox et al., 2015) was able to demonstrate mortality at high doses of LAIV, which has not yet been described for any sciIAV, suggesting that sciIAV is more safe than LAIV. Intranasal immunization is a desirable delivery method to prevent infection with IAV because it leads to the generation of a mucosal immune response, creating an immune barrier at the site of potential infection (Kohlmeier and Woodland, 2009). Indeed, sciIAV priming elicits not only a robust systemic humoral response but also a mucosal immune response, as anti-influenza IgG and IgA Abs were found in nasal washes and bronchial alveolar lavage (Katsura et al., 2012; Victor et al., 2012). Furthermore, sciIAV vaccination protected mice against homologous challenge for up to 4 months after vaccination (Powell et al., 2012) (Fig. 4B). Similar to infection with WT IAV, sciIAV immunization also leads to recruitment of influenza-specific CD8 T cells to the lungs (Baker et al., 2013; Guo et al., 2014; Katsura et al., 2012; Powell et al., 2012; Uraki et al., 2013), which is likely to be the main contributor of immunity against heterologous influenza challenge (Baker et al., 2013; Guo et al., 2014). Two doses (prime and boost) of sciIAV are required to elicit sterilizing immunity, as shown by an absence of challenge virus recovered from animals (Baker et al., 2013; Katsura et al., 2012; Powell et al., 2012; Victor et al., 2012). However, one dose of sciIAV ΔHA is sufficient to prevent lethal heterosubtypic challenge (Guo et al., 2014). Moreover ferrets, which have upper respiratory physiology more closely related to humans than mice and can transmit influenza via aerosol droplets, were also protected by sciIAV against infection and transmitting virus (Baker et al., 2013). Vaccination with sciIAV in swine was also shown to be protective and immunogenic (Masic et al., 2013; Pyo and Zhou, 2014).
Infection with influenza leads to a type 1 skewed adaptive immune response (Kohlmeier and Woodland, 2009) so sciIAV can potentially be used as a vector to protect against other upper-respiratory tract pathogens. Evidence for this was first provided by showing the presence of anti-GFP Abs in mice following immunization with a GFP-expressing sciIAV ΔPB2 (Victor et al., 2012). Furthering this data, when a reporter gene from a sciIAV was replaced by an inactive HA gene from a heterologous IAV, both mice and swine were protected against heterologous influenza virus infections (Masic et al., 2013; Uraki et al., 2013). However, no NAbs were detected against the heterologous strain in mice even though they were detected in swine. Whether these bivalent sciIAV had different protective efficacies than reporter-expressing sciIAV was not addressed (Masic et al., 2013; Uraki et al., 2013). Amenability of sciIAV as a bivalent vaccine was further shown when Streptococcus pneumoniae surface protein A (PspA), HPIV-3 HN protein, or RSV F protein replaced the reporter gene in sciIAV (Fonseca et al., 2014; Katsura et al., 2014; Kobayashi et al., 2013). All of these vaccines could induce Abs and reduce the burden against the heterologous pathogen while retaining their ability to protect against influenza challenge. The use of sciIAV has also been demonstrated as a vector for the exogenous expression of small RNAs in cellular and in vivo systems, and for gene knock-down with therapeutic purposes (Schmid et al., 2014) (Fig. 4B). Schmid et al. evaluated the use of a sciIAV as a delivery tool for both coding and non-coding RNA, demonstrating that these small RNAs are expressed and functional in cultured cells, primary cells and in infected mice, opening the possibility of using sciIAVs as a delivery tool of therapeutic value.
For vaccine or vaccine vector applications, it is important to consider the viral gene replaced when generating sciIAV. Although the polymerase (PB2, PB1 and PA) segments may afford more flexibility to include larger transgenes, the inability of virus to encode for more polymerase during the single round of infection should theoretically decrease total viral gene expression. For example, it was shown that a single dose of sciIAV ΔHA or ΔNA could protect against lethal influenza challenge (Guo et al., 2014; Shinya et al., 2004). However, Victor et al. demonstrated that one dose of sciIAV ΔPB2 was only as efficacious as inactivated virus (Victor et al., 2012). Additionally, it was also demonstrated that in situ sciIAV replication is required, because inactivated sciIAV did not confer full protection (Baker et al., 2013; Katsura et al., 2012; Victor et al., 2012).
In summary, sciIAV has been used as a safe and effective vaccine in several animal models and can elicit humoral and cellular responses, representing a feasible alternative to current influenza vaccines. In this regard, sciIAVs combine the advantages (safety of the IIV and better immunogenicity of the LAIV) and avoid disadvantages (poor immunogenicity of the IIV and safety of the LAIV) of current influenza vaccination approaches. However, although this virus elicited protective and therapeutic immunity in animal studies, not human trials has been performed yet. It is important to consider the sizable regulatory requirements of developing human vaccines, which would be more severe in the case of sciIAV because complementing cell lines would similarly need to be approved for vaccine production (Perdue et al., 2011). Importantly, MDCK cells have recently been given FDA approval for use in vaccine virus production, but stable virus protein-expressing MDCK cells would need a corresponding approval (Hussain et al., 2010). Moreover, there are other limitations associated to vaccine manufacturing. Given that, a system for large-scale production must be available, and the sciIAV engineered should be genetically stable in multiple passages in order to achieve commercial quantities of vaccine.
3.4 Studying the biology of influenza virus using sciIAV
3.4.1 Co-infections and viral reassortment
Antigenic shift occurs in IAV when a reassortment event generates a human-transmissible strain that contains novel HA or NA segments, against which a majority of humans are immunologically naïve (Shaw and Palese, 2013). This has directly led to at least three IAV pandemics: H2N2 in 1957, H3N2 in 1968, and pH1N1 in 2009 (Garten et al., 2009; Shaw and Palese, 2013). By using transgenes with unique fluorescent protein emission spectra, sciIAVs have been used to study IAV co-infection and segment specific packaging hierarchy (Bodewes et al., 2012; Inagaki et al., 2012). Inagaki et al. showed that rescue transfections of sciIAV ΔNA or ΔPB2, when there was equal competition for a GFP or DsRed transgene, resulted in few bi-fluorescent protein-expressing sciIAV plaques. These results suggest that most virions specifically package eight viral segments, because only 0.6–1.6% of plaques were both GFP and DsRed positive. To evaluate the ability of IAV to co-infect cells in vitro, GFP or mCherry sciIAV ΔNA viruses were used to infect A549 or MDCK cells (Bodewes et al., 2012). Using flow cytometry and confocal microscopy, the authors found that although an MOI of 0.1 did not lead to many GFP and mCherry double positive cells, infecting with an MOI of 3 led to a co-infection rate of 2% in A549 and 4–10% in MDCK cells. Both of these studies exemplify the extreme selectivity to incorporate only eight vRNA segments and infrequency of co-infection at low MOIs.
