Abstract
Central sensitivity syndromes (CSSs) represent a heterogeneous group of disorders (e.g., fibromyalgia [FM], irritable bowel syndrome [IBS], chronic headache, temporomandibular disorders [TMDs], pelvic pain syndromes) that share common symptoms, with persistent pain being the most prominent feature.
Although the etiology and pathophysiology of CSSs are currently incompletely understood, central sensitization has emerged as one of the significant mechanisms. Given that there are currently no known cures for CSSs, people living with these disorders must learn to cope with and manage their symptoms throughout their lives. Medical interventions alone have not proven to be sufficient for helping people with CSSs manage their symptoms. A biopsychosocial perspective that considers the ways that biological, psychological, and social factors work independently and jointly to affect a person's experience is the most effective conceptualization and guide for effective treatment. In this article, we discuss several psychological and social features that may influence the experience of a person with CSS and their symptom management, regardless of their specific diagnosis. We highlight the longitudinal aspect of adjustment to illness, the distinction between psychosocial factors as causes of symptoms versus modifiers and perpetuators of symptoms, dispel the notion that all patients with the same diagnosis are a homogeneous group (the “patient-uniformity myth”), and acknowledge the importance of environmental and situational context on symptom management for individuals with any CSS.
Keywords: Central sensitivity syndromes (CSS), chronic pain, biopsychosocial, psychological factors, social factors, fear avoidance, post-traumatic stress disorder, cognitive-behavior therapy
A number of common and prevalent disorders (e.g., fibromyalgia [FM], irritable bowel syndrome [IBS], chronic headache, temporomandibular disorders [TMDs], pelvic pain syndromes) appear to overlap and share a set of common features although their primary body locations differ. Historically, this set of disorders has been somewhat isolated from each other with some dependence on the medical specialist from whom treatment was sought – FM treated by rheumatologists, IBS by gastroenterologists, headache by neurologists, TMD by dentists, and pelvic syndromes by urologists and gynecologists. Over the past decade there has been a growing recognition that there is significant overlap among these disorders with persistent pain as a predominant feature, along with multiple and numerous comorbid symptoms such as fatigue, sleep problems, dizziness, cognitive problems (e.g., difficulties with attention, memory, concentration), depression, anxiety, and irritability. In one study with FM patients, the mean number of symptoms reported by patients was 22 [1]. There has been a growing recognition that a shared mechanism may underlie each of them, namely, a lowered threshold for the perception of sensory information. As a group these disorders have come to be labeled as central sensitivity syndromes (CSS) [2]. Since pain seems to be a central feature of all of the CSSs, we will focus most of our discussion on this “core” symptom. In addition to potential neurophysiological overlap, individuals with this set of overlapping disorders often share a set of important psychological (e.g., depression, anxiety, anger) and social (e.g., interpersonal distress) features [3].
Although the etiology and pathophysiology of CSSs are currently incompletely understood, central sensitization has emerged as one of the significant mechanisms. Genetics play an important role and in a subgroup of patients, psychological biology is contributory to the syndromes [2, 4]. Currently available treatments for CSSs are empirical, palliative, and provide only modest benefits. Those with the diagnosis often have no observable signs of illness or injury, and hence often feel stigmatized by the health care systems, significant others, and employers. Thus, those with CSS have chronic conditions and will have to adapt and accommodate their lives accordingly; they will have to learn to self-manage their symptoms and their lives despite the presence of distressing symptoms and skepticism. Other articles in this issue address the inherent pathophysiology, shared neurophysiological mechanisms, and treatment of CSSs. In this article we will focus on the psychosocial and behavioral factors that transcend the specific diagnosis and that characterize those with CSSs regardless of the location of their primary symptom(s) and that impact on adjustment and response to the symptoms and treatments prescribed.
A Biopsychosocial Approach
As noted, CSSs, regardless of the specific diagnosis or primary location(s) is a complex problem that exerts an influence on the individuals' subjective experience and is influenced by that person's prior experiences [5]. Historical conceptualizations have assumed a direct, linear relationship among the sensory experience, tissue pathology, subjective experience, and response. From this traditional biomedical perspective each symptom should have a specific physiological cause, and treatments should target the cause and alleviate the symptoms. Subjective reports of symptoms in the absence of any identified biological cause are, often viewed derogatorily and deemed psychogenic. Such a unidimensional, dichotomy -- somatogenic—psychogenic -- framework of understanding symptoms leaves little room to consider the interplay between biological and psychological, and contextual factors and their evolution and interactions overtime. Although there are a number of shortcomings to this dated view, many people, laypeople and medical providers alike, continue to conceptualize various CSSs, whatever the presenting symptoms, in this way.
One of the major failings of a unidimensional model of symptoms is its inability to account for a variety of commonly seen illness-related phenomena, especially as it relates to many CSSs [6]. For example, individuals with the same symptom onset may provide vastly discrepant reports of symptom causality and severity; treatments prescribed for the same symptoms may have vastly different responses by people with ostensibly the same diagnosis; and individuals with identifiable causal pathology may report no symptoms. Conversely those without any identifiable physical pathology may report comparable symptom severity to those with objectively determined pathology. These clinical scenarios suggest that the experience of symptoms associated with CSS are multifaceted, and requires a broader conceptualization than the biomedical model has promulgated. Rather than relying solely on biophysical perspectives and intervention, an integrated, biopsychosocial approach to understanding patients and management of their symptoms has proven to be an effective strategy for symptom relief and control, if not cure [7-8].
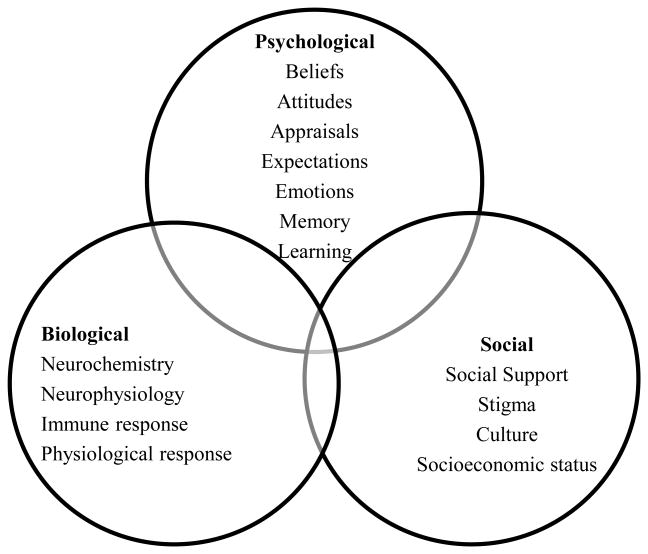
A biopsychosocial perspective takes into account the myriad psychological, social, and contextual factors, in conjunction with biological influences that contribute to the experience, maintenance, and exacerbation of symptoms, as well as response to symptoms and treatments. Perhaps most importantly, such an approach suggests that in order to adequately care for individuals with CSS, despite the specific diagnosis, psychological and social factors, in addition to biological ones, must each be considered and addressed [7-8]. It is important to emphasize, however, that these factors are not uniquely associated with CSSs, but rather are relevant to all chronic diseases. Figure 1 provides a schematic representation of the biopsychosocial model, showcasing the overlapping dimensions of biological, psychological, and social factors that affect the experience of CSSs. It is important to acknowledge that CSSs are chronic conditions, and thus the interplay of physical, psychosocial, and behavioral factors will evolve over time and should not be viewed as static.
Figure 1. Biopsychosocial Model of Chronic Pain.
Key Considerations
Before delving into the discussion of psychological and social aspects associated with CSSs, we highlight several points that must be considered whenever the topic of CSS treatments is raised. We believe that without first addressing these topics, the relationships between biological, psychological, and social components may be misinterpreted. These points include recognizing the longitudinal aspect of patient (not just symptom) management and adjustment to illness, the distinction between psychosocial factors as causes of symptoms versus modifiers of symptoms, identifying and dispelling the patient-uniformity myth (e.g., all patients with the same diagnosis are a homogeneous group requiring a common treatment approach), and acknowledging the importance of environmental context.
