Abstract
This clinical study investigated plasma NT-proBNP levels as a potential predictor of heart failure in pediatric patients with sepsis. Plasma NT-ProBNP levels of 211 pediatric patients with sepsis and 126 healthy children were measured. Patients were stratified as with heart failure (HF) or without heart failure (non-HF). Patients were graded as having sepsis, severe sepsis, or septic shock. The optimal cut-off values of plasma NT-ProBNP for heart failure were determined by analyzing the receiver operating characteristic (ROC). In the HF, non-HF and control groups, the median plasma NT-proBNP levels were 3640, 656, and 226 ng/L, respectively. For all patients with sepsis, the optimal diagnostic cut-off value was 1268 ng/L for differentiating heart failure. In the severe sepsis patients and septic shock patients, the optimal diagnostic cut-off values were 1368 ng/L and 1525 ng/L, respectively. This report is the first one to reveal that NT-proBNP may predict heart failure in children with sepsis. It provides an important clinical reference for the diagnosis of heart failure in pediatric patients with sepsis, and enables monitoring septic children for cardiac involvement.
Introduction
Plasma NT-proBNP levels are often used as a biological marker of heart failure [1–8]. Heart failure may be diagnosed in adults younger than 50 years when NT-proBNP levels are >450 ng/L [9], and in children up to 14 years if ≥500 ng/L [10]. Higher-than-normal NT-proBNP levels are also a sign of systolic and diastolic dysfunction [11–14]. However, although the association between sepsis and cardiac dysfunction is well established [15], the response of plasma NT-proBNP to a septic condition has not been clearly demonstrated. Furthermore, patients with sepsis often have cardiovascular or other organ dysfunction, but a specific correlation between heart failure and NT-ProBNP in these patients is not clear. Therefore, when sepsis is present it is difficult to determine the relevancy of NT-proBNP for diagnosing heart failure.
This study delineated the association between plasma NT-proBNP levels and sepsis in children, and determined the appropriate cut-off values of plasma NT-proBNP for predicting heart failure in pediatric patients with sepsis.
Materials and Methods
The Ethics Committee of Affiliated Shunde Women and Children’s Hospital of Jinan University and First Affiliated Hospital of Zhongshan University approved the study. All participants provided their written informed consent before the start of the research. We also obtained the written informed consent from the next of kin, caretakers, or guardians on behalf of the children enrolled in our study.
Subjects
All subjects were recruited between June 2010 and June 2014 from Affiliated Shunde Women and Children’s Hospital of Jinan University and First Affiliated Hospital of Zhongshan University. Sepsis was defined by the International Consensus Conference on Pediatric Sepsis (2005) [16]. Heart failure was rated by the modified Ross score. In detail, the modified Ross scores for heart failure were: 0–2, none; 3–6, mild; 7–9, moderate; and 10–12, severe (Table 1) [17, 18]. Sepsis and heart failure were diagnosed by clinicians and confirmed by their senior colleagues without reference to NT-proBNP data.
Table 1. Modified Ross Score.
| 0 | +1 | +2 | ||
|---|---|---|---|---|
| Diaphoresis | Head only | Head & body a | Head & body b | |
| Tachypnea | Rare | Several times | Frequent | |
| Physical examination | Breathing | Normal | Retractions | Dyspnea |
| Respiratory rate, breathes/min | 0–1 y | <50 | 50–60 | >60 |
| 1–6 y | <35 | 35–45 | >45 | |
| 7–10 y | <25 | 25–35 | >35 | |
| 11–14 y | <18 | 18–28 | >28 | |
| Heart rate, beats/min | 0–1 y | <160 | 160–170 | >170 |
| 1–6 y | <105 | 105–115 | >115 | |
| 7–10 y | <90 | 90–100 | >100 | |
| 11–14 y | <80 | 80–90 | >90 | |
| Hepatomegaly size, cm | <2 | 2–3 | >3 |
Total score: 0–2 (no HF), 3–6 (mild HF), 7–9 (moderate HF), 10–12 (severe HF)
a At exertion
b at rest
We consecutively enrolled 211 pediatric patients with sepsis. In complex congenital heart disease, the natriuretic peptide cut-offs for heart failure could differ from that of patients with structurally normal hearts, and therefore patients with complex congenital heart disease were excluded. Also excluded were patients with Kawasaki disease, nephropathy syndrome, nephritis, coronary heart disease, genetic cardiomyopathy, or pulmonary arterial hypertension disease, because these conditions affect plasma NT-proBNP levels. A control group of 126 healthy children were recruited.
