Abstract
Background
Neuroinflammatory signaling may contribute to the pathophysiology of chronic anxiety disorders. Previous work showed that repeated social defeat (RSD) in mice promoted stress-sensitization that was characterized by the recurrence of anxiety following sub-threshold stress 24 days after RSD. Furthermore, splenectomy following RSD prevented the recurrence of anxiety in stress-sensitized (SS) mice. We hypothesize that the spleen of RSD-exposed mice became a reservoir of primed monocytes that were released following neuroendocrine activation by sub-threshold stress.
Methods
Mice were subjected to sub-threshold stress (i.e., single cycle of social defeat) 24 days after RSD, and immune and behavioral measures were taken.
Results
Sub-threshold stress 24 days after RSD re-established anxiety-like behavior that was associated with egress of Ly6Chi monocytes from the spleen. Moreover, splenectomy prior to RSD blocked monocyte trafficking to the brain and prevented anxiety-like behavior following sub-threshold stress. Splenectomy, however, had no effect on monocyte accumulation or anxiety when determined 14 hours after RSD. In addition, splenocytes cultured 24 days after RSD exhibited a primed inflammatory phenotype. Peripheral sympathetic inhibition prior to sub-threshold stress blocked monocyte trafficking from the spleen to the brain and prevented the re-establishment of anxiety in RSD-sensitized mice. Last, β-adrenergic antagonism also prevented splenic monocyte egress after acute stress.
Conclusion
The spleen served as a unique reservoir of primed monocytes that were readily released following sympathetic activation by sub-threshold stress that promoted the re-establishment of anxiety. Collectively, the long-term storage of primed monocytes in the spleen may have a profound influence on recurring anxiety disorders.
Keywords: Stress, Anxiety, Microglia, Neuroinflammation, PTSD, Macrophages
Introduction
Psychological stress contributes to the development and exacerbation of mental health disturbances, especially chronic anxiety disorders [1–4]. This is an important phenomenon because chronic anxiety disorders are the most common psychiatric illness affecting nearly 1 in 3 individuals over their life span [5, 6]. Bidirectional communication between the brain and immune system contributes to the etiology of many psychiatric symptoms and disorders in relation to psychological stress [7–11]. Broadly, chronic psychosocial stress is associated with a sequela of immunological changes that are often correlated with poor mental health outcomes. Many of these immunological changes are related to increased accumulation of primed monocytes that have increased potential for inflammatory signaling [12, 13] and are resistant to the anti-inflammatory effects of glucocorticoids (GCs) [14, 15]. Moreover, many of the pro-inflammatory effects of stress can be attributed to enhanced monocytopoiesis in the bone marrow that results in the selective accumulation of the Ly6Chi monocyte subset [13, 16]. Ly6Chi monocytes have a higher inflammatory capacity compared to their more mature immunoregulatory Ly6Clo counterparts [17, 18]. Additionally, there is evidence that this monocytic immune activation contributes to psychiatric illness in humans, as reviewed by Beumer et al. [19]. For example, increased perivascular brain-macrophages were observed in depressed patients who committed suicide [20]. Moreover, PTSD symptoms significantly correlated with pro-inflammatory NFκB signaling in leukocytes and with GC-resistance in monocytes [21, 22]. Thus, these clinical data provide key evidence that links stress, monocytes, and mood disorders.
Repeated social defeat (RSD) in mice recapitulates key immunological and behavioral deficits [23, 24] associated with psychosocial stress in humans. For example, RSD increased monocytopoiesis in the bone marrow that caused selective accumulation of Ly6Chi monocytes in circulation, spleen, and brain [25, 26]. The accumulation of Ly6Chi monocytes during RSD promoted a pro-inflammatory leukocyte “transcriptional fingerprint” that was similar to that observed in human populations [13]. Similarly, RSD promotes a primed monocyte phenotype characterized by exaggerated inflammatory response to ex vivo innate immune challenge that is resistant to inhibition by GCs [27]. Additionally, the development of prolonged anxiety-like behavior that is detectable up to 8 days after RSD [28] is dependent upon sympathetic activation of the immune system [13, 25, 27]. Further studies revealed that the development of prolonged anxiety-like behavior was specifically dependent on monocyte accumulation in the brain following RSD [29]. Taken together, monocyte trafficking to the brain represent a novel axis of immune-to-brain signaling that promotes prolonged behavioral responses to stress [30, 31].
Recent evidence shows that RSD caused long-term sensitization that caused mice to have exaggerated immunological and behavioral responses following subsequent exposure to an acute stressor [28]. In this study, RSD-exposed mice were termed “stress-sensitized” because they exhibited exaggerated responses to an otherwise sub-threshold stressor. For instance, exposure to a single cycle of social defeat 24 days after RSD re-established monocyte trafficking and anxiety-like behavior without affecting these parameters in naïve, non-stressed controls [28]. Notably, splenectomy in stress-sensitized mice prevented the re-establishment of monocyte trafficking and anxiety-like behavior 24 days after RSD. These data were interpreted to indicate that monocyte trafficking from the spleen to the brain promoted the re-establishment of anxiety in stress-sensitized mice. However, it is currently unclear if the spleen is unique in its ability to store these releasable monocytes. In immunological studies, other immune organs were capable of storing myeloid cells, but the spleen was unique in its capacity to functionally contribute monocytes to distant inflammatory sites [32–35].
Based on these collective data, the objective of this study was to test the hypothesis that the spleen of RSD-exposed mice serves as a unique reservoir of primed monocytes that are released following sympathetic outflow in response to an acute stressor. Here, we provide several lines of evidence that the spleen is unique in its capacity to maintain and release a population of primed monocytes 24 days after RSD. Moreover, sub-threshold stress mice caused this pool of primed monocytes to traffic to the brain and promote the recurrence of anxiety-like behavior. Furthermore, inhibition of the peripheral sympathetic nervous system during sub-threshold stress blocked spleen-to-brain monocyte trafficking and prevented the recurrence of anxiety in stress-sensitized mice. These novel studies reveal that the spleen is capable of maintaining long term neuroimmune sensitization that can regulate behavioral responses many days after the initial sensitizing event.
Materials and Methods
Mice
Male C57BL/6 (6–8 weeks old) and CD-1 (retired breeders) mice were purchased from Charles River Laboratories (Wilmington, MA). C57BL/6 mice were housed in cohorts of three per cage. All procedures were in accordance with the NIH Guidelines and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
Repeated social defeat (RSD)
Mice were subjected to RSD as previously reported [29] and as described in Supplementary Materials. In brief, an aggressive intruder male CD-1 mouse was introduced into cages of established male cohorts (three per cage) of C57BL/6 mice for 6 consecutive nights. During each cycle, submissive behaviors were observed to ensure that the resident mice showed subordinate behavior. As previously described [28], to study the sensitizing effects of RSD, mice were either exposed to control (naïve) or RSD conditions (stress-sensitized). Then, 24 days later naïve and stress-sensitized (SS) mice were subjected to an additional cycle of social defeat. All behavior and biological measures were obtained 14 h after the final cycle. This time point was selected because both HPA and SNS activation following social defeat return to baseline within 14 hours [27].