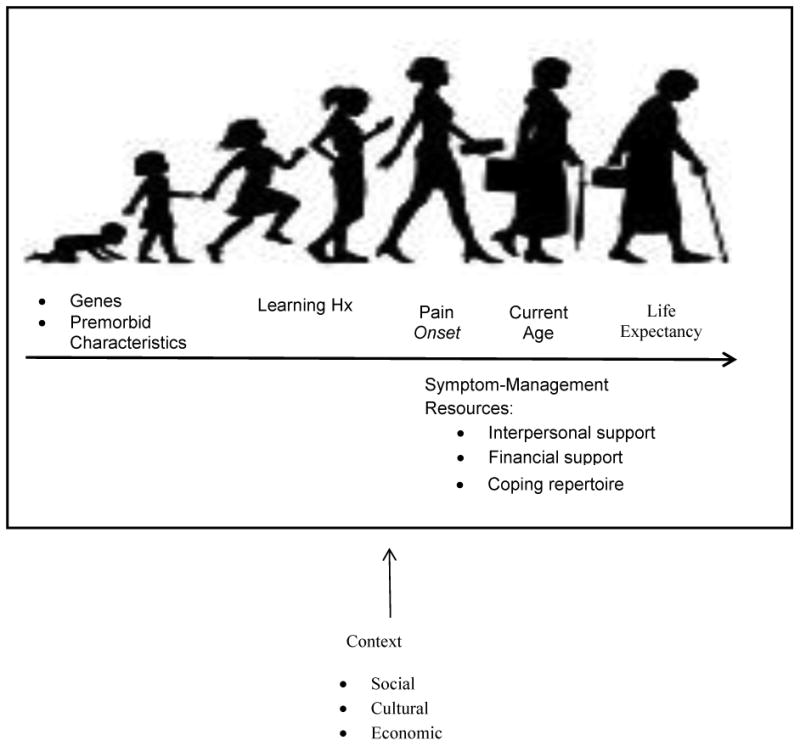
Many CSSs develop in late adolescence and continue for decades. Given the persistence of these syndromes, it is important to keep in mind a longitudinal or life span perspective (Figure 2). Prior to syndrome onset, each person has a unique genetic composition and social learning history that preceded their condition; they seek an understanding of the cause of their symptoms, and go on a quest for a “cure.” Fortunately, they will not likely die from the symptoms of CSSs, but each person is faced with the task of finding a way to continue on with his or her life despite their symptoms as these will likely persist over extended periods of time – years even decades. Although providers may develop relationships with their patients and may believe that they have a full understanding of their patients' experiences, it is important to note that providers only witness “snapshots” of their patients' lives. The vast majority of important events occur outside the provider's presence, and without his or her knowledge. By the time that a patient enters the doctor's office, he or she has likely lived with his or her symptoms for many years, and has learned how to cope with pain and other symptoms to the best of his or her ability. Patient responses have been reinforced, thinking styles have been ingrained, and relationships with others as they assimilate and accommodate their lives to the presence of symptoms have evolved. In order to best serve these patients, providers must be mindful of their patients' unique learning histories, experiences, and life circumstances that guide their current presentation.
Figure 2. Longitudinal Perspective.
Throughout this article, we highlight psychological and social factors that influence the way that individuals with CSSs may experience their illnesses. Although we take care to emphasize a biopsychosocial approach to symptoms and to discuss the evidence for such a conceptualization, some maintain a perspective that implicates psychosocial features as direct causes of symptoms. Even in cases in which symptoms occur after some psychological or social perturbation is reported, there is little empirical evidence to support the notion that any psychosocial factor is the sole cause of CSS symptoms [6,9]. Moreover, many who experience comparable events do not develop symptoms. These predisposing factors do appear to have an impact on perceptions of symptoms and the behaviors of some individuals in response to their symptoms. There is little question, however, that psychosocial factors may intensify and maintain symptoms. Living with a persistent set of symptoms as characterized by CSSs is challenging, and the importance and weight of psychosocial factors will vary as individuals adapt and adjust to their persistence. The distinction between the causal and moderating and modulating roles of psychosocial factors is critical for understanding and appropriately treating patients. It is vital that this message be shared with patients who may have preconceived notions about the role of psychosocial factors in their lives and may resist consideration of them if they perceive that these factors are being presented to invalidate their experiences of physical symptoms and contribute to the stigma often associated with these conditions.
Although there are common features of CSSs, individuals who experience them represent a heterogeneous group, and this is true both across CSS conditions and within them [1]. Each of the psychological and social factors that we detail in this article may be relevant for some patients, and may be less relevant for others, and the weight of importance of these factors may vary both across and within those with the same CSS diagnosis as these evolve over time (Figure 1). Although characterization of patient populations eases the burden of classification, strengthens the search for common underlying mechanisms, and helps to direct treatment plans, it is important that providers not fall victim to the “patient uniformity” or “patient-homogeneity” myth. In fact, years of research have consistently demonstrated that no single treatment works for everyone despite comparable diagnoses [8]. Individuals presenting with identical CSS diagnoses exhibit a great deal of variability in the degree of pain, number and nature of physical symptoms, and psychological distress that they experience. Thus, they require a person-centered approach by their medical providers help patients manage their symptoms [10-11]. Though an eye for common themes and shared experiences among patients certainly aids in providing good health care, individual assessments must be made with each patient.
Psychological Disorders and Chronic Pain
In this section, we provide an overview of the relationship between several psychiatric conditions and the common symptom across CSSs -- chronic pain. However, an important caveat to reiterate is that psychological disorders may develop subsequent to symptom onset and should not necessarily be viewed as being causal. It is also important to note that the formal diagnosis of a mental disorder requires more than the endorsement of a cluster of specific symptoms, which may be reflected in behaviors, emotion regulation, or thinking styles. Beyond this, the diagnosis of a mental disorder demonstrates significant impairment in functioning along with a clinically significant level of distress. These responses can be observed in some individuals with CSS, not by all.
Depression
In the U.S. general population, approximately 7% of adults are diagnosed with depression [12]. Depression is often associated with poor treatment adherence and more intensive and expensive medical follow-up [13-14]. Chronic pain and depression have a significant relationship, and this link has been validated in several epidemiological studies [15]. The prevalence of chronic pain is higher among individuals diagnosed with depression, and conversely, the prevalence of depression among individuals with chronic pain is also elevated. In fact, depression is the most commonly reported psychiatric condition associated with chronic pain, with the prevalence varying between 5% to 22% in population-based studies [15]. In clinical studies, the prevalence of patients being treated for chronic pain ranges is significantly higher from 40%-60% [16]. Depression appears to exacerbate the pain experience, and is a significant determinant of pain-related disability [17-18]. Given the range and severity of symptoms experienced by individuals with CSSs, it is reasonable to ask “why aren't they all depressed?” One study of FM patients that investigated this question determined that depressive symptoms were most likely to arise when patients felt that symptoms had a greater impact on their lives and that they were helpless with little they could do to control their symptoms and their lives [19].
Several studies have attempted to address the “chicken and egg” question when it comes to depression and chronic pain --does depression precede pain or does pain precede depression? The extant literature supports the notion that depression tends to arise after the development of chronic pain [20-21]. However, it would be incorrect to assume that the presence of depression has no influence on pain perception and response. Although it is not indicated as the sole cause of pain, longitudinal studies have demonstrated that depression is a risk factor for the development of subsequent chronic pain [22]. Others have hypothesized that the relationship is not direct, and that it is mediated by how patients view their pain [19]. In further support of this, Lackner and colleagues found that among individuals diagnosed with IBS, the relationship between depression and abdominal pain severity could be partially explained by the propensity of those with higher depression scores to have catastrophic thoughts about their pain (e.g., “I can't handle this pain.”) [23].
Still, it is important to note that a close relationship between pain and depression does not necessitate a causal connection, even if one predates the other. Given the complex association between depression and chronic pain, some have suggested foregoing attempts to determine causal order, instead accepting that the two exist in a mutually reinforcing relationship, and highlighting the need to treat both [24].
Anxiety
People with chronic pain often report being anxious and worried about their pain, its impact on their lives, general health and the future. Having unexplained pain, as is often the case with CSSs, further complicates patients' anxiety and fear. There is a strong evidence base to support the influence of anxiety on pain perception. In a sample of individuals who responded to a web-based survey of FM, more than half endorsed having symptoms of anxiety [25]. These studies suggest that intense anxiety is associated with a decrease in individuals' perceived pain tolerance and an increase in pain perception [26]. Cross-sectional epidemiological studies demonstrate a relationship between anxiety disorders and chronic pain. Roughly 18% of American adults are diagnosed with an anxiety disorder [27]. The prevalence of any anxiety disorder is twice as high for chronic pain patients when compared to those without chronic pain [28].
As is the case with depression, research supports a bidirectional relationship between anxiety and chronic pain. In a sample of outpatients receiving care in a tertiary pain clinic, Knaster and colleagues found that a quarter of the participants met diagnostic criteria for an anxiety disorder, most of which (75%) had been present before the onset of pain [29]. Further, among those with anxiety disorders, chronic pain is associated with a worse, more chronic course of anxiety [30]. Chronic pain grade, a measure of pain intensity and pain-related disability, was also found to be a significant predictor of the onset of anxiety disorders in a sample without a prior history of depression or anxiety disorder, providing evidence that pain also contributes to the later development of anxiety disorders [31].
Post-traumatic stress disorder (PTSD)
The lifetime prevalence of PTSD in the United States is approximately 8%, while the one year prevalence rate is just under 4% [27,32]. Most studies of chronic pain and PTSD have focused on PTSD symptoms, and not on a formal diagnosis. Available data suggest that the prevalence of PTSD is approximately 10% among chronic pain patients, though that estimate increases to nearly 50% when PTSD symptoms are considered in place of a formal psychiatric diagnosis [33]. Evidence for the temporal onset of PTSD versus chronic pain is mixed. Given that PTSD requires a person to be exposed to a traumatic event, which in and of itself may cause pain (e.g., traumatic brain injury, spinal cord injury), it is not surprising to note that in some cases, the stress response precedes chronic pain. For example, Jenewein and others found that PTSD symptoms was the only baseline factor that predicted persistent pain three years after participants were involved in serious accidents [34]. However, this direction of effects does not appear to be the case for all individuals with chronic pain, and it may be especially unlikely for people who have chronic pain that is not caused by a traumatic injury. In a study of patients with FM, Hauser and colleagues found that while 66% of patients reported that traumatic life events and symptoms of PTSD began before their FM started, 30% reported that FM began first, and 4% reported that FM symptoms and PTSD symptoms began within the same year [35].
Substance misuse and dependence
The relationship between substance misuse and chronic pain is one that many medical providers have expressed concern about in their clinical practices. Meanwhile, rates of opioid prescription for the treatment of chronic non-cancer pain have risen in the United States [36-37]. Providers may be reticent to prescribe medications, especially opioid medication, to individuals with chronic pain for fear of misuse, addiction potential, or diversion [38]. On the other hand, chronic pain patients who have a history of substance abuse may be more resistant to other medical interventions as a result of a higher tolerance to opioids [39]. Boscarino and colleagues found that among a large sample of participants undergoing long-term opioid therapy for non-cancer pain, nearly 35% met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) diagnostic criteria for an opioid-use disorder in their lifetime [40]. This proportion represents a high-end estimate of the range of prevalence rates (3% to 48%) suggested by Morasco and others in their systematic review of rates of illicit drug use among chronic non-cancer pain patients [41].
In their review of 38 studies to address the prevalence, clinical characteristics, and treatment outcomes of chronic non-cancer pain patients with comorbid substance use disorder relative to those without substance problems, Morasco and colleagues found that no pattern of demographic, pain-related, or psychiatric variables consistently distinguished between pain patients with and without a history of SUD. The authors noted chronic pain patients with SUD were more likely to be prescribed a higher dose of medication than pain patients without SUD, and were somewhat more likely to misuse them. They concluded that there was little evidence for differences in treatment response across the two groups, although the authors highlighted that the quality of this data was low [41].
As it stands, clear-cut clinical guidance for managing chronic pain with comorbid SUD is lacking. Methodologically rigorous studies are needed to further examine the role of SUD in pain patients' care and treatment response. Novel and customizable approaches to pain management that employ interventions beyond standard opioid treatment may also be especially beneficial in this population.
Common Cognitive and Affective Features that Contribute to Greater Emotional Distress and Physical Symptoms
Although rates of psychiatric conditions (e.g., major depression, anxiety disorders) are higher among patients experiencing chronic pain than people without chronic pain, not all of these individuals will meet full diagnostic criteria for mental illness. Despite this, many CSS patients share a particular set of thinking styles and affective features that may serve to exacerbate their pain and make it more difficult to cope with their symptoms. We emphasize that these thinking styles and affective features vary in presence and intensity across individuals. However, in this section we highlight several aspects of cognitive styles and emotional reactions that are commonly seen among chronic pain patients, and have been consistently shown to have an effect on their pain, disability, activity, other symptoms, and treatment response.
Anger
People with CSS often report feelings of anger and frustration about issues specifically related to the symptoms that they experience and the associated stigma they perceive. They are likely to be upset about the intensity of their symptoms, the lack of clarity about their causes and cure, challenges within the health care system, difficult interactions with others who may appear dismissive of their symptoms, and cost of treatment. Anger can be conceptualized as having several dimensions including the anger experience, anger expressions, and the target of angry feelings.
Anger, while often thought of as a negative emotion, is not necessarily maladaptive. Anger can be an adaptive response when it is the result of a perceived slight or injustice. Research supports the notion that the relationship between anger expression and symptoms is more complex than previously thought. For example, Materazzo and colleagues studied the relationship between anger expression and headache severity among migraine headache patients in a two-week long prospective study. They found that higher anger expression scores at baseline predicted greater subsequent mean daily headache severity [42]. In contrast to this finding, Venable and colleagues found no relationship between anger expression scores and headache frequency or severity in their study of 65 women with recurrent tension-type or mixed tension-type/migraine headache [43].
Research suggests that how anger is modulated (e.g., expressed, inhibited) may have a greater impact on patients' symptoms and their functioning than how angry they are in a given situation (e.g., level of state anger) [44]. Anger expression is thought to be related to symptoms along two different pathways. In the first, it is hypothesized that people who demonstrate their anger through verbal or physical means and who also show a higher sensitivity to symptoms may have deficits in their endogenous inhibitory mechanisms. In support of this model, findings among chronic pain patients and pain-free adults suggest that people who are high in anger expressiveness have deficits in their endogenous opioid function, which results in increased pain sensitivity [45]. In the second pathway, muscle tension as a result of anger arousal is viewed as a cause of increased pain [46]. This symptom-specific reactivity model has been supported in studies of adults with chronic lower back pain [47-48]. Inhibiting anger also appears to contribute to increased pain, and it is common among pain patients. In their early study, Pilowsky and Spence found that 54% of chronic pain patients reported that they internalized or “bottled up” their anger instead of expressing it [49]. Inhibited anger expression is related to depression, pain interference, and the frequency of pain behaviors [50].
Symptom appraisals and symptom beliefs
The way that a person views and interprets a situation can have a profound influence on their experience, and this is true regardless of the objective facts of the situation. Indeed, many clinicians recognize that their patients' perspectives on symptoms associated with the same medical condition may vary a great deal, once again highlighting their heterogeneity. Whereas symptom appraisal refers to the meaning that a person ascribes to their symptoms (e.g., “This pain means that something is wrong”), symptom beliefs reflect larger assumptions about a person's experience that shape how they interpret events. Symptom beliefs develop over time, and incorporate the individual's unique learning history. A person's symptom beliefs are a key determinant of their appraisal of symptoms, symptom severity, symptom impact, and responses.
Symptoms can be appraised in different ways by the range of people who experience them. Benedetti and colleagues found that when they induced pain in a group of pain-free adults, those who were told that the pain was indicative of an “adverse event” demonstrated a lower pain tolerance than those who were informed that the pain was “beneficial to the muscles” [51]. It does not take a formal, controlled study to demonstrate the influence of beliefs about symptoms on emotion and behavior. As an example, it would be unusual to expect that a person who attributed their headache to being “hung over” from having too much alcohol the night before would feel and behave the same way as a person who attributed their (similarly intense) headache to being a sign of a brain tumor. Individual interpretation of the same event matters and will likely lead to differences in emotional arousal (fear, irritation) and the behavior selected to respond (do nothing, lie down, rush to the emergency department).
Patients with physical symptoms may hold beliefs that lead to worse coping, magnification of symptoms, and functional decline [52-53]. Beliefs about symptoms may not only disrupt short-term participation in tasks, but may also have an effect on future behavior. Symptom appraisal and beliefs have been identified as key determinants of adjustment. For example, holding negative beliefs about pain or the expectation that pain may arise from certain activities may lead to avoidance of these activities, even if engaging in them may be helpful in terms of pain management or general quality of life. Although many specific beliefs about pain have been studied, the following have been identified as particularly maladaptive in managing chronic pain: pain is a signal of damage; activity should be avoided when pain is present, pain leads to disability, pain is uncontrollable, and pain is a permanent condition [54-56].
