Abstract
The aim of this study was to identify factors associated with phylogenetic clustering among people with recently acquired hepatitis C virus (HCV) infection. Participants with available sample at time of HCV detection were selected from three studies; the Australian Trial in Acute Hepatitis C, the Hepatitis C Incidence and Transmission Study - Prison and Community. HCV RNA was extracted and Core to E2 region of HCV sequenced. Clusters were identified from maximum likelihood trees with 1000 bootstrap replicates using 90% bootstrap and 5% genetic distance threshold. Among 225 participants with available Core-E2 sequence (ATAHC, n=113; HITS-p, n=90; and HITS-c, n=22), HCV genotype prevalence was: G1a: 38% (n=86), G1b: 5% (n=12), G2a: 1% (n=2), G2b: 5% (n=11), G3a: 48% (n=109), G6a: 1% (n=2) and G6l 1% (n=3). Of participants included in phylogenetic trees, 22% of participants were in a pair/cluster (G1a-35%, 30/85, mean maximum genetic distance =0.031; G3a-11%, 12/106, mean maximum genetic distance =0.021; other genotypes-21%, 6/28, mean maximum genetic distance =0.023). Among HCV/HIV co-infected participants, 50% (18/36) were in a pair/cluster, compared to 16% (30/183) with HCV mono-infection (P=<0.001). Factors independently associated with phylogenetic clustering were HIV co-infection [vs. HCV mono-infection; adjusted odds ratio (AOR) 4.24; 95%CI 1.91, 9.39], and HCV G1a infection (vs. other HCV genotypes; AOR 3.33, 95%CI 0.14, 0.61). HCV treatment and prevention strategies, including enhanced antiviral therapy, should be optimised. The impact of targeting of HCV treatment as prevention to populations with higher phylogenetic clustering, such as those with HIV co-infection, could be explored through mathematical modelling.
Keywords: people who inject drugs, prison, hepatitis C virus, molecular epidemiology, human immunodeficiency virus, gay and bisexual men
Graphical abstract
1. Introduction
The burden of hepatitis C virus (HCV) infection continues to grow, despite targeted public health strategies to prevent transmission (Sacks-Davis, Horyniak et al. 2012). There is a high incidence of HCV infection among people who inject drugs (PWID) (Maher, Li et al. 2007, Page, Hahn et al. 2009) and an increasing incidence of HCV infection has been observed among human immunodeficiency virus (HIV) positive gay and bisexual men (Danta, Brown et al. 2007, van de Laar, Pybus et al. 2009). Ongoing HCV transmission in these groups suggests a clear need for further characterisation of factors influencing HCV transmission. This need is particularly pertinent due to the development of new therapies for the treatment of HCV infection, which while being highly curative (>90% sustained virological response), well tolerated and likely to have a short treatment duration (8–12 weeks) (Grebely, Matthews et al. 2013), also carry considerable financial burden. More detailed characterisation of the transmission of HCV infection, in particular amongst those with acute and recently acquired infection, is needed to guide HCV prevention strategies, including treatment as prevention (Martin, Vickerman et al. 2013, Grebely and Dore 2014).
Characterizing acute HCV transmission has historically been difficult as it is often asymptomatic and there is limited public health surveillance infrastructure to monitor populations at risk of infection, who are often marginalised and burdened by stigma (Treloar, Rance et al. 2014). Traditional epidemiological studies of acute infection tend to measure factors associated with acquisition rather than transmission, and are often complicated by multiple risk factors and overlapping modes of acquisition (Matthews, Pham et al. 2011, Mahony, Donnan et al. 2013). However, novel molecular epidemiological methods used to study HIV transmission (Pillay, Rambaut et al. 2007, Lewis, Hughes et al. 2008) have provided unique insights into the groups most at risk of transmission and are now beginning to shed light on the transmission dynamics of HCV (Pybus, Cochrane et al. 2005). It has been demonstrated that phylogenetic clustering of HCV is associated with social-injecting networks (Sacks-Davis, Daraganova et al. 2012), sexual networks (Bradshaw, Jacka et al. 2014), HIV co-infection (van de Laar, Pybus et al. 2009, Matthews, Pham et al. 2011), HCV seroconversion and recent receptive syringe borrowing (Jacka, Applegate et al. 2014, Cunningham, Jacka et al. 2015). Although behavioural risk factors linked to transmission of HCV in HIV positive gay and bisexual men have been identified (Danta, Brown et al. 2007, van de Laar, Pybus et al. 2009, Matthews, Pham et al. 2011), epidemiological factors associated with transmission clusters of acute and recently acquired HCV infection have not been well characterized.