Guanethidine Treatment
Twenty four hours prior to acute social defeat, mice were injected subcutaneously with either vehicle or 50 mg/kg guanethidine (Santa Cruz Biotechnology, Dallas, TX). Injection regimen was based on a previous report [36].
Anxiety-like behavior
Anxiety-like behavior was determined using open-field activity as previously reported [29] and as described in Supplementary Materials.
Isolation of cells from bone marrow, spleen, blood, and brain
Tissues were collected immediately following CO2 asphyxiation. Cells from BM, spleen, and blood were isolated as previously described [26, 27]. CD11b+ brain cells were enriched by Percoll density gradient as previously reported [29]. See Supplementary Materials for details.
Statistical analysis
To determine significant main effects and interactions between main factors, data were analyzed using two-way ANOVA using the General Linear Model procedures of SAS (Cary, NC). ANOVA results are presented in figure legends. When there was a main effect of experimental treatment or a treatment interaction effect, differences between means were evaluated by an F-protected t-test using the Least-Significant Difference procedure of SAS. All data are expressed as treatment means ± SEM.
Results
Recurrence of anxiety-like behavior in stress-sensitized mice was associated with Ly6Chi monocyte egress from the spleen
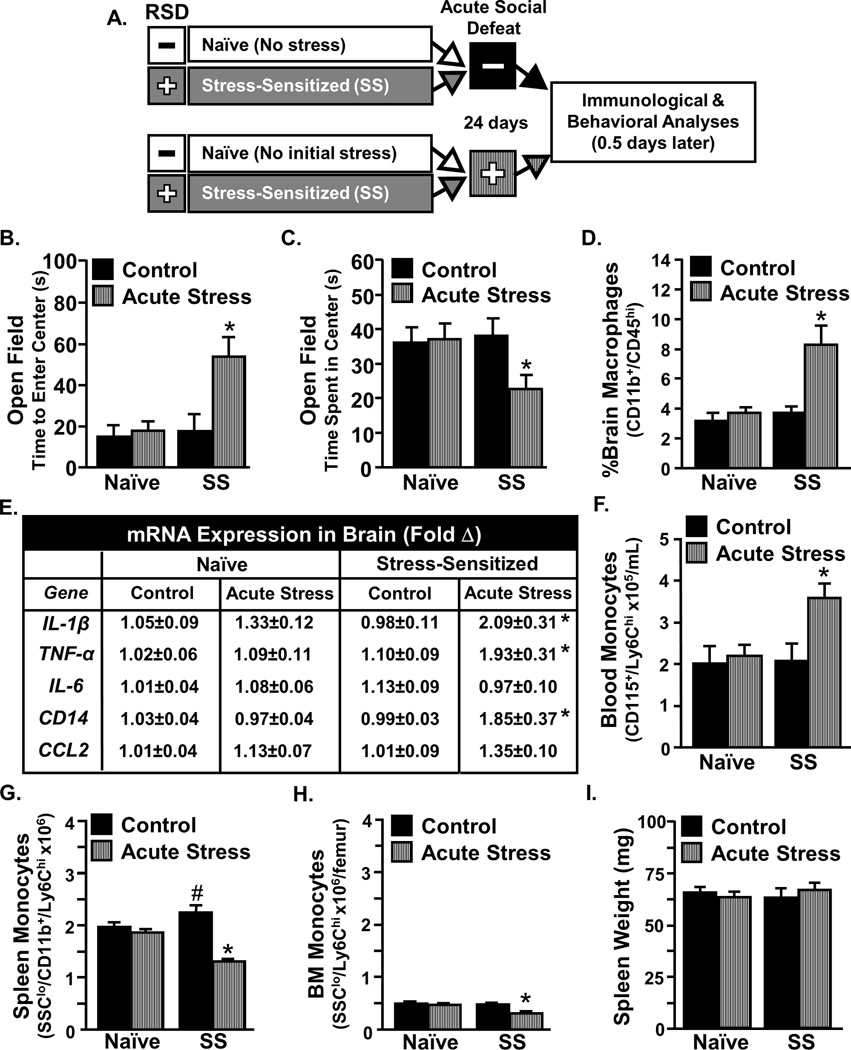
Our previous study showed that removal of the spleen after RSD prevented both monocyte trafficking to the brain and the recurrence of anxiety-like behavior in stress-sensitized (SS) mice [28]. To further examine the possible release of monocytes from the spleen in response to acute stress, the following experimental design was used. Fig.1A illustrates that mice were stress-sensitized by 6 cycles of social defeat (SS) or left undisturbed (Naïve). Twenty four days later, mice were exposed to acute social defeat (acute stress) or left alone as controls. Congruent with our previous findings [28], acute social defeat promoted the recurrence of anxiety-like behavior that was associated with increased monocyte trafficking to the brain. For instance, SS mice exposed to acute stress took longer to enter the center (Fig.1B, p<0.05) and spent less time in the center of the open field (Fig.1C, p<0.05). Additionally, acute stress increased the accumulation of macrophages in the brain (Fig.1D, p<0.05) and increased mRNA expression of IL-1β, TNF-α, and CD14 in the brain of SS mice (Fig.1E, all p<0.05).
Figure 1. Acute stress in stress-sensitized (SS) mice caused re-establishment of anxiety-like behavior that was associated with release monocytes from the spleen.
A) Male C57BL/6 mice were stress-sensitized (SS) by 6 repeated cycles of social defeat or left undisturbed as controls (Naïve). Mice were subjected to acute social defeat 24 days later and anxiety-like behavior and biochemical analyses were completed 14 h later. Stress-Sensitized mice exposed to acute social defeat exhibited anxiety-like behavior in the open field with B) increased time to enter the center (interaction, F1,42=4.52, p<0.05) and C) reduced time spent in the center (tendency for interaction, F1,44=2.98, p<0.10). D) Acute social defeat in SS mice increased percentage of macrophages associated with the brain (interaction, F1,21=8.22, p<0.05) and E) increased Ly6Chi monocytes in circulation (main effect of SS, F1,19=4.47, p≤0.05; tendency for interaction, F1,19=2.67, p≤0.1). F) Several inflammatory mediators were determined in a coronal brain section and acute social defeat increased mRNA expression of IL-1β in SS mice (F1,36=10.55, p<0.01; interaction, F1,36=3.66, p≤0.05), CCL2 (F1,36=5.42, p<0.05), TNF (interaction, F1,39=4.23, p<0.05), and CD14 (interaction, F1,39=4.46, p<0.05). The relative number of Ly6Chi monocytes was determined in the G) spleen and H) bone marrow. Acute stress reduced the number of monocytes in both the spleen (interaction, F1,18=8.35, p≤0.01) and bone marrow (interaction, F1,18=9.82, p≤0.01) of SS mice. I) Spleen weight was determined and shown as a percentage of body mass. Bars represent the mean ± SEM. Means with asterisk (*) are significantly different from CON (p<0.05) according to F-protected post hoc analysis.