Beliefs and expectations about symptoms are often well-forged, entrenched, and difficult to change. The unwavering nature of beliefs is reasonable when we consider that people tend to behave in ways that reinforce their belief system. However, beliefs about illness are modifiable with appropriate intervention. In a study of patients with IBS, researchers found that a cognitive-behavioral intervention that addressed beliefs about IBS symptoms resulted in positive changes in several cognitive domains (e.g., illness attribution, illness perception), which were associated with reduced IBS symptom severity and improved social adjustment (e.g., work, social activities, leisure activities) six months later. Interestingly, changes in beliefs were a stronger predictor of better functioning than improvements in depression and anxiety symptoms after the intervention [57]. This suggests that in addition to addressing how chronic pain patients feel, providers should also inquire about patients' beliefs.
Catastrophic thinking and fear-avoidance
Catastrophizing represents a particularly unhelpful style of negative thinking that greatly affects symptoms and disability. Catastrophic thinking is defined as having an exaggerated negative view of one's problems such that the person expects the worst possible outcome to occur [58]. Although it has been conceptualized as both an attitudinal set given its reliance on cognitions and as a form of coping given its potential to elicit support, there is overwhelming evidence that catastrophic thinking is largely detrimental [58-59].
Investigators have proposed a fear-avoidance model to incorporate the role of catastrophizing and negative symptom beliefs on illness-behavior [60]. According to this model, behaviorally, anxiety is often expressed as an escape or the avoidance of a negative stimulus, along with a hypervigilance directed towards the feared situation in order to prevent the unwanted outcome. For example, people with chronic pain may attempt to avoid pain by severely restricting their activity levels. In doing so, they remain nervous about the potential for over-exertion and focused on the possibility of worsening their pain, which maintains their level of anxiety, hypervigilance, and disability. In practice, this reduction in activity may actually serve to sustain or exacerbate chronic pain, contrary to the individual's intent, and may result in even more activity restriction and subsequently, functional limitations due to physical deconditioning. Numerous studies have demonstrated that fear plays a key role in how individuals respond to symptoms and these responses can in turn influence symptom persistence and magnitude [61].
The role of catastrophic thinking in pain perception has been explored in a variety of studies with a range of different methodologies. Experimental laboratory studies with pain-free adults and with pain patients, case-controlled cohort studies, and longitudinal studies have all demonstrated a relationship between catastrophizing and pain [62-64]. Cross-sectional studies have found that catastrophizing is associated with increased pain, increased illness behavior, and worse physical and psychological functioning. Additionally, longer-term effects of catastrophizing have also been observed. Catastrophic thinking was associated with reported pain intensity and disability, along with the progression of both, 18 months later in a sample of adults with TMDs [64]. To the benefit of many chronic pain patients, several investigators have exhibited the therapeutic efficacy of reducing catastrophic thinking and of limiting avoidance behaviors on symptom improvement [65-66].
Hypervigilance
Hypervigilance for symptoms represents an attentional bias that many people with CSSs exhibit. An attentional bias is a selective attention towards specific information, most often in relation to threatening information. People who experience recurrent symptoms may develop a pattern of behavior in which they are constantly on high alert for future pain. This hypervigilance may lead patients to avoid activity all together or they may stop activity at the first sign of symptoms as suggested in the fear- avoidance model described earlier [60,67].
Hypervigilance for pain has been associated with increased pain intensity, disability, and pain-related health care utilization in a variety of populations of patients with chronic pain (e.g., fibromyalgia, rheumatoid arthritis, back pain) [67]. It is important to bear in mind that for the individual with the symptoms, being hypervigilant to pain makes perfect sense – paying attention is an important survival mechanism. This is true in the short-term; in order to protect ourselves against threats, we must attend to them. To promote a healthy amount of attention to symptoms, CSS patients may require ongoing education about the difference between monitoring symptoms and excessive focus on symptoms.
Perceived control versus helplessness
People with chronic symptoms may believe that the symptoms that they experience are unpredictable and completely beyond their control. This perspective may be especially likely among individuals with a CSS diagnosis, as many of these syndromes are associated with an ebb and flow in intensity, which persists despite many attempts to remediate their symptoms. The way that individuals view their ability to manage their symptoms is directly associated with how they actually cope with their illness. People who believe that they have an influence on their symptoms, whether it be the duration, frequency, intensity or unpleasantness, may be more motivated to engage in problem-solving to manage their symptoms. As might be expected, research supports the notion that individuals who exhibit a greater sense of control over their symptoms and symptom management function better [19, 54,68]. In general, individuals who are low in perceived control, and feel helpless as a result, report worse outcomes, including greater pain intensity, and poorer physical and psychological adjustment to their illness [19,69].
The influence of perceived control on symptoms may be more complex than previously conceptualized, especially with regard to conditions that are more difficult to treat. There is evidence to support that in some cases when actual control is low, repeated attempts to control or eliminate symptoms may result in increased worry, catastrophic thinking, and other unhelpful approaches [70-71]. Instead, it appears that a balanced perspective that includes recognizing specific behaviors that a patient can engage in to reduce their symptoms (e.g., stress management, exercise, sleep hygiene), along with the recognition that their symptoms may not be completely eradicated and they will have to accommodate their lives to this fact may be the best approach.
Self-efficacy
Perceived control is closely related to one's self-efficacy in a given domain. Whereas perceived control is described as a person's perception of whether they can exert an influence on an outcome, self-efficacy refers to the extent to which a person believes that they can successfully perform the necessary tasks needed to achieve the desired outcome in a given situation [72]. For example, an individual with FM may believe that she has some control over managing her pain via increased physical activity (perceived control), but may feel that she does not have the tools in order to do (self-efficacy). Self-efficacy is not a global trait; instead, it changes in reference to specific situations and behaviors. That is, a person will not have high self-efficacy across the board; they may have high self-efficacy for a specific behavior (e.g., performing a particular exercise) or set of behaviors (e.g., engaging in social activities).
Efficacy judgments are made based upon four pieces of information about the person's capacities: their own past performance at that task or a similar task, the performance of others who are thought to be similar to the person, messages from others that the person is capable of performing the task, and the person's perception of his state of physiological arousal [73]. Low self-efficacy for pain management is associated with disability in both cross-sectional and longitudinal studies [74-76]. The relationship between pain intensity and subsequent physical and psychological difficulties is mediated by self-efficacy beliefs [74,77]. While high baseline self-efficacy for pain control predicts better outcomes for people with chronic pain, improvements in self-efficacy beliefs is also a strong predictor of better therapeutic outcomes with regard to pain management [78-79].
Importantly, for individuals to develop high self-efficacy with regard to their symptom management, they must first have an experience of mastery in that domain. In clinical practice, this may mean allowing patients to perform tasks in gradual, small increments rather than being expected to make large gains immediately. Returning to our example of an FM patient with low self-efficacy for increasing her physical activity, a provider may request that the patient begin by engaging in two minutes of planned walking per day instead of beginning by advising her to exercise for 20 minutes daily. As she experiences success in adding small breaks for walking into her day, the patient may be more likely to believe that she can increase and sustain this activity over time.
Psychological (in)flexibility
Psychological flexibility refers to one's ability to act in accordance with his or her own values, even in the midst of interfering thoughts, feelings, or bodily sensations [80]. In acting in alignment with one's values, individuals may persist in behaviors that move them toward their broader goals, and may need to change behaviors that move them further away from their values. Studies of psychological flexibility demonstrate that higher flexibility is adaptive. Higher psychological flexibility has been consistently associated with better psychological outcomes and higher quality of life [81]. Low levels of psychological flexibility, also known as psychological inflexibility, are associated with avoidant coping methods like denial, behavioral disengagement, and self-blame [82].
In the context of patients with CSS, psychological flexibility appears as physical symptoms plus quality of life. For example, psychological flexibility may be manifested by a person's ability and dedication to maintain involvement in meaningful activities or a person's acceptance that they will live despite some level of symptoms throughout their lives, while still striving for a full life. On the opposite end of the spectrum, psychological inflexibility may be evident among patients who withdraw from their favorite activities as a result of believing that they cannot be active in hobbies and experience symptoms. These patients may also obsessively seek ways to eradicate their symptoms completely, often spending a great deal of time in the search to remove pain, and to the detriment of actively engaging in their lives. Obviously the issue of balance becomes paramount. The Serenity Prayer attributed to the theologian Reinhold Niebhur captures this quite well – “Give me the serenity to accept this things I cannot change, the courage to change the things I can, and the wisdom to know the difference.”
Social Aspects of Pain
The experience of pain is largely a private, highly personal, and subjective experience. However, there are social features associated with pain. People with chronic pain do not exist in a vacuum in their daily lives -- they are parents, children, partners, employees, and more. That is, they are an integral part of many complex socioenvironmental systems. People with CSS rarely suffer in complete silence, and their lives, affected by the symptoms that they live with, influence the lives of those around them. In this section, we detail several constructs within the social realm of the biopsychosocial model that influence and are influenced by chronic symptoms.
Social learning
The social-learning perspective provides a framework for how CSS patients' symptom behaviors (e.g., actions, verbalizations, facial expressions) may develop and be maintained over time. In short, a person's learning history, including the sum of responses that their behavior elicits from others over time, guides their behavior. Specifically, behaviors that result in pleasant outcomes (e.g., positive reinforcement) or the removal of unpleasant outcomes (e.g., negative reinforcement) are maintained while behaviors that result in negative outcomes (positive punishment) or the removal of positive outcomes (e.g., negative punishment) are terminated [83].
The social learning process begins in childhood when attitudes about health, symptoms, and responses first develop. These ideas about symptoms are learned from parents, cultural influences, and the broader environment. Social learning has been put forth as a precipitator of the onset of some CSS conditions. For example, in retrospective studies of pain-free adults and adults with IBS, higher rates of reinforcement of pain behaviors and modeling of illness behaviors during childhood have been found for IBS patients versus pain-free adults, suggesting that there is a relationship between the presence of the syndrome and people's learning histories [84]. However, these retrospective findings do not support causal claims about social learning and the presence of symptoms. Although there is no evidence to support social learning as the only, or even primary, cause of any CSS conditions, there is evidence that the symptom experience is shaped by learning. A plethora of studies show that in laboratory settings and in field settings, patients' pain responses can be shaped by the behavior of others [85].
Symptom-related behaviors that communicate pain, distress, and suffering and that have garnered positive rewards are maintained, whereas those that resulted in negative consequences (e.g., criticism) are terminated. For instance, an individual with chronic pelvic pain who feels unsupported by her family may receive more attention and outward support from them when she exhibits more symptomatic behaviors; this may encourage the patient to continue to display, and possibly exaggerate, her symptoms in order to experience the support that she desires. It is important to note that people do not necessarily engage in this type of behavior in order to be manipulative or with malicious intent and it may not even be consciously motivated. Instead, the person is exhibiting a normal and expected pattern of behavior (e.g., social learning) that includes maladaptive components (e.g., reinforced pain behavior). Purely behavioral treatments (e.g., operant behavioral programs) that focus on changing pain responses by altering the type and frequency of reinforcement have been found to be modestly effective at reducing pain and pain behaviors, along with improving general functioning [86]. However, in order to achieve the greatest gains in chronic pain management, these behavioral treatments are best employed as one component of a more comprehensive treatment program [87].
Social stigma and skepticism
Individuals with CSSs, regardless of age, race, and other demographic identifiers, often report that stigma arising from others' responses to their illnesses is a relevant issue in their lives. Stigma is most often defined as the identification of negative characteristics that distinguish a person as being different and separate from the normative group [88]. Stigma may lead to direct discrimination by others and often leads to a loss in social status [89]. Social stigma is comprised of several constructs including the perception of stigma, internalization of stigma (e.g., self-stigmatization), and enacted stigma by others (e.g., discrimination).
Chronic illnesses have varying possibilities for stigma depending on the traits of the illness and on how people perceive it. For example, stigma has frequently been discussed in the context of diseases that may be contracted due to stigmatized behaviors (e.g., unprotected sex/injection drug use in HIV/AIDS, smoking in lung disease) or are infectious (e.g., HIV/AIDS). Individuals diagnosed with CSS may experience stigma as a result of several characteristics. First, the elusive etiology of CSS conditions may increase others' skepticism about the validity of the condition as a medical phenomenon that is not solely psychogenic. Second, the invisible nature of pain can make it difficult for others to believe that there is anything wrong with the patient; people with CSSs often note hearing phrases like “you look so healthy.” Third, the cyclical nature of symptoms and symptom flare-ups in these conditions may reinforce others' skepticism (e.g., “But she was fine yesterday.”). Indeed, in a qualitative study of women with chronic fatigue syndrome and FM, women reported that they primarily felt stigmatized by others' questioning the veracity of their symptoms [90]. Last, the symptoms associated with CSS and responses to help manage it (e.g., limiting some activity, pacing) may increase the burden of duty on others, which may lead to resentment and shaming by individuals who are not limited by symptoms.
Stigma has indirect, but negative implications for psychological wellbeing, quality of life, and health maintenance. A substantial literature highlights that stigma can result in decreased self-esteem and higher rates of depression [89]. This can lead to reduced engagement and follow-up in health care [91]. In addition to the intrapersonal effects of stigma, there are interpersonal effects as well. The interpersonal effects of stigma result from others' reactions to the person's medical condition. For instance, CSS patients may have the experience of being excluded from a friend's outing because the friend is uncertain about how to help them address their symptoms. Although this behavior is not meant to stigmatize or hurt the patient, it has the effect of distancing the patient from others who are “healthy.”
CSS patients are not immune from feeling stigmatized by their medical providers. The private features of the experience of symptoms, especially without an easily diagnosable basis, can make it difficult for providers to know how to appropriately treat CSS patients. This, coupled with care that may be perceived as sub-par by patients who continue to have persistent symptoms after medical intervention, can further complicate the patient-provider relationship [92]. Even when they do not intend to stigmatize their patients, providers' ambivalence about how to effectively manage their patients' symptoms can be experienced by the patient as hostile, blaming, disbelief, or even discriminatory. For example, patients with chronic pain have characterized their interactions with their pain care providers as “strenuous,” “complicated,” and “heavy” [93]. Emerging research suggests that shared decision making interventions, in which providers and patients collaborate to make health care choices together by taking into account the best evidence and patients' values and preferences, can help increase physicians' self-efficacy and confidence in treating chronic pain, and can improve relationships with these patients [94]. Bieber and colleagues noted that in a study including physicians and their patients with FM, a shared decision making intervention led to more productive interactions (rated by both patients and physicians), reductions in physicians' negative feelings about patients with FM, improvements in coping among patients, and patient's more active engagement in their treatment plans, even though their pain scores did not change [95].
Social support
Social support is one of the most frequently cited psychosocial factors that influences health outcomes. A critical mass of evidence has accumulated demonstrating that social ties and feeling cared for by others in one's network are positively associated with mental health, physical health, and lifespan [96-98]. Importantly, more research has studied perceptions of social support, rather than actual social support received. In their influential work, Cohen and Willis found that there was a stronger association between perceived social support and health behaviors than between actual support and health behaviors [99]. They noted that in order for available resources to be effectively utilized by a person, he or she must first perceive them to be available. In strong support of the impact of these perceptions on health, Holt-Lunstad and colleagues determined that perceived social supported was related to significantly lower risk for all-cause mortality, even after statistically controlling for other potential confounding variables like demographics and health behaviors in their meta-analysis [100].
In CSSs, the influence of social support and adjustment to illness is not straightforward. Most evidence supports the standard role of social support with pain patients who report high levels of social support endorsing less distress, less intense pain, and better overall adjustment [101-102]. However, contradictory findings show that greater social support can be associated with increased pain severity and increases in pain behavior [103-104]. Why might these conflicting findings exist? The primary explanation offered is that the type of social support available is critical. In studies that show deleterious effects of social support, the support is often conceptualized as support through increased attention and solicitousness that may actually reinforce symptoms and pain behaviors [103]. Still, the overarching message in the literature is that believing that a person is a valued member of a social network and that they are able to receive assistance from others when needed is helpful for managing pain.
Social support appears to have a positive influence on pain through several routes. People may learn about adaptive coping strategies to manage their pain from others in their lives, and may be encouraged to try new techniques or to sustain engagement in care by others. For example, a supportive wife might encourage her husband who suffers from migraines to ask for a referral for specialty services. Patients may also effectively use their relationships with their support systems to express their frustrations, which could have the effect of reducing negative emotions (e.g., anger, sadness) and pain.
Integrating Psychosocial Factors into Chronic Pain Treatment
As outlined above, emotional, cognitive, and social factors all play important roles in the experience and maintenance of chronic pain, and engagement in and response to treatments. Prior to providing care for the patient with CSS, clinicians must gain a thorough understanding of the person including their symptoms and history, extending beyond medical histories and current symptoms alone to include psychological and social factors. Several brief self-report measures exist to assess individual syndromes that represent CSSs (e.g., FM, chronic fatigue, and IBS), and many of these measures include brief assessment of psychological distress, most commonly anxiety and depression [105-108]. However, over-reliance on measures of specific diagnoses may neglect important comorbidities among CSSs, especially as overlap among CSSs is common, and assessing individual conditions using separate measures may be overly time consuming and burdensome in practice [2,4]. The Central Sensitization Inventory (CSI) is one instrument that was recently developed to help clinicians evaluate patients with CSSs. It assesses the presence and extent of key features of CSS in an efficient manner [109]. In addition to primary CSS-related symptoms, the CSI also briefly assesses psychological symptoms including anxiety, depression, panic disorder, PTSD, and childhood trauma. In preliminary studies, the CSI has been shown to have good psychometric properties, and the ability to distinguish between CSS patients and non-patient controls [109, 110]. Integrating a measure of CSSs that also assesses psychological functioning into standard practice can aid providers in both diagnosing their patients and guiding them to appropriate care, which may often include psychological intervention (see article by Williams in this issue).
Once a CSS diagnosis has been established, there is a wide array of psychological interventions to manage pain and other symptoms associated with CSS, many with empirically established support [111]. The most common goals of psychological interventions for pain management are to reduce pain intensity, increase physical functioning, improve mood, sleep, and relationships, enhance patients' quality of life, return to normal daily activities, and to help control medication use. Self-regulatory approaches to pain control often teach a particular skill or technique to help manage pain, and include practices like relaxation training, hypnosis or biofeedback. Aside from isolated techniques, there is evidence for the effectiveness of particular forms of therapy for pain management. It is critically important for medical providers to recognize that psychological treatment does not entail a single approach or modality. There are many schools of thought, along with their own theories, models, and techniques that guide the way that a therapist interacts with a patient. Some of these theories are implicated in the effective treatment of pain management, and some are not. For instance, there is support for behaviorally based therapies, cognitive-behavioral therapy, and mindfulness based therapy for pain control [112]. These therapies use different approaches to provide some distinct and some overlapping skills to help patients better cope with their pain.
Meta-analyses and systematic reviews have been conducted to examine the effects of psychological treatments and in particular, cognitive-behavior therapy (CBT; see article by Williams in this issue) for a number of pain conditions (e.g., arthritis pain, cancer pain, CLBP) [111, 113]. In general they demonstrate that CBT produces significant decreases in pain (typically small to medium effect sizes) and significant improvements in indices of adjustment to pain (e.g., activity, depression, anxiety, self-confidence, maladaptive beliefs). However, the results are not consistent across studies, which may relate to the specific content, mode of delivery, duration of treatment, and extent of therapist training. Most commonly, psychological approaches are viewed as important components integrated within more comprehensive approaches, especially for patients with complex medical and psychosocial problems.
Concluding Thoughts
The experience of CSSs is a multifactorial one, including physiological, behavioral, cognitive, affective, and social features. Failure to recognize each of these factors will result in an incomplete understanding of a person's symptoms. Given the complexity of CSSs and their related symptoms, along with the dynamic interaction of all of these features, the biopsychosocial model has been accepted as the primary approach to understanding and managing pain [9]. The intent of the present article was to highlight psychological and social factors relevant to the experience, exacerbation, and maintenance of pain and response to treatments. As we stressed, psychological and social factors may exacerbate a patient's clinical presentation, but they rarely are the sole cause of the symptoms. With this in mind, providers must recognize that in order to treat the CSS patient's symptoms, they must attempt to understand the whole unique person – his or her history, environment, and resources, and not just their symptoms or disorder.
Acknowledgments
The contribution of Dr. Adams was supported by the National Institute on Aging (5T32AG027677).
Contributor Information
Leah M. Adams, Group Health Research Institute
Dennis C. Turk, University of Washington
References
- 1.Wilson HD, Robinson JP, Turk DC. Toward the identification of symptom patterns in people with fibromyalgia. Arthritis Car Res. 2009;61:527–534. doi: 10.1002/art.24163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum. 2008;37:339–352. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Van Houdenhove B, Luyten P. Fibromyalgia and related syndromes characterized by stress intolerance and pain hypersensitivity: do we need a new nosology? Curr Rheumatol Rev. 2007;3(4):304–308. [Google Scholar]
- 4.Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Flor H, Turk DC. Chronic pain: an integrated biobehavioral approach. Seattle: IASP Press; 2011. [Google Scholar]
- 6.Sperber AD, Drossman DA. Irritable bowel syndrome: a multidimensional disorder cannot be understood or treated from a unidimensional perspective. Therap Adv Gastroenterol. 2012;5:387–393. doi: 10.1177/1756283X12460420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatchel RJ, Peng YB, Peters ML, et al. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 8.Lumley MA, Cohen JL, Borszcz GS, et al. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol. 2011;67:942–968. doi: 10.1002/jclp.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassett AL, Clauw DJ. Does psychological stress cause chronic pain? Psychiatr Clin North Am. 2011;34:579–594. doi: 10.1016/j.psc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Stratz T, Schneider M, Joos TO, et al. Pathophysiology and treatment of fibromyalgia syndrome. Curr Rheumatol Rev. 2007;3(2):167–170. [Google Scholar]
- 11.Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet. 2011;377(9784):2226–2235. doi: 10.1016/S0140-6736(11)60402-9. [DOI] [PubMed] [Google Scholar]
- 12.Substance Abuse and Mental Health Services Administration. Results from the 2012 national survey on drug use and health: mental health findings. Rockville: Substance Abuse and Mental Health Services; 2013. (NSDUH Series H-47). HHS Publication No. (SMA) 13-4805. [Google Scholar]
- 13.Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26:1175–1182. doi: 10.1007/s11606-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13:7–23. doi: 10.31887/DCNS.2011.13.1/wkaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 16.Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull. 1996;119:95–110. [Google Scholar]
- 17.Tripp DA, VanDenKerkhof EG, McAlister M. Prevalence and determinants of pain and pain-related disability in urban and rural settings in southeastern Ontario. Pain Res Manage. 2006;11:225–233. doi: 10.1155/2006/720895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall AM, Kamper SJ, Maher CG, et al. Symptoms of depression and stress mediate the effect of pain on disability. Pain. 2011;152:1044–1051. doi: 10.1016/j.pain.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Okifuji A, Turk DC, Sherman JJ. Evaluation of the relationship between depression and fibromyalgia syndrome: why aren't all patients depressed? J Rheum. 2000;27:212–219. [PubMed] [Google Scholar]
- 20.Brown GK. A causal analysis of chronic pain and depression. J Abnorm Psychol. 1990;99:127–137. doi: 10.1037//0021-843x.99.2.127. [DOI] [PubMed] [Google Scholar]
- 21.Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review Clin J Pain. 1997;13:116–137. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Jarvik JG, Hollingworth W, Heagerty PJ, et al. Three-year incidence of low back pain in an initially asymptomatic cohort: clinical and imaging risk factors. Spine. 2005;30:1541–1548. doi: 10.1097/01.brs.0000167536.60002.87. [DOI] [PubMed] [Google Scholar]
- 23.Lackner JM, Quigley BM, Blanchard EB. Depression and abdominal pain in IBS patients: the mediating role of catastrophizing. Psychosom Med. 2004;66:435–441. doi: 10.1097/01.psy.0000126195.82317.46. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Wu J, Bair MJ. Reciprocal relationship between pain and depression: a 12-month analysis in primary care. J Pain. 2011;12:964–973. doi: 10.1016/j.jpain.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett RM, Jones J, Turk DC, et al. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27. doi: 10.1186/1471-2474-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz J, Page MG, Fashler S, et al. Chronic pain and the anxiety disorders: epidemiology, mechanisms and models of comorbidity and treatment. In: Marchand S, Saravane D, Gaumond I, editors. Mental health and pain: somatic and psychiatric components of pain in mental health. Paris: Springer-Verlag; 2014. pp. 119–155. [Google Scholar]
- 27.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R) Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- 29.Knaster P, Karlsson H, Estlander A, et al. Psychiatric disorders as assessed with SCID in chronic pain patients: the anxiety disorders precede the onset of pain. Gen Hosp Psychiatry. 2012;34:46–52. doi: 10.1016/j.genhosppsych.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Gerrits MMJG, Vogelzangs N, van Oppen P, et al. Impact of pain on the course of depressive and anxiety disorders. Pain. 2012;153:429–436. doi: 10.1016/j.pain.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Gerrits MMJG, van Oppen P, van Marwijk HWJ, et al. Pain and the onset of depressive and anxiety disorders. Pain. 2014;155:53–59. doi: 10.1016/j.pain.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 33.Asmundson GJ, Coons MJ, Taylor S, et al. PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. Can J Psychiatry. 2002;47:930–937. doi: 10.1177/070674370204701004. [DOI] [PubMed] [Google Scholar]
- 34.Jenewein J, Moergeli H, Wittmann L, et al. Development of chronic pain following severe accidental injury. Results of a 3-year follow-up study J Psychosom Res. 2009;66:119–126. doi: 10.1016/j.jpsychores.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Hauser W, Galek A, Erbsloh-Moller B, et al. Posttraumatic stress disorder in fibromyalgia syndrome: prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain. 2013;154:1216–1223. doi: 10.1016/j.pain.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 36.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Upshur CC, Luckmann RS, Savageau JA. Primary care provider concerns about management of chronic pain in community clinic populations. J Gen Intern Med. 2006;21:652–655. doi: 10.1111/j.1525-1497.2006.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pud D, Cohen D, Lawental E, et al. Opioids and abnormal pain perception: new evidence from a study of chronic opioid addicts and healthy subjects. Drug Alcohol Depend. 2006;82:218–223. doi: 10.1016/j.drugalcdep.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Boscarino JA, Rukstalis MR, Hoffman SN, et al. Prevalence of prescription opioid-use disorder among chronic pain patients: comparison of the DSM-5 vs. DSM-4 diagnostic criteria J Addict Dis. 2011;30:185–194. doi: 10.1080/10550887.2011.581961. [DOI] [PubMed] [Google Scholar]
- 41.Morasco BJ, Gritzner S, Lewis L, et al. Systematic review of prevalence, correlates, and treatment outcomes for chronic non-cancer pain in patients with comorbid substance use disorder. Pain. 2011;152:488–497. doi: 10.1016/j.pain.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Materazzo F, Cathart S, Pritchard D. Anger, depression, and coping interactions in headache activity and adjustment: a controlled study. J Psychosom Res. 2000;49:69–75. doi: 10.1016/s0022-3999(00)00144-6. [DOI] [PubMed] [Google Scholar]
- 43.Venable VL, Carlson CR, Wilson J. The role of anger and depression in recurrent headache. Headache. 2001;41:21–30. doi: 10.1046/j.1526-4610.2001.111006021.x. [DOI] [PubMed] [Google Scholar]
- 44.van Middendorp H, Lumley MA, Moerbeek M, et al. Effects of anger and anger regulation styles on pain in daily life of women with fibromyalgia: a diary study. Eur J Pain. 2010;14:176–182. doi: 10.1016/j.ejpain.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Bruehl S, Chung OY, Burns JW, et al. Trait anger expressiveness and pain-induced beta-endorphin release: support for the opioid dysfunction hypothesis. Pain. 2002;130:208–215. doi: 10.1016/j.pain.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flor H, Turk DC, Birbaumer N. Assessment of stress-related psychophysiological reactions in chronic back pain patients. J Consult Clin Psychol. 1985;53:354–364. doi: 10.1037//0022-006x.53.3.354. [DOI] [PubMed] [Google Scholar]
- 47.Burns JW. Arousal of negative emotions and symptom-specific reactivity in chronic low back pain patients. Emotion. 2006;6:309–319. doi: 10.1037/1528-3542.6.2.309. [DOI] [PubMed] [Google Scholar]
- 48.Burns JW, Bruehl S, Quartana PJ. Anger management style and hostility among chronic pain patients: effects on symptom-specific physiological reactivity during anger-and sadness-recall interviews. Psychosom Med. 2006;68:786–793. doi: 10.1097/01.psy.0000238211.89198.e4. [DOI] [PubMed] [Google Scholar]
- 49.Pilowsky I, Spence N. Pain, anger, and illness behavior. J Psychosom Res. 1976;20:411–416. doi: 10.1016/0022-3999(76)90003-9. [DOI] [PubMed] [Google Scholar]
- 50.Bruehl S, Chung OY, Burns JW. Anger expression and pain: an overview of findings and possible mechanisms. J Behav Med. 2006;29:593–606. doi: 10.1007/s10865-006-9060-9. [DOI] [PubMed] [Google Scholar]
- 51.Benedetti F, Thoen W, Blancahrd C, et al. Pain as a reward: changing the meaning of pain from negative to positive co-activates opioid and cannabinoid systems. Pain. 2013;154:361–367. doi: 10.1016/j.pain.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Leventhal H, Brissette I, Leventhal EA. The common-sense model of self-regulation of health and illness. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behavior. London: Routledge; 2003. pp. 42–65. [Google Scholar]
- 53.Moss-Morris R, Chalder T. Illness perceptions and levels of disability in patients with chronic fatigue syndrome and rheumatoid arthritis. J Psychosom Res. 2003;55:305–308. doi: 10.1016/s0022-3999(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 54.Turner JA, Jensen MP, Romano JM. Do beliefs, coping, and catastrophizing independently predict functioning in patients with chronic pain? Pain. 2000;85:115–125. doi: 10.1016/s0304-3959(99)00259-6. [DOI] [PubMed] [Google Scholar]
- 55.Balderson BHK, Lin EHB, Von Korff M. The management of pain-related fear in primary care. In: Asmundson GJ, Vlaeyen JWS, Crombez G, editors. nderstanding and treating fear of pain. Oxford; Oxford University Press; 2004. pp. 267–292. [Google Scholar]
- 56.Ihlebaek C, Eriksen HR. Are the “myths” of low back pain alive in the general Norwegian population? Scan J Public Health. 2003;31:395–398. doi: 10.1080/14034940210165163. [DOI] [PubMed] [Google Scholar]
- 57.Chilcot J, Moss-Morris R. Changes in illness-related cognitions rather than distress mediate improvements in irritable bowel syndrome (IBS) symptoms and disability following a brief cognitive behavioural therapy intervention. Behav Res Ther. 2013;51:690–695. doi: 10.1016/j.brat.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan MJL, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 59.Severeijns R, Vlaeyen JW, van den Hout MA. Do we need a communal coping model of pain catastrophizing? An alternative explanation Pain. 2004;111:226–229. doi: 10.1016/j.pain.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 60.Vlaeyen JWS, Kole-Snijders AMJ, Rotteveel AM, et al. The role of fear of movement/(re)injury in pain disability. J Occupational Rehabil. 1995;5:235–252. doi: 10.1007/BF02109988. [DOI] [PubMed] [Google Scholar]
- 61.Leeuw M, Goossens MEJB, Linton SJ, et al. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30:77–93. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 62.Edwards RR, Bingham CO, Bathon J, et al. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Care Res. 2006;55:325–332. doi: 10.1002/art.21865. [DOI] [PubMed] [Google Scholar]
- 63.Swinkels-Meewisse IEJ, Roelofs J, Oostendorp RAB, et al. Acute low back pain: pain-related fear and pain catastrophizing influence physical performance and perceived disability. Pain. 2006;120:36–43. doi: 10.1016/j.pain.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Velly AM, Look JO, Carlson C, et al. The effect of catastrophizing and depression on chronic pain-a prospective cohort study of temporomandibular muscle and joint pain disorders. Pain. 2011;152:2377–2383. doi: 10.1016/j.pain.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 65.Jensen MP, Turner JA, Romano JM. Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. J Consult Clin Psychol. 2001;69:655–662. doi: 10.1037//0022-006x.69.4.655. [DOI] [PubMed] [Google Scholar]
- 66.Craske MG, Wolitzky-Taylor KB, Labus J, et al. A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations. Behav Res Ther. 2011;49:413–421. doi: 10.1016/j.brat.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crombez G, van Damme S, Eccleston C. Hypervigilance to pain: an experimental and clinical analysis. Pain. 2005;116:4–7. doi: 10.1016/j.pain.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 68.Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain. 2007;127:276–286. doi: 10.1016/j.pain.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Keefe FJ, Rumble ME, Scipio CD, et al. Psychological aspects of persistent pain: current state of the science. J Pain. 2004;5:195–211. doi: 10.1016/j.jpain.2004.02.576. [DOI] [PubMed] [Google Scholar]
- 70.Crombez G, Eccleston C, de Vlieger P, et al. Is it better to have controlled and lost than never to have controlled at all? An experimental investigation of control over pain Pain. 2008;137:631–639. doi: 10.1016/j.pain.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 71.McCracken LM, Carson JW, Eccleston C, et al. Acceptance and change in the context of chronic pain. Pain. 2004;109:4–7. doi: 10.1016/j.pain.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 73.Bandura A. Self-efficacy: the exercise of control. New York: Freeman; 1997. [Google Scholar]
- 74.Arnstein P. The mediation of disability by self efficacy in different samples of chronic pain patients. Disabil Rehabil. 2000;22:794–801. doi: 10.1080/09638280050200296. [DOI] [PubMed] [Google Scholar]
- 75.French DJ, Holroyd KA, Pinell C, et al. Perceived self-efficacy and headache-related disability. Headache. 2000;40:647–656. doi: 10.1046/j.1526-4610.2000.040008647.x. [DOI] [PubMed] [Google Scholar]
- 76.Costa LC, Maher CG, McAuley JH, et al. Self-efficacy is more important than fear of movement in the relationship between pain and disability in chronic low back pain. Eur J Pain. 2011;15:213–219. doi: 10.1016/j.ejpain.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 77.Woby SR, Urmston M, Watson PJ. Self-efficacy mediates the relation between pain-related fear and outcome in chronic low back pain patients. Eur J Pain. 2007;11:711–718. doi: 10.1016/j.ejpain.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 78.Beal CC, Stuifbergen AK, Brown A. Predictors of a health promoting lifestyle in women with fibromyalgia syndrome. Psychol Health Med. 2009;14:343–353. doi: 10.1080/13548500902730093. [DOI] [PubMed] [Google Scholar]
- 79.Wells-Federman C, Arnstein P, Caudill M. Nurse-led pain management program: effect on self-efficacy, pain intensity, pain-related disability, and depressive symptoms in chronic pain patients. Pain Management Nursing. 2002;3:131–140. doi: 10.1053/jpmn.2002.127178. [DOI] [PubMed] [Google Scholar]
- 80.Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes, and outcomes. Behav Res Ther. 2006;44:1–25. doi: 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 81.Kashdan TB, Rottenberg J. Psychological flexibility as a fundamental aspect of health. Clin Psychol Rev. 2010;30:865–878. doi: 10.1016/j.cpr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wicksell RK, Renofalt J, Olsson GL, et al. Avoidance and cognitive fusion-central components in pain related disability? Development and preliminary validation of the Psychological Inflexibility in Pain Scale (PIPS) Eur J Pain. 2008;12:491–500. doi: 10.1016/j.ejpain.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 83.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs: Prentice Hall; 1986. [Google Scholar]
- 84.Lowman BC, Drossman DA, Cramer EM, et al. Recollection of childhood events in adults with irritable bowel syndrome. J Clin Gastroenterol. 1987;9:324–330. doi: 10.1097/00004836-198706000-00017. [DOI] [PubMed] [Google Scholar]
- 85.Hermann C. Modeling, social learning in pain. In: Schmidt R, Willis W, editors. The encyclopedia of pain. Heidelberg: Springer Publishing; 2013. pp. 1894–1898. [Google Scholar]
- 86.Eccleston C, Williams ACDC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2009;(2) doi: 10.1002/14651858.CD007407.pub2. Art. No.: CD007407. [DOI] [PubMed] [Google Scholar]
- 87.Sanders SH. Operant therapy with pain patients: evidence for its effectiveness. Semin Pain Med. 2003;1:90–98. [Google Scholar]
- 88.Goffman E. Stigma: the management of spoiled identity. Harmondsworth: Penguin; 1963. [Google Scholar]
- 89.van Brakel WH. Measuring health-related stigma-a literature review. Psychol Health Med. 2006;11:307–334. doi: 10.1080/13548500600595160. [DOI] [PubMed] [Google Scholar]
- 90.Asbring P, Narvanen AL. Women's experiences of stigma in relation to chronic fatigue syndrome and fibromyalgia. Qual Health Res. 2002;12:148–160. doi: 10.1177/104973230201200202. [DOI] [PubMed] [Google Scholar]
- 91.Earnshaw VA, Quinn DM. The impact of stigma in healthcare on people living with chronic illnesses. J Health Psychol. 2012;17:157–168. doi: 10.1177/1359105311414952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tait RC, Chibnall JT, Kalauokalani D. Provider judgments of patients in pain: seeking symptom certainty. Pain Med. 2009;10:11–34. doi: 10.1111/j.1526-4637.2008.00527.x. [DOI] [PubMed] [Google Scholar]
- 93.Werner A, Malterud K. It is hard work behaving as a credible patient: encounters between women with chronic pain and their doctors. Soc Sci Med. 2003;57:1409–1419. doi: 10.1016/s0277-9536(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 94.Bieber C, Nicolai J, Hartmann M, et al. Training physicians in shared decision-making-who can be reached and what is achieved. Patient Educ Couns. 2009;77:48–54. doi: 10.1016/j.pec.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 95.Bieber C, Muller KG, Blumenstiel K, et al. Long-term effects of a shared decision-making intervention on physician-patient interaction and outcome in fibromyalgia: a qualitative and quantitative 1 year follow-up of a randomized controlled trial. Patient Educ Couns. 2006;63:357–366. doi: 10.1016/j.pec.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 96.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23:207–218. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
- 97.Callaghan P, Morrissey J. Social support and health: a review. J Adv Nurs. 1993;8:203–210. doi: 10.1046/j.1365-2648.1993.18020203.x. [DOI] [PubMed] [Google Scholar]
- 98.Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- 99.Cohen S, Wills TA. Stress, social support and the buffering hypothesis. Psychol Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- 100.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lackner JM, Brasel AM, Quigley BM. The ties that bind: perceived social support, stress, and IBS in severely affected patients. Neurogastroeterol Motil. 2010;22:893–900. doi: 10.1111/j.1365-2982.2010.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lopez-Martinez AE, Esteve-Zarazaga R, Ramirez-Maestre C. Perceived social support and coping responses are independent variables explaining pain adjustment among chronic pain patients. J Pain. 2008;9:373–379. doi: 10.1016/j.jpain.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Romano JM, Jensen MP, Turner JA, et al. Chronic pain patient-partner interactions: further support for a behavioural model of chronic pain. Behav Ther. 2000;31:415–440. [Google Scholar]
- 104.Flor H, Kerns RD, Turk DC. The role of spouse reinforcement, perceived pain, and activity levels of chronic pain patients. J Psychosom Res. 1987;106:453–460. doi: 10.1016/0022-3999(87)90082-1. [DOI] [PubMed] [Google Scholar]
- 105.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18(5):728–733. [PubMed] [Google Scholar]
- 106.Smets EMA, Garssen B, Bonke B, et al. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 107.Reme SE, Darnley S, Kennedy T, et al. The development of the irritable bowel syndrome-behavioral responses questionnaire. J Psychosom Res. 2010;69(3):319–325. doi: 10.1016/j.jpsychores.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 108.Bennett RM, Friend R, Jones KD, et al. The revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis Res Care. 2009;11:R120. doi: 10.1186/ar2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mayer TG, Neblett R, Cohen H, et al. The development and psychometric validation of the Central Sensitization Inventory (CSI) Pain Pract. 2012;12(4):276–285. doi: 10.1111/j.1533-2500.2011.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neblett R, Cohen H, Choi Y, et al. The Central Sensitization Inventory (CSI): establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain. 2013;14(5):438–445. doi: 10.1016/j.jpain.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;(2) doi: 10.1002/14651858.CD007407.pub2. Art. No.: CD007407. doi:10.1002/14651858.CD007407.pub 3. [DOI] [PubMed] [Google Scholar]
- 112.Kerns RD, Sellinger J, Goodin BR. Psychological treatment of chronic pain. Ann Rev Clin Psychol. 2011;7:411–434. doi: 10.1146/annurev-clinpsy-090310-120430. [DOI] [PubMed] [Google Scholar]
- 113.Hoffman BM, Papas RK, Chatkoff DK, et al. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol. 2007;26:1–9. doi: 10.1037/0278-6133.26.1.1. [DOI] [PubMed] [Google Scholar]