The aim of this study was to investigate phylogenetic clustering of HCV and associated factors among individuals with acute or recently acquired HCV infection in Australia.
2. Methods
2.1 Study population and design
Data and specimens from three studies of recently acquired HCV in Australia were used for this study. The Australian Trial in Acute Hepatitis C (ATAHC) was a multicentre, prospective study of recent HCV recruited between 2004 and 2007 (Dore, Hellard et al. 2010). The Hepatitis C Incidence and Transmission Study - prison (HITS-p) was a study of prison inmates at-risk of HCV infection in correctional centres recruited between 2005 and 2014 (Teutsch, Luciani et al. 2010). The Hepatitis C Incidence and Transmission Study - community (HITS-c) was a study of community-based people who inject drugs (PWID) at risk of HCV infection, which recruited between 2008 and 2014 (White, Dore et al. 2014).
For inclusion, participants from these cohorts had to have acute or recently acquired HCV defined by an initial positive anti-HCV antibody test and either (1) a negative anti-HCV antibody test within 2 years prior to the initial positive anti-HCV test or (2) acute clinical hepatitis (either jaundice or alanine aminotransferase [ALT] >400 IU/mL) within 12 months of the initial positive anti-HCV result. Participants also had to have a HCV RNA positive plasma sample, with the first available sample following the detection of acute HCV selected. All participants provided written informed consent and protocols were approved by appropriate Human Research Ethics Committees.
The estimated date of infection was calculated for subjects who presented with acute clinical hepatitis as six weeks prior to onset of symptoms. For subjects identified by recent positive HCV antibody test with a negative test in the prior two years, the estimated date of infection was calculated as the midpoint the between the first positive test and the last negative test.
2.2 Detection and quantification of HCV RNA
Qualitative HCV RNA testing was performed using the Versant TMA assay (Bayer, Australia; <10 IU/mL; ATAHC) or COBAS AmpliPrep/COBAS TaqMan HCV assay (Roche, Branchburg, NJ; <15 IU/mL; HITS-p, HITS-c). Quantitative HCV RNA testing was performed using the Versant HCV RNA 3.0 (Bayer, Australia; <615 IU/mL; ATAHC) or COBAS AmpliPrep/COBAS TaqMan HCV assay (Roche; <15 IU/mL; HITS-p). HCV genotyping (Versant LiPa1 or LiPa2, Bayer, Australia) was performed on all participants with detectable HCV RNA at first HCV detection.
2.3 HCV RNA sequencing
HCV RNA was extracted from EDTA plasma using QIAamp viral extraction mini kit (#52906, QIAGEN, Limburg, NL). Reverse transcription and polymerase chain reaction (PCR) amplification of a region of the HCV genome encoding Core, Envelope-1 (E1) and the beginning of Envelope-2 (E2) was performed to generate a 1,404 base pair (bp) amplicon (nucleotides 347–1750 in H77 reference sequence [GenBank ascension no. NC_004102]) using a method previously described (Lamoury, Jacka et al. 2015). PCR amplicons were sequenced by Sanger sequencing and sequence chromatograms were processed using RECall: a fully automated sequence analysis pipeline (Woods, Brumme et al. 2012). Subtypes were determined by constructing a subtyping tree using the panel of reference sequences classified by Smith et al (Smith, Bukh et al. 2014) (Supplementary Figure 1.).
2.4 Phylogenetics
Phylogenetic trees of the Core-E2 fragment were inferred separately for major subtypes and minor genotype groups (1a, 1b, 2a/c, 3a and 6a/l) using maximum-likelihood analysis implemented in RAxML (Stamatakis, Ludwig et al. 2005) through the CIPRES Science Gateway (Miller, Pfeiffer et al. 2010) under the General Time Reversible model of nucleotide substitution with a gamma shaped distribution of rate variation across sites (GTR+G). JModelTest (Guindon and Gascuel 2003, Darriba, Taboada et al. 2012) was used to determine the most appropriate model of nucleotide substitution. Reference sequences obtained from the Los Alamos National Laboratory HCV database (Kuiken, Richardson et al. 2004) and from previous sequencing studies (Jacka, Applegate et al. 2014, Cunningham, Jacka et al. 2015) were included to support identification of “local” clusters (Hué, Pillay et al. 2005). All sequences were aligned using pair-wise alignment in ClustalX prior to phylogenetic analysis (Larkin, Blackshields et al. 2007).