Next, the peripheral origin of increased brain macrophages was explored. Acute stress increased the number of circulating Ly6Chi monocytes in SS mice (Fig.1F, p<0.05) but not in naïve mice. Moreover, the increase in circulating monocytes was associated with increased release of monocytes from the spleen (Fig 1G, p<0.05) and bone marrow of SS mice (Fig.1H, p<0.05). Neither stress-sensitization nor acute stress altered spleen weight (Fig.1I). These results indicate that the spleen is the primary source of monocyte accumulation in SS mice following exposure to acute stress.
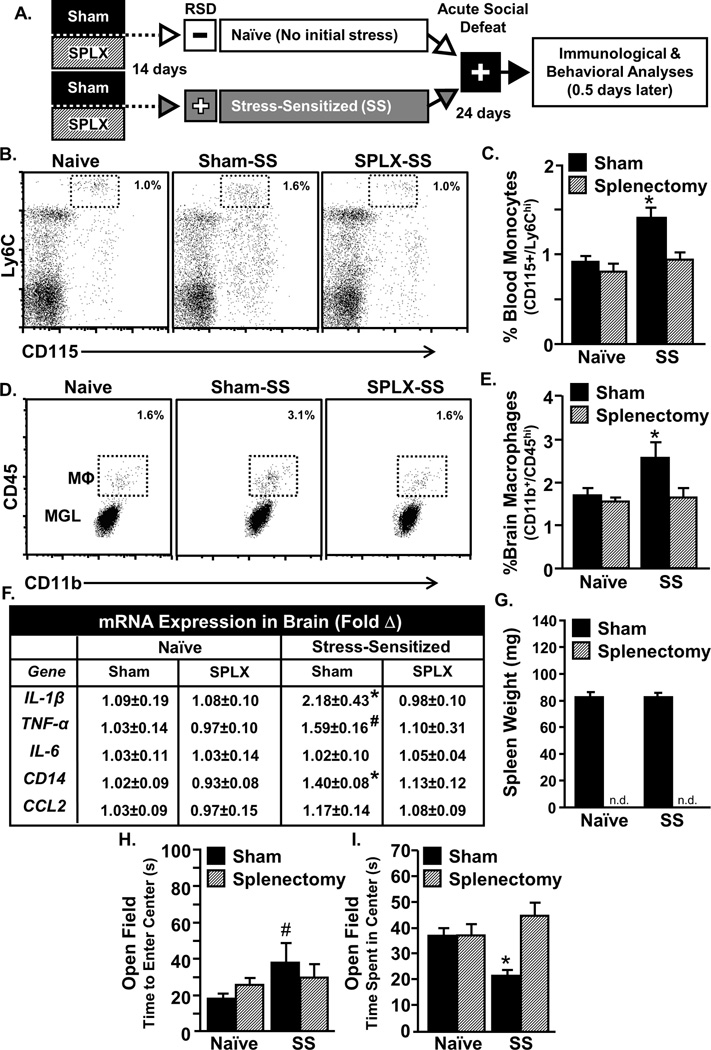
Splenectomy prior to RSD prevented recurrence of monocyte trafficking and anxiety-like behavior following acute stress in sensitized mice
Our previous study showed that removal of the spleen after RSD prevents monocyte accumulation in the brain following acute stress 24 days later [28]. It is possible, however, that other immune compartments may compensate for the spleen following splenectomy and function as alternative myeloid reservoirs [35]. Thus, the next objective was to determine if other immune reservoirs compensated for the spleen during stress. To do this, mice were splenectomized 14 days prior to RSD and then exposed to acute social defeat 24 days later (Fig.2A). Splenectomy prior to RSD prevented the recurrence of monocyte trafficking and anxiety-like behavior in SS mice. For instance, acute stress in sham-treated SS increased Ly6Chi monocytes in circulation (Fig.2B–C, p<0.05), increased brain-macrophages (Fig.2D–E, p<0.05), and increased IL-1β, TNF-α, and CD14 mRNA expression in the brain (Fig.2F, all p<0.1), all of which were prevented by splenectomy (Fig.2B–F). Moreover, prevention of monocyte trafficking to the brain corresponded with prevention of the recurrence of anxiety-like behavior. For instance, Sham-SS mice tended to take longer to enter the center (Fig.2H, p=0.1) compared to naïve Sham mice. Moreover, Sham-SS mice also had reduced time spent in the center of the open field compared to all other groups (Fig.2I, p<0.05). This anxiety-like behavior, however, was undetected in splenectomized SS mice (Fig.2H&I). These data are interpreted to indicate that other immune compartments were unable to compensate for the spleen and act as functional reservoirs of releasable monocytes following RSD.
Figure 2. Splenectomy prior to stress-sensitization prevented re-establishment of monocyte trafficking and anxiety-like behavior following subsequent exposure to acute stress.
A) Male C57BL/6 mice were subjected to sham or splenectomy (SPLX) surgery and were allowed to recover for 14 days. Mice were then stress-sensitized (SS) by 6 repeated cycles of social defeat or left undisturbed as controls (Naïve). Twenty four days later, mice were subjected to acute social defeat, anxiety-like behavior and biochemical analyses were completed 14h later. B) Representative flow Bi-variate dot plots of CD115 and Ly6C labeling of blood cells. C) The percentage of Ly6Chi monocytes was determined in blood. Monocytes in circulation were increased by acute stress in SS mice (F1,25=11.9, p<0.05) and this effect was blocked by splenectomy (tendency for interaction, F1,25=3.3, p<0.1). D) Representative flow Bi-variate dot plots of CD11b and CD45 labeling on enriched brain macrophages (MΦ) and microglia (MGL). E) The percentage of brain macrophages was determined and they were increased by acute stress (F1,25=2.9, p<0.1) and this effect was blocked by splenectomy (tendency for interaction, F1,25=3.52, p<0.1). F) Several inflammatory mediators were determined in a coronal brain section and acute social defeat increased mRNA expression of IL-1b (F1,18=2.4, p<0.1), TNFa (F1,18=2.83, p<0.1) and CD14 (F1,18=7.92, p<0.05) in Sham mice but not SPLX mice. G) Spleen weight is shown. Stress-sensitized Sham mice exhibited anxiety-like behavior in the open field with increased time to enter the center (H; tendency for main effect of sensitization, F1,25=3.2, p<0.1) and reduced time spent in the center (I; interaction effect, F1,25=6.5, p<0.05). Bars represent the mean ± SEM. Means with asterisk (*) are significantly different from CON (p<0.05) and means with (#) tended to be different from CON (p<0.1), according to F-protected post hoc analysis.