Sepsis patients were divided into those with heart failure (HF group, n = 66) and those without heart failure (non-HF group, n = 145). Patients were also categorized as having sepsis, severe sepsis, or septic shock.
Laboratory assays
The plasma NT-proBNP levels were measured and interpreted in a blind manner. Measurement of the NT-proBNP levels was fully automated to eliminate human error. A 2-mL venous blood sample was collected in a tube containing EDTA-K2. Blood samples from the sepsis patients were drawn on admission to the hospital; samples from the control group were drawn during routine check-ups.
The plasma NT-ProBNP concentration was measured by a RAMP fluorescence quantitative analyzer (Model YZB/CAN 91001, Canada Response Biomedical, Burnaby, Canada). The serum C reaction protein (CRP) concentration was measured using the turbidity method, and procalcitonin (PCT) was measured by using an automatic biochemical analyzer (Roche Diagnostics, model p800, Basel, Switzerland). Tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL6) concentrations were measured with a chemical luminescence immunity analyzer (Siemens Medical Diagnostic Products, model Lknf1, Berlin, Germany).
Heart failure was diagnosed if the patient had a Ross score between 3 and 12, ejection fraction was < 50% (systolic heart), or E peak/A peak was < 1 (diastolic heart failure). The cut-off value of NT-ProBNP for heart failure diagnosis was estimated from the receiver operating characteristic (ROC) curve for plasma NT-ProBNP.
Statistical analyses
The statistical analyses were performed with SPSS version 17.0 statistical software (SPSS, Chicago, IL, USA). Figures were generated using GraphPad software. (GraphPad Software, La Jolla, USA).
K-S testing was used to investigate the distribution of plasma NT-ProBNP levels in both the experimental and control groups. The results showed that distributions were not normal (Z = 2.701, P < 0.001; Z = 1.402, P = 0.041). The Kruskal Wallis test was used to compare the plasma NT-proBNP levels in patients of the sepsis HF, sepsis non-HF, and healthy control groups; and for the plasma NT-proBNP levels for patients with mild, moderate, and severe HF. Spearman’s test was applied for the correlation analysis between plasma NT-proBNP levels and heart rate, breath rate, liver enlargement, ejection fraction, and modified Ross score in all HF patients.
ROC curves were employed to determine the optimal cut-off values of plasma NT-ProBNP for heart failure in patients with sepsis, severe sepsis, or septic shock. The area under the ROC curve (AUC), sensitivity, specificity, positive and negative likelihood ratios, and 95% confidence intervals were calculated for the cutoff values. A probability of ≤ 0.05 was taken as significant.
Results
The ages and genders of the sepsis (n = 211) and control (n = 126) groups were statistically similar (Table 1). Among the 211 sepsis patients, 85, 74, and 52 were determined to have sepsis, severe sepsis, and septic shock, respectively. Of the 211 patients, 66 had HF (31.3%), and 145 (68.7%) were in the non-HF group. Of the 66 HF patients, 6 (7.1%) had sepsis, 25 (33.8%) had severe sepsis, and 35 (67.3%) had septic shock. All HF patients had left ventricle systolic dysfunction (systolic HF) with an ejection fraction < 50% (50–75% is considered normal), and no diastolic HF (ratios of the E-to-A peaks were >1 and < 3; 1–3 considered normal) in the heart Doppler examination [19, 20]. Gender- and age-matched healthy subjects (65 boys and 61 girls) had an average age of 4.1 years.
The primary symptoms of sepsis included severe lower respiratory tract infection (n = 89), meningoencephalitis (n = 29), viral myocarditis (n = 23), severe salmonella enteritidis (n = 9), severe pancreatitis (n = 9), toxic bacillary dysentery (n = 8), cellulitis (n = 8), severe entero virus 71 infection (n = 7), liver abscess (n = 6), infective endocarditis (n = 5), postoperative appendicitis (n = 5), cholangitis (n = 4), severe burn complicated by infection (n = 3), severe trauma (n = 2), lung abscess (n = 2), and urinary tract infection (n = 2).
