Abstract
BACKGROUND
Community-acquired pneumonia is a leading infectious cause of hospitalization and death among U.S. adults. Incidence estimates of pneumonia confirmed radio-graphically and with the use of current laboratory diagnostic tests are needed.
METHODS
We conducted active population-based surveillance for community-acquired pneumonia requiring hospitalization among adults 18 years of age or older in five hospitals in Chicago and Nashville. Patients with recent hospitalization or severe immunosuppression were excluded. Blood, urine, and respiratory specimens were systematically collected for culture, serologic testing, antigen detection, and molecular diagnostic testing. Study radiologists independently reviewed chest radiographs. We calculated population-based incidence rates of community-acquired pneumonia requiring hospitalization according to age and pathogen.
RESULTS
From January 2010 through June 2012, we enrolled 2488 of 3634 eligible adults (68%). Among 2320 adults with radiographic evidence of pneumonia (93%), the median age of the patients was 57 years (interquartile range, 46 to 71); 498 patients (21%) required intensive care, and 52 (2%) died. Among 2259 patients who had radio-graphic evidence of pneumonia and specimens available for both bacterial and viral testing, a pathogen was detected in 853 (38%): one or more viruses in 530 (23%), bacteria in 247 (11%), bacterial and viral pathogens in 59 (3%), and a fungal or mycobacterial pathogen in 17 (1%). The most common pathogens were human rhinovirus (in 9% of patients), influenza virus (in 6%), and Streptococcus pneumoniae (in 5%). The annual incidence of pneumonia was 24.8 cases (95% confidence interval, 23.5 to 26.1) per 10,000 adults, with the highest rates among adults 65 to 79 years of age (63.0 cases per 10,000 adults) and those 80 years of age or older (164.3 cases per 10,000 adults). For each pathogen, the incidence increased with age.
CONCLUSIONS
The incidence of community-acquired pneumonia requiring hospitalization was highest among the oldest adults. Despite current diagnostic tests, no pathogen was detected in the majority of patients. Respiratory viruses were detected more frequently than bacteria. (Funded by the Influenza Division of the National Center for Immunizations and Respiratory Diseases.)
Pneumonia is a leading infectious cause of hospitalization and death among adults in the United States,1,2 with medical costs exceeding $10 billion in 2011.3 Routine administration of the pneumococcal conjugate vaccine in children has resulted in an overall reduction in the rate of invasive disease and pneumonia among adults, owing to herd immunity.4-8 The last U.S. population–based incidence estimates of hospitalization due to community-acquired pneumonia were made in the 1990s,9 before the availability of the pneumococcal conjugate vaccine and more sensitive molecular and antigen-based laboratory diagnostic tests. Thus, contemporary population-based etiologic studies involving U.S. adults with pneumonia are needed.10-13
The Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study was a prospective, multicenter, population-based, active surveillance study. Radio-graphic confirmation and extensive diagnostic methods were used to determine the incidence and microbiologic causes of community-acquired pneumonia requiring hospitalization among U.S. adults.
METHODS
ACTIVE POPULATION-BASED SURVEILLANCE
From January 1, 2010, to June 30, 2012, adults 18 years of age or older were enrolled at three hospitals in Chicago (John H. Stroger, Jr., Hospital of Cook County, Northwestern Memorial Hospital, and Rush University Medical Center) and at two in Nashville (University of Tennessee Health Science Center–Saint Thomas Health and Vanderbilt University Medical Center). We sought to enroll all eligible adults; therefore, trained staff screened adults for enrollment at least 18 hours per day, 7 days per week. Written informed consent was obtained from all the patients or their caregivers before enrollment. The study protocol was approved by the institutional review board at each participating institution and at the CDC. Weekly teleconferences, enrollment reports, data audits, and annual study-site visits were conducted to ensure uniform procedures among the study sites. Patients or their caregivers provided demographic and epidemiologic data, and medical charts were abstracted for clinical data. All the authors vouch for the accuracy and completeness of the data and analyses reported and for the fidelity of the study to the protocol. All the authors made the decision to submit the manuscript for publication.
Adults were eligible for enrollment if they were admitted to a study hospital on the basis of a clinical assessment by the treating clinician; resided in the study catchment area (see the Supplementary Appendix, available with the full text of this article at NEJM.org); had evidence of acute infection, defined as reported fever or chills, documented fever or hypothermia, leukocytosis or leukopenia, or new altered mental status; had evidence of an acute respiratory illness, defined as new cough or sputum production, chest pain, dyspnea, tachypnea, abnormal lung examination, or respiratory failure; and had evidence consistent with pneumonia as assessed by means of chest radiography by the clinical team within 48 hours before or after admission.
Patients were excluded if they had been hospitalized recently (<28 days for immunocompetent patients and <90 days for immunosuppressed patients), had been enrolled in the EPIC study within the previous 28 days, were functionally dependent nursing home residents,14 or had a clear alternative diagnosis (see the Supplementary Appendix). Patients were also excluded if they had undergone tracheotomy, if they had a percutaneous endoscopic gastrostomy tube, if they had cystic fibrosis, if they had cancer with neutropenia, if they had received a solid-organ or hematopoietic stem-cell transplant within the previous 90 days, if they had active graft-versus-host disease or bronchiolitis obliterans, or if they had human immunodeficiency virus infection with a CD4 cell count of less than 200 per cubic millimeter.10
SPECIMEN COLLECTION
Blood samples, acute-phase serum specimens, urine samples, and nasopharyngeal and oropharyngeal swabs were obtained from the patients as soon as possible after presentation (see the Supplementary Appendix). In the case of patients with a productive cough, sputum was obtained. Pleural fluid, endotracheal aspirates, and broncho-alveolar-lavage samples that had been obtained for clinical care were analyzed for the study. Only specimens obtained within 72 hours before or after admission were included, except for pleural fluid, which was included if it was obtained within 7 days after admission. Patients were asked to return 3 to 10 weeks after enrollment for convalescent-phase serum collection.