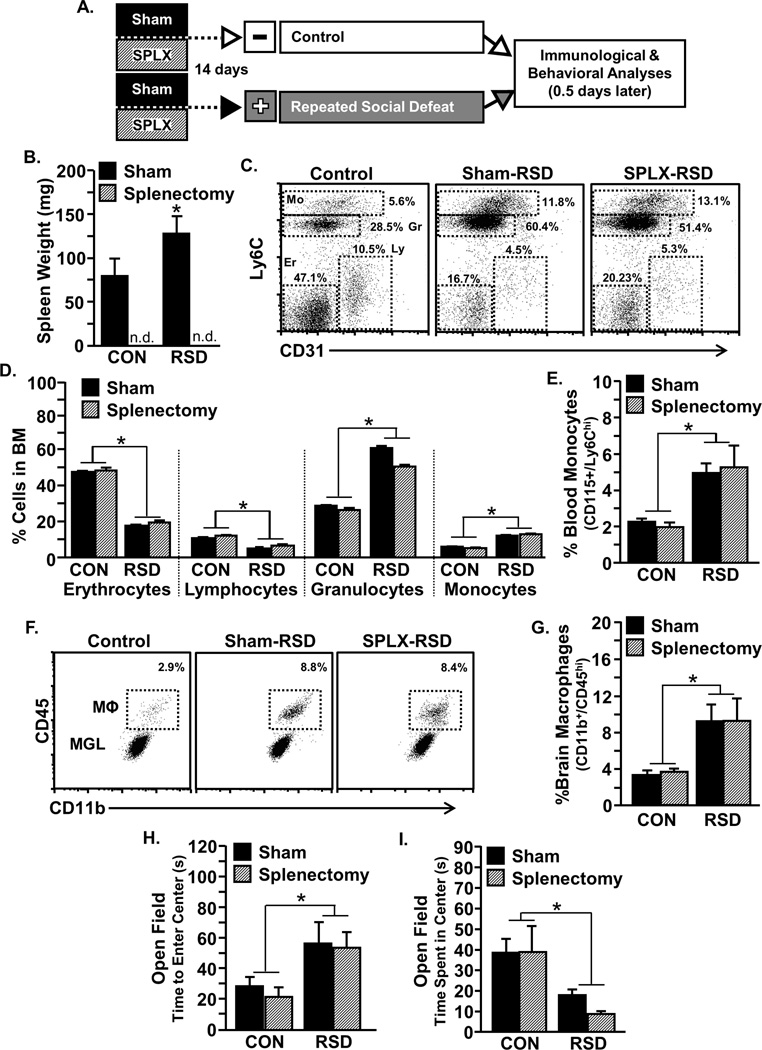
Splenectomy did not influence monocyte trafficking or anxiety-like behavior 14 hours after RSD
Our data indicate that monocyte release and anxiety-like behavior following acute stress in SS mice was dependent on the spleen (Fig.2). It is possible that the spleen was also necessary for some of the primary immune and behavioral responses to the initial exposure to RSD. To address this, mice were splenectomized prior to RSD, and behavioral and biological measures were determined 14 hours after the final cycle (Fig.3A). Consistent with substantial accumulation of primed myeloid cells in the spleen [26], RSD increased spleen weight in sham mice (Fig. 3B; p<0.05). Overall, Figure 3 shows that the primary immune and behavioral responses evident 14 h after RSD were unaltered by splenectomy. For example, there was main effect of RSD on myelopoiesis with increased monocytes and granulocytes and decreased lymphocytes and erythrocytes in the bone marrow (Fig.3C–D; all p<0.05) that was unaffected by the splenectomy. In addition, splenectomy did not prevent increased Ly6Chi monocytes in circulation following RSD (Fig.3E), did not affect the accumulation of macrophages in the brain (Fig.3F–G), and did not prevent increased brain cytokine mRNA expression of IL-1β, IL-6, TNF-α, CD14, and CCL2 (data not shown). Nor did splenectomy block the development of anxiety-like behavior 14 hours after RSD (Fig. 3H&I). Taken together the spleen was not required for the primary immune and behavioral response to RSD observed 14 hours after the last cycle.
Figure 3. Splenectomy did not influence myelopoiesis, monocyte redistribution, or the establishment of anxiety-like behavior following initial exposure to RSD.
A) Male C57BL/6 mice were subjected to sham or splenectomy (SPLX) surgery and were allowed to recover for 14 days. Mice were then exposed to repeated social defeat (RSD) or left undisturbed as controls (CON), and 14 hrs after the final cycle, anxiety-like behavior was assessed in the open field. Subsequently, brain, blood, and bone marrow were collected for analysis. B) RSD increased spleen weight in sham mice (p<0.05). Spleen weights were not detectable (n.d.) in splenectomized mice. C) Representative flow Bi-variate dot plots of CD31 and Ly6C labeling on bone marrow cells is shown. D) Independent of splenectomy, RSD decreased erythrocytes (F1,17=124.5, p<0.0001) and lymphocytes (F1,17=129.2, p<0.01) and increased monocytes (F1,17=120.0, p<0.0001) and granulocytes (F1,17=144.9, p<0.0001) in bone marrow. E) RSD increased percent Ly6Chi monocytes in circulation independent of splenoctomy (F1,18=12.0, p<0.01). F) Representative flow Bi-variate dot plots of CD11b and CD45 labeling on enriched brain macrophages (MΦ) and microglia (MGL). G) RSD increased percent brain macrophages (F1,18=10.4, p<0.01). RSD increased anxiety-like behavior in the open field with increased time to enter the center (H; F1,23=8.2, p<0. 01) and decreased time spent in the center (I; F1,23=10.2 p<0. 01). Abbreviations: Mo, Monocytes; Gr, Granulocytes; Ly, Lymphocytes; Er, Erythrocytes. Bars represent the mean ± SEM. Means with asterisk (*) are significantly different from CON (p<0.05) and means with (#) tended to be different from CON (p<0.1), according to F-protected post hoc analysis.