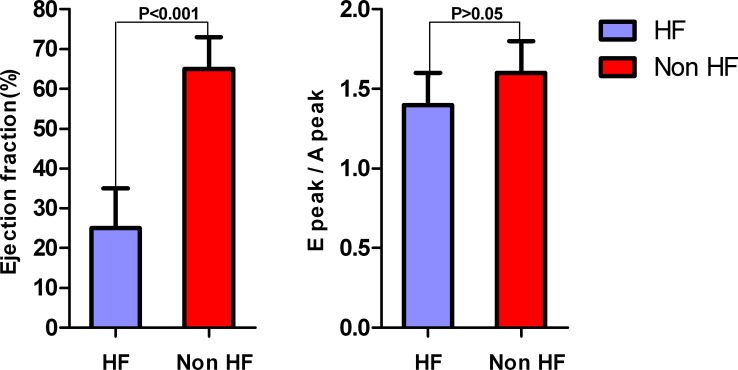
The ultrasonic cardiogram results at first administration are shown in Table 2. The ejection fractions in HF and non-HF patients were 25% ± 10.4% and 65% ± 7.5%, respectively, a significant difference (P < 0.001). The E peak/A peak of the 2 groups were normal and not significantly different (1.4 ± 0.2 cf. 1.6 ± 0.2). The results suggested a left ventricle systolic dysfunction in all HF patients (Fig 1).
Table 2. Characteristics of pediatric sepsis patients at administration.
| All patients | HF | Non-HF | P | |
|---|---|---|---|---|
| Number of subjects | 211 | 66 | 145 | — |
| Age, y | 3.6 ± 1.3 | 3.6 ± 1.5 | 3.6 ± 1.2 | 0.25 |
| Male % | 51.5 | 51.3 | 51.7 | 0.86 |
| NT-proBNP, ng/L | 1396 | 3640 | 656 | <0.001 |
| Body temperature, °C | 39 ± 0.7 | 39 ± 0.8 | 39 ± 0.5 | 0.58 |
| Heart rate, beats/min | 120 ± 15 | 145 ± 20 | 95 ± 10 | <0.05 |
| Breath rate, breathes/min | 45 ± 5 | 55 ± 6 | 35 ± 4 | <0.05 |
| Liver enlargement, cm | 2 ± 1.0 | 4 ± 0.5 | 1 ± 0.5 | <0.001 |
| Plasma glucose, mmol/L | 8.7 ± 1.8 | 9.6 ± 1.7 | 7.8 ± 1.8 | 0.17 |
| White blood cell increase, ×109/ L | 15 ± 2.7 | 16 ± 3.1 | 14 ± 2.3 | 0.47 |
| White blood cell decrease, ×109/ L | 3.0 ± 0.8 | 3.0 ± 0.7 | 3.0 ± 0.9 | 0.28 |
| Platelet, 10 × 109/ L | 7.4 ± 0.3 | 5.3 ± 0.2 | 9.4 ± 0.4 | <0.05 |
| Hemoglobin, g/L | 69 ± 14 | 62 ± 12 | 87 ± 17 | 0.07 |
| Serum total bilirubin, mmol/L | 77 ± 23.3 | 98 ± 33.8 | 55 ± 12.7 | <0.05 |
| Alanine transaminase, U/L | 59 ± 17 | 82 ± 15 | 36 ± 18 | <0.001 |
| C reaction protein, mg/L | 39 ± 6.4 | 48 ± 11.2 | 29 ± 0.6 | <0.05 |
| Procalcitonin, mg/L | 14 ± 1.1 | 16 ± 2.6 | 11 ± 0.5 | <0.001 |
| Blood lactic acid, mmol/L | 5 ± 0.9 | 6 ± 1.2 | 4 ± 0.6 | <0.05 |
| Activated partial thromboplastin time, s | 70 ± 14.8 | 95 ± 12.5 | 45 ± 17.1 | <0.001 |
| TNF-α, pg/mL | 42 ± 15.2 | 52 ± 15.3 | 31 ± 16.1 | <0.05 |
| IL6, pg/mL | 61 ± 11.3 | 77 ± 11.9 | 44 ± 10.6 | <0.05 |
| Ejection fraction, % | 45 ± 9.5 | 25 ± 10.4 | 65 ± 8.5 | <0.001 |
| E peak /A peak | 1.5± 0.2 | 1.4± 0.2 | 1.6± 0.2 | 0.12 |
| Arterial oxygen saturation, % | 44 ± 14.9 | 33 ± 16.4 | 55 ± 13.4 | <0.05 |
| Systolic blood pressure, mmHg | 89 ± 11.5 | 85 ± 11.6 | 92 ± 12.5 | 0.42 |
| Urine volume, mg·kg-1·min-1 | 0.3 ± 0.05 | 0.2 ± 0.05 | 0.4 ± 0.05 | <0.05 |
| Capillary filling, s | 4 ± 1.0 | 5 ± 1.0 | 3 ± 1.0 | <0.05 |
Fig 1. In the heart Doppler examination, the ejection fractions in HF and non-HF patients with sepsis were 25% ± 10.4% and 65% ± 7.5%, respectively, a significant difference (P < 0.001); the E peak/A peak of the 2 groups were normal and not significantly different (1.4 ± 0.2 cf. 1.6 ± 0.2).