RADIOGRAPHIC CONFIRMATION
Initial enrollment was based on the clinical interpretation of chest radiographs that were obtained at admission. Inclusion in the final study analyses required independent confirmation by a board-certified chest radiologist who reviewed all the chest radiographs and computed tomographic scans obtained within 48 hours before or after admission; these radiologists, who are coauthors of the study, were unaware of the clinical data. Radiographic evidence of pneumonia was defined as the presence of consolidation (a dense or fluffy opacity with or without air bronchograms), other infiltrate (linear and patchy alveolar or interstitial densities), or pleural effusion.5,15,16
CONTROLS
From November 1, 2011, to June 30, 2012, a convenience sample of asymptomatic adults from the Nashville study catchment area who presented for nonacute care to a general medicine clinic at Vanderbilt University Medical Center was enrolled weekly. Nasopharyngeal and oropharyngeal swabs were obtained to assess the prevalence of respiratory pathogens among asymptomatic adults. Exclusion criteria were the same as those for the adults with pneumonia, except that controls were also excluded if they had fever or respiratory symptoms within 14 days before or after enrollment (on the basis of information obtained during a telephone interview) or had received live attenuated influenza vaccination within 7 days before enrollment.
LABORATORY TESTING
Bacterial culture was performed, with the use of standard techniques, on blood samples, pleural fluid, high-quality sputum samples and endotracheal aspirates, and quantified bronchoalveolar-lavage specimens.17,18 A real-time polymerase-chain-reaction (PCR) assay for legionella was performed on sputum regardless of the quality of the sample.19,20 PCR assays targeting Enterobacteriaceae, Haemophilus influenzae, pseudomonas, Staphylococcus aureus, Streptococcus anginosus, S. mitis, S. pneumoniae, and S. pyogenes were also performed on pleural fluid.21-23 Urinary antigen testing was performed for the detection of Legionella pneumophila and S. pneumoniae (BinaxNOW, Alere).20,24
A PCR assay was performed on nasopharyngeal and oropharyngeal swabs with the use of CDC-developed methods for the detection of adenovirus; Chlamydophila pneumoniae; coronaviruses 229E, HKU1, NL63, and OC43; human metapneumovirus (HMPV); human rhinovirus; influenza A and B viruses; Mycoplasma pneumoniae; parainfluenza virus types 1, 2, and 3; and respiratory syncytial virus (RSV).19,25,26 Serologic testing for adenovirus, HMPV, influenza A and B viruses, parainfluenza viruses, and RSV was performed on available paired acute-phase and convalescent-phase serum specimens (see the Supplementary Appendix).27-32 The results of microbiologic testing that was conducted for clinical care, including testing for fungi or mycobacteria, were also obtained.
PATHOGEN DETECTION
A bacterial pathogen was determined to be present if gram-positive or gram-negative bacteria (except for L. pneumophila) were detected in a blood sample, sputum, endotracheal aspirate, bronchoalveolar-lavage specimen, or pleural fluid by means of culture or in pleural fluid by means of PCR assay; if C. pneumoniae or M. pneumoniae was detected in a nasopharyngeal or oropharyngeal swab by means of PCR assay; if L. pneumophila was detected in sputum by means of PCR assay; or if L. pneumophila or S. pneumoniae was detected in urine by means of antigen detection. Selected bacteria were considered to be contaminants.
A viral pathogen was determined to be present if adenovirus, coronavirus, HMPV, human rhinovirus, influenza virus, parainfluenza virus, or RSV was detected in a nasopharyngeal or oropharyngeal swab by means of PCR assay or if a pathogen-specific antibody titer was increased by a factor of 4 or more between the acute-phase serum specimen and the convalescent-phase serum specimen for all viruses except human rhinovirus and coronaviruses. Fungal or mycobacterial detections were determined according to clinical guidelines (see the Supplementary Appendix).
STATISTICAL ANALYSIS
Annual incidence rates were calculated from July 1, 2010, to June 30, 2011, and from July 1, 2011, to June 30, 2012. The number of enrolled adults with radiographic evidence of pneumonia was adjusted, according to age group, for the proportion of eligible adults enrolled at each study site and for the estimated proportion of admissions of adults for pneumonia to study hospitals in the catchment area (i.e., the market share, which was based on discharge-diagnosis codes). The adjusted number was then divided by the U.S. Census population estimates in the catchment area for the corresponding year (see the Supplementary Appendix).33
We calculated pathogen-specific incidence rates by multiplying the total incidence of pneumonia by the proportion of each pathogen detected among adults with radiographic evidence of pneumonia who had samples available for the detection of both bacterial and viral pathogens. Bootstrap methods with 10,000 samples were used to calculate 95% confidence intervals.
RESULTS
STUDY POPULATION
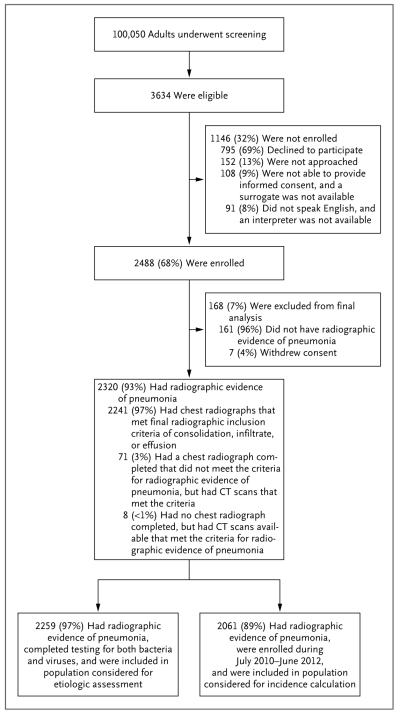
Of 3634 eligible adults, 2488 (68%) were enrolled (Fig. 1). As compared with persons who were eligible but not enrolled, the enrolled patients were significantly less likely to be 65 years of age or older and less likely to require invasive mechanical ventilation; in addition, a smaller proportion of the enrolled patients subsequently died (Table S1 in the Supplementary Appendix).
Figure 1. Screening, Eligibility, and Enrollment of Patients with Pneumonia.
CT denotes computed tomography.
Of 2488 enrolled patients, 2320 (93%) had radiographic evidence of pneumonia (Fig. 1). The median age of the patients with radiographic evidence of pneumonia was 57 years (interquartile range, 46 to 71) (Table 1), and the median length of hospital stay was 3 days (interquartile range, 2 to 6). A total of 498 patients (21%) required admission to the intensive care unit (ICU), 131 (6%) required invasive mechanical ventilation, and 52 (2%) died during hospitalization.
Table 1.