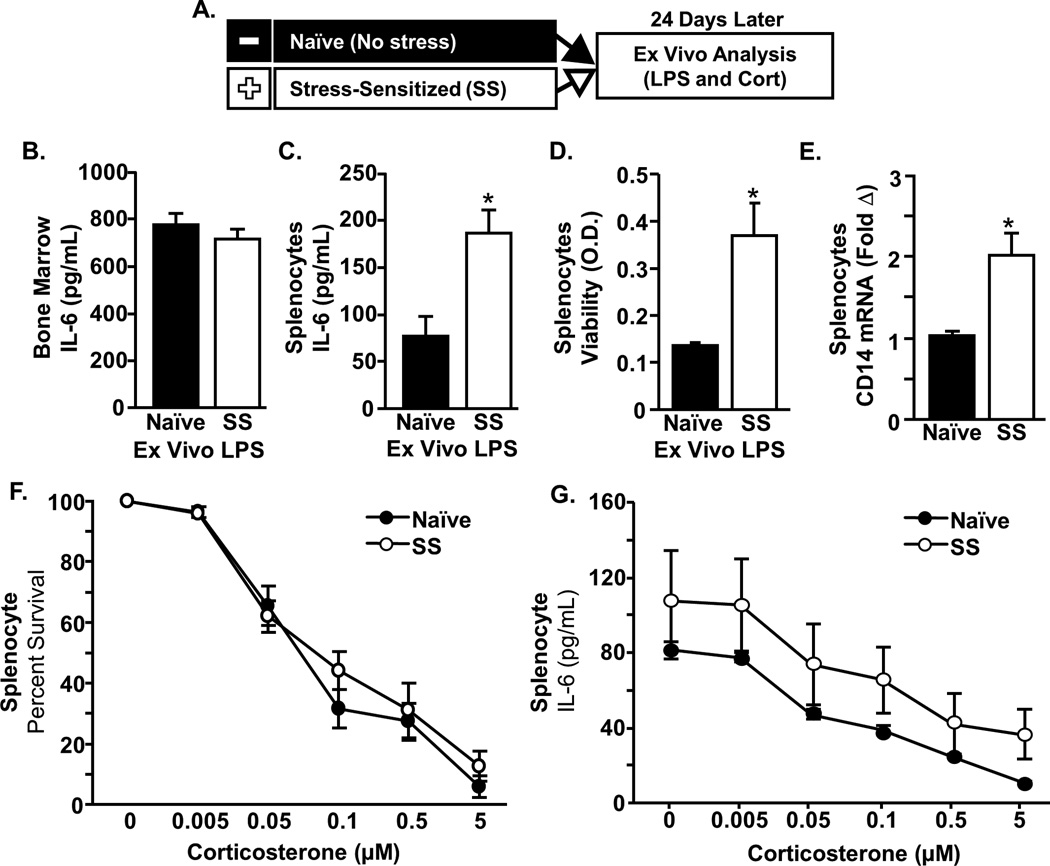
RSD increased the accumulation of primed myeloid cells in the spleen that exhibited exaggerated inflammatory response to ex vivo mitogen challenge
Data presented here demonstrate that the spleen is necessary for the maintenance of a releasable pool of monocytes following RSD. Previous reports indicated that RSD increased release and trafficking of BM-derived monocyte-lineage cells that are both primed and GC-insensitive [26, 37, 38]. For instance, our previous results showed that primed monocytes seed the spleen and retain a GC-insensitive phenotype for at least 8 days after RSD [39]. Nonetheless, the presence and phenotype of monocytes in the spleen 24 days after RSD is unknown. To address this, cytokine responses to LPS and sensitivity to GCs were assessed in spleen and bone marrow cells 24 days after RSD (Fig.4A). IL-6 production following ex vivo LPS stimulation of BM was not different between groups (Fig.4B). However, splenocytes from SS mice produced more IL-6 following LPS stimulation compared to cells from naïve mice (Fig.4C, P<0.05). Additionally, there was increased cell viability in response to LPS stimulation in splenocytes from SS mice compared to those from naïve mice (Fig. 4D p<0.05). This exaggerated splenocyte response to LPS stimulation was associated with enhanced baseline mRNA expression of CD14 (Fig. 4E, p<0.05) but not TLR4 (data not shown). Next, to determine if this primed phenotype was associated with GC-insensitivity in SS mice, the effect of increasing corticosterone concentrations on LPS-induced IL-6 production and cell viability was determined. Fig.4F&G show that increasing corticosterone concentrations reduced cell viability (Fig.4F, p<0.05) and IL-6 production (Fig.4G, p<0.05) independent of stress-sensitization. Thus, primed but not GC-insensitive monocytes were maintained in the spleen for at least 24 days after RSD.
Figure 4. Stress-sensitization resulted in the accumulation of primed splenocytes with an enhanced response to ex vivo mitogen challenge.
A) Male C57BL/6 mice were stress-sensitized (SS) by 6 repeated cycles of social defeat or left undisturbed as controls (Naïve). Twenty four days later, spleen and bone marrow (BM) cells were cultured ex vivo in the presence of lipopolysaccharide (LPS) and corticosterone (cort). IL-6 protein was determined in the cell supernatants collected 18 h after LPS in BM and Splenocytes. B) IL-6 secretion was similar between groups in bone marrow cells. C) The LPS induced IL-6 secretion was higher in splenocytes cultured from SS mice compared to naïve mice (p<0.01). D) Cell viability of LPS-stimulated splenocytes was increased in SS mice (p<0.05). E) mRNA expression of CD14 in SS splenocytes was higher than naïve (p<0.01). Next, ex vivo cultures from spleen were stimulated with LPS for 48 h in the presence of increasing concentrations of corticosterone and cell viability and IL-6 concentrations were determined. F) There was a main effect of corticosterone on viability (F5,36=6.84, p<0.0001) and G) on IL-6 production F5,36=4.42, p<0.005) that was independent of stress. F&G) There was also a main effect of stress on viability (F1,36=9.63, p<0.005) and IL-6 production (F1,36=4.69, p<0.05). Bars represent the mean ± SEM. Means with asterisk (*) are significantly different from CON (p<0.05) according to F-protected post hoc analysis.
Sympathetic inhibition prevented monocyte trafficking and the recurrence of anxiety-like behavior in SS mice
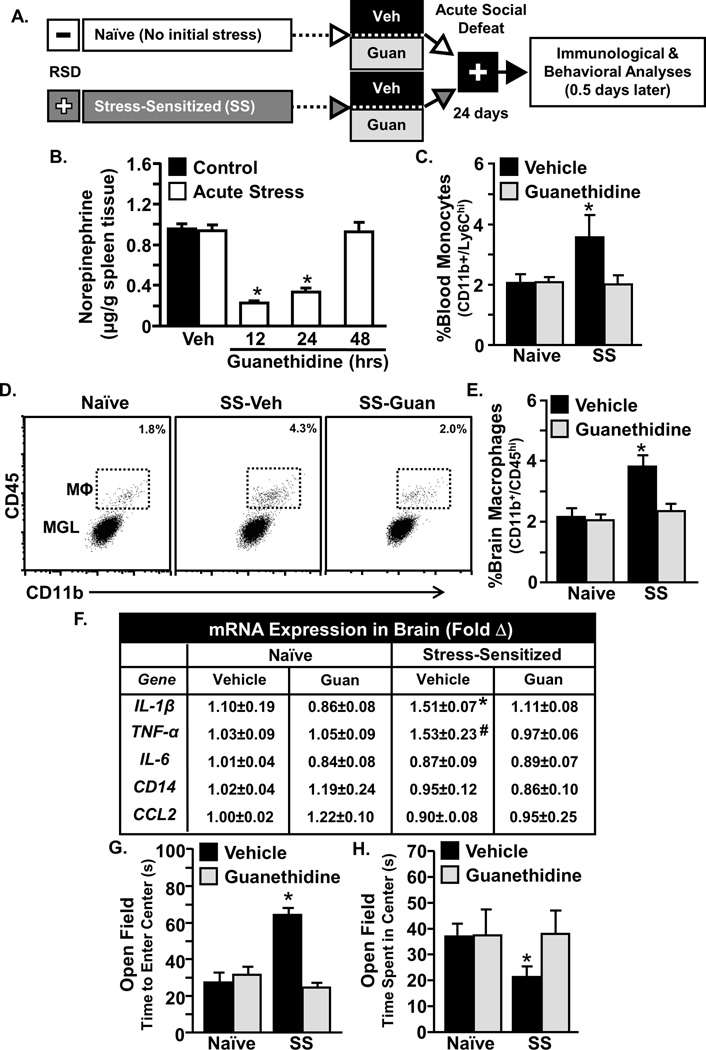
Data shown here indicate that the spleen is uniquely responsible for the increased availability of primed and releasable monocytes 24 days after RSD. Despite this, the physiological signaling pathway that initiates release of monocytes from the spleen in response to acute stress was unknown. Previous studies demonstrate a role for the SNS in the release of splenic myeloid cells [40]. Therefore, guanethidine, a peripheral sympathetic inhibitor, was used because it is a non CNS active drug that prevents the release of norepinephrine (NE) by vesicular displacement [41]. In this manner, guanethidine inhibits SNS activation in a dose-dependent manner under both homeostasis and stress.
Therefore, the effect of acute stress in RSD-sensitized mice was determined following guanethidine intervention (Fig.5A). Fig.5B confirms that guanethidine intervention significantly reduced splenic NE at 12 and 24 hours after injection in mice exposed to acute stress (Fig. 5J; p<0.05). As expected, acute stress increased Ly6Chi monocytes in circulation (Fig.5C, p<0.05) and increased CD45hi brain-macrophages in SS mice but not naïve mice (Fig.5D&E, p<0.05). This redistribution of monocytes, however, was undetected in guanethidine-treated SS mice (Fig.5C–E). Similarly, acute stress increased brain mRNA expression of IL-1β and TNF-α in vehicle-treated SS mice (Fig.3F, both p<0.05). This was also prevented by guanethidine treatment. Moreover, blockade of monocyte trafficking to the brain with guanethidine corresponded with prevention of anxiety-like behavior in SS mice. For instance, acute stress in vehicle-treated SS mice increased time to enter the center (Fig.5G, p<0.05) and reduced time spent in the center of the open field (Fig.5H, p<0.05), and neither of these behaviors were observed in guanethidine treated SS mice (Fig.5G&H). To further address the role of the SNS in the release of monocytes from the spleen, the effect of pretreatment with propranolol, a beta adrenergic receptor antagonist was determined. Propranolol pretreatment (1 h) prior to acute stress in SS mice enhanced monocyte retention in the spleen (p<0.05; Fig. S1A) and reduced the presence of Ly6Chi monocytes in circulation (Fig.S1B). Taken together, sympathetic inhibition prevented spleen-to-brain monocyte trafficking, and this corresponded with attenuated anxiety-like behavior and reduced neuroinflammatory signaling following acute stress exposure.
Figure 5. Guanethidine blocked primed monocyte trafficking from the spleen to the brain and prevented the re-establishment of anxiety in stress sensitized mice.
A) Male C57BL/6 mice were stress-sensitized (SS) by 6 repeated cycles of social defeat or left undisturbed as controls (Naïve). Mice were pretreated with guanethidine (Guan) or Vehicle (Veh) prior to acute social defeat. Fourteen hours after acute social defeat, anxiety-like behavior and biochemical analyses were completed. B) Splenic norepinephrine was determined immediately following acute stress at 12, 24, & 48 following guanethidine injections (50 mg/kg). C) The percentage of Ly6Chi monocytes was determined in blood. Acute stress increased percent Ly6Chi monocytes in SS-Veh mice but not naïve mice (interaction effect, F1,46=4.35, p<0.05). D) Representative flow Bi-variate dot plots of CD11b and CD45 labeling on enriched brain macrophages (MΦ) and microglia (MGL). E) The percentage of brain macrophages was determined and they were increased by acute stress (F1,46=14.66, p<0.0005) and this effect was blocked by guanethidine (F1,46=7.72, p<0.01). F) Several inflammatory mediators were determined in a coronal brain section and acute social defeat increased mRNA expression of IL-1b (F1,22=6.46, p<0.05) and TNFa (F1,22=3.18, p<0.1) in SS-Veh mice but not SS-Guan mice. G&H) SS vehicle treated mice exhibited anxiety-like behavior in the open field with increased time to enter the center (G; F1,46=2.42, p≤0.1; main effect of splenectomy; F1,46=5.66, p<0.05) and reduced time spent in the center (H; tendency for interaction effect, F1,46=2.42, p≤0.1). Bars represent the mean ± SEM. Bars represent the mean ± SEM. Means with asterisk (*) are significantly different from CON (p<0.05) and means with (#) tended to be different from CON (p<0.1), according to F-protected post hoc analysis.
Discussion
The results presented here demonstrate a novel and critical role for the spleen in the maintenance of stress-sensitization that persisted for 24 days after the initial sensitizing, stressful event. First, the recurrence of anxiety-like behavior was associated with increased monocyte trafficking from the spleen and increased macrophage accumulation in the brain. Next, novel data shown here indicate that the spleen was indispensable for the maintenance of primed and releasable monocytes 24 days after RSD. For example, splenectomy prior to stress-sensitization blocked monocyte re-distribution and prevented the recurrence of anxiety in stress-sensitized mice. Notably, no other organ acted as a compensatory reservoir. Additionally, splenectomy prior to RSD did not attenuate the primary immune and behavioral response to RSD observed 14 hours after the final cycle. Thus, the spleen was necessary for the maintenance of releasable monocytes 24 days after RSD but was not necessary for the initial production and trafficking of primed monocytes or the initial development of anxiety immediately after RSD. In addition, the splenic monocytes retained a primed but not GC-insensitive phenotype in stress-sensitized mice. This was interpreted to indicate that RSD primed and mobilized monocyte-lineage cells that persisted in the spleen for 24 days following cessation of the stressor. Further work addressed physiological signals that contributed to the release of monocytes from the spleen. These studies showed that pretreatment with the SNS-inhibitor, guanethidine, prevented monocyte trafficking and anxiety in stress-sensitized mice. Thus, we interpret these data to mean that sympathetic initiation of monocyte trafficking from the spleen to the brain promoted the recurrence of anxiety-like behavior in sensitized mice.
An important finding in this study was that stress-sensitization following RSD was associated with an altered myeloid composition of the spleen. First, there was a tendency for increased Ly6Chi monocytes in the spleen that persisted 24 days after exposure to RSD. Second, accumulation of monocytes in circulation and brain following acute stress in stress-sensitized mice was associated with a robust reduction in the number of Ly6Chi monocytes in the spleen. This is consistent with egress of Ly6Chi monocytes from the spleen that accumulated in circulation and brain. This re-distribution of splenic monocytes characterized here resembles studies of myocardial infarction that revealed that monocyte redistribution from the spleen contributed to myocardial pathogenesis [32]. Notably, acute stress in stress-sensitized mice also reduced the number of Ly6Chi monocytes in the BM. Nonetheless, our previous work [28] and data presented here show that cells from the spleen but not the BM are critical for increased trafficking of primed monocytes in stress-sensitized mice. Importantly, these splenic monocytes released by acute stress contribute to the recurrence of anxiety in sensitized mice. For instance, splenectomy blocked monocyte trafficking to the brain with acute stress and this corresponded with the prevention of anxiety in the open field (latency and time spent in the center).
The splenectomy studies presented here provide evidence that the spleen is not required for primary immune and behavioral responses to RSD, but rather, the spleen is necessary for the maintenance of releasable monocytes 24 days after RSD. This is an important distinction, because it implicates the BM, not the spleen, in the initial production and accumulation of monocytes immediately following RSD. These results are consistent with other studies of RSD and chronic unpredictable stress that demonstrated increased production of myeloid cells in the BM [16, 26]. In addition, RSD suppressed T-cell production in the BM independent of splenectomy. Others have reported that T-cells are capable of regulating behavior following social defeat stress [42]. This further supports the hypothesis that immune-derived signals are important regulators of stress-related behaviors. Data from RSD indicated that the monocytes that accumulate with stress are primed to be more inflammatory in response to challenges (e.g., LPS) and less sensitive to the anti-inflammatory effects of GCs [27]. Thus, we hypothesize that RSD mobilizes primed monocytes that seed the spleen and contribute to the maintenance of releasable monocytes with the ability to traffic in the brain and promote anxiety in stress-sensitized mice.
Related to the above points, data here support the hypothesis that splenic monocytes from stress-sensitized mice are inherently more reactive to neuroendocrine or immune stimulation. For instance, cells that persist in the spleen 24 days after RSD appear to have a primed profile with increased IL-6 secretion following ex vivo LPS stimulation. In contrast, BM cells from stress-sensitized mice were not more sensitive to LPS stimulation. It is important to mention that these experiments were completed with whole splenocytes, but we attribute these affects to monocytes. This is supported by previous studies showing that monocytes/macrophages were the primary cells that responded to LPS stimulation in ex vivo splenocyte cultures [43]. Although stress-sensitized mice retained a primed monocyte phenotype, they did not retain the GC-insensitive phenotype that is observed for up to eight days after RSD [27, 39]. We interpret these data to indicate that the spleen maintains a population of primed monocytes following stress-sensitization and that these cells can traffic to the brain and promote the recurrence of anxiety following acute stress many days later. Despite the evidence provided here, it is possible that enhanced splenic monocyte trafficking observed in stress-sensitized mice is mediated by neuroendocrine sensitization and was unrelated to immunomodulation. For example, fear conditioning in stress-sensitized mice might contribute to exaggerated neuroendocrine response to the acute stressor, resulting in sufficient stimulation to cause the release of splenic monocytes that traffic to the brain and promote anxiety. Nonetheless, priming of splenic monocytes was observed independent of neuronal mediation. For instance, splenic myeloid cells demonstrated increased CD14 mRNA expression and enhanced IL-6 production following ex vivo LPS stimulation. Thus persistent splenic priming was observed independent of neuroendocrine sensitization.
Another important finding was that the release of primed monocytes from the spleen of stress-sensitized mice after acute social defeat was dependent activation of the β-adrenergic receptors of the SNS. Our previous work with RSD shows that monocyte redistribution is dependent on SNS activation [25, 27, 44]. In addition, activation of the SNS specifically has been implicated in splenic monocyte egress [40]. Moreover, SNS inhibition, but not adrenalectomy, prevented myeloid redistribution following social defeat in rats [45]. While there is evidence that GCs are important for transient leukocyte redistribution following acute stress [46], data here show a primary role for the SNS in the egress of primed splenic monocytes in sensitized mice. The SNS can interact with the spleen either through circulating epinephrine or norepinephrine released from the adrenal medulla or through direct sympathetic innervation [47]. Here, we confirmed that guanethidine intervention prior to acute stress depleted splenic NE 12 and 24 hours post-injection. These results mirror previous reports that s.c. guanethidine substantially reduced splenic NE for more than 24 hours (e.g., Cass & Spriggs et al 1961). Our guanethidine intervention studies showed that monocyte release from the spleen was dependent on SNS activation. In addition, pretreatment with propranolol, a β-adrenergic receptor antagonist, prior to acute stress in SS mice enhanced monocyte retention in the spleen and reduced the presence of Ly6Chi monocytes in circulation. It should be noted that the relative contribution of circulating NE vs. direct splenic innervation cannot be discerned with data presented here. For instance, guanethidine prevents NE release in both tissue and circulation [41], and others have implicated circulating NE in splenic monocyte egress [40]. Taken together, monocyte egress from the spleen with acute stress was dependent upon SNS activation of β-adrenergic receptors.
Data here show that guanethidine blocked accumulation of Ly6Chi monocytes in circulation and blocked macrophage trafficking in the brain. In addition, this blockade corresponded with prevention of anxiety-like behavior in stress-sensitized mice. This point is of particular interest because it reveals a clinically relevant pharmacological strategy to attenuate maladaptive behaviors related to peripheral immunological sensitization. Although underappreciated, it has been reported that β-adrenergic antagonists (i.e., beta-blockers) have chronic anxiolytic effects in certain clinical populations [48] that may be related to interactions with the immune system. Thus, studies here provide a biological mechanism that supports the use of sympathetic inhibitors to abrogate recurring anxiety promoted by monocyte redistribution. Thus, we conclude that activation of the SNS is the key parameter for the release of primed monocytes from the spleen of stress-sensitized mice.