3.4.2 Influenza virus tropism and host range
Receptor-binding of IAV is one of the main determinants of viral tropism and host specificity, where avian-tropic viruses preferentially bind to α2,3 linked SA receptors and human strains typically bind α2,6 linkages (Rogers et al., 1983a; Rogers et al., 1983b; Yoon et al., 2014). To investigate attachment and post-attachment events with mammalian and avian cells, GFP-expressing sciIAV ΔNA were used with three different HA subtypes (H1, H3 and H5) (Rimmelzwaan et al., 2007). For virus attachment, the authors used a sciIAV where the transmembrane region of NA is fused to GFP and thus GFP is incorporated into virions. Flow cytometric analysis of cells bound by virus or productively infected with sciIAV showed that H1 and H3 viruses bind human A549 cells better than quail QT6 cells (6.7 and 35.0 compared to 1.9 and 0.8 percent, respectively) (Rimmelzwaan et al., 2007). Furthermore, H5 sciIAV bound QT6 cells better than H1 and H3 viruses (39.2, 1.9, and 0.8 percent, respectively). It is surprising to note, however, that more than 50% of QT6 cells expressed GFP 16 hours post-treatment in all conditions, despite the inability to detect frequent initial human-tropic virus attachment (Rimmelzwaan et al., 2007).
To evaluate the requirement of HA as the receptor-binding protein, Hooper et al. used reverse genetics transfections to rescue a sciIAV ΔPB1 in which the receptor binding function of HA was abolished (BindMut) (Hooper and Bloom, 2013). To the surprise of the authors, a virus arose that contained a mutation in NA (G147R) that conferred receptor-binding functionality to NA. In a follow up study, the authors again used sciIAV ΔPB1 in elegant MN assays that included oseltamivir and found that NA G147R’s ability to bind receptor is dependent on its sialidase activity, presumably because sialidase activity is dependent on receptor engagement (Hooper et al., 2015).
To identify a bat cell line permissive to H1N1 virus infection, Poole et al. utilized GFP-expressing sciIAV ΔHA for single-round infections at various MOIs (Poole et al., 2014). Prior to this work, this important potential reservoir for IAV lacked experimental evidence suggesting permissiveness to human IAVs (Mehle, 2014). The permissive bat cell line, Tb 1 Lu, that was identified to support H1N1 replication and infection did so through binding of sialic acid-linked receptors, as treatment of cells with receptor destroying enzyme prior to sciIAV ΔHA inoculation inhibited subsequent GFP expression (Poole et al., 2014). With reports that continually suggest new host species for IAV, sciIAV can be used to evaluate virus tropism to and permissivity of new species (Yoon et al., 2014).
3.4.3 Inhibition of influenza A and B intertypic reassortment
Despite the continual co-circulation of IAV and IBV in humans and the ability of each virus to reassort within the same type, intertypic reassortments have never been documented in nature or in vitro (McCullers et al., 2004; McCullers et al., 1999; Shaw and Palese, 2013; Xu et al., 2004). In fact, a competitive interference at the level of viral transcription between both virus species has been reported (Aoki et al., 1984; Gotlieb and Hirst, 1954; Kaverin et al., 1983). To demonstrate the interference of IAV mediated by IBV, Wantichang et al. co-infected cells with DsRed-expressing sciIAV ΔNA and either of two IBV strains (B/Lee/40 and B/Maryland/2/59) (Wanitchang et al., 2012). In agreement with previous studies (Aoki et al., 1984), the authors used flow cytometry to show that DsRed expression was markedly lower in IBV, but not IAV (WT PR8) co-infected cells (Wanitchang et al., 2012). Cells that overexpressed IBV NP prior to sciIAV ΔNA infection were refractory to high DsRed expression post-infection, demonstrating that IBV NP was sufficient to interfere with IAV replication. This work, along with follow-up studies, demonstrated the ability of IBV NP to interfere with IAV replication (Jaru-ampornpan et al., 2014; Wanitchang et al., 2013), but it does not explain the inability to rescue intertypic reassortant viruses in the absence of influenza B NP. In fact, using GFP-expressing sciIAV ΔHA, Baker et al. demonstrated, complementation of sciIAV ΔHA with full-length IBV HA via MDCK-HA cells (Baker et al., 2014). Following the hypothesis that certain homologs of IBV can function in IAV, the authors demonstrated that packaging signal discrepancies were sufficient to limit IAV and IBV intertypic reassortment, rather than incompatibilities in the function of viral proteins. It remains to be determined whether or not IBV uses similar packaging signals to those described to IAV. Similarities and differences between packaging signals of IAV and IBV will also need to be compared.
3.5 Conclusions and future directions
In this review, we summarize and discuss the biology and applications of the sciIAVs described to date. The recombinant viruses and methods described herein do not cover all potential sciIAV uses in influenza laboratories. Mini-replicon systems – co-expression of IAV RdRp and NP that drive transcription of a viral-like RNA encoding a reporter gene – are commonly used to study IAV genome replication and gene transcription (Shaw and Palese, 2013). Reporter-expressing sciIAVs can similarly be used for quantitative analysis of RdRp activity and are advantageous over mini-genome approaches in that cells are reconstituted with all the proteins of IAV. Moreover, the use of sciIAV is also applicable to translational research and has been demonstrated as a screening platform to identify specific or broadly-reactive NAbs, antiviral compounds, or host proteins or RNAs involved in IAV replication, including highly pathogenic IAVs normally confined to BSL-3+ facilities. The sciIAV platform can also be used as a next-generation IAV vaccine or vaccine vector for other respiratory pathogens (Fig. 4). It will be interesting to continue research with sciIAV as an efficacious vaccine or vaccine vector because it is capable of expressing several antigens or immune-modulators during a single round of infection. There is also great value in using sciIAV to perform forward evolution studies safely and with a quantitative read-out that is compatible with HTS. Not only can mutants be identified readily that confer a certain growth phenotype, but also these studies can be conducted in a self-contained system, significantly alleviating concerns about escape of so called gain-of-function mutants. Other future areas of sciIAV application include, but are not limited to, virus tropism, the analysis of viral reassortments during replication, or immunological responses to the first round of infection. In conclusion, there are potential advantages and disadvantages associated to sciIAV. However, the abundant safety, economic, and biological advantages of sciIAV highlight their promising future in basic and applied influenza research or as therapies. The use of these bio-contained viruses is desirable when studying basic in vitro biology of highly pathogenic IAV.
Highlights.
Influenza A virus infections cause both seasonal epidemics and occasional pandemics.
SciIAVs are defective in viral replication or propagation.
Biocontained sciIAV can be used to study highly pathogenic influenza A viruses at BSL-2.
Expression of a reporter gene has allowed the use of sciIAVs in HTS settings.
SciIAVs have been used as potential safe vaccines or delivery vectors in mammals.
Acknowledgments
SFB was supported by the University of Rochester Immunology Training Grant AI 007285-26. WD was supported by NIH/NHLBI T32 HL066988. Influenza research to LM-S was partially funded by the NIAID Centers of Excellence for Influenza Research and Surveillance (CEIRS HHSN266200700008C) and the 2014 University of Rochester Research Award.