The results suggested a left ventricle systolic dysfunction in all heart failure patients with sepsis.
The severity of heart failure was evaluated on the basis of ejection fraction and results showed that there were 14 (21.2%), 19 (28.8%), and 33 (50%) patients with mild, moderate, and severe heart failure, respectively. Patients with moderate or severe heart failure were among the patients with severe sepsis or septic shock.
The plasma NT-proBNP levels were not normally distributed (P < 0.001) and therefore the median was used. There was a positive correlation between plasma NT-proBNP levels and heart rate (r = 0.754, P < 0.001), breath rate (r = 0.659, P < 0.001), and liver enlargement (r = 0.651, P < 0.001). NT-proBNP levels negatively correlated with ejection fraction in these sepsis patients (r = –0.782, P < 0.001); the F-test for linear regression reached statistical significance (P < 0.001; Table 3).
Table 3. The correlation between plasma NT-proBNP level and heart rate, breathe rate, liver enlargement, and Ejection fraction in the all heart failure patients.
| Related factors | r | P | F | R |
|---|---|---|---|---|
| Heart rate, beats/min | 0.754 | 0.000 | 77.65 | 0.573 |
| Breath rate, breathes/min | 0.659 | 0.000 | 56.86 | 0.494 |
| Liver enlargement, cm | 0.651 | 0.000 | 56.36 | 0.486 |
| Ejection fraction, % | –0.782 | 0.000 | 78.87 | 0.588 |
In the 66 septic patients with HF, the median plasma NT-proBNP levels of mild, moderate, and severe HF were 1348.22 ng/L (n = 32), 3472.15 ng/L (n = 19), and 4236.23 ng/L (n = 11), respectively; the differences among them were significant (x2 = 41.13, P < 0.001). The plasma NT-proBNP level rises with the increasing modified Ross score; there was a positive correlation between NT-proBNP level and modified Ross score (r = 0.679, P < 0.001).
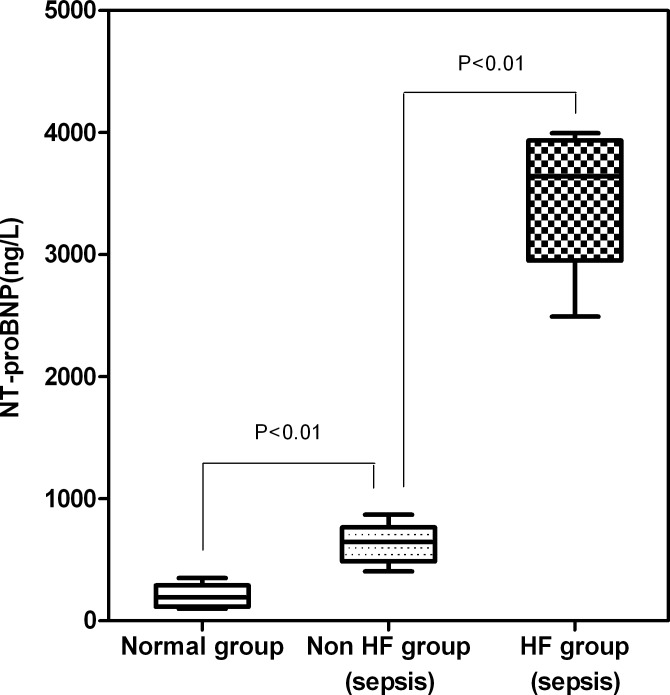
The median plasma NT-proBNP levels of the sepsis HF, sepsis non-HF, and healthy control groups were 3885 ng/L, 656 ng/Land 226 ng/L, respectively; the differences among them were significant (χ2 = 41.87, P < 0.001; Fig 2). Among the sepsis HF patients, the median plasma NT-proBNP levels of those with severe sepsis and those with septic shock were 4565 ng/L and 5872 ng/L, respectively.
Fig 2. The median plasma NT-proBNP levels of the sepsis HF, sepsis non-HF, and healthy control groups were 3885 ng/L, 656 ng/Land 226 ng/L, respectively; the differences among them were significant (χ2 = 41.87, P < 0.001).