The final fragment analysed was 1104 bp long following the removal of the hypervariable region one (HVR1) of E2 and gaps created by alignment. HVR1 was removed based on a previous finding that inclusion of this region leads to decreased ability to identify pairs and clusters due to extreme genetic variation seen between individuals in this region (Lamoury, Jacka et al. 2015). The robustness of the resulting tree was assessed using a rapid bootstrap algorithm with 1000 replicates, and clusters were identified using ClusterPicker software (Ragonnet-Cronin, Hodcroft et al. 2013). A sensitivity analysis was performed by varying the genetic distance threshold between 1.5–5% in ClusterPicker, with and without 90% bootstrap threshold, to determine the effect this had on the identification of factors associated with clustering (Supplementary Table 1 and 2).
2.5 Study outcome
The primary study outcome was phylogenetic clustering of HCV infections, as defined by two or more participants with HCV genome sequence within the bootstrap and genetic distance threshold cut off. A pair was defined as two participants with HCV genome sequence within the bootstrap and genetic distance threshold cut off and a cluster was defined by three or more participants with HCV genome sequence within the bootstrap and genetic distance threshold cut off.
2.6 Statistical analyses
Unadjusted logistic regression analysis was used to identify factors associated with being in a pair/cluster. Factors hypothesised to be associated with HCV pairing or clustering that were assessed included: age (Page, Morris et al. 2013), female sex (vs. male sex) (Dore, Law et al. 2003), HIV infection (Danta, Brown et al. 2007, Urbanus, van de Laar et al. 2009, van de Laar, Pybus et al. 2009, Matthews, Pham et al. 2011), recent injection drug use (defined as injecting in the last 3–6 months) (Maher, Li et al. 2007, Aitken, Lewis et al. 2008, Sacks-Davis, Daraganova et al. 2012), incarceration ever (Hellard, Hocking et al. 2004) and current incarceration (Hellard, Hocking et al. 2004). All variables with P<0.20 in the unadjusted analysis were considered in the adjusted logistic regression model, using a backwards stepwise approach with factors sequentially eliminated according to the result of a likelihood ratio test. To account for potential unmeasured confounding introduced by the different cohort characteristics, adjusted logistic regression analysis was performed using mixed modelling, with a random intercept for cohort. For all analyses, statistically significant differences were assessed at P<0.05; P-values are two-sided. All analyses were performed using STATA software (version 12.1; StataCorp L.P., College Station, Texas, USA).
3. Results
3.1 Study population
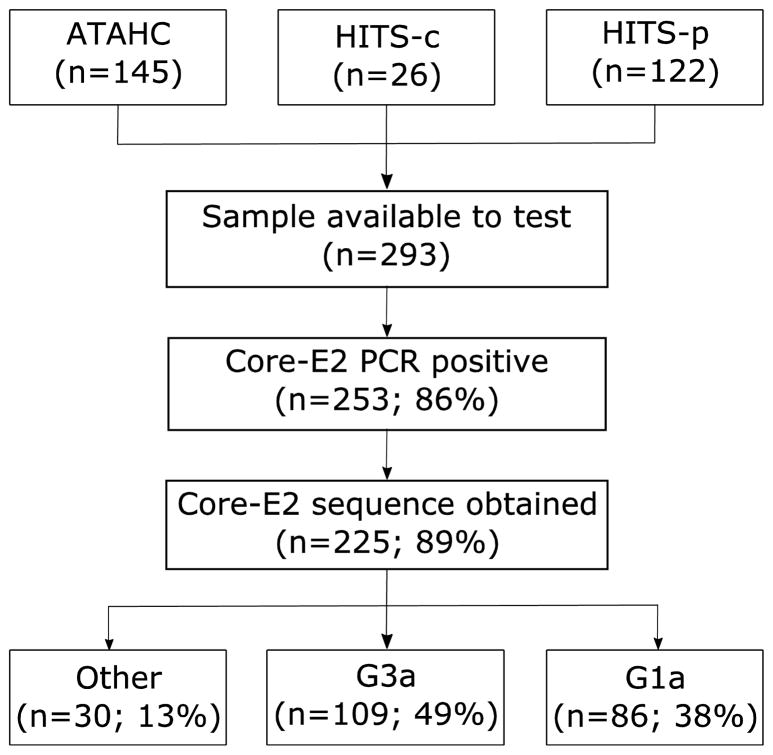
Overall, 293 participants were initially included in this study (Figure 1). Core-E2 was amplifiable in 86% (n=253), with sequence obtainable in 89% (n=219) of those participants (ATAHC, n=113; HITS-p, n=90; and HITS-c, n=22). HCV genotype prevalence was: G1a: 38% (n=86), G1b: 5% (n=12), G2a: 1% (n=2), G2b: 5% (n=11), G3a: 48% (n=109), G6a: 1% (n=2) and G6l 1% (n=3).