Abbreviations
- ACIP
Advisory Committee on Immunization Practices
- Ab
antibody
- att
attenuated
- BSL
biosafety level
- ca
cold-adapted
- CPE
cytopathic effect
- DI
defective interfering
- dsRed
Discosoma red fluorescent protein
- ELISA
enzyme-linked immunosorbent assay
- Fluc
Firefly luciferase
- FFU
focus forming units
- FDA
Food and Drug Administration
- GFP
green fluorescent protein
- HA
hemagglutinin
- HN
hemagglutinin-neuraminidase
- HI
hemagglutination inhibition
- HAU
hemagglutinating units
- HTS
high-throughput screening
- IFA
immunofluorescence
- IIV
inactivated influenza vaccine
- IAV
Influenza A virus
- IBV
Influenza B virus
- IRES
internal ribosome entry site
- LAIV
live attenuated influenza vaccine
- MDCK
Madin-Darby canine kidney
- MN
microneutralization
- mRFP
monomeric red fluorescent protein
- MOI
multiplicity of infection
- NA
neuraminidase
- NAb
neutralizing antibody
- NCR
non-coding regions
- NEP
nuclear export protein
- nt
nucleotide
- PFU
plaque forming units
- PRNT
plaque reduction neutralization test
- Pol I
polymerase I
- Pol II
polymerase II
- RIV
recombinant influenza vaccine
- RFP
red fluorescent protein
- Rluc
Renilla luciferase
- RdRp
RNA-dependent RNA polymerase
- SA
sialic acid
- sciIAV
single-cycle infectious Influenza A Virus
- ts
temperature-sensitive
- VLP
virus-like particle
- vRNP
viral ribonucleoprotein
- vRNA
viral RNA
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Wkly Epidemiol Rec; Assays for neutralizing antibody to influenza viruses. Report of an informal scientific workshop; Dresden. 18–19 March 2003; pp. 290–293. [PubMed] [Google Scholar]
- Abe T, Fujimori T. Reporter mouse lines for fluorescence imaging. Dev Growth Differ. 2013;55(4):390–405. doi: 10.1111/dgd.12062. [DOI] [PubMed] [Google Scholar]
- Ambrose CS, Dubovsky F, Yi T, Belshe RB, Ashkenazi S. The safety and efficacy of live attenuated influenza vaccine in young children with asthma or prior wheezing. Eur J Clin Microbiol Infect Dis. 2012;31(10):2549–2557. doi: 10.1007/s10096-012-1595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H, Nishiyama Y, Tsurumi T, Shibata M, Ito Y, Seo H, Yoshii S, Maeno K. Mechanism of interference between influenza A/WSN and B/Kanagawa viruses. J Gen Virol. 1984;65(Pt 8):1385–1393. doi: 10.1099/0022-1317-65-8-1385. [DOI] [PubMed] [Google Scholar]
- Baker SF, Guo H, Albrecht RA, Garcia-Sastre A, Topham DJ, Martinez-Sobrido L. Protection against lethal influenza with a viral mimic. J Virol. 2013;87(15):8591–8605. doi: 10.1128/JVI.01081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SF, Nogales A, Finch C, Tuffy KM, Domm W, Perez DR, Topham DJ, Martinez-Sobrido L. Influenza A and B virus intertypic reassortment through compatible viral packaging signals. J Virol. 2014;88(18):10778–10791. doi: 10.1128/JVI.01440-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SF, Nogales A, Santiago FW, Topham DJ, Martinez-Sobrido L. Competitive detection of influenza neutralizing antibodies using a novel bivalent fluorescence-based microneutralization assay (BiFMA) Vaccine. 2015 doi: 10.1016/j.vaccine.2015.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare AS, Webster RG. Replication of avian influenza viruses in humans. Arch Virol. 1991;119(1–2):37–42. doi: 10.1007/BF01314321. [DOI] [PubMed] [Google Scholar]
- Belshe RB, Ambrose CS, Yi T. Safety and efficacy of live attenuated influenza vaccine in children 2–7 years of age. Vaccine. 2008;26(Suppl 4):D10–16. doi: 10.1016/j.vaccine.2008.06.083. [DOI] [PubMed] [Google Scholar]
- Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K, Treanor J, Zangwill K, Hayden FG, Bernstein DI, Kotloff K, King J, Piedra PA, Block SL, Yan L, Wolff M. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis. 2000;181(3):1133–1137. doi: 10.1086/315323. [DOI] [PubMed] [Google Scholar]
- Beyleveld G, White KM, Ayllon J, Shaw ML. New-generation screening assays for the detection of anti-influenza compounds targeting viral and host functions. Antiviral Res. 2013;100(1):120–132. doi: 10.1016/j.antiviral.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom JD, Gong LI, Baltimore D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science. 2010;328(5983):1272–1275. doi: 10.1126/science.1187816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom JD, Nayak JS, Baltimore D. A computational-experimental approach identifies mutations that enhance surface expression of an oseltamivir-resistant influenza neuraminidase. PLoS One. 2011;6(7):e22201. doi: 10.1371/journal.pone.0022201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodewes R, Nieuwkoop NJ, Verburgh RJ, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Use of influenza A viruses expressing reporter genes to assess the frequency of double infections in vitro. J Gen Virol. 2012;93(Pt 8):1645–1648. doi: 10.1099/vir.0.042671-0. [DOI] [PubMed] [Google Scholar]
- Bottini A, De SK, Baaten BJ, Wu B, Barile E, Soonthornvacharin S, Stebbins JL, Bradley LM, Chanda SK, Pellecchia M. Identification of small molecules that interfere with H1N1 influenza A viral replication. ChemMedChem. 2012;7(12):2227–2235. doi: 10.1002/cmdc.201200453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA. 2006;295(8):891–894. doi: 10.1001/jama.295.8.joc60020. [DOI] [PubMed] [Google Scholar]
- Bussow K. Stable mammalian producer cell lines for structural biology. Curr Opin Struct Biol. 2015;32:81–90. doi: 10.1016/j.sbi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25(39–40):6852–6862. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- CDC. Update: infections with a swine-origin influenza A (H1N1) virus--United States and other countries, April 28, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(16):431–433. [PubMed] [Google Scholar]
- CDC. Estimates of deaths associated with seasonal influenza --- United States, 1976–2007. MMWR Morb Mortal Wkly Rep. 2010;59(33):1057–1062. [PubMed] [Google Scholar]
- Chen W, Calvo PA, Malide D, Gibbs J, Schubert U, Bacik I, Basta S, O’Neill R, Schickli J, Palese P, Henklein P, Bennink JR, Yewdell JW. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med. 2001;7(12):1306–1312. doi: 10.1038/nm1201-1306. [DOI] [PubMed] [Google Scholar]
- Chou YY, Vafabakhsh R, Doganay S, Gao Q, Ha T, Palese P. One influenza virus particle packages eight unique viral RNAs as shown by FISH analysis. Proc Natl Acad Sci U S A. 2012;109(23):9101–9106. doi: 10.1073/pnas.1206069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua MA, Schmid S, Perez JT, Langlois RA, Tenoever BR. Influenza A virus utilizes suboptimal splicing to coordinate the timing of infection. Cell Rep. 2013;3(1):23–29. doi: 10.1016/j.celrep.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A, Baker SF, Nogales A, Martinez-Sobrido L, Dewhurst S. Development of a mouse-adapted live attenuated influenza virus that permits in vivo analysis of enhancements to the safety of live attenuated influenza virus vaccine. J Virol. 2015;89(6):3421–3426. doi: 10.1128/JVI.02636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel) 2003;115:63–73. [PubMed] [Google Scholar]
- de Jong MD, Tran TT, Truong HK, Vo MH, Smith GJ, Nguyen VC, Bach VC, Phan TQ, Do QH, Guan Y, Peiris JS, Tran TH, Farrar J. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353(25):2667–2672. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, Martin E, Gurunathan S, Nathan R, Greenberg DP, Tornieporth NG, Decker MD, Talbot HK. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- Dos Santos Afonso E, Escriou N, Leclercq I, van der Werf S, Naffakh N. The generation of recombinant influenza A viruses expressing a PB2 fusion protein requires the conservation of a packaging signal overlapping the coding and noncoding regions at the 5′ end of the PB2 segment. Virology. 2005;341(1):34–46. doi: 10.1016/j.virol.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Dudek T, Knipe DM. Replication-defective viruses as vaccines and vaccine vectors. Virology. 2006;344(1):230–239. doi: 10.1016/j.virol.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Essere B, Yver M, Gavazzi C, Terrier O, Isel C, Fournier E, Giroux F, Textoris J, Julien T, Socratous C, Rosa-Calatrava M, Lina B, Marquet R, Moules V. Critical role of segment-specific packaging signals in genetic reassortment of influenza A viruses. Proc Natl Acad Sci U S A. 2013;110(40):E3840–3848. doi: 10.1073/pnas.1308649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DT, Bricker JE, Sanford HB, Lang S, Carville A, Richardson BA, Piatak M, Jr, Lifson JD, Mansfield KG, Desrosiers RC. Immunization of macaques with single-cycle simian immunodeficiency virus (SIV) stimulates diverse virus-specific immune responses and reduces viral loads after challenge with SIVmac239. J Virol. 2005;79(12):7707–7720. doi: 10.1128/JVI.79.12.7707-7720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig L, Soyka J, Buda S, Buchholz U, Dehnert M, Haas W. Avian influenza A(H5N1) in humans: new insights from a line list of World Health Organization confirmed cases, September 2006 to August 2010. Euro Surveill. 2011;16(32) [PubMed] [Google Scholar]
- Flick R, Neumann G, Hoffmann E, Neumeier E, Hobom G. Promoter elements in the influenza vRNA terminal structure. RNA. 1996;2(10):1046–1057. [PMC free article] [PubMed] [Google Scholar]
- Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, Garcia-Sastre A. Rescue of influenza A virus from recombinant DNA. J Virol. 1999;73(11):9679–9682. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor E, Pritlove DC, Brownlee GG. Characterization of the RNA-fork model of virion RNA in the initiation of transcription in influenza A virus. J Virol. 1995;69(7):4012–4019. doi: 10.1128/jvi.69.7.4012-4019.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca W, Ozawa M, Hatta M, Orozco E, Martinez MB, Kawaoka Y. A recombinant influenza virus vaccine expressing the F protein of respiratory syncytial virus. Arch Virol. 2014;159(5):1067–1077. doi: 10.1007/s00705-013-1932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier E, Moules V, Essere B, Paillart JC, Sirbat JD, Cavalier A, Rolland JP, Thomas D, Lina B, Isel C, Marquet R. Interaction network linking the human H3N2 influenza A virus genomic RNA segments. Vaccine. 2012a;30(51):7359–7367. doi: 10.1016/j.vaccine.2012.09.079. [DOI] [PubMed] [Google Scholar]
- Fournier E, Moules V, Essere B, Paillart JC, Sirbat JD, Isel C, Cavalier A, Rolland JP, Thomas D, Lina B, Marquet R. A supramolecular assembly formed by influenza A virus genomic RNA segments. Nucleic Acids Research. 2012b;40(5):2197–2209. doi: 10.1093/nar/gkr985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K, Fujii Y, Noda T, Muramoto Y, Watanabe T, Takada A, Goto H, Horimoto T, Kawaoka Y. Importance of both the coding and the segment-specific noncoding regions of the influenza A virus NS segment for its efficient incorporation into virions. J Virol. 2005;79(6):3766–3774. doi: 10.1128/JVI.79.6.3766-3774.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K, Ozawa M, Iwatsuki-Horimoto K, Horimoto T, Kawaoka Y. Incorporation of influenza A virus genome segments does not absolutely require wild-type sequences. J Gen Virol. 2009;90(Pt 7):1734–1740. doi: 10.1099/vir.0.010355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y, Goto H, Watanabe T, Yoshida T, Kawaoka Y. Selective incorporation of influenza virus RNA segments into virions. Proc Natl Acad Sci U S A. 2003;100(4):2002–2007. doi: 10.1073/pnas.0437772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Chou YY, Doganay S, Vafabakhsh R, Ha T, Palese P. The influenza A virus PB2, PA, NP, and M segments play a pivotal role during genome packaging. J Virol. 2012;86(13):7043–7051. doi: 10.1128/JVI.00662-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Palese P. Rewiring the RNAs of influenza virus to prevent reassortment. Proc Natl Acad Sci U S A. 2009;106(37):15891–15896. doi: 10.1073/pnas.0908897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325(5937):197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini R, Amicizia D, Lai PL, Panatto D. Clinical and socioeconomic impact of seasonal and pandemic influenza in adults and the elderly. Hum Vaccin Immunother. 2012;8(1):21–28. doi: 10.4161/hv.8.1.17622. [DOI] [PubMed] [Google Scholar]
- Gavazzi C, Isel C, Fournier E, Moules V, Cavalier A, Thomas D, Lina B, Marquet R. An in vitro network of intermolecular interactions between viral RNA segments of an avian H5N2 influenza A virus: comparison with a human H3N2 virus. Nucleic Acids Research. 2013a;41(2):1241–1254. doi: 10.1093/nar/gks1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi C, Yver M, Isel C, Smyth RP, Rosa-Calatrava M, Lina B, Moules V, Marquet R. A functional sequence-specific interaction between influenza A virus genomic RNA segments. Proc Natl Acad Sci U S A. 2013b;110(41):16604–16609. doi: 10.1073/pnas.1314419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen WP. Emerging infections: pandemic influenza. Epidemiol Rev. 1996;18(1):64–76. doi: 10.1093/oxfordjournals.epirev.a017917. [DOI] [PubMed] [Google Scholar]
- Gog JR, dos Afonso ES, Dalton RM, Leclercq I, Tiley L, Elton D, von Kirchbach JC, Naffakh N, Escriou N, Digard P. Codon conservation in the influenza A virus genome defines RNA packaging signals. Nucleic Acids Res. 2007;35(6):1897–1907. doi: 10.1093/nar/gkm087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlieb T, Hirst GK. The experimental production of combination forms of virus. III. The formation of doubly antigenic particles from influenza A and B virus and a study of the ability of individual particles of X virus to yield two separate strains. J Exp Med. 1954;99(4):307–320. doi: 10.1084/jem.99.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H, Muramoto Y, Noda T, Kawaoka Y. The genome-packaging signal of the influenza A virus genome comprises a genome incorporation signal and a genome-bundling signal. J Virol. 2013;87(21):11316–11322. doi: 10.1128/JVI.01301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, Karron RA, Walter EB Centers for Disease C and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63(32):691–697. [PMC free article] [PubMed] [Google Scholar]
- Gubareva LV, Kaiser L, Hayden FG. Influenza virus neuraminidase inhibitors. Lancet. 2000;355(9206):827–835. doi: 10.1016/S0140-6736(99)11433-8. [DOI] [PubMed] [Google Scholar]
- Guo H, Baker SF, Martinez-Sobrido L, Topham DJ. Induction of CD8 T cell heterologous protection by a single dose of single-cycle infectious influenza virus. J Virol. 2014;88(20):12006–12016. doi: 10.1128/JVI.01847-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai R, Garcia-Sastre A, Swayne DE, Palese P. A reassortment-incompetent live attenuated influenza virus vaccine for protection against pandemic virus strains. J Virol. 2011;85(14):6832–6843. doi: 10.1128/JVI.00609-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SC. Viral membrane fusion. Virology. 2015;479–480C:498–507. doi: 10.1016/j.virol.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay AJ, Wolstenholme AJ, Skehel JJ, Smith MH. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985;4(11):3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden FG, de Jong MD. Emerging influenza antiviral resistance threats. J Infect Dis. 2011;203(1):6–10. doi: 10.1093/infdis/jiq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Mullarkey CE, Miller MS. Measuring the neutralization potency of influenza A virus hemagglutinin stalk/stem-binding antibodies in polyclonal preparations by microneutralization assay. Methods. 2015 doi: 10.1016/j.ymeth.2015.04.037. [DOI] [PubMed] [Google Scholar]
- Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336(6088):1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg RP, Pope AJ. High-throughput screening: new technology for the 21st century. Curr Opin Chem Biol. 2000;4(4):445–451. doi: 10.1016/s1367-5931(00)00110-1. [DOI] [PubMed] [Google Scholar]
- Hirsch AJ. The use of RNAi-based screens to identify host proteins involved in viral replication. Future Microbiol. 2010;5(2):303–311. doi: 10.2217/fmb.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GK. The Quantitative Determination of Influenza Virus and Antibodies by Means of Red Cell Agglutination. J Exp Med. 1942;75(1):49–64. doi: 10.1084/jem.75.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Neumann G, Hobom G, Webster RG, Kawaoka Y. “Ambisense” approach for the generation of influenza A virus: vRNA and mRNA synthesis from one template. Virology. 2000a;267(2):310–317. doi: 10.1006/viro.1999.0140. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A. 2000b;97(11):6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper KA, Bloom JD. A mutant influenza virus that uses an N1 neuraminidase as the receptor-binding protein. J Virol. 2013;87(23):12531–12540. doi: 10.1128/JVI.01889-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper KA, Crowe JE, Jr, Bloom JD. Influenza viruses with receptor-binding N1 neuraminidases occur sporadically in several lineages and show no attenuation in cell culture or mice. J Virol. 2015;89(7):3737–3745. doi: 10.1128/JVI.00012-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horimoto T, Kawaoka Y. Pandemic threat posed by avian influenza A viruses. Clin Microbiol Rev. 2001;14(1):129–149. doi: 10.1128/CMR.14.1.129-149.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TS, Palese P, Krystal M. Determination of influenza virus proteins required for genome replication. J Virol. 1990;64(11):5669–5673. doi: 10.1128/jvi.64.11.5669-5673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt AC, Holien JK, Parker M, Kelso A, Barr IG. Zanamivir-resistant influenza viruses with a novel neuraminidase mutation. J Virol. 2009;83(20):10366–10373. doi: 10.1128/JVI.01200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain AI, Cordeiro M, Sevilla E, Liu J. Comparison of egg and high yielding MDCK cell-derived live attenuated influenza virus for commercial production of trivalent influenza vaccine: in vitro cell susceptibility and influenza virus replication kinetics in permissive and semi-permissive cells. Vaccine. 2010;28(22):3848–3855. doi: 10.1016/j.vaccine.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson EC, Curran MD, Read EK, Gog JR, Digard P. Mutational analysis of cis-acting RNA signals in segment 7 of influenza A virus. J Virol. 2008;82(23):11869–11879. doi: 10.1128/JVI.01634-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson EC, von Kirchbach JC, Gog JR, Digard P. Genome packaging in influenza A virus. J Gen Virol. 2010;91(Pt 2):313–328. doi: 10.1099/vir.0.017608-0. [DOI] [PubMed] [Google Scholar]
- Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, Li C, Kawakami E, Yamada S, Kiso M, Suzuki Y, Maher EA, Neumann G, Kawaoka Y. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012;486(7403):420–428. doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki A, Goto H, Kakugawa S, Ozawa M, Kawaoka Y. Competitive incorporation of homologous gene segments of influenza A virus into virions. J Virol. 2012;86(18):10200–10202. doi: 10.1128/JVI.01204-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AMQ. International Committee on Taxonomy of Viruses. Virus taxonomy : classification and nomenclature of viruses : ninth report of the International Committee on Taxonomy of Viruses. x. Academic Press; London ; Waltham, MA: 2012. p. 1327. [Google Scholar]
- Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science. 2012;337(6091):199–204. doi: 10.1126/science.1222213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaru-ampornpan P, Narkpuk J, Wanitchang A, Jongkaewwattana A. Nucleoprotein of influenza B virus binds to its type A counterpart and disrupts influenza A viral polymerase complex formation. Biochem Biophys Res Commun. 2014;443(1):296–300. doi: 10.1016/j.bbrc.2013.11.100. [DOI] [PubMed] [Google Scholar]
- Jia B, Ng SK, DeGottardi MQ, Piatak M, Yuste E, Carville A, Mansfield KG, Li W, Richardson BA, Lifson JD, Evans DT. Immunization with single-cycle SIV significantly reduces viral loads after an intravenous challenge with SIV(mac)239. PLoS Pathog. 2009;5(1):e1000272. doi: 10.1371/journal.ppat.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao RY, Yang D, Lau LS, Tsui WH, Hu L, Dai J, Chan MP, Chan CM, Wang P, Zheng BJ, Sun J, Huang JD, Madar J, Chen G, Chen H, Guan Y, Yuen KY. Identification of influenza A nucleoprotein as an antiviral target. Nat Biotechnol. 2010;28(6):600–605. doi: 10.1038/nbt.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura H, Iwatsuki-Horimoto K, Fukuyama S, Watanabe S, Sakabe S, Hatta Y, Murakami S, Shimojima M, Horimoto T, Kawaoka Y. A replication-incompetent virus possessing an uncleavable hemagglutinin as an influenza vaccine. Vaccine. 2012;30(42):6027–6033. doi: 10.1016/j.vaccine.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura H, Piao Z, Iwatsuki-Horimoto K, Akeda Y, Watanabe S, Horimoto T, Oishi K, Kawaoka Y. A bivalent vaccine based on a replication-incompetent influenza virus protects against Streptococcus pneumoniae and influenza virus infection. J Virol. 2014;88(22):13410–13417. doi: 10.1128/JVI.01205-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverin NV, Varich NL, Sklyanskaya EI, Amvrosieva TV, Petrik J, Vovk TC. Studies on heterotypic interference between influenza A and B viruses: a differential inhibition of the synthesis of viral proteins and RNAs. J Gen Virol. 1983;64(Pt 10):2139–2146. doi: 10.1099/0022-1317-64-10-2139. [DOI] [PubMed] [Google Scholar]
- Keech M, Beardsworth P. The impact of influenza on working days lost: a review of the literature. Pharmacoeconomics. 2008;26(11):911–924. doi: 10.2165/00019053-200826110-00004. [DOI] [PubMed] [Google Scholar]
- Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis. 2006;12(1):9–14. doi: 10.3201/eid1201.051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian ML. Hemagglutination assay for the avian influenza virus. Methods Mol Biol. 2008;436:47–52. doi: 10.1007/978-1-59745-279-3_7. [DOI] [PubMed] [Google Scholar]
- Knipe DM, Howley PM. Fields virology. 6. Vol. 2. Wolters Kluwer/Lippincott Williams & Wilkins Health; Philadelphia, PA: 2013. [Google Scholar]
- Kobayashi H, Iwatsuki-Horimoto K, Kiso M, Uraki R, Ichiko Y, Takimoto T, Kawaoka Y. A replication-incompetent influenza virus bearing the HN glycoprotein of human parainfluenza virus as a bivalent vaccine. Vaccine. 2013;31(52):6239–6246. doi: 10.1016/j.vaccine.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier JE, Woodland DL. Immunity to respiratory viruses. Annu Rev Immunol. 2009;27:61–82. doi: 10.1146/annurev.immunol.021908.132625. [DOI] [PubMed] [Google Scholar]
- Konig R, Stertz S, Zhou Y, Inoue A, Hoffmann HH, Bhattacharyya S, Alamares JG, Tscherne DM, Ortigoza MB, Liang Y, Gao Q, Andrews SE, Bandyopadhyay S, De Jesus P, Tu BP, Pache L, Shih C, Orth A, Bonamy G, Miraglia L, Ideker T, Garcia-Sastre A, Young JA, Palese P, Shaw ML, Chanda SK. Human host factors required for influenza virus replication. Nature. 2010;463(7282):813–817. doi: 10.1038/nature08699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015;14(3):167–182. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- Lackenby A, Hungnes O, Dudman SG, Meijer A, Paget WJ, Hay AJ, Zambon MC. Emergence of resistance to oseltamivir among influenza A(H1N1) viruses in Europe. Euro Surveill. 2008;13(5) doi: 10.2807/ese.13.05.08026-en. [DOI] [PubMed] [Google Scholar]
- Lamb RA, Choppin PW. The gene structure and replication of influenza virus. Annu Rev Biochem. 1983;52:467–506. doi: 10.1146/annurev.bi.52.070183.002343. [DOI] [PubMed] [Google Scholar]
- Lanza AM, Kim do S, Alper HS. Evaluating the influence of selection markers on obtaining selected pools and stable cell lines in human cells. Biotechnol J. 2013;8(7):811–821. doi: 10.1002/biot.201200364. [DOI] [PubMed] [Google Scholar]
- Laursen NS, Wilson IA. Broadly neutralizing antibodies against influenza viruses. Antiviral Res. 2013;98(3):476–483. doi: 10.1016/j.antiviral.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver WG, Air GM, Webster RG, Gerhard W, Ward CW, Dopheide TA. Antigenic drift in type A influenza virus: sequence differences in the hemagglutinin of Hong Kong (H3N2) variants selected with monoclonal hybridoma antibodies. Virology. 1979;98(1):226–237. doi: 10.1016/0042-6822(79)90540-3. [DOI] [PubMed] [Google Scholar]
- Laver WG, Webster RG. Ecology of influenza viruses in lower mammals and birds. Br Med Bull. 1979;35(1):29–33. doi: 10.1093/oxfordjournals.bmb.a071537. [DOI] [PubMed] [Google Scholar]
- Lee PS, Yoshida R, Ekiert DC, Sakai N, Suzuki Y, Takada A, Wilson IA. Heterosubtypic antibody recognition of the influenza virus hemagglutinin receptor binding site enhanced by avidity. Proc Natl Acad Sci U S A. 2012;109(42):17040–17045. doi: 10.1073/pnas.1212371109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Hong Y, Parslow TG. cis-Acting packaging signals in the influenza virus PB1, PB2, and PA genomic RNA segments. J Virol. 2005;79(16):10348–10355. doi: 10.1128/JVI.79.16.10348-10355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luytjes W, Krystal M, Enami M, Parvin JD, Palese P. Amplification, expression, and packaging of foreign gene by influenza virus. Cell. 1989;59(6):1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- Maassab HF, Bryant ML. The development of live attenuated cold-adapted influenza virus vaccine for humans. Rev Med Virol. 1999;9(4):237–244. doi: 10.1002/(sici)1099-1654(199910/12)9:4<237::aid-rmv252>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Marsh GA, Hatami R, Palese P. Specific residues of the influenza A virus hemagglutinin viral RNA are important for efficient packaging into budding virions. J Virol. 2007;81(18):9727–9736. doi: 10.1128/JVI.01144-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh GA, Rabadan R, Levine AJ, Palese P. Highly conserved regions of influenza a virus polymerase gene segments are critical for efficient viral RNA packaging. J Virol. 2008;82(5):2295–2304. doi: 10.1128/JVI.02267-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Sobrido L, Cadagan R, Steel J, Basler CF, Palese P, Moran TM, Garcia-Sastre A. Hemagglutinin-pseudotyped green fluorescent protein-expressing influenza viruses for the detection of influenza virus neutralizing antibodies. J Virol. 2010;84(4):2157–2163. doi: 10.1128/JVI.01433-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masic A, Pyo HM, Babiuk S, Zhou Y. An eight-segment swine influenza virus harboring H1 and H3 hemagglutinins is attenuated and protective against H1N1 and H3N2 subtypes in pigs. J Virol. 2013;87(18):10114–10125. doi: 10.1128/JVI.01348-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullers JA, Saito T, Iverson AR. Multiple genotypes of influenza B virus circulated between 1979 and 2003. J Virol. 2004;78(23):12817–12828. doi: 10.1128/JVI.78.23.12817-12828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullers JA, Wang GC, He S, Webster RG. Reassortment and insertion-deletion are strategies for the evolution of influenza B viruses in nature. J Virol. 1999;73(9):7343–7348. doi: 10.1128/jvi.73.9.7343-7348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehle A. Unusual influenza A viruses in bats. Viruses. 2014;6(9):3438–3449. doi: 10.3390/v6093438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- Monto AS, McKimm-Breschkin JL, Macken C, Hampson AW, Hay A, Klimov A, Tashiro M, Webster RG, Aymard M, Hayden FG, Zambon M. Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrob Agents Chemother. 2006;50(7):2395–2402. doi: 10.1128/AAC.01339-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto Y, Takada A, Fujii K, Noda T, Iwatsuki-Horimoto K, Watanabe S, Horimoto T, Kida H, Kawaoka Y. Hierarchy among viral RNA (vRNA) segments in their role in vRNA incorporation into influenza A virions. J Virol. 2006;80(5):2318–2325. doi: 10.1128/JVI.80.5.2318-2325.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefkens I, Garcia JM, Ling CS, Lagarde N, Nicholls J, Tang DJ, Peiris M, Buchy P, Altmeyer R. Hemagglutinin pseudotyped lentiviral particles: characterization of a new method for avian H5N1 influenza sero-diagnosis. J Clin Virol. 2007;39(1):27–33. doi: 10.1016/j.jcv.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Neumann G, Brownlee GG, Fodor E, Kawaoka Y. Orthomyxovirus replication, transcription, and polyadenylation. Curr Top Microbiol Immunol. 2004;283:121–143. doi: 10.1007/978-3-662-06099-5_4. [DOI] [PubMed] [Google Scholar]
- Neumann G, Kawaoka Y. Generation of influenza A virus from cloned cDNAs--historical perspective and outlook for the new millenium. Rev Med Virol. 2002;12(1):13–30. doi: 10.1002/rmv.332. [DOI] [PubMed] [Google Scholar]
- Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez DR, Donis R, Hoffmann E, Hobom G, Kawaoka Y. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A. 1999;96(16):9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann G, Whitt MA, Kawaoka Y. A decade after the generation of a negative-sense RNA virus from cloned cDNA - what have we learned? J Gen Virol. 2002;83(Pt 11):2635–2662. doi: 10.1099/0022-1317-83-11-2635. [DOI] [PubMed] [Google Scholar]
- Noda T, Kawaoka Y. Structure of influenza virus ribonucleoprotein complexes and their packaging into virions. Rev Med Virol. 2010;20(6):380–391. doi: 10.1002/rmv.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales A, Baker SF, Martinez-Sobrido L. Replication-competent influenza A viruses expressing a red fluorescent protein. Virology. 2015;476:206–216. doi: 10.1016/j.virol.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmit SE, Victor JC, Rotthoff JR, Teich ER, Truscon RK, Baum LL, Rangarajan B, Newton DW, Boulton ML, Monto AS. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355(24):2513–2522. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Maeda J, Iwatsuki-Horimoto K, Watanabe S, Goto H, Horimoto T, Kawaoka Y. Nucleotide sequence requirements at the 5′ end of the influenza A virus M RNA segment for efficient virus replication. J Virol. 2009;83(7):3384–3388. doi: 10.1128/JVI.02513-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Victor ST, Taft AS, Yamada S, Li C, Hatta M, Das SC, Takashita E, Kakugawa S, Maher EA, Neumann G, Kawaoka Y. Replication-incompetent influenza A viruses that stably express a foreign gene. J Gen Virol. 2011;92(Pt 12):2879–2888. doi: 10.1099/vir.0.037648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health. 2013;103(3):e43–51. doi: 10.2105/AJPH.2012.301137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue ML, Arnold F, Li S, Donabedian A, Cioce V, Warf T, Huebner R. The future of cell culture-based influenza vaccine production. Expert Rev Vaccines. 2011;10(8):1183–1194. doi: 10.1586/erv.11.82. [DOI] [PubMed] [Google Scholar]
- Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69(3):517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- Pizzorno A, Abed Y, Boivin G. Influenza drug resistance. Semin Respir Crit Care Med. 2011;32(4):409–422. doi: 10.1055/s-0031-1283281. [DOI] [PubMed] [Google Scholar]
- Poole DS, Yu S, Cai Y, Dinis JM, Muller MA, Jordan I, Friedrich TC, Kuhn JH, Mehle A. Influenza A virus polymerase is a site for adaptive changes during experimental evolution in bat cells. J Virol. 2014;88(21):12572–12585. doi: 10.1128/JVI.01857-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell TJ, Silk JD, Sharps J, Fodor E, Townsend AR. Pseudotyped influenza A virus as a vaccine for the induction of heterotypic immunity. J Virol. 2012;86(24):13397–13406. doi: 10.1128/JVI.01820-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers DC, Fries LF, Murphy BR, Thumar B, Clements ML. In elderly persons live attenuated influenza A virus vaccines do not offer an advantage over inactivated virus vaccine in inducing serum or secretory antibodies or local immunologic memory. J Clin Microbiol. 1991;29(3):498–505. doi: 10.1128/jcm.29.3.498-505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo HM, Zhou Y. Protective efficacy of intranasally administered bivalent live influenza vaccine and immunological mechanisms underlying the protection. Vaccine. 2014;32(30):3835–3842. doi: 10.1016/j.vaccine.2014.04.065. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008;453(7195):615–619. doi: 10.1038/nature06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon S, Baker SF, Sahler JM, Kim N, Feldsott EA, Serhan CN, Martinez-Sobrido L, Topham DJ, Phipps RP. The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? J Immunol. 2014;193(12):6031–6040. doi: 10.4049/jimmunol.1302795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Baars M, Claas EC, Osterhaus AD. Comparison of RNA hybridization, hemagglutination assay, titration of infectious virus and immunofluorescence as methods for monitoring influenza virus replication in vitro. J Virol Methods. 1998;74(1):57–66. doi: 10.1016/s0166-0934(98)00071-8. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Nieuwkoop NJ, de Mutsert G, Boon AC, Kuiken T, Fouchier RA, Osterhaus AD. Attachment of infectious influenza A viruses of various subtypes to live mammalian and avian cells as measured by flow cytometry. Virus Res. 2007;129(2):175–181. doi: 10.1016/j.virusres.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Verburgh RJ, Nieuwkoop NJ, Bestebroer TM, Fouchier RA, Osterhaus AD. Use of GFP-expressing influenza viruses for the detection of influenza virus A/H5N1 neutralizing antibodies. Vaccine. 2011;29(18):3424–3430. doi: 10.1016/j.vaccine.2011.02.082. [DOI] [PubMed] [Google Scholar]
- Rodrigo WW, de la Torre JC, Martinez-Sobrido L. Use of single-cycle infectious lymphocytic choriomeningitis virus to study hemorrhagic fever arenaviruses. J Virol. 2011;85(4):1684–1695. doi: 10.1128/JVI.02229-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GN, Paulson JC, Daniels RS, Skehel JJ, Wilson IA, Wiley DC. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983a;304(5921):76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- Rogers GN, Pritchett TJ, Lane JL, Paulson JC. Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: selection of receptor specific variants. Virology. 1983b;131(2):394–408. doi: 10.1016/0042-6822(83)90507-x. [DOI] [PubMed] [Google Scholar]
- Rossman JS, Lamb RA. Influenza virus assembly and budding. Virology. 2011;411(2):229–236. doi: 10.1016/j.virol.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, Fukuda K, Cox NJ, Katz JM. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37(4):937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk JE. A Simplified Procedure for Titrating Hemagglutinating Capacity of Influenza-Virus and the Corresponding Antibody. The Journal of Immunology. 1944;49(2):87–98. [Google Scholar]
- Sandbulte MR, Westgeest KB, Gao J, Xu X, Klimov AI, Russell CA, Burke DF, Smith DJ, Fouchier RA, Eichelberger MC. Discordant antigenic drift of neuraminidase and hemagglutinin in H1N1 and H3N2 influenza viruses. Proc Natl Acad Sci U S A. 2011;108(51):20748–20753. doi: 10.1073/pnas.1113801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S, Zony LC, tenOever BR. A versatile RNA vector for delivery of coding and noncoding RNAs. J Virol. 2014;88(4):2333–2336. doi: 10.1128/JVI.03267-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw ML, Palese P. Fields Virology. 6. Lippincott Williams and Wilkins; Philadelphia: 2013. Orthomyxoviridae. [Google Scholar]
- Shih FF, Cerasoli DM, Caton AJ. A major T cell determinant from the influenza virus hemagglutinin (HA) can be a cryptic self peptide in HA transgenic mice. Int Immunol. 1997;9(2):249–261. doi: 10.1093/intimm/9.2.249. [DOI] [PubMed] [Google Scholar]
- Shinya K, Fujii Y, Ito H, Ito T, Kawaoka Y. Characterization of a neuraminidase-deficient influenza a virus as a potential gene delivery vector and a live vaccine. J Virol. 2004;78(6):3083–3088. doi: 10.1128/JVI.78.6.3083-3088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi K, Mitamura K, Sakai-Tagawa Y, Goto H, Sugaya N, Kawaoka Y. High frequency of resistant viruses harboring different mutations in amantadine-treated children with influenza. J Infect Dis. 2003;188(1):57–61. doi: 10.1086/375799. [DOI] [PubMed] [Google Scholar]
- Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459(7250):1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- Stephenson I, Das RG, Wood JM, Katz JM. Comparison of neutralising antibody assays for detection of antibody to influenza A/H3N2 viruses: an international collaborative study. Vaccine. 2007;25(20):4056–4063. doi: 10.1016/j.vaccine.2007.02.039. [DOI] [PubMed] [Google Scholar]
- Stephenson I, Wood JM, Nicholson KG, Zambon MC. Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian influenza haemagglutinin. J Med Virol. 2003;70(3):391–398. doi: 10.1002/jmv.10408. [DOI] [PubMed] [Google Scholar]
- Su CY, Cheng TJ, Lin MI, Wang SY, Huang WI, Lin-Chu SY, Chen YH, Wu CY, Lai MM, Cheng WC, Wu YT, Tsai MD, Cheng YS, Wong CH. High-throughput identification of compounds targeting influenza RNA-dependent RNA polymerase activity. Proc Natl Acad Sci U S A. 2010;107(45):19151–19156. doi: 10.1073/pnas.1013592107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16(3):265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Winkelmann ER, Mason PW. Construction and characterization of a single-cycle chimeric flavivirus vaccine candidate that protects mice against lethal challenge with dengue virus type 2. J Virol. 2009;83(4):1870–1880. doi: 10.1128/JVI.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DC. Perspectives on replication-incompetent nasal influenza virus vaccines. Expert Rev Vaccines. 2012;11(8):907–909. doi: 10.1586/erv.12.64. [DOI] [PubMed] [Google Scholar]
- Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12(1):15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger JK, Morens DM. Pandemic influenza--including a risk assessment of H5N1. Rev Sci Tech. 2009;28(1):187–202. doi: 10.20506/rst.28.1.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temperton NJ, Hoschler K, Major D, Nicolson C, Manvell R, Hien VM, Ha do Q, de Jong M, Zambon M, Takeuchi Y, Weiss RA. A sensitive retroviral pseudotype assay for influenza H5N1-neutralizing antibodies. Influenza Other Respir Viruses. 2007;1(3):105–112. doi: 10.1111/j.1750-2659.2007.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, Turmelle AS, Moran D, Rogers S, Shi M, Tao Y, Weil MR, Tang K, Rowe LA, Sammons S, Xu X, Frace M, Lindblade KA, Cox NJ, Anderson LJ, Rupprecht CE, Donis RO. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A. 2012;109(11):4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Johnson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013;9(10):e1003657. doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran V, Moser LA, Poole DS, Mehle A. Highly sensitive real-time in vivo imaging of an influenza reporter virus reveals dynamics of replication and spread. J Virol. 2013;87(24):13321–13329. doi: 10.1128/JVI.02381-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor JJ, Betts RF. Evaluation of live, cold-adapted influenza A and B virus vaccines in elderly and high-risk subjects. Vaccine. 1998;16(18):1756–1760. doi: 10.1016/s0264-410x(98)00136-4. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Schiff GM, Hayden FG, Brady RC, Hay CM, Meyer AL, Holden-Wiltse J, Liang H, Gilbert A, Cox M. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA. 2007;297(14):1577–1582. doi: 10.1001/jama.297.14.1577. [DOI] [PubMed] [Google Scholar]
- Uraki R, Kiso M, Iwatsuki-Horimoto K, Fukuyama S, Takashita E, Ozawa M, Kawaoka Y. A novel bivalent vaccine based on a PB2-knockout influenza virus protects mice from pandemic H1N1 and highly pathogenic H5N1 virus challenges. J Virol. 2013;87(14):7874–7881. doi: 10.1128/JVI.00076-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeki TM, Cox NJ. Global concerns regarding novel influenza A (H7N9) virus infections. N Engl J Med. 2013;368(20):1862–1864. doi: 10.1056/NEJMp1304661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese JN, McKimm-Breschkin JL, Caldwell JB, Kortt AA, Colman PM. The structure of the complex between influenza virus neuraminidase and sialic acid, the viral receptor. Proteins. 1992;14(3):327–332. doi: 10.1002/prot.340140302. [DOI] [PubMed] [Google Scholar]
- Victor ST, Watanabe S, Katsura H, Ozawa M, Kawaoka Y. A replication-incompetent PB2-knockout influenza A virus vaccine vector. J Virol. 2012;86(8):4123–4128. doi: 10.1128/JVI.06232-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanitchang A, Narkpuk J, Jaru-ampornpan P, Jengarn J, Jongkaewwattana A. Inhibition of influenza A virus replication by influenza B virus nucleoprotein: an insight into interference between influenza A and B viruses. Virology. 2012;432(1):194–203. doi: 10.1016/j.virol.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Wanitchang A, Narkpuk J, Jongkaewwattana A. Nuclear import of influenza B virus nucleoprotein: Involvement of an N-terminal nuclear localization signal and a cleavage-protection motif. Virology. 2013;443(1):59–68. doi: 10.1016/j.virol.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Watanabe S, Noda T, Fujii Y, Kawaoka Y. Exploitation of nucleic acid packaging signals to generate a novel influenza virus-based vector stably expressing two foreign genes. J Virol. 2003;77(19):10575–10583. doi: 10.1128/JVI.77.19.10575-10583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56(1):152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Pandemic (H1N1) 2009–update 112, Global Alert and Response (GAR) Vol. 2015. World Health Organization; 2010. [Google Scholar]
- WHO. Manual for the laboratory diagnosis and virological surveillance of influenza. 2011. [Google Scholar]
- WHO. Serological detection of avian influenza A(H7N9)virus infections by modified horse red blood cells haemagglutination-inhibition assay. 2013. [Google Scholar]
- WHO. Avian Influenza Fact Sheet. 2014a. [Google Scholar]
- WHO. Influenza (Seasonal) Fact sheet No. 211. 2014b. [Google Scholar]
- WHO. Risk assessment of human infection with avian influenza A(H7N9) virus. 2015. [DOI] [PubMed] [Google Scholar]
- Wise HM, Hutchinson EC, Jagger BW, Stuart AD, Kang ZH, Robb N, Schwartzman LM, Kash JC, Fodor E, Firth AE, Gog JR, Taubenberger JK, Digard P. Identification of a novel splice variant form of the influenza A virus M2 ion channel with an antigenically distinct ectodomain. PLoS Pathog. 2012;8(11):e1002998. doi: 10.1371/journal.ppat.1002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Lindstrom SE, Shaw MW, Smith CB, Hall HE, Mungall BA, Subbarao K, Cox NJ, Klimov A. Reassortment and evolution of current human influenza A and B viruses. Virus Res. 2004;103(1–2):55–60. doi: 10.1016/j.virusres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Ye S, Evans JG, Stambas J. Influenza reverse genetics: dissecting immunity and pathogenesis. Expert Rev Mol Med. 2014;16:e2. doi: 10.1017/erm.2014.4. [DOI] [PubMed] [Google Scholar]
- Yen HL, Webster RG. Pandemic influenza as a current threat. Curr Top Microbiol Immunol. 2009;333:3–24. doi: 10.1007/978-3-540-92165-3_1. [DOI] [PubMed] [Google Scholar]
- Yoon SW, Webby RJ, Webster RG. Evolution and ecology of influenza a viruses. Curr Top Microbiol Immunol. 2014;385:359–375. doi: 10.1007/82_2014_396. [DOI] [PubMed] [Google Scholar]