The median plasma NT-proBNP levels were 656 ng/L (465–2150 ng/L) in non-HF sepsis patients, 1350 ng/L (1200–3054 ng/L) in non-HF severe sepsis patients, and 1425 ng/L (1250–3225 ng/L) in non-HF septic shock patients. The ejection fraction and E peak/A peak were normal in all non-HF sepsis patients.
Among the 211 patients, 187 recovered (average hospitalization was 12.5 days) and 24 died (average hospitalization was 5.5 days), including 10 with severe sepsis (4.74%) and 14 with septic shock (6.64%). Plasma NT-proBNP levels of the surviving and deceased patients were not significantly different (4486 ng/L cf. 4618 ng/L in patients with severe sepsis; 4774 ng/L cf. 4951 ng/ L in patients with septic shock).
Among the 66 septic patients with HF, 17 patients died. In the 49 surviving patients, the average recovery time for HF and sepsis were 1.5 days and 14.5 days, respectively; the median plasma NT-proBNP levels were 3000.15 ng/L before treatment, 1685.22 ng/L after recovery of HF, and 329.35 ng/L after recovery of sepsis, which showed the potential effects of sepsis on NT- proBNP; the differences among them and between any two comparisons (before treatment and after HF recovery, before treatment and after sepsis recovery, and after HF and sepsis recovery) were significant (P < 0.001 for all; Table 4).
Table 4. Comparison of plasma NT-proBNP levels before treament and after HF and sepsis recovery, ng/L.
| NT-proBNP | Z-value | P-value | |
|---|---|---|---|
| Before treatment | 3000.15 | –3.862 | 0.000 |
| After HF recovered | 1685.22 | ||
| Before treatment | 3000.15 | –5.238 | 0.000 |
| After sepsis recovered | 329.35 | ||
| After HF recovered | 1685.22 | –4.159 | 0.000 |
| After sepsis recovered | 329.35 |
In 187 surviving patients, the median plasma NT-proBNP level was 986 ng/L before treatment and significantly lower (312 ng/L) after treatment (P < 0.001).
Sicker patients with severe sepsis (n = 74) and with septic shock (n = 52) required more fluid for treatment. Patients were stratified as group A or group B, according to the total per capita infusion in the first week, as follows. Patients with severe sepsis were considered in the A1 or B1 group if they received ≤6000 mL or >6000 mL infusions, respectively. Patients with septic shock were in the A2 or B2 group if they received ≤9000 mL or >9000 mL. In the A1 and B1 groups, NT-proBNP levels were 2056 ng/L and 2462 ng/L, respectively (P = 0.09). In the A2 and B2 groups, NT-proBNP levels were 3168 ng/L and 3719 ng/L (P = 0.08). There was no significant difference between patients receiving high volume and lower volume transfusions.
Among the sepsis patients, plasma NT-proBNP levels positively correlated with CRP (r = 0.544), procalcitonin (r = 0.815), TNF-α (r = 0.712), and IL6 (r = 0.682; P < 0.001 for all).
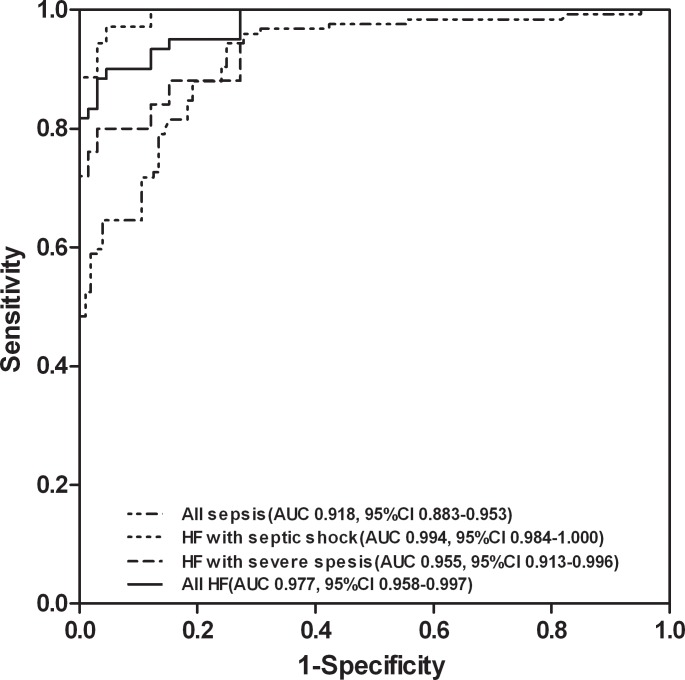
To estimate the diagnostic accuracy of plasma NT-proBNP levels to detect heart failure in all sepsis patients, severe sepsis patients and septic shock patients, ROC curve analysis was employed (Fig 3; Table 5). The optimal diagnostic cut-off value for heart failure was 1268 ng/L (positive likelihood ratio = 11.9) sepsis patients, 1368 ng/L (positive likelihood ratio = 13.2) in severe sepsis patients, and 1525 ng/L (positive likelihood ratio = 21.4) in patients with septic shock (Table 5). The diagnostic sensitivity and specificity were shown in Table 5.