Figure 1.
Flowchart of sources of participant sequences.
The characteristics of those with obtainable HCV sequencing (n = 219), and characteristics stratified by cohort, are shown in Table 1. Cohort differences included a higher proportion with HIV infection in ATAHC, a higher proportion of people ever or currently incarcerated in HITS-p (all subjects). The median age was 29 (interquartile range 24–36), 69% were male, 77% were Caucasian and 16% were HIV positive. Among people with HIV infection (n=36), all participants reported homosexual exposure as a risk factor for HIV acquisition.
Table 1.
Characteristics of participants with available Core-Envelope 2 region sequence according to cohort
| Characteristic Total n (%) |
Overall (n = 225) | ATAHC (n = 113) | HITS-p (n = 90) | HITS-c (n = 22) |
|---|---|---|---|---|
| Age (median years, Q2–Q3 ) | 29 (24–36) | 33 (26–40) | 27 (23–31) | 26 (22–32) |
| Female sex | 70 (31%) | 30 (27%) | 32 (36%) | 8 (36%) |
| Unstable housing‡ | 101 (45%) | 8 (7%) | 90 (100%) | 3 (14%) |
| Caucasian ethnicity | 173 (77%) | 101 (89%) | 57 (63%) | 15 (68%) |
| HCV Genotype | ||||
| 1a | 86 (38%) | 56 (50%) | 26 (29%) | 4 (18%) |
| 1b | 12 (5%) | 8 (7%) | 2 (2%) | 2 (9%) |
| 2 | 13 (6%) | 3 (3%) | 9 (10%) | 1 (5%) |
| 3a | 109 (48%) | 45 (40%) | 50 (56%) | 14 (64%) |
| 6 | 5 (2%) | 1 (1%) | 3 (3%) | 1 (5%) |
| HIV infection | 36 (16%) | 36 (32%) | 0 (0%) | 0 (0%) |
| Homosexual HIV acquisition** | 36 (100%) | 36 (100%) | NA | NA |
| Injection drug use | ||||
| Ever | 204 (90%) | 92 (81%) | 90 (100%) | 22 (100%) |
| Recent* † | 130 (60%) | 41 (38%) | 69 (77%) | 20 (91%) |
| Incarceration | ||||
| Ever | 114 (50%) | 19 (17%) | 90 (100%) | 5 (23%) |
| Currently† | 92 (41%) | 0 (0%) | 90 (100%) | 2 (9%) |
Percentages indicate column percentages
Defined as living in prison, a shelter or hostel, or having no fixed address in the last 6 months
Within last 3–6 months prior to sample date
Among total population
Among people with HIV co-infection
NA not available
3.2 Phylogenetic cluster composition
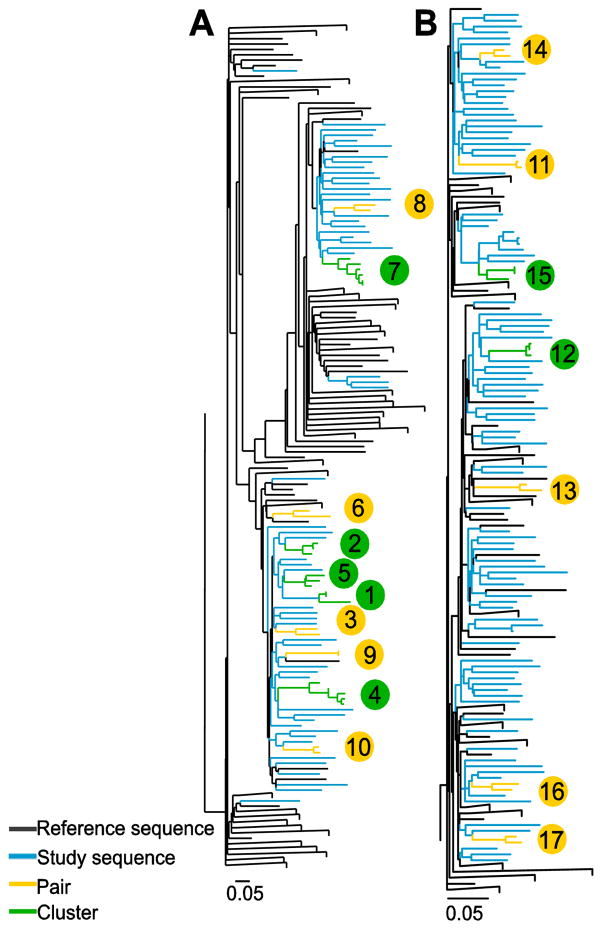
Phylogenetic trees of the core to E2 region (minus HVR1) were constructed for each HCV subtype, HCV genotypes 1a (n=85) and 3a (n=106) are shown in Figure 2A and 2B respectively (the phylogenetic trees for all other genotypes shown in Supplementary Figures 1A, 1B & 1C). Reference sequences obtained from the Los Alamos National Laboratory HCV database (Kuiken, Richardson et al. 2004) and from previous sequencing studies (Jacka, Applegate et al. 2014, Cunningham, Jacka et al. 2015) were included in all trees (G1a; n=635, G1b; n=221, Gt2a/c; n=65, G3a; n=98, G6a/l; n=25).
Figure 2.
Maximum-likelihood phylogenetic trees of a 1104 bp region encompassing the Core to E2 (minus HVR1) region were generated for HCV (A) genotype (G) 1a and (B) G3a from people with recently acquired HCV infections in Australia between 2004 and 2014. The tree for G1a contained 85 sequences from study participants and 635 reference sequences. The tree for G3a contained 106 sequences from study participants and 98 reference sequences. Trees were inferred separately using RAxML with the GTR+G nucleotide substitution model. Participants in pairs (n = 2, yellow) and clusters (n > 2, green) are differentiated from non-clustered study participants (blue) and reference sequences (black) using ClusterPicker with a bootstrap threshold of ≥90% and genetic distance cut off of ≤5%. Large clades containing only reference sequences were collapsed. Scale bars indicate nucleotide substitutions per site. Clusters and pairs are numbered (bubbles are colour coded to represent either cluster [green] or pair [yellow]) and this corresponds to the clusters and pairs represented in Figure 4.
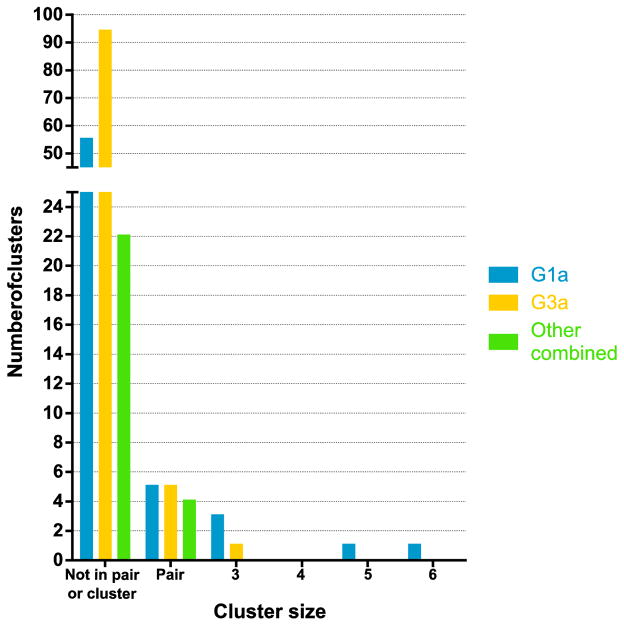
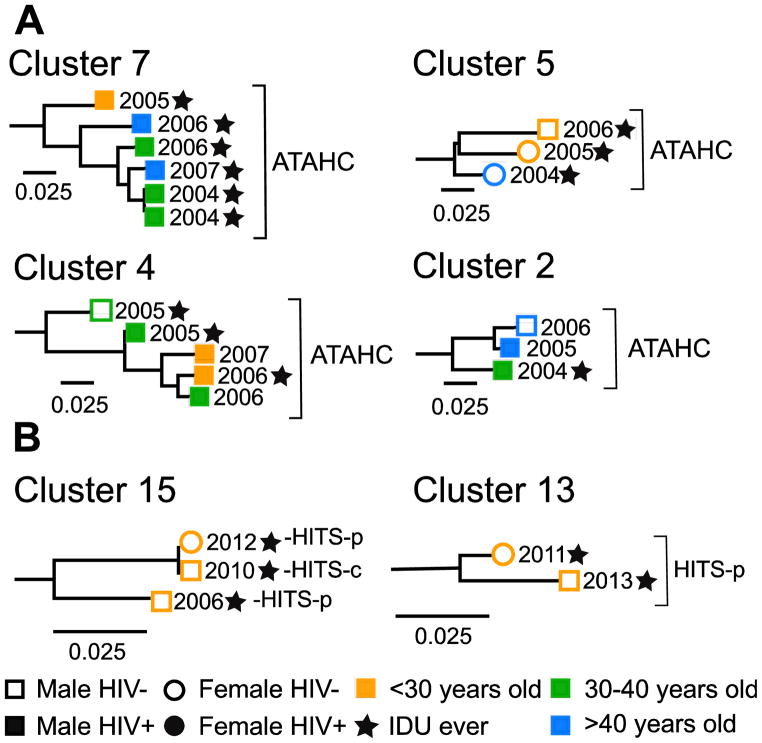
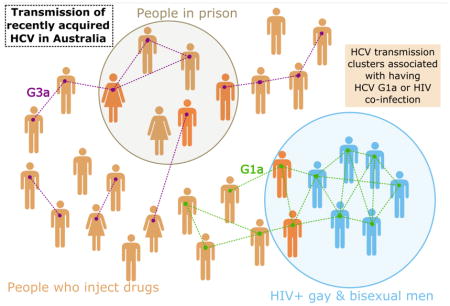
Overall, 22% of participants were grouped in a pair or cluster in the phylogenetic trees. Among participants with HCV G1a, 35% were in a pair or cluster (30/85) with mean maximum genetic distance = 0.031. Among participants with HCV G3a, 11% were in a pair or cluster (12/106) with a mean maximum genetic distance = 0.021. Of HCV/HIV co-infected participants, 50% (18/36) were in a pair/cluster, compared to 16% (30/183) of those with HCV mono-infection (Table 2). Clusters ranged in size from three to six participants and the distribution of pairs and clusters differed between genotypes (Figure 3). As seen in Figure 4A, some clusters displayed discrete characteristics depending on the genotype [e.g. only G1a clusters (clusters 2, 4 & 7) contained individuals with HIV co-infection]. However some clusters also demonstrated mixing of characteristics, such as male and female sex (Cluster 13, Figure 4B), being in prison and in the community (Cluster 15, Figure 4B) and being HIV positive and HIV negative (Clusters 2 and 4, Figure 4A).
Table 2.
Logistic regression of factors associated with being in a phylogenetic pair/cluster (defined using ClusterPicker with 5% genetic distance and 90% bootstrap support) for participants with recently acquired HCV infection in Australia between 2004 and 2014. Only factors with P<0.2 in the unadjusted analysis were included in the adjusted model and only those included in the adjusted model are presented in this table.
| Overall | Not cluster | Pair/cluster | Membership in cluster n ≥ 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Unadjusted | Adjusted | |||||||
| Total n (%) | (n= 219) | (n=171) | (n=48 ) | Odds ratio | 95% CI | P | Adjusted odds ratio | 95% CI | P |
| Age >30 (vs. ≤30 years) | 99 (45%) | 73 (43%) | 26 (54%) | 1.66 | 0.87, 3.18 | 0.126 | - | - | - |
| Female sex (vs. male sex) | 69 (32%) | 58 (34%) | 11 (23%) | 0.58 | 0.28, 1.23 | 0.159 | - | - | - |
| HIV infection (vs. no HIV) | 36 (16%) | 18 (11%) | 18 (38%) | 5.28 | 2.46, 11.33 | <0.001 | 4.24 | 1.91, 9.39 | <0.001 |
| Incarceration currently (vs. not) | 89 (41%) | 79 (46%) | 10 (21%) | 3.22 | 0.15, 0.67 | 0.003 | - | - | - |
| Recent* injection drug use (vs. none) | 124 (57%) | 105 (61%) | 19 (40%) | 0.42 | 0.22, 0.83 | 0.012 | - | - | - |
| HCV G1a (vs. other genotypes) | 85 (39%) | 55 (32%) | 30 (63%) | 4.00 | 0.12, 0.50 | <0.001 | 3.33 | 0.14, 0.61 | 0.001 |
Percentages indicate column percentages
Within last 3–6 months prior to sample date
Abbreviations: Confidence interval (CI)
Figure 3.
Distribution of number unclustered participants, participants in pairs (n = 2 tips in clade) and clusters (n > 2 tips in clade) for G1a (blue), G3a (yellow) and other genotypes (green) (using ClusterPicker with 5% genetic distance and 90% bootstrap threshold) for participants with recently acquired HCV infection in Australia between 2004 and 2014.
Figure 4.
Examples of pairs and clusters for HCV (A) genotype 1a and (B) genotype 3a from people with recently acquired HCV infections in Australia between 2004 and 2014. Clusters were identified using ClusterPicker with 5% genetic distance and 90% bootstrap support from maximum-likelihood phylogenetic trees of a 1104 bp region encompassing the Core to E2 (minus HVR1) region of HCV. Numbers at tips represent year when subject acquired HCV infection. Scale bars indicate nucleotide substitutions per site. Clusters and pairs are numbered corresponding to the clusters and pairs represented in Figure 2. Additional figures of all other clusters and pairs identified are included in Supplementary Materials Figure 3.
3.3 Factors associated with membership in pair/clusters
Among 219 participants with an available HCV sequence that was able to be aligned, 28 (13%) and 20 (9%) were found to be in a pair and cluster, respectively. In unadjusted logistic regression analyses, membership in a pair/cluster was associated with HIV infection (vs. HCV mono-infection), recent injection drug use (vs.none), being incarcerated at the time of sampling (vs. not) and having HCV G1a (vs. other HCV genotypes) (Table 2). In the adjusted logistic regression model, HIV co-infection (vs. HCV mono-infection; adjusted odds ratio [AOR] 4.24; 95% confidence interval [CI] 1.91, 9.39), and HCV G1a infection (vs. other HCV genotypes; AOR3.33 95% CI 0.14, 0.61) remained independently associated with pair or cluster membership (Table 2).
3.4 Clusters with membership of three or more participants
Seven clusters of three or more closely related HCV sequences were identified (Figure 3, Figure 4A Clusters 2, 4, 5 and 7 and Figure 4B Cluster 15) and the sequences included belonged to participants with similar characteristics, with distinctions between age, sex, HIV infection, history of injecting drug use and imprisonment.
4. Discussion
This study characterized phylogenetic clustering among cohorts of people with recently acquired HCV in Australia between 2004 and 2014. Overall, 22% of participants were identified as being in a pair/cluster. HIV coinfection and G1a were independently associated with being in a pair/cluster. These findings identify participant characteristics that may be associated with a greater potential for HCV transmission. Strategies for the delivery of prevention and treatment interventions to groups with high transmission potential could be explored to reduce transmission of HCV.
Overall, one-fifth of recently acquired HCV infections in this study demonstrated phylogenetic clustering. This is consistent with other studies in HCV, but is approximately 10% lower than previously demonstrated prevalence of phylogenetic clustering of HCV in Australia (Aitken, McCaw et al. 2004) and other countries (Urbanus, van de Laar et al. 2009, Jacka, Applegate et al. 2014). The lower observed proportion with clustering in the current study could be due to the inclusion of only acute or recently infected individuals, rather than considering transmissions from chronically infected individuals. Although the results of this study are not too dissimilar to previous reports, caution should be made when comparing the results of phylogenetic studies, given differences in study eligibility criteria and recruitment methods, study follow-up, the genetic diversity of the region of the HCV genome used for analysis, cut-offs used to define pairs and clusters and the phylogenetic methodology employed.
This study found that HIV/HCV co-infection was independently associated with HCV phylogenetic clustering. In this study, HIV infection was exclusively acquired homosexually. Behavioural risk factors linked to transmission of HCV in HIV positive gay and bisexual men have been identified previously (Danta, Brown et al. 2007, van de Laar, Pybus et al. 2009, Matthews, Pham et al. 2011), however our finding is particularly novel, given this is the first study to examine phylogenetic clustering in recently acquired HCV. Furthermore, epidemiological factors associated with transmission clusters of recently acquired HCV infection have not been well characterized. Although there is no direct evidence for sexual transmission of HCV among HIV positive gay and bisexual men, especially given many also had a history of injecting drug use, our results suggest that HCV is being transmitted among people with HIV within more closely related social and behavioural networks. It was previously demonstrated that among HIV positive gay and bisexual men with HCV infection, 84% of HCV infections demonstrated phylogenetic clustering (van de Laar, Pybus et al. 2009). This proportion is higher than that observed in the current study, however the method used to define clustering was more relaxed, which could account for this disparity. The extremely high proportion (50%) of people with HIV/acute HCV co-infection in this study in a pair/cluster is consistent with relatively rapid emergence of the HCV epidemic in this group, providing a basis for targeting of resources to HCV treatment and prevention in people with HIV.
In this study, the majority of participants were infected with HCV genotype 1a (38%) and genotype 3a infection (48%), consistent with previous data showing an increased prevalence of HCV G1a and G3a in PWID globally (Pybus, Cochrane et al. 2005). Despite G3a infection being most prevalent in this study, G1a infection was independently associated with phylogenetic clustering. This association could be due to a founder effect which is the result of reduced diversity when only a handful of variants establish the initial infection in a population (Pybus, Cochrane et al. 2005). Founder effects are diminished over time, so it is possible that this association is particular to this study, given it is looking in particular at acute and recently acquired infections in a specific time period. Subsequent analyses are planned to shed further light on whether this is the case.
This study demonstrated that transmission of recently acquired HCV infection is linked to complex patterns of risk behaviours. This was seen in some clusters that contained individuals with multiple risk factors for acquisition of HCV, such as having both a history of injecting drug use and being HIV positive, indicating bridging between communities can occur. These findings support previous work demonstrating the co-existence of risk behaviours, such as injecting drug use and high-risk sexual practices, within networks of HIV-infected gay and bisexual men (Danta and Rodger 2011, Matthews, Pham et al. 2011). Although many clusters had discrete characteristics, there was also mixing of characteristics within clusters, such as clusters containing incarcerated males and females, community based PWID and incarcerated PWID and HIV negative and HIV positive individuals. Presently, there is insufficient evidence to conclude what role, if any, bridging between infected populations has on onward transmission of HCV. However these findings illustrate there is a need to further investigate the transmission of HCV among both PWID and HIV positive individuals, to characterise clusters with mixed characteristics and the possible effect of bridging between communities.
This study is one of few globally investigating HCV transmission and factors associated with phylogenetic clustering in a sample this size of recently acquired or acute infection. It gives a foundation on which transmission in recently acquired and acute infection can be evaluated in the future, which will be important to monitor the growth of the HCV transmission clusters, especially in HIV positive gay and bisexual men, and to evaluate the impact of new treatment therapies and strategies. However, this study also has several limitations. The cohorts were not random samples of the eligible populations. All HIV-infected participants in this study were from the Australian Trial in Acute Hepatitis C (ATAHC Study), which was recruited through an Australian network of tertiary hospitals (n=13) and general practice/primary care clinics (n=3). As such, it is possible that there is some bias towards a higher proportion with HCV clustering among people with HIV, than would be expected in the general population with acute HCV infection, given that recruitment was somewhat limited to geographical areas with higher cases of HIV/HCV co-infection (e.g. Sydney and Melbourne). Therefore, these findings may not be generalizable to the broader population of people with recently acquired HCV infection in Australia. While there were significant differences in participant characteristics between the study cohorts, study cohort was adjusted for in the multivariate analyses, so factors associated with clustering should be applicable across cohorts.
Additionally, using clustering as a measure of transmission has several limitations. This analysis was not intended to identify direct transmission, as the entire infected population was not sampled meaning individuals involved in transmission chains were likely unsampled; therefore the direction of transmission cannot be inferred. Instead, factors associated with membership in a pair/cluster for the entire study sample were determined, rather than for individual transmission chains. It is important also to note that due to the method used to estimate the date of infection for subjects in this study, the estimated date of infection could be up to 12 months earlier or later than their actual date of infection.
5. Conclusions
Directly-acting interferon-free HCV therapies continue to become increasingly available and hepatitis C treatment as prevention strategies may now be feasible in many settings (Martin, Vickerman et al. 2015). Therefore, understanding factors that may be associated with increased risk of transmission are needed to help guide the implementation of prevention and treatment as prevention programs and to inform public health interventions at a population level. Potential strategies for enhanced prevention could include broader Needle and Syringe Program coverage and enhanced HCV prevention education among gay and bisexual men (e.g. to better understand risks for HCV acquisition and safer injecting and sexual practices). Research to better understand social networks and their role in HCV transmission among populations of gay and bisexual men may help to design targeted strategies towards those at higher risk of transmission (e.g. a “bring a friend” treatment strategy which has proposed for PWID) (Hellard, McBryde et al. 2015). These results further demonstrate the ongoing transmission of HCV among HIV positive gay and bisexual men. The discrete clustering of HIV positive gay and bisexual men in this study suggests that targeted prevention and treatment of HCV in this risk group could reduce onward HCV transmission, therefore further studies are needed to investigate the feasibility and effectiveness of enhanced prevention programs including hepatitis C Treatment as Prevention in the setting of HCV/HIV co-infection.
Supplementary Material
Highlights.
We characterized the phylogenetics of recent HCV in Australia from 2004–2014.
22% of participants were identified as being in a phylogenetic pair/cluster.
HIV coinfection & G1a were independently associated with being in a pair/cluster.
These factors may be associated with a greater potential for HCV transmission.
HCV prevention & treatment interventions could be targeted to those with HIV.
Acknowledgments
Funding: This work was supported by the United States National Institutes of Health [R01 DA 15999-01] and the Australian Government Department of Health. The views expressed in this publication do not necessarily represent the position of the Australian Government. JG is supported by a National Health and Medical Research Council (NHMRC) Career Development Fellowship, GD is supported by a NHMRC Practitioner Research Fellowship and LM is supported by a NHMRC Senior Research Fellowship.
The cