Abstract
Pulmonary vein stenosis (PVS) is rare condition characterized by a challenging diagnosis and unfavorable prognosis at advance stages. At present, injury from radiofrequency ablation for atrial fibrillation has become the main cause of the disease. PVS is characterized by a progressive lumen size reduction of one or more pulmonary veins that, when hemodynamically significant, may raise lobar capillary pressure leading to signs and symptoms such as shortness of breath, cough, and hemoptysis. Image techniques (transesophageal echocardiography, computed tomography, magnetic resonance and perfusion imaging) are essential to reach a final diagnosis and decide an appropriate therapy. In this regard, series from referral centers have shown that surgical and transcatheter interventions may improve prognosis. The purpose of this article is to review the etiology, assessment and management of PVS.
Keywords: Pulmonary vein stenosis, Pulmonary vein stenosis etiology, Pulmonary vein stenosis causes, Pulmonary vein stenosis diagnosis, Pulmonary vein stenosis management, Pulmonary vein stenosis treatment
Core tip: Several papers in literature focus either on the causes, diagnosis or treatment of pulmonary vein stenosis. However this is simple yet complete and updated review of all these matters that may guide physician’s decision making when facing a suspected or confirmed case of this unusual disease.
INTRODUCTION
Despite pulmonary vein stenosis (PVS) is an uncommon entity (estimated incidence about 2-3 cases per year in large centers)[1] its morbidity and mortality rates are high at advance stages[2]. The condition, linked in the past to congenital heart diseases in childhood and mediastinal processes (i.e., tumors) in adults, is nowadays firstly associated to injury from radiofrequency ablation (PVA) for atrial fibrillation (AF). It is essential to consider the possibility of the disease in patients at-risk to guarantee early detection (image techniques play a key role in this regard) and treatment. The aim of this article is to review the etiology, assessment and management of PVS.
ETIOLOGY
Congenital PVS
Congenital PVS is an exceptional abnormality (0.4% of congenital heart diseases) consequence of a failed incorporation of the common right and/or left PV into the left atrium (LA) during the embryologic development of the vessel that leads to partial or complete obliteration of the PVs on one or both sides[3]. From a histological point of view its main feature is an overgrowth of connective tissue with medial hypertrophy and intimal fibrosis which results in obstruction. Even though diagnosis is usually made within the first 3 years of life, it may be delayed till adulthood in some cases[3]. Congenital PVS is frequently associated (50%) with other cardiac defects[4,5], hence imaging examination protocols applied to patients with congenital heart diseases should include a systematic evaluation of the PVs (Table 1).
Table 1.
Causes of pulmonary vein stenosis
| Congenital |
| Cardiac defects associated: |
| Total anomalous pulmonary venous return |
| Septal defects |
| Transposition of the great vessels |
| Acquired |
| Pulmonary vein ablation |
| Sarcoidosis |
| Neoplasm |
| Fibrosing mediastinitis |
| Post cardiovascular surgery |
ACQUIRED PVS
PVA for AF
At the present time PVA for AF has become the principal cause of PVS. Incidence derived from recent studies reaches a mean and median of 2% and 3.1%, respectively. These figures represent a significant reduction in comparison with those reported in pioneer series (mean: 6.3% and median: 5.4%, estimated from papers published between 1999 and 2004)[5]. Main factors contributing to this finding are operator experience and improvements in the procedure [changing of ablation site from the PVs antra to ostia, reduction of temperature applied to tissue, cryoablation and intracardiac echocardiography (ICE) guidance][2]. However real occurrence of PVS is probably underestimated as screening is only performed within the first 3 mo in some centers (it has been demonstrated that PVS can occur over this time period)[6] and asymptomatic patients are not always imaged.
Mediastinal processes
Extrinsic compression by lymphadenopathies or granulomatous involvement may cause PVS in sarcoidosis[7,8].
Fibrosing mediastinitis, a rare complication of tuberculosis and Histoplasma capsulatum infection, characterized by uncontrolled fibrosis around the affected mediastinal lymph nodes, may lead to invasion and obstruction of the surrounding PVs[9].
Neoplasm adjacent to the PVs may cause stenosis due to compression or infiltration[10,11].
Cardiovascular surgery
Clinically significant PVS in pediatric population is most frequently seen after total anomalous pulmonary venous return repair (estimated incidence approximately equal 10%)[12,13]. Obliteration may be localized either at the level of the anastomosis of the PV into the LA or further into the center of the vessel. Isolated cases of PV injury leading to obstruction after myxoma resection[14], suture repair of a PV cannulation site[15] and lung transplantation[16] can be found in literature.
ASSESSMENT
PVS may be symptomatic when vein caliber is reduced significantly (> 50% stenosis), as a consequence of a raise in lobar wedge pressure, or lung perfusion is decreased by > 20%-25%[17-19]. Clinical manifestations, which in case of PVA normally appear 3-6 mo after the procedure, are clearly related to the number of PVs affected and include progressive exertional dyspnea, cough, chest pain (frequently following a pleuritic profile) and hemoptysis. Chest X ray may demonstrate signs of congestion (peribronchovascular and septal thickening, Kerlley B lines, alveolar edema) either diffuse or localized (mimicking other processes such as pneumonia), depending on the PVs involve[6]. Other findings can be found depending on the cause of the stenosis (i.e., lung size reduction in congenital PV atresia, a thoracic mass in the case of a tumor, mediastinal calcifications in fibrosing mediatinitis or calcified mediastinal lymph nodes in sarcoidosis). As the clinical picture is nonspecific, collateral flow development may mitigate symptoms[17], and occasionally physicians do not bear in mind the possibility of the disease, the diagnosis is commonly missed or delayed. Therefore screening with available imaging modalities in patients at risk (especially those with history of PVA) who develop respiratory symptoms is warranted.
Echocardiography
Transesophageal echocardiography (TEE) is a useful tool for PV investigation. Studies have shown high diagnosis accuracy for detection of PVS after PVA (sensitivity: 82%-100%, specificity: 95%-100%) compared to other techniques [computed tomography (CT), magnetic resonance imaging (MRI) and angiography][20]. Advantages of TEE are its wide availability, avoidance of radiation exposure, low cost, and applicability to patients with ferromagnetic implanted devices (i.e., pacemakers, defibrillators). There is no standard definition of PVS, nevertheless it seems that an increased maximum PV Doppler flow velocity (> 1.1 m/s) combined with color Doppler turbulence may be a reliable index[21] (Figure 1). TEE poses however noteworthy limitations including need of sedation, technical difficulties to visualize all PVs if performed by not experienced operators, non-volumetric but planar acquisition, inadequate assessment of paracardial structures, imprecise delimitation of LA-PVs junction (makes PVs ostial size a non-reliable anatomical parameter) and, even low, risk of esophageal perforation and pulmonary aspiration. 3D TEE may overcome some of these limitations.
Figure 1.

Transesophageal echocardiography of a patient with a recent left lung transplantation and severe congestion in the graft. A: Narrowing in the common trunk of the left PVs at the level of the sutures (arrow). Color Doppler and Continuous Wave Doppler demonstrate turbulent flow (B) and high velocity (C: peak velocity 2.4 m/s; peak gradient 23 mmHg) across the vessel which is consistent with a significant stenosis. A stent was successfully implanted at the level of the stenosis (D: “en face” 3D echo image view; arrow). Laminar flow (E) and normal velocities (F: peak velocity 0.8 m/s; Peak gradient 2.5 mmHg) were seen after the procedure.
ICE has been successfully used to guide PVA and evaluate PV ostial narrowing. The invasive nature of the technique restricts however its use to patients undergoing a redo PVA. Although diagnostic accuracy of ICE has not been investigated, an increased peak Doppler flow velocity over 1.6 m/s is consistent with PVS according to initial experiences[22].
CT
CT allows assessment of the extension of mediastinal neoplastic and non tumoral diseases infiltrating or compressing the PVs and enables the diagnosis of PVS after PVA by directly depicting vessel diameter (significant stenosis > 50%)[3,4] (Figure 2). Although the choice of CT protocol depends on daily practice in every center ECG gated scanning improves quality and allows postprocessing with 3D reconstruction software which permits better evaluation of the PVs ostia. The main benefits of CT are short examination time, multiplanar views and high spatial resolution, whereas disadvantages include patient exposition to ionizing radiation and need of intravenous iodine contrast agents that might impair renal function in vulnerable individuals. PVs (typically the left inferior) can be compressed between the LA and the descending aorta appearing stenotic (pseudostenosis). Differential diagnosis can be made measuring PV caliber in every phase of the cardiac cycle (fixed in cases of true PVS and variable in pseudostenosis) or imaging the patient in prone position (this maneuver eliminates LA compression and therefore pseudoestenosis) when either a multiphase scan has not been performed or findings are inconclusive[23].
Figure 2.

Computed tomography of a patient undergone radiofrequency ablation two months before and recent onset of dyspnea on exertion. A: Absent of contrast (arrow) in the left lower pulmonary vein (complete occlusion); B: Extensive infiltrate within the left lung (arrow) cause by localized edema; C and D: After stent implantation (arrows) flow was successfully restored.
MRI
MRI is diagnostic in most cases by analyzing PV anatomy (MR angiography) and flow dynamics (MR phase contrast imaging; velocity and gradients across the vessel)[24,25] (Figure 3). This modality can be also used to evaluate congenital cardiopathies and processes in the vicinity of the heart associated with a PVS (i.e., neoplasm). The main advantage of MRI over CT is that it does not expose the patient to radiation. Nevertheless drawbacks are considerable: Spatial resolution is lower than CT, it is contraindicated in patients with implanted non-compatible metal devices, and it may not be possible to perform in individuals with claustrophobia, unable to cooperate, large body habitus or severe renal impairment when gadolinium contrast is needed. Additionally, scanning time is considerably long.
Figure 3.
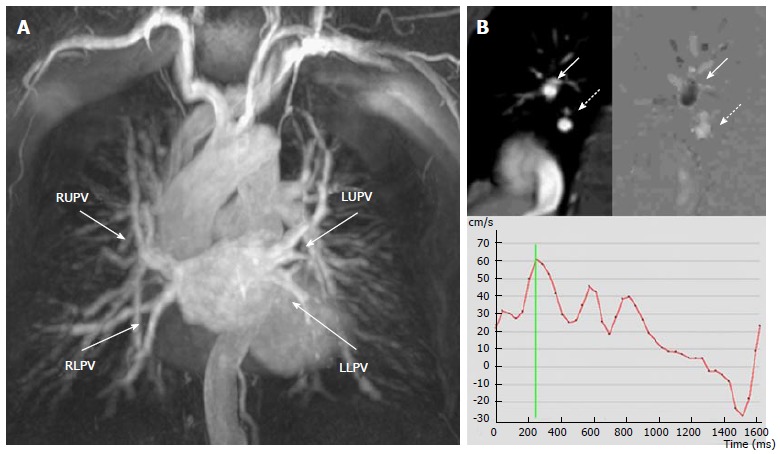
Magnetic resonance scan of a patient with a radiofrequency ablation procedure one month before, mild hemoptysis and fever. A: Angiography shows normal caliber of the four PVs; B: Phase contrast imaging of the right lower PV. Top left: right pulmonary artery (arrow) and right lower PV (dashed arrow). Top right: Flow map. Black or white signal depends on the direction of the flow. The PV “white flow” (dashed arrow) compares with the opposite direction of flow in the pulmonary artery seen in the same image that is “black” (arrow). Bottom: the resulting velocity-time curve demonstrates normal flow morphology and velocities in the PV. Significant PVS was excluded. RUPV: Right upper pulmonary vein; RLPV: Right lower pulmonary vein; LUPV: Left upper pulmonary vein; LLPV: Left lower pulmonary vein; PV: Pulmonary vein.
Perfusion imaging
Perfusion of a pulmonary lobe draining to a PV with a significant stenosis may be decreased and detected using radionuclide quantitative pulmonary flow imaging (TC99m macroaggregated albumin) (Figure 4). This test however is not valuable for an etiological diagnosis of a PVS, may be altered in other pathologies that decreased lobar perfusion (i.e., pulmonary thromboembolism), is not suitable for detection of < 50% stenosis[26] and may be inaccurate if significant compensating ipsilateral PV flow is present. Moreover, even small, it implicates radiation exposure (Table 2).
Figure 4.

Radionuclide lung ventilation/perfusion scan performed three months after radiofrequency ablation in a patient with shortness of breath. A and B: Normal ventilation; C and D marked hypoperfusion within the left lung consistent with significant left PV stenosis which was demonstrated on a CT scan. PV: Pulmonary vein; CT: Computed tomography.
Table 2.
Advantages of imaging modalities used for pulmonary vein stenosis evaluation
| TEE | CT | MRI | VQ | |
| Availability | Yes | Yes | No | No |
| Non-invasive | No1 | Yes | Yes | Yes |
| Caliber assessment | No | Yes | Yes | No |
| Functional assessment | Yes | No | Yes | Yes |
| Evaluation of surrounding tissues | No | Yes | Yes | No |
| Radiation avoidance | Yes | No | Yes | No |
TEE is generally considered a semi-invasive technique. TEE: Transesophagueal echocardiography; CT: Computed tomography; MRI: Magnetic resonance imaging; VQ: Ventilation perfusion scan.
Summing up, clinical manifestations and imaging test are the key elements in PVS assessment. Choice of imaging modality depends on availability, experience, and patient characteristics. Despite current guidelines do not provide a recommendation for frequency and duration of imaging screening in case of PVA most electrophysiology (EP) labs suggest a follow up test within 3-6 mo after the procedure in order to detect significant iatrogenic PVS at an early stage and avoid its sequelae.
MANAGEMENT
PVS in pediatric population
Mild and asymptomatic PVS may not need intervention; clinical and image surveillance is advised as the disease can evolve over time. Surgery is the preferred approach in most congenital or acquired significant symptomatic PVS. The conventional interventions (Figure 5) include: (1) endarterectomy (excision of the stenotic ring and direct anastomosis of the PV to the LA endocardium); and (2) pericardial patch venoplasty (resection of the stenotic tissue and patch anastomosis to enlarge the tightened segment). The newer sutureless marsupialization technique (the pericardium surrounding the affected PV is directly attached to the LA so direct stiches over the cut edges of the vessel are avoided) can help to prevent deformation of the suture line and reduce tissue growth stimulus decreasing therefore restenosis risk[27]. Overall, published surgical outcomes are modest; only half of cases are free from reintervention or death at 5 years[27,28]. Pneumectomy may be mandatory in cases of severe or uncontrolled hemoptysis and lung transplantation has been performed in patients with relentless PVS progression and severe pulmonary hypertension[29]. There is limited experience with percutaneous interventions in childhood; angioplasty is technically challenging (high pressures are needed to released stenosis and in case of stent implantation prosthesis should allow future expansion to adult dimension (> 12 mm) and results are suboptimal (repeated dilatations are frequently needed as instent restenosis rate is high)[30].
Figure 5.
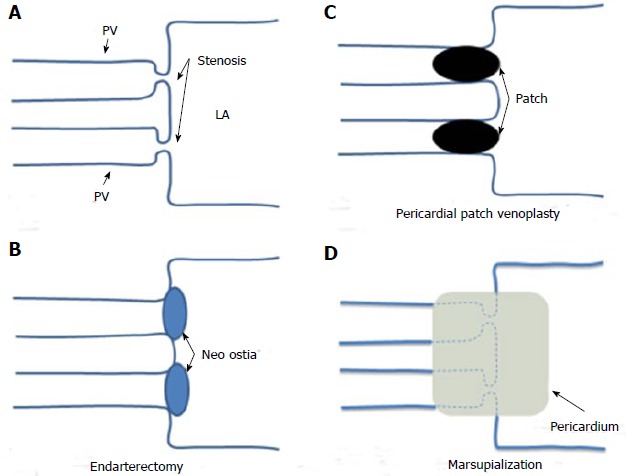
Surgical techniques for pulmonary veins. A: Schematic representation of a bilateral pulmonary vein stenosis at the ostia of the vessels; B: Endarterectomy; the stenotic tissue has been excised and the PVs directly anastomosed to the LA; C: Pericardial patch venoplasty; the stenotic tissue has been resected and a pericardial patch anastomosis has been used to enlarge the tightened ostia of the vessels; D: Sutureless marsupialization: the veins ostia have been incised longitudinally, excess fibrotic tissue has been excised and in situ pericardial flaps have been sewn directly to the left atrium so direct stiches over the cut edges of the pulmonary veins are avoid. PV: Pulmonary vein; LA: Left atrium.
PVS in adult population
Transcatheter therapy is the most common chosen approach (Figure 6). While evidence of treatment of PVS due to extrinsic compression, infiltration or cardiac surgery is restricted to cases reports in literature[16] several small studies have evaluated the efficacy of percutaneous interventions for PVS after PVA. There are discrepancies among EP labs about management of asymptomatic PVS. Despite most authors recommend clinical and imaging monitoring every 3-6 mo in patients with 50%-85% stenosis, some promote angioplasty if a single stenosis > 75%[17] and others in cases of a cumulative stenosis index (average stenosis of the PVs of one site) > 75%[18]. Main arguments for early intervention are: Inadequate recovery of lung perfusion at advance stages caused by fixed venoconstriction leading to permanent pulmonary hypertension; and fast progression to PV occlusion in some cases which may be difficult to amend. Regarding the technique itself stenting appears better than isolated balloon venoplasty in terms of vessel restenosis (60% vs 36% for PV over 8 mm)[5]. Mid to long term patency is directly related to vessel size with higher rates of restenosis observed in PV < 1 cm. Drug eluting stents may have a better restenosis profile than conventional bare metal stent however studies regarding their use this scenario are scarce[31].
Figure 6.
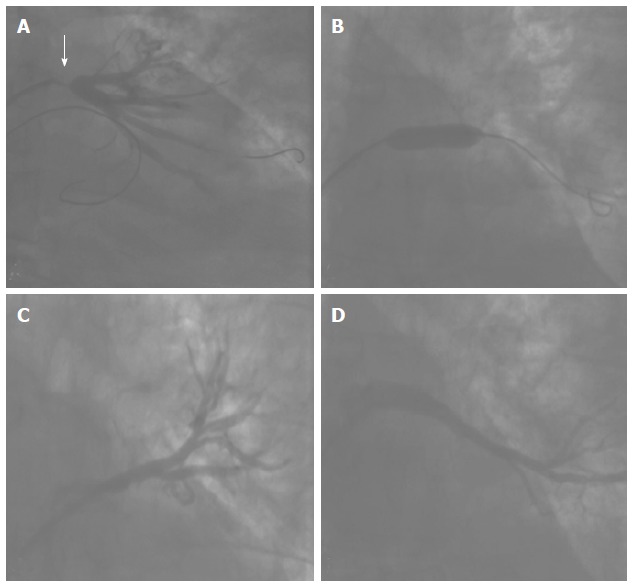
Stent implantation in a pulmonary vein stenosis. A: Angiography showing a critical stenosis in the ostium of the left lower pulmonary vein; B: Bare metal stent release; C and D: Final result. The stenosis was resolved. Normal flow can be seen in the main superior (C) and inferior (D) branches of the vein.
Limited data about antithrombotic regimes are available: (1) anticoagulation with warfarin, with an international normalized ratio target of 2-3, is generally recommended for at least 12 mo in the case of stents > 1 cm and indefinitely for those smaller[19]; (2) dual antiplatelet therapy, added to anticoagulation, with aspirin plus clopidogrel is usually prescribed for a minimum of 3 mo, however optimal duration is not known; and (3) new oral anticoagulants (dabigatran, rivaroxaban, apixaban) or antiaggregants (prasugrel, ticagrelor) have not been tested.
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 2, 2015
First decision: August 22, 2015
Article in press: November 25, 2015
P- Reviewer: Ennezat PV, Peteiro J S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Latson LA, Prieto LR. Congenital and acquired pulmonary vein stenosis. Circulation. 2007;115:103–108. doi: 10.1161/CIRCULATIONAHA.106.646166. [DOI] [PubMed] [Google Scholar]
- 2.Maan A, Shaikh AY, Mansour M, Ruskin JN, Heist EK. Complications from catheter ablation of atrial fibrillation: a systematic review. Crit Pathw Cardiol. 2011;10:76–83. doi: 10.1097/HPC.0b013e318224b7bd. [DOI] [PubMed] [Google Scholar]
- 3.Porres DV, Morenza OP, Pallisa E, Roque A, Andreu J, Martínez M. Learning from the pulmonary veins. Radiographics. 2013;33:999–1022. doi: 10.1148/rg.334125043. [DOI] [PubMed] [Google Scholar]
- 4.Edwards JE. Congenital stenosis of pulmonary veins. Pathologic and developmental considerations. Lab Invest. 1960;9:46–66. [PubMed] [Google Scholar]
- 5.Rostamian A, Narayan SM, Thomson L, Fishbein M, Siegel RJ. The incidence, diagnosis, and management of pulmonary vein stenosis as a complication of atrial fibrillation ablation. J Interv Card Electrophysiol. 2014;40:63–74. doi: 10.1007/s10840-014-9885-z. [DOI] [PubMed] [Google Scholar]
- 6.Packer DL, Keelan P, Munger TM, Breen JF, Asirvatham S, Peterson LA, Monahan KH, Hauser MF, Chandrasekaran K, Sinak LJ, et al. Clinical presentation, investigation, and management of pulmonary vein stenosis complicating ablation for atrial fibrillation. Circulation. 2005;111:546–554. doi: 10.1161/01.CIR.0000154541.58478.36. [DOI] [PubMed] [Google Scholar]
- 7.Padia SA, Budev M, Farver CF, Mohammed TL. Intravascular sarcoidosis presenting as pulmonary vein occlusion: CT and pathologic findings. J Thorac Imaging. 2007;22:268–270. doi: 10.1097/RTI.0b013e3180437e3f. [DOI] [PubMed] [Google Scholar]
- 8.Gomes M, Bendaoud S, Wemeau-Stervinou L, Faivre JB, Duhamel A, Wallaert B, Remy J, Remy-Jardin M. Prevalence of Venoatrial Compression by Lymphadenopathy in Sarcoidosis. J Thorac Imaging. 2015;30:268–273. doi: 10.1097/RTI.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 9.Albers EL, Pugh ME, Hill KD, Wang L, Loyd JE, Doyle TP. Percutaneous vascular stent implantation as treatment for central vascular obstruction due to fibrosing mediastinitis. Circulation. 2011;123:1391–1399. doi: 10.1161/CIRCULATIONAHA.110.949180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamzeh I, Rashid A, Shaib F, Dawn B. Pulmonary vein stenosis due to a compressive malignant tumor detected by transesophageal echocardiography. Circulation. 2011;123:349–350. doi: 10.1161/CIRCULATIONAHA.110.958082. [DOI] [PubMed] [Google Scholar]
- 11.Morjaria JB, Choong CK, Amsha K, Stewart S, Wells FC, Rintoul RC. Small cell lung cancer mimicking a pulmonary venous angiosarcoma. Thorax. 2009;64:827–828. doi: 10.1136/thx.2008.109306. [DOI] [PubMed] [Google Scholar]
- 12.Caldarone CA, Najm HK, Kadletz M, Smallhorn JF, Freedom RM, Williams WG, Coles JG. Relentless pulmonary vein stenosis after repair of total anomalous pulmonary venous drainage. Ann Thorac Surg. 1998;66:1514–1520. doi: 10.1016/s0003-4975(98)00952-7. [DOI] [PubMed] [Google Scholar]
- 13.Hancock Friesen CL, Zurakowski D, Thiagarajan RR, Forbess JM, del Nido PJ, Mayer JE, Jonas RA. Total anomalous pulmonary venous connection: an analysis of current management strategies in a single institution. Ann Thorac Surg. 2005;79:596–606; discussion 596-606. doi: 10.1016/j.athoracsur.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Rosetti M, Tighe DA, Chandok D, Gammie JS, Griffith BP, Folland ED. An unusual cause of pulmonary vein stenosis: a case report and review of the literature. Echocardiography. 2006;23:685–688. doi: 10.1111/j.1540-8175.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 15.Booher AM, Bach DS. Acquired pulmonary vein stenosis: one problem, two mechanisms. J Am Soc Echocardiogr. 2010;23:904.e1–904.e3. doi: 10.1016/j.echo.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Pazos-López P, Piñeiro-Portela M, Bouzas-Mosquera A, Peteiro-Vázquez J, Vázquez-Gonzalez N, Rueda-Nuñez F, Duro-Tacón J, Fernández-Prado R, Martínez-Sapiña MJ, Castro-Beiras A. Images in cardiovascular disease. Pulmonary vein stenosis after lung transplantation successfully treated with stent implantation. Circulation. 2010;122:2745–2747. doi: 10.1161/CIRCULATIONAHA.110.973370. [DOI] [PubMed] [Google Scholar]
- 17.Holmes DR, Monahan KH, Packer D. Pulmonary vein stenosis complicating ablation for atrial fibrillation: clinical spectrum and interventional considerations. JACC Cardiovasc Interv. 2009;2:267–276. doi: 10.1016/j.jcin.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Di Biase L, Fahmy TS, Wazni OM, Bai R, Patel D, Lakkireddy D, Cummings JE, Schweikert RA, Burkhardt JD, Elayi CS, et al. Pulmonary vein total occlusion following catheter ablation for atrial fibrillation: clinical implications after long-term follow-up. J Am Coll Cardiol. 2006;48:2493–2499. doi: 10.1016/j.jacc.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 19.Baranowski B, Saliba W. Our approach to management of patients with pulmonary vein stenosis following AF ablation. J Cardiovasc Electrophysiol. 2011;22:364–367. doi: 10.1111/j.1540-8167.2010.01981.x. [DOI] [PubMed] [Google Scholar]
- 20.Stavrakis S, Madden GW, Stoner JA, Sivaram CA. Transesophageal echocardiography for the diagnosis of pulmonary vein stenosis after catheter ablation of atrial fibrillation: a systematic review. Echocardiography. 2010;27:1141–1146. doi: 10.1111/j.1540-8175.2010.01250.x. [DOI] [PubMed] [Google Scholar]
- 21.Yu WC, Hsu TL, Tai CT, Tsai CF, Hsieh MH, Lin WS, Lin YK, Tsao HM, Ding YA, Chang MS, et al. Acquired pulmonary vein stenosis after radiofrequency catheter ablation of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2001;12:887–892. doi: 10.1046/j.1540-8167.2001.00887.x. [DOI] [PubMed] [Google Scholar]
- 22.Ren JF, Marchlinski FE, Callans DJ, Zado ES. Intracardiac Doppler echocardiographic quantification of pulmonary vein flow velocity: an effective technique for monitoring pulmonary vein ostia narrowing during focal atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2002;13:1076–1081. doi: 10.1046/j.1540-8167.2002.01076.x. [DOI] [PubMed] [Google Scholar]
- 23.Shroff GS, Guirguis MS, Ferguson EC, Oldham SA, Kantharia BK. Re: CT imaging of complications of catheter ablation for atrial fibrillation. A reply. Clin Radiol. 2014;69:e369. doi: 10.1016/j.crad.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Kluge A, Dill T, Ekinci O, Hansel J, Hamm C, Pitschner HF, Bachmann G. Decreased pulmonary perfusion in pulmonary vein stenosis after radiofrequency ablation: assessment with dynamic magnetic resonance perfusion imaging. Chest. 2004;126:428–437. doi: 10.1378/chest.126.2.428. [DOI] [PubMed] [Google Scholar]
- 25.Dill T, Neumann T, Ekinci O, Breidenbach C, John A, Erdogan A, Bachmann G, Hamm CW, Pitschner HF. Pulmonary vein diameter reduction after radiofrequency catheter ablation for paroxysmal atrial fibrillation evaluated by contrast-enhanced three-dimensional magnetic resonance imaging. Circulation. 2003;107:845–850. doi: 10.1161/01.cir.0000048146.81336.1d. [DOI] [PubMed] [Google Scholar]
- 26.Nanthakumar K, Mountz JM, Plumb VJ, Epstein AE, Kay GN. Functional assessment of pulmonary vein stenosis using radionuclide ventilation/perfusion imaging. Chest. 2004;126:645–651. doi: 10.1378/chest.126.2.645. [DOI] [PubMed] [Google Scholar]
- 27.Shi G, Zhu Z, Chen H, Zhang H, Zheng J, Liu J. Surgical repair for primary pulmonary vein stenosis: Single-institution, midterm follow-up. J Thorac Cardiovasc Surg. 2015;150:181–188. doi: 10.1016/j.jtcvs.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Devaney EJ, Chang AC, Ohye RG, Bove EL. Management of congenital and acquired pulmonary vein stenosis. Ann Thorac Surg. 2006;81:992–995; discussion 995-996. doi: 10.1016/j.athoracsur.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Mendeloff EN, Spray TL, Huddleston CB, Bridges ND, Canter CB, Mallory GB. Lung transplantation for congenital pulmonary vein stenosis. Ann Thorac Surg. 1995;60:903–906; discussion 907. doi: 10.1016/0003-4975(95)00543-t. [DOI] [PubMed] [Google Scholar]
- 30.Tomita H, Watanabe K, Yazaki S, Kimura K, Ono Y, Yagihara T, Echigo S. Stent implantation and subsequent dilatation for pulmonary vein stenosis in pediatric patients: maximizing effectiveness. Circ J. 2003;67:187–190. doi: 10.1253/circj.67.187. [DOI] [PubMed] [Google Scholar]
- 31.De Potter TJ, Schmidt B, Chun KR, Schneider C, Malisius R, Nuyens D, Ouyang F, Kuck KH. Drug-eluting stents for the treatment of pulmonary vein stenosis after atrial fibrillation ablation. Europace. 2011;13:57–61. doi: 10.1093/europace/euq419. [DOI] [PubMed] [Google Scholar]