Overall, the current studies provide evidence that the spleen contributes to long term neuroimmune sensitization capable of regulating behavioral responses many days after a sensitizing stressful event. For example, the spleen acted as unique reservoir for maintaining primed monocytes following exposure to RSD. These primed monocytes were readily releasable following neuroendocrine activation by acute stress 24 days after RSD. Neuroendocrine activation by acute stress caused primed monocytes to traffic to the brain and promote the recurrence of anxiety in sensitized mice. This phenomenon may be relevant because persistent or recurring behavioral complications observed in several psychiatric populations are associated with immune activation [49]. Thus, recurring behavioral complications associated with psychological stress may be related to splenic monocyte re-distribution. Collectively, these findings reveal novel neuroimmune mechanisms that may be implicated in recurring anxiety disorders.
Supplementary Material
Acknowledgements
This study was supported by National Institute of Mental Health (NIMH) grants R01-MH093473 and R01-MH097243 to J.F.S. D.B.M. and B.L.J. were supported by a National Institute of Dental and Craniofacial Research Training Grant T32-DE014320. The authors would also like to thank Dr. Kelley Madden and Ryan Dawes (University of Rochester) for their technical assistance measuring norepinephrine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. J Consult Clin Psychol. 2003;71(4):692–700. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- 2.Faravelli C, Pallanti S. Recent Life Events and Panic Disorder. Am J Psychiat. 1989;146(5):622–626. doi: 10.1176/ajp.146.5.622. [DOI] [PubMed] [Google Scholar]
- 3.Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch Gen Psychiat. 2003;60(8):789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- 4.Kendler KS, Karkowski LM, Prescott CA. Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. The Journal of nervous and mental disease. 1998;186(11):661–669. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiat. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JRT, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiat. 1999;60(7):427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- 7.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37(1):137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105(2):751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun. 2012;25(1):6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 2011;108(7):3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110(41):16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109(16):5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller GE, Murphy ML, Cashman R, Ma R, Ma J, Arevalo JM, et al. Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain Behav Immun. 2014;41:191–199. doi: 10.1016/j.bbi.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172(7):4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 18.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beumer W, Gibney SM, Drexhage RC, Pont-Lezica L, Doorduin J, Klein HC, et al. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. J Leukoc Biol. 2012;92(5):959–975. doi: 10.1189/jlb.0212100. [DOI] [PubMed] [Google Scholar]
- 20.Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun. 2011;26(1):13–17. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- 22.Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40. doi: 10.1186/1471-244X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med. 2013;19(3):337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christoffel DJ, Golden SA, Heshmati M, Graham A, Birnbaum S, Neve RL, et al. Effects of inhibitor of kappaB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology. 2012;37(12):2615–2623. doi: 10.1038/npp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31(17):6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol. 2004;148(1–2):106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. β-adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, et al. Re-establishment of Anxiety in Stress-Sensitized Mice Is Caused by Monocyte Trafficking from the Spleen to the Brain. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33(34):13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Monocyte Trafficking to the Brain with Stress and Inflammation: A Novel Axis of Immune-to-Brain Communication that Influences Mood and Behavior. Frontiers in Neuroscience. doi: 10.3389/fnins.2014.00447. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reader BF, Jarrett BL, Mckim DB, Godbout JP, Sheridan JF. Peripheral and Central Effects of Repeated Social Defeat Stress: Monocyte Trafficking, Microglial Activation, and Anxiety. Neuroscience. doi: 10.1016/j.neuroscience.2015.01.001. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ajmo CT, Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, et al. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86(10):2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012;7(4):1017–1024. doi: 10.1007/s11481-012-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donello JE, Guan Y, Tian M, Cheevers CV, Alcantara M, Cabrera S, et al. A peripheral adrenoceptor-mediated sympathetic mechanism can transform stress-induced analgesia into hyperalgesia. Anesthesiology. 2011;114(6):1403–1416. doi: 10.1097/ALN.0b013e31821c3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav. 2001;39(4):247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- 38.Engler H, Engler A, Bailey MT, Sheridan JF. Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. J Neuroimmunol. 2005;163(1–2):110–119. doi: 10.1016/j.jneuroim.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Avitsur R, Stark JL, Dhabhar FS, Padgett DA, Sheridan JF. Social disruption-induced glucocorticoid resistance: kinetics and site specificity. J Neuroimmunol. 2002;124(1–2):54–61. doi: 10.1016/s0165-5728(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 40.Ajmo CT, Jr, Collier LA, Leonardo CC, Hall AA, Green SM, Womble TA, et al. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol. 2009;218(1):47–55. doi: 10.1016/j.expneurol.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freis ED. Guanethidine. Prog Cardiovasc Dis. 1965;8(2):183–193. doi: 10.1016/s0033-0620(65)80008-1. [DOI] [PubMed] [Google Scholar]
- 42.Brachman RA, Lehmann ML, Maric D, Herkenham M. Lymphocytes from Chronically Stressed Mice Confer Antidepressant-Like Effects to Naive Mice. Journal of Neuroscience. 2015;35(4):1530–1538. doi: 10.1523/JNEUROSCI.2278-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. Am J Physiol Regul Integr Comp Physiol. 2001;280(6):R1799–R1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- 44.Powell MJ, Thompson SA, Tone Y, Waldmann H, Tone M. Posttranscriptional regulation of IL-10 gene expression through sequences in the 3'-untranslated region. J Immunol. 2000;165(1):292–296. doi: 10.4049/jimmunol.165.1.292. [DOI] [PubMed] [Google Scholar]
- 45.Engler H, Dawils L, Hoves S, Kurth S, Stevenson JR, Schauenstein K, et al. Effects of social stress on blood leukocyte distribution: the role of alpha- and beta-adrenergic mechanisms. Journal of Neuroimmunology. 2004;156(1–2):153–162. doi: 10.1016/j.jneuroim.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells--from barracks to boulevards to battlefields: a tale of three hormones--Curt Richter Award winner. Psychoneuroendocrinology. 2012;37(9):1345–1368. doi: 10.1016/j.psyneuen.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21(6):736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Battes LC, Pedersen SS, Oemrawsingh RM, van Geuns RJ, Al Amri I, Regar E, et al. Beta blocker therapy is associated with reduced depressive symptoms 12 months post percutaneous coronary intervention. J Affect Disorders. 2012;136(3):751–757. doi: 10.1016/j.jad.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 49.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163(9):1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.