Fig 3. Comparing ROC curves for plasma NT-proBNP in all pediatric patients with sepsis, HF patients with severe sepsis, HF patients with septic shock, and all HF patients with sepsis.
Table 5. ROC analysis determining viability of plasma NT-ProBNP levels for differentiating heart failure in sepsis patients.*.
| All | Severe sepsis | Septic shock | |
|---|---|---|---|
| Subjects, n | 211 | 74 | 52 |
| Optimal cut-off, ng/L | 1268 | 1368 | 1525 |
| AUC | 0.977 (0.958–0.997) | 0.955 (0.913–0.996) | 0.994 (0.984–1.000) |
| Sensitivity | 0.900 (0.795–0.962) | 0.800 (0.593–0.932) | 0.971 (0.851–0.999) |
| Specificity | 0.924 (0.832–0.975) | 0.939 (0.852–0.983) | 0.955 (0.873–0.991) |
| Positive likelihood ratio | 11.9 | 13.2 | 21.4 |
* Reported as value (95% CI), unless otherwise noted
Discussion
This study investigated whether plasma NT-proBNP levels can be used to predict heart failure in pediatric patients with sepsis. The plasma NT-proBNP levels of pediatric patients with sepsis (including patients with heart failure) were evaluated and compared with age- and gender-matched healthy children. Using plasma NT-proBNP as an index for the diagnosis of heart failure has been accepted and widely used; the commonly used diagnostic cut-off points (>450 ng/L for adults and >500 ng/L for children), however, may not be appropriate for septic patients. This study found that NT-proBNP levels in sepsis patients were significantly higher than that of non-sepsis patients, suggesting the need to determine the appropriate diagnostic cut-off points for heart failure in septic patients that will be conducive to the management of septic patients with heart failure. We found that septic patients with heart failure had a left ventricle systolic dysfunction and plasma NT-ProBNP levels were significantly elevated in septic patients (with or without heart failure), relative to the healthy control group. NT-ProBNP levels were also significantly higher in patients with heart failure compared with those without heart failure. The optimal cut-off value of NT-ProBNP for heart failure in all patients was 1268 ng/L, and the diagnostic sensitivity, specificity, and accuracy were 90.0%, 92.4%, and 0.912%, respectively, with a positive likelihood ratio of 11.9. These results support plasma NT-ProBNP as a valid predictor of heart failure in children with sepsis.
Myocardial dysfunction is a common complication in septic patients. Caksen et al. [21] observed pericardial effusion in 20% of pediatric patients with sepsis caused by Staphylococcus aureus. In this study, the median plasma NT-proBNP level of sepsis patients without heart failure (656 ng/L) was significantly higher than that of healthy children (226 ng/L). This is also higher than the 500 ng/L for diagnosing heart failure in general pediatric patients [10]. In the 49 surviving patients with heart failure, the plasma NT-proBNP levels were significantly reduced but still as high as 1685.22 ng/L after heart failure recovery. However, plasma NT-proBNP levels decreased to normal (329.35 ng/L) after sepsis recovery, which showed the potential effect of sepsis on NT-proBNP levels.
This study suggests that sepsis may contribute to heart failure. These findings are consistent with those of previous reports that NT-proBNP strongly correlates with systolic dysfunction and diastolic dysfunction in patients with severe sepsis or septic shock. Other studies have shown that high NT-proBNP was associated with mortality in adult patients with severe sepsis [22], or in pediatric patients with severe sepsis or septic shock [23]. A meta-analysis further showed that an elevated NT-proBNP is a strong predictor of high mortality in septic patients [24].