Characteristics of Adults with Community-Acquired Pneumonia Requiring Hospitalization.
| Characteristic | Adults with Radiographic Evidence of Pneumonia (N = 2320) |
|---|---|
| Race or ethnic group — no. (%)* | |
| Non-Hispanic white | 1086 (47) |
| Non-Hispanic black | 898 (39) |
| Hispanic | 243 (10) |
| Other | 93 (4) |
| Age group — no. (%) | |
| 18–49 yr | 701 (30) |
| 50–64 yr | 787 (34) |
| 65–79 yr | 517 (22) |
| ≥80 yr | 315 (14) |
| Duration from illness onset to hospital presentation — days† | |
| Median | 4 |
| Interquartile range | 2–7 |
| Any underlying condition — no. (%)‡ | 1817 (78) |
| Chronic lung disease | 968 (42) |
| Chronic heart disease | 810 (35) |
| Immunosuppression | 685 (30) |
| Diabetes mellitus | 597 (26) |
| Status regarding receipt of vaccine or treatment — no./total no. (%)§ | |
| Seasonal influenza vaccination | 448/1898 (24) |
| Pneumococcal vaccination in adults ≥65 yr of age | 308/704 (44) |
| Outpatient antibiotic use | 249/2232 (11) |
| Inpatient antibiotic use | 2287/2320 (99) |
| Radiographic finding — no. (%)¶ | |
| Consolidation | 1447 (62) |
| Alveolar or interstitial infiltrate | 920 (40) |
| Pleural effusion | 714 (31) |
| Pneumonia severity index∥ | |
| Median | 76 |
| Interquartile range | 52–103 |
| Risk class — no. (%) | |
| 1–3 | 1510 (65) |
| 4 | 606 (26) |
| 5 | 204 (9) |
Race and ethnic group were self-reported.
Data were missing for three patients.
Any underlying medical condition included chronic lung disease (asthma, chronic obstructive pulmonary disease, or obstructive sleep apnea), chronic heart disease (i.e., coronary artery disease or congestive heart failure, but not hypertension), immunosuppression (either due to chronic condition or medication, cancer [but not skin cancer], or human immunodeficiency virus [HIV] infection with a CD4 cell count of >200 per cubic millimeter), diabetes mellitus, chronic kidney disease (with or without dialysis), neurologic disorders (epilepsy, cerebral palsy, dementia, or history of stroke), chronic liver disease (hepatitis, cirrhosis, or hepatic failure), and splenectomy. The specific conditions that affected at least 25% of patients are listed here. Among the 685 patients with immunosuppression, 416 had nondermatologic cancer (but did not have neutropenia), 381 had immunosuppression due to a chronic condition or a medication, and 68 had HIV infection with a CD4 cell count of more than 200 per cubic millimeter. The groups were not mutually exclusive.
Data were based on verified vaccine information and not self-report. For influenza vaccine, the season was defined as September 1 through August 31 of the following year, and the percentage of patients vaccinated was based on the 1898 of 2320 adults (82%) for whom information was available. For pneumococcal polysaccharide vaccine, the percentage of patients vaccinated was based on 704 of 832 adults (85%) who were 65 years of age or older and had available information. For both vaccines, patients were considered to be vaccinated if they had received the vaccine at least 2 weeks before admission. Outpatient antibiotics were defined as those received within 5 days before admission. Inpatient antibiotics were defined as those received at any point during the hospitalization.
The categories were not mutually exclusive and therefore do not sum to 100%. Only 23 adults (1%) had pleural effusion alone. Among all the patients with pleural effusion, 481 had unilateral pleural effusion, 232 had bilateral pleural effusion, and 1 had unknown status. Among the 853 patients in whom a pathogen was detected, 65% had consolidation, 38% had infiltrate, and 26% had pleural effusion. There were radiographic differences between the 530 patients who had only viruses detected and the 247 who had only bacteria detected with respect to consolidation (59% vs. 73%), infiltrate (42% vs. 31%), and pleural effusion (23% vs. 33%).
The pneumonia severity index is a clinical prediction rule for mortality related to community-acquired pneumonia that is based on sex, age, nursing home status, mental status, heart rate, respiratory rate, blood pressure, temperature, selected underlying medical conditions, selected laboratory values, and the presence or absence of pleural effusion.34 The index is divided into five classes, with higher class indicating greater risk. Class 1 to 3 indicates a low risk of death, class 4 moderate risk, and class 5 high risk.
DETECTION OF PATHOGENS
Nasopharyngeal and oropharyngeal swabs were obtained from 2272 of the 2320 adults (98%) with radiographic evidence of pneumonia, blood for culturing from 2103 (91%), a specimen for urinary antigen detection from 1973 (85%), a sputum specimen from 960 (41%) (of whom 272 had a high-quality specimen), paired serum specimens from 859 (37%), a bronchoalveolar-lavage specimen from 84 (4%), a pleural-fluid specimen from 78 (3%), and an endotracheal aspirate from 4 (<1%). Whereas 78% of the blood-culture specimens were obtained before the administration of antibiotic agents, 12% or less of other specimen types were obtained before the administration of antibiotics. Bacteria were detected significantly more frequently in blood cultures collected before the administration of antibiotics than in those collected after antibiotic use (7% vs. 3%, P = 0.002), but this was not true for other specimen types (Table S2 in the Supplementary Appendix).
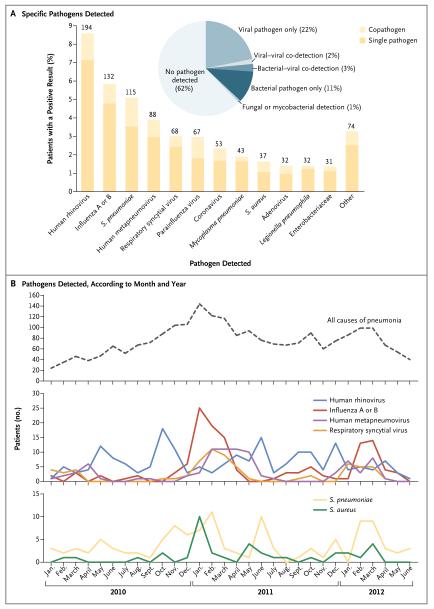
Diagnostic results for both bacteria and viruses were available for 2259 adults (97%) with radio-graphic evidence of pneumonia. A pathogen was detected in 853 of these patients (38%): one or more viruses were detected in 530 patients (23%), one or more bacteria in 247 (11%), both bacterial and viral pathogens in 59 (3%), and fungi or mycobacteria in 17 (1%) (Fig. 2A). The most common pathogens detected were human rhinovirus (in 9% of patients), influenza virus (in 6%), and S. pneumoniae (in 5%) (Fig. 2A, and Tables S3, S4, and S5 in the Supplementary Appendix). HMPV, influenza A and B viruses, RSV, S. aureus, and S. pneumoniae had similar peaks during the winter seasons (Fig. 2B). Three pathogens were detected more commonly in patients in the ICU than in patients not in the ICU: S. pneumoniae (8% vs. 4%), S. aureus (5% vs. 1%), and Enterobacteriaceae (3% vs. 1%) (P<0.001 for all comparisons) (Table S6 in the Supplementary Appendix).
Figure 2. Pathogen Detection among U.S. Adults with Community-Acquired Pneumonia Requiring Hospitalization, 2010–2012.
Panel A shows the numbers (above the bars) and percentages of all adults in whom a specific pathogen was detected. A total of 966 pathogens were detected in 853 of 2259 hospitalized adults with radiographic evidence of pneumonia who had tests available for the detection of both bacterial and viral pathogens. The proportions of viral, viral–viral, bacterial–viral, bacterial, and fungal or mycobacterial pathogens detected and no pathogen detected are shown in the pie chart. Percentages may not sum as expected owing to rounding. A total of 76 pathogens other than those listed here were detected in 74 patients, including Haemophilus influenzae (in 12 patients), Chlamydophila pneumoniae (in 9), Mycobacterium tuberculosis (in 8), pseudomonas (in 8), Streptococcus pyogenes (in 7), viridans streptococci (in 7), other streptococcus species (in 7), nontuberculous mycobacterial species (in 4), fusobacterium (in 3), Pneumocystis jirovecii (in 3), and bacteroides, coccidioides, histoplasma, pasteurella, both H. influenzae and Neisseria meningitidis, and both viridans streptococci and other streptococcus species (in 1 each). Panel B shows, according to month and year, the number of hospitalized adults who had pneumonia from any cause; patients in whom human rhinovirus, influenza virus, human metapneumovirus, and respiratory syncytial virus were detected; and patients in whom S. pneumoniae and Staphylococcus aureus were detected. Note the difference in scale of the y axis for each line graph.
Among 262 controls, 6 (2%) were not available for follow-up and 18 (7%) were excluded from the analysis because of fever or respiratory symptoms after enrollment (Table S7 in the Supplementary Appendix). A pathogen was detected less frequently in nasopharyngeal and oropharyngeal swabs obtained from 238 asymptomatic controls than in swabs obtained from 192 patients with pneumonia who were enrolled during the same period in the same catchment area (2% vs. 27%, P<0.001); this was true for each pathogen individually, including human rhinovirus (1% vs. 11%, P<0.001).
INCIDENCE
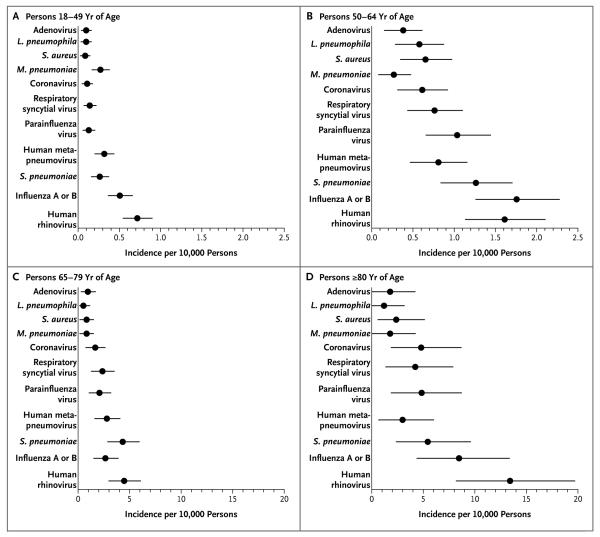
Among 2320 adults with radiographic evidence of pneumonia, 2061 (89%) were enrolled from July 1, 2010, to June 30, 2012. The annual incidence of community-acquired pneumonia requiring hospitalization was 24.8 cases (95% confidence interval, 23.5 to 26.1) per 10,000 adults. The incidence overall and for each pathogen increased with increasing age (Table 2). The incidences of influenza and of S. pneumoniae were almost 5 times as high among adults 65 years of age or older than among younger adults, and the incidence of human rhinovirus was almost 10 times as high among adults 65 years of age or older than among younger adults (Fig. 3).
Table 2.
Estimated Annual Incidence Rates of Hospitalization for Community-Acquired Pneumonia, According to Year of Study, Study Site, Age Group, and Pathogen Detected.*
| Variable | Incidence of Pneumonia-Related Hospitalization (95% CI) |
|---|---|
| no. of cases per 10,000 adults per yr | |
|
| |
| Year of study† | |
|
| |
| Yr 1 and 2 | 24.8 (23.5–26.1) |
|
| |
| Yr 1 | 29.2 (27.2–31.2) |
|
| |
| Yr 2 | 20.6 (19.1–22.3) |
|
| |
| Study site | |
|
| |
| Chicago | 26.7 (25.0–28.4) |
|
| |
| Nashville | 21.9 (20.0–23.8) |
|
| |
| Age group | |
|
| |
| 18–49 yr | 6.7 (6.1–7.3) |
|
| |
| 50–64 yr | 26.3 (24.1–28.7) |
|
| |
| 65–79 yr | 63.0 (56.4–70.3) |
|
| |
| ≥80 yr | 164.3 (141.9–189.3) |
|
| |
| Pathogen detected‡ | |
|
| |
| Human rhinovirus | 2.0 (1.7–2.3) |
|
| |
| Influenza A or B virus | 1.5 (1.3–1.8) |
|
| |
| Streptococcus pneumoniae | 1.2 (1.0–1.4) |
|
| |
| Human metapneumovirus | 0.9 (0.7–1.2) |
|
| |
| Parainfluenza virus | 0.8 (0.6–1.0) |
|
| |
| Respiratory syncytial virus | 0.7 (0.5–0.9) |
|
| |
| Coronavirus | 0.6 (0.4–0.7) |
|
| |
| Mycoplasma pneumoniae | 0.5 (0.4–0.7) |
|
| |
| Staphylococcus aureus | 0.4 (0.3–0.6) |
|
| |
| Legionella pneumophila | 0.4 (0.2–0.5) |
|
| |
| Adenovirus | 0.4 (0.2–0.5) |
Analyses were based on 10,511,911 person-years of observation.
Annual incidence rates were calculated from July 1, 2010, to June 30, 2011, for year 1 and from July 1, 2011, to June 30, 2012, for year 2 and represent the 2061 of 2320 adults (89%) who had radiographic evidence of pneumonia and were enrolled during that time.
Pathogen-specific incidence was calculated for the 2004 adults who had radio-graphic evidence of pneumonia during the incidence period and who had at least one specimen available for both bacterial and viral testing. Pathogen-specific incidence was only calculated for pathogens that were detected in more than 1% of adults.
Figure 3. Estimated Annual Pathogen-Specific Incidence Rates of Community-Acquired Pneumonia Requiring Hospitalization, According to Age Group.
Circles indicate the annual number of hospitalizations for pneumonia per 10,000 adults, and the horizontal lines 95% confidence intervals. Rates are based on 10,511,911 person-years of observation. Note the differences in the scale of the x axis for the groups of patients 65 to 79 years of age and 80 years of age or older, as compared with the groups of patients 18 to 49 years of age and 50 to 64 years of age.
DISCUSSION
The EPIC study was a large, contemporary, prospective, population-based study of community-acquired pneumonia in hospitalized adults in the United States. The estimated incidences of hospitalization for pneumonia among adults 50 to 64 years of age, 65 to 79 years of age, and 80 years of age or older were approximately 4, 9, and 25 times as high, respectively, as the incidence among adults 18 to 49 years of age. Pathogens were detected in 38% of the patients, with viruses detected in 27% and bacteria in 14%. Human rhinovirus, influenza virus, and S. pneumoniae were the most commonly detected pathogens, with the highest burden occurring among older adults.
The annual incidence of community-acquired pneumonia requiring hospitalization that we observed — 24.8 cases per 10,000 adults — is similar to the incidence of 26.7 cases per 10,000 adults that was observed in a prospective study conducted in Ohio in 1991.9 Methodologic differences in enrollment criteria, methodologic differences in incidence estimations, changes or differences in demographic characteristics, and changes in the provision of and access to health care preclude direct comparisons of incidence between the studies.9,10,33 Our estimates are lower than the rates of pneumonia requiring hospitalization that are based on claims data, owing to our exclusion of recently hospitalized or severely immunocompromised patients and the increased specificity of radiographic confirmation in our case definition.1,2,6
Despite our efforts to use more sensitive and specific diagnostic methods than had been available previously,11-13,19-29 pathogens were detected in only 38% of adults. Possible reasons for such few detections include an inability to obtain lower respiratory tract specimens, antibiotic use before specimen collection, insensitive diagnostic tests for known pathogens, a lack of testing for other recognized pathogens (e.g., coxiella), unidentified pathogens, and possible noninfectious causes (e.g., aspiration pneumonitis).10,13,35,36 We may also have missed preceding microbiologic insults that triggered a subsequent hospitalization for pneumonia. Nevertheless, our pathogen-detection yield is within the range (20 to 76%) of the yield in other etiologic studies of pneumonia in adults, although it is closer to the lower end of the range9,37-43 and contrasts with the detection yield in the pediatric EPIC study, in which pathogens were detected in 81% of children who had been hospitalized with community-acquired pneumonia.44 The low pathogen-detection yield among adults who were hospitalized for pneumonia highlights the need for more sensitive diagnostic methods and innovative discovery of pathogens.45
Viruses were detected in 27% of the patients. Human rhinovirus was the most commonly detected virus in patients with pneumonia but was rarely detected in asymptomatic controls, a finding similar to that in other studies.37,46 Although our understanding of human rhinovirus remains incomplete, these data suggest a role for human rhinovirus in adult pneumonia.35,47 Influenza virus was the second most common pathogen detected, despite mild influenza seasons during the study.48 The incidence of influenza virus was almost twice that of any other pathogen (except for human rhinovirus) among adults 80 years of age or older, which underscores the need for improvements in influenza-vaccine uptake and effectiveness.49
Together, HMPV, RSV, parainfluenza viruses, coronaviruses, and adenovirus were detected in 13% of the patients, a proportion similar to those found in other PCR-based etiologic studies of pneumonia in adults (11 to 28%).11,37-41 Among adults 80 years of age or older, the incidence of RSV, parainfluenza virus, and coronavirus each was similar to that of S. pneumoniae. Our study adds to the growing evidence of the contribution of viruses to hospitalizations of adults, high-lighting the usefulness of molecular methods for the detection of respiratory pathogens.35,47,50,51 The lower circulation of respiratory viruses in the 2011–2012 season (Fig. 2B), as compared with the previous year, probably contributed to the lower incidence of pneumonia in that year and is supported by national surveillance data.48
Bacterial pathogens were detected in 14% of the patients. S. pneumoniae was the most commonly detected bacterium, with an incidence that was almost 5 times as high among adults 65 years of age or older as among younger adults. The prevalence of pneumococcal disease of 5% that was observed in our study was lower than the 13% found by Marston et al.9 Although both studies used sputum culture, our study included only high-quality sputum specimens for the detection of non-legionella bacteria, which probably improved specificity but decreased sensitivity.17 Bacterial cultures, especially in the context of antimicrobial use, are insensitive.13,52 Urinary antigen tests for pneumococcus, which were not available for the study by Marston et al., were responsible for the majority (67%) of pneumococcal detections in our study. These tests are more sensitive than blood culture and improve the detection of nonbacteremic pneumococcal pathogens with a reported sensitivity of 70 to 80% and a specificity of more than 90%.9,13,24,52 Moreover, the indirect protection of adults as a result of pediatric pneumococcal vaccination in the United States probably contributed to the lower observed incidence of pneumococcal infection in our study than in the study by Marston et al.4-9 Our estimates provide an important benchmark to monitor the effect of the 2014 vaccination recommendations for the 13-valent pneumococcal conjugate vaccine in persons 65 years of age or older.53
M. pneumoniae, L. pneumophila, and C. pneumoniae combined were detected in 4% of the adults. Marston et al. found that these pathogens were identified in 10% of patients with definite cases of pneumonia and in 44% of those with possible cases.9 Although both our study and that of Marston et al. used urinary antigen tests for L. pneumophila, the detections of M. pneumoniae and C. pneumoniae in the study by Marston et al. relied exclusively on serologic testing, which has less specificity than the PCR assay used in our study.9,19,20,54-56
Overall S. aureus was detected in 2% of adults — a lower rate than that of S. pneumoniae or viruses. The low prevalences of Enterobacteriaceae (1%) and other gram-negative bacteria were probably due to the exclusion of patients with known risk factors for these bacteria (e.g., patients with recent hospitalization, patients with severe immunosuppression, and functionally dependent nursing home residents), which is consistent with the contemporary concept of community-acquired pneumonia.10 S. pneumoniae, S. aureus, and Enterobacteriaceae were significantly more common among severely ill patients, accounting for 16% of the detected pathogens among patients in the ICU, as compared with 6% among patients not in the ICU.
This study has limitations. First, we were not able to enroll every eligible patient; patients who were 65 years of age or older and those who were undergoing invasive mechanical ventilation were less likely to be enrolled; a greater proportion of nonenrolled patients than of enrolled patients died during hospitalization. Although the incidence calculations were adjusted for the enrollment differences according to age, market share data on severity were not available and prevented the calculations of severity-related incidence.
Second, specimens were not obtained from patients who were not enrolled, and among those who were enrolled, not all specimen types were available, which could have led to underestimation or overestimation of the pathogen-specific rates, including the rates associated with severe disease and older age. Owing to ethical and feasibility considerations, invasive procedures to obtain specimens directly from the lung were not usually performed, which may have reduced the microbiologic yield.35 However, 97% of the adults had at least one specimen type available for bacterial and viral detection.
Third, the sensitivities and specificities of the available diagnostic tests were imperfect. Although the majority of blood-culture samples were collected before the administration of antibiotics, the yield was lower in the samples collected after the administration of antibiotics. Thus, despite extensive testing, pathogens may have been missed. Moreover, molecular detection of viruses and atypical bacteria in the nasopharynx and oropharynx does not necessarily indicate causation and could represent infection that is limited to the upper respiratory tract or convalescent-phase shedding. Similarly, urinary pneumococcal antigen can be present for weeks after the onset of pneumococcal pneumonia, and recent pneumococcal vaccination can lead to false positive results.52
Fourth, we were not able to enroll asymptomatic controls for the entire study period or in both Chicago and Nashville, so it is possible that we missed detecting pathogens that were more commonly circulating at other times or in other places. However, as in other studies,37,40,46 pathogens were rarely detected among adult asymptomatic controls, which suggests that the respiratory viruses and atypical bacteria that were identified in patients with pneumonia may have contributed to disease.
Fifth, the clinical and radiographic features of pneumonia overlap with those of other syndromes, including chronic lung disease and congestive heart failure, such that even strict definitions may not accurately distinguish among these entities, resulting in potential misclassification. However, this situation is consistent with the real-world challenges of diagnosing pneumonia and of its subsequent management. Finally, data from our five urban hospitals (three academic, one public, and one community), although inclusive of diverse communities, may not be representative of the entire U.S. adult population or generalizable to other settings, since the circulation of respiratory pathogens varies according to geographic region, timing, and other factors.
In conclusion, the burden of community-acquired pneumonia requiring hospitalization among adults is substantial and is markedly higher among the oldest adults. Although pathogens were not detected in the majority of patients, respiratory viruses were more frequently detected than bacteria, which probably reflects the direct and indirect benefit of bacterial vaccines and relatively insensitive diagnostic tests. These data suggest that improving the coverage and effectiveness of recommended influenza and pneumococcal vaccines and developing effective vaccines and treatments for HMPV, RSV, and parainfluenza virus infection could reduce the burden of pneumonia among adults. Further development of new rapid diagnostic tests that can accurately identify and distinguish among potential pneumonia pathogens is needed.45
Supplementary Material
Acknowledgments
Supported by the Influenza Division of the National Center for Immunizations and Respiratory Diseases at the CDC through cooperative agreements with each study site. The study was based on a competitive research funding opportunity.
Dr. Self reports receiving fees for serving on an advisory board from BioFire Diagnostics, research supplies from CareFusion, grant support through his institution from bioMérieux, Affinium Pharmaceuticals, Astute Medical, Crucell Holland, Thermo Fisher Scientific, Pfizer, Rapid Pathogen Screening, Venaxis, and Cempra, and holding a pending patent (13/632,874) related to a sterile blood-culture collection system; Dr. Wunderink, receiving consulting fees from Roche, the Medicines Company, Vical, Cubist Pharmaceuticals, Bayer, Cerexa, and Visterra; Dr. Grijalva, receiving consulting fees from Pfizer; Dr. Anderson, receiving grant support through his institution from MedImmune, editorial support for an unrelated study from Roche, and consulting fees from AbbVie; Dr. Courtney, receiving fees through his institution from Thermo Fisher Scientific; Dr. Chappell, holding a patent (U.S. 8,293,498 B2) related to a system for generation of viable reovirus from cloned complementary DNA and a pending patent (13/639,564) related to reovirus vaccines and methods of use therefor; Dr. Arnold, receiving grant support from GlaxoSmithKline; Dr. Ampofo, receiving fees through his institution from GlaxoSmithKline and Cubist Pharmaceuticals for the enrollment of patients in other studies, and collaborating with BioFire Diagnostics on grants funded by the National Institutes of Health; Dr. Katz, receiving grant support through her institution from Juvaris BioTherapeutics and GlaxoSmithKline; Dr. Pavia, receiving fees for serving on an advisory board from BioFire Diagnostics, fees for the preparation of educational material from Medscape, and royalties from Antimicrobial Therapy; and Dr. Edwards, serving on a data and safety monitoring board for Novartis for which her institution receives fees.
We thank the patients who consented to participate in this study; Suzette Bartley, Bernie Beall, Nicole Burcher, Robert Davidson, Michael Dillon, Barry Fields, Phalasy Juieng, and Shelley Magill of the CDC; Bharat Reddy Dhanireddy and Pinal Modi of John H. Stroger, Jr., Hospital of Cook County; Alison Chevrier, Ashraf Luqman, Cecilia Scholcoff, and Jill Sears of Northwestern Memorial Hospital; Alexander Baldridge, Kelly Harris, Rajiv Midha, and Denetra Smith of University of Tennessee Health Science Center–Saint Thomas Health; and Heather Diggs, Regina Ellis, Timothy Girard, Charity Graves, Angela Harbeson, Deborah Hunter, Donna Jones, Romina Libster, Karen Miller, Kelly Moser, Deborah Myers, Rabon Lee Smalling, Tanya Steinback, Scott Taylor, and Sandy Yoder of the Vanderbilt University Medical Center.
APPENDIX
The authors’ full names and academic degrees are as follows: Seema Jain, M.D., Wesley H. Self, M.D., M.P.H., Richard G. Wunderink, M.D., Sherene Fakhran, M.D., M.P.H., Robert Balk, M.D., Anna M. Bramley, M.P.H., Carrie Reed, Ph.D., Carlos G. Grijalva, M.D., M.P.H., Evan J. Anderson, M.D., D. Mark Courtney, M.D., James D. Chappell, M.D., Ph.D., Chao Qi, Ph.D., Eric M. Hart, M.D., Frank Carroll, M.D., Christopher Trabue, M.D., Helen K. Donnelly, R.N., B.S.N., Derek J. Williams, M.D., M.P.H., Yuwei Zhu, M.D., Sandra R. Arnold, M.D., Krow Ampofo, M.D., Grant W. Waterer, M.B., B.S., Ph.D., Min Levine, Ph.D., Stephen Lindstrom, Ph.D., Jonas M. Winchell, Ph.D., Jacqueline M. Katz, Ph.D., Dean Erdman, Dr.P.H., Eileen Schneider, M.D., M.P.H., Lauri A. Hicks, D.O., Jonathan A. McCullers, M.D., Andrew T. Pavia, M.D., Kathryn M. Edwards, M.D., and Lyn Finelli, Dr.P.H., for the CDC EPIC Study Team
The authors’ affiliations are as follows: the Centers for Disease Control and Prevention, Atlanta (S.J., A.M.B., C.R., M.L., S.L., J.M.W., J.M.K., D.E., E.S., L.A.H., L.F.); Vanderbilt University School of Medicine (W.H.S., C.G.G., J.D.C., F.C., D.J.W., Y.Z., K.M.E.) and University of Tennessee Health Science Center–Saint Thomas Health (C.T.), Nashville, and Le Bonheur Children’s Hospital (S.R.A., J.A.M.), University of Tennessee Health Science Center (S.R.A., J.A.M.), and St. Jude Children’s Research Hospital (J.A.M.), Memphis — all in Tennessee; Northwestern University Feinberg School of Medicine (R.G.W., E.J.A., D.M.C., C.Q., E.M.H., H.K.D., G.W.W.), John H. Stroger, Jr., Hospital of Cook County (S.F.), and Rush University Medical Center (R.B.) — all in Chicago; University of Utah Health Sciences Center, Salt Lake City (K.A., A.T.P.); and University of Western Australia, Perth (G.W.W.).
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
The authors’ full names, academic degrees, and affiliations are listed in the Appendix.
REFERENCES
- 1.Pfuntner A, Wier LM, Stocks C. HCUP statistical brief #162. Agency for Healthcare Research and Quality; Rockville, MD: 2013. Most frequent conditions in U.S. hospitals, 2011. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. [PubMed] [Google Scholar]
- 2.Health, United States, 2012: with special features on emergency care. National Center for Health Statistics; Hyattsville, MD: 2013. pp. 297–9. [PubMed] [Google Scholar]
- 3.Pfuntner A, Wier LM, Steiner C. HCUP statistical brief #168. Agency for Healthcare Research and Quality; Rockville, MD: 2013. Costs for hospital stays in the United States, 2011. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb168-Hospital-Costs-United-States-2011.pdf. [PubMed] [Google Scholar]
- 4.Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369:1179–86. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- 5.Nelson JC, Jackson M, Yu O, et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008;26:4947–54. doi: 10.1016/j.vaccine.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CGUS. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–63. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–51. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 8.Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 9.Marston BJ, Plouffe JF, File TM, Jr, et al. Incidence of community-acquired pneumonia requiring hospitalization: results of a population-based active surveillance study in Ohio. Arch Intern Med. 1997;157:1709–18. [PubMed] [Google Scholar]
- 10.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavia AT. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis. 2011;52(Suppl 4):S284–S289. doi: 10.1093/cid/cir043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caliendo AM. Multiplex PCR and emerging technologies for the detection of respiratory pathogens. Clin Infect Dis. 2011;52(Suppl 4):S326–S330. doi: 10.1093/cid/cir047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartlett JG. Diagnostic tests for agents of community-acquired pneumonia. Clin Infect Dis. 2011;52(Suppl 4):S296–S304. doi: 10.1093/cid/cir045. [DOI] [PubMed] [Google Scholar]
- 14.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies in the aged: the index of ADL: a standardized measure of biological and psychological function. JAMA. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 15.Cherian T, Mulholland EK, Carlin JB, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83:353–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Watt JP, Moïsi JC, Donaldson RLA, et al. Measuring the incidence of adult community-acquired pneumonia in a Native American community. Epidemiol Infect. 2010;138:1146–54. doi: 10.1017/S0950268809991464. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett RC. Medical microbiology: quality, cost and clinical relevance. John Wiley; New York: 1974. pp. 24–31. [Google Scholar]
- 18.Pollock HM, Hawkins EL, Bonner JR, Sparkman T, Bass JB., Jr Diagnosis of bacterial pulmonary infections with quantitative protected catheter cultures obtained during bronchoscopy. J Clin Microbiol. 1983;17:255–9. doi: 10.1128/jcm.17.2.255-259.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thurman KA, Warner AK, Cowart KC, Benitez AJ, Winchell JM. Detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella spp. in clinical specimens using a single-tube multiplex real-time PCR assay. Diagn Microbiol Infect Dis. 2011;70:1–9. doi: 10.1016/j.diagmicrobio.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murdoch DR. Diagnosis of Legionella infection. Clin Infect Dis. 2003;36:64–9. doi: 10.1086/345529. [DOI] [PubMed] [Google Scholar]
- 21.Carvalho MGS, Tondella ML, McCaustland K, et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J Clin Microbiol. 2007;45:2460–6. doi: 10.1128/JCM.02498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaschke AJ, Heyrend C, Byington CL, et al. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30:289–94. doi: 10.1097/INF.0b013e3182002d14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blaschke AJ, Heyrend C, Byington CL, et al. Rapid identification of pathogens from positive blood cultures by multiplex polymerase chain reaction using the FilmArray system. Diagn Microbiol Infect Dis. 2012;74:349–55. doi: 10.1016/j.diagmicrobio.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murdoch DR, Laing RTR, Mills GD, et al. Evaluation of a rapid immunochromatographic test for detection of Streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J Clin Microbiol. 2001;39:3495–8. doi: 10.1128/JCM.39.10.3495-3498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberg GA, Schnabel KC, Erdman DD, et al. Field evaluation of TaqMan Array Card (TAC) for the simultaneous detection of multiple respiratory viruses in children with acute respiratory infection. J Clin Virol. 2013;57:254–60. doi: 10.1016/j.jcv.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dare RK, Fry AM, Chittaganpitch M, Sawanpanyalert P, Olsen SJ, Erdman DD. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays. J Infect Dis. 2007;196:1321–8. doi: 10.1086/521308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawatwong P, Chittaganpitch M, Hall H, et al. Serology as an adjunct to polymerase chain reaction assays for surveillance of acute respiratory virus infections. Clin Infect Dis. 2012;54:445–6. doi: 10.1093/cid/cir710. [DOI] [PubMed] [Google Scholar]
- 28.Feikin DR, Njenga MK, Bigogo G, et al. Additional diagnostic yield of adding serology to PCR in diagnosing viral acute respiratory infections in Kenyan patients 5 years of age and older. Clin Vaccine Immunol. 2013;20:113–4. doi: 10.1128/CVI.00325-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manual for the laboratory diagnosis and virological surveillance of influenza. World Health Organization; Geneva: 2011. pp. 43–77. http://www.who.int/influenza/gisrs_laboratory/manual_diagnosis_surveillance_influenza/en. [Google Scholar]
- 30.Monto AS, Maassab HF. Ether treatment of type B influenza virus antigen for the hemagglutination inhibition test. J Clin Microbiol. 1981;13:54–7. doi: 10.1128/jcm.13.1.54-57.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kendal AP, Cate TR. Increased sensitivity and reduced specificity of hemagglutination inhibition tests with ether-treated influenza B/Singapore/222/79. J Clin Microbiol. 1983;18:930–4. doi: 10.1128/jcm.18.4.930-934.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiore AE, Bridges CB, Katz JM, Cox NJ. Inactivate influenza vaccines. In: Plot-kin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed Elsevier; Philadelphia: 2012. pp. 257–93. [Google Scholar]
- 33.Vintage 2012 postcensal estimates of the resident population of the United States by year, county, single-year of age, bridged race, Hispanic origin, and sex. National Center for Health Statistics; Atlanta: 2012. http://www.cdc.gov/nchs/nvss/bridged_race.htm. [Google Scholar]
- 34.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 35.Karhu J, Ala-Kokko TI, Vuorinen T, Ohtonen P, Syrjälä H. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin Infect Dis. 2014;59:62–70. doi: 10.1093/cid/ciu237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanspa MJ, Peyrani P, Wiemken T, Wilson EL, Ramirez JA, Dean NC. Characteristics associated with clinician diagnosis of aspiration pneumonia: a descriptive study of afflicted patients and their outcomes. J Hosp Med. 2015;10:90–6. doi: 10.1002/jhm.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jennings LC, Anderson TP, Beynon KA, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42–8. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 38.Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202–9. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest. 2008;134:1141–8. doi: 10.1378/chest.08-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lieberman D, Shimoni A, Shemer-Avni Y, Keren-Naos A, Shtainberg R, Lieberman D. Respiratory viruses in adults with community-acquired pneumonia. Chest. 2010;138:811–6. doi: 10.1378/chest.09-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Templeton KE, Scheltinga SA, van den Eeden WC, Graffelman AW, van den Broek PJ, Claas EC. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345–51. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charles PGP, Whitby M, Fuller AJ, et al. The etiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis. 2008;46:1513–21. doi: 10.1086/586749. [DOI] [PubMed] [Google Scholar]
- 43.Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619–28. doi: 10.1056/NEJMra1312885. [DOI] [PubMed] [Google Scholar]
- 44.Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–45. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caliendo AM, Gilbert DN, Ginocchio CC, et al. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis. 2013;57(Suppl 3):S139–S170. doi: 10.1093/cid/cit578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fry AM, Lu X, Olsen SJ, et al. Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS One. 2011;6(3):e17780. doi: 10.1371/journal.pone.0017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruuskanen O, Järvinen A. What is the real role of respiratory viruses in severe community-acquired pneumonia? Clin Infect Dis. 2014;59:71–3. doi: 10.1093/cid/ciu242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.FluView: a weekly influenza surveillance report prepared by the Influenza Division. Centers for Disease Control and Prevention; Atlanta: http://www.cdc.gov/flu/weekly. [Google Scholar]
- 49.Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2014–2015. MMWR Morb Mortal Wkly Rep. 2014;63:691–7. [PMC free article] [PubMed] [Google Scholar]
- 50.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–59. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 51.Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168:2489–96. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Werno AM, Murdoch DR. Medical microbiology: laboratory diagnosis of invasive pneumococcal disease. Clin Infect Dis. 2008;46:926–32. doi: 10.1086/528798. [DOI] [PubMed] [Google Scholar]
- 53.Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2014;63:822–5. [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar S, Hammerschlag MR. Acute respiratory infection due to Chlamydia pneumoniae: current status of diagnostic methods. Clin Infect Dis. 2007;44:568–76. doi: 10.1086/511076. [DOI] [PubMed] [Google Scholar]
- 55.Nilsson AC, Björkman P, Persson K. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol. 2008;8:93. doi: 10.1186/1471-2180-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nir-Paz R, Michael-Gayego A, Ron M, Block C. Evaluation of eight commercial tests for Mycoplasma pneumoniae antibodies in the absence of acute infection. Clin Microbiol Infect. 2006;12:685–8. doi: 10.1111/j.1469-0691.2006.01469.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.