This study showed that sicker patients with sepsis required more fluid for treatment, which did not increase NT-proBNP levels significantly (P > 0.05), and affect NT-proBNP for diagnosis of septic heart failure.
This study shows that NT-proBNP is associated with the severity of sepsis but is not a predictor of death in patients with severe sepsis or septic shock.
The cardiovascular response to septic shock is peripheral vasodilatation, resulting from myocardial depression and ventricular dilation, possibly caused by endotoxins from Gram-negative bacteria [25]. In our study, NT-proBNP levels positively correlated with the inflammatory molecules CRP, procalcitonin, TNF-α and IL6, suggesting that the inflammatory response may contribute to cardiac dysfunctions and indirectly elevates circulatory NT-proBNP levels. It is noteworthy that a previous study showed that in vitro exposure to TNF-α or IL6 isolated from serum of humans with septic shock induced depression of rat cardiac myocyte contractile function, and these two factors appeared to have a synergistic effect [26].
In the present study, the cut-off value of NT-proBNP for heart failure in patients with septic shock (1525 ng/L) was significantly higher than that of patients with severe sepsis (1368 ng/L), suggesting that septic shock has a greater influence on NT-proBNP levels than sepsis does. This could be due to inflammatory factors released during septic shock. Further investigation is warranted to elucidate the mechanism underlying the correlation between plasma NT-proBNP and measured inflammatory factors.
Conclusion
This report is the first one to reveal that NT-proBNP may predict heart failure in children with sepsis. In addition, it provides an important clinical reference for the diagnosis of heart failure in pediatric patients with sepsis, and enables monitoring septic children for cardiac involvement.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by the Department of Science and Technology of Guangdong Province in China (grant no. 2011B031800134) and the Bureau of Science and Technology of Foshan City in China (grant no. 201008189). These funders were not involved in the study design, or the collection, analysis, or interpretation of the data, in the writing of the report, or in the decision to submit the article for publication.
References
- 1.deFilippi CR, de Lemos JA, Tkaczuk AT, Christenson RH, Carnethon MR, Siscovick DS, et al. Physical activity, change in biomarkers of myocardial stress and injury, and subsequent heart failure risk in older adults. J Am Coll Cardiol 2012. 60; 2539–2547. 10.1016/j.jacc.2012.08.1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eurlings LW, Sanders-van Wijk S, van Kraaij DJ, van Kimmenade R, Meeder JG, Kamp O, et al. Risk stratification with the use of serial N-terminal pro-B-type natriuretic peptide measurements during admission and early after discharge in heart failure patients: post hoc analysis of the PRIMA study. J Card Fail 2014. 20; 881–890. 10.1016/j.cardfail.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 3.Gruson D, Ahn SA, Lepoutre T, Rousseau MF. Measurement of NT-proBNP with LOCI technology in heart failure patients. Clin Biochem 2012. 45; 171–174. 10.1016/j.clinbiochem.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 4.Guazzi M, Boracchi P, Labate V, Arena R, Reina G. Exercise oscillatory breathing and NT-proBNP levels in stable heart failure provide the strongest prediction of cardiac outcome when combining biomarkers with cardiopulmonary exercise testing. J Card Fail 2012. 18; 313–320. 10.1016/j.cardfail.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 5.Iqbal N, Alim KS, Aramin H, Iqbal F, Green E, Higginbotham E, et al. Novel biomarkers for heart failure. Expert Rev Cardiovasc Ther 2013. 11; 1155–1169. 10.1586/14779072.2013.832476 [DOI] [PubMed] [Google Scholar]
- 6.Madamanchi C, Alhosaini H, Sumida A, Runge MS. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol 2014. 176; 611–617. 10.1016/j.ijcard.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popelova J, Kotaska K, Cerny S, Prokopova M, Rubacek M. Range and distribution of NT-proBNP values in stable corrected congenital heart disease of various types. Can J Cardiol 2012. 28; 471–476. 10.1016/j.cjca.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 8.Seronde MF, Gayat E, Logeart D, Lassus J, Laribi S, Boukef R, et al. Comparison of the diagnostic and prognostic values of B-type and atrial-type natriuretic peptides in acute heart failure. Int J Cardiol 2013. 168; 3404–3411. 10.1016/j.ijcard.2013.04.164 [DOI] [PubMed] [Google Scholar]
- 9.Johns MC, Stephenson C. Amino-terminal pro-B-type natriuretic peptide testing in neonatal and pediatric patients. Am J Cardiol 2008. 101; 76–81. 10.1016/j.amjcard.2007.11.028 [DOI] [PubMed] [Google Scholar]
- 10.Lin CW, Zeng XL, Zhang JF, Meng XH. Determining the optimal cutoff values of plasma N-terminal pro-B-type natriuretic peptide levels for the diagnosis of heart failure in children of age up to 14 years. J Card Fail 2014. 20; 168–173. 10.1016/j.cardfail.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 11.McGrady M, Reid CM, Shiel L, Wolfe R, Boffa U, Liew D, et al. NT-proB natriuretic peptide, risk factors and asymptomatic left ventricular dysfunction: results of the SCReening Evaluation of the Evolution of New Heart Failure study (SCREEN-HF). Int J Cardiol 2013. 169; 133–138. 10.1016/j.ijcard.2013.08.089 [DOI] [PubMed] [Google Scholar]
- 12.Pellicori P, Kallvikbacka-Bennett A, Zhang J, Khaleva O, Warden J, Clark AL, et al. Revisiting a classical clinical sign: jugular venous ultrasound. Int J Cardiol 2014. 170; 364–370. 10.1016/j.ijcard.2013.11.015 [DOI] [PubMed] [Google Scholar]
- 13.Wong DT, George K, Wilson J, Manlhiot C, McCrindle BW, Adeli K, et al. Effectiveness of serial increases in amino-terminal pro-B-type natriuretic peptide levels to indicate the need for mechanical circulatory support in children with acute decompensated heart failure. Am J Cardiol 2011. 107; 573–578. 10.1016/j.amjcard.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 14.Xue Y, Chan J, Sakariya S, Maisel A. Biomarker-guided treatment of congestive heart failure. Congest Heart Fail 2010. 16 Suppl 1; S62–67. 10.1111/j.1751-7133.2010.00169.x [DOI] [PubMed] [Google Scholar]
- 15.Flynn A, Chokkalingam Mani B, Mather PJ. Sepsis-induced cardiomyopathy: a review of pathophysiologic mechanisms. Heart Fail Rev 2010. 15; 605–611. 10.1007/s10741-010-9176-4 [DOI] [PubMed] [Google Scholar]
- 16.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatr Crit Care Med. 2005, 6(1):2–8. [DOI] [PubMed] [Google Scholar]
- 17.Laer S, Mir TS, Behn F, Eiselt M, Scholz H, Venzke A, et al. Carvedilol therapy in pediatric patients with congestive heart failure: a study investigating clinical and pharmacokinetic parameters. American heart journal 2002; 143(5):916–22. [DOI] [PubMed] [Google Scholar]
- 18.Ross RD. The Ross Classification for Heart Failure in Children After 25 Years: A Review and an Age-Stratified Revision. PediatrCardiol. 2012. 33:1295–1300. [DOI] [PubMed] [Google Scholar]
- 19.Moutinho MA, Colucci FA, Alcoforado V, Tavares LR, Rachid MB, Rosa ML, et al. Heart failure with preserved ejection fraction and systolic dysfunction in the community. Arq Bras Cardiol 2008. 90; 132–137. [DOI] [PubMed] [Google Scholar]
- 20.Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation 2000. 101; 2118–2121. [DOI] [PubMed] [Google Scholar]
- 21.Caksen H, Uzum K, Yuksel S, Basriustunbas H, Ozturk MK, Narin N. Cardiac findings in childhood staphylococcal sepsis. Jpn Heart J 2002. 43; 9–11. [DOI] [PubMed] [Google Scholar]
- 22.Zhang XH, Dong Y, Chen YD, Zhou P, Wang JD, Wen FQ. Serum N-terminal pro-brain natriuretic peptide level is a significant prognostic factor in patients with severe sepsis among Southwest Chinese population. Eur Rev Med Pharmacol Sci 2013. 17; 517–521. [PubMed] [Google Scholar]
- 23.Samransamruajkit R, Uppala R, Pongsanon K, Deelodejanawong J, Sritippayawan S, Prapphal N. Clinical outcomes after utilizing surviving sepsis campaign in children with septic shock and prognostic value of initial plasma NT-proBNP. Indian J Crit Care Med 2014. 18; 70–76. 10.4103/0972-5229.126075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang F, Wu Y, Tang L, Zhu W, Chen F, Xu T, et al. Brain natriuretic peptide for prediction of mortality in patients with sepsis: a systematic review and meta-analysis. Crit Care 2012. 16; R74 10.1186/cc11331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med 1993. 328; 1471–1477. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 1996. 183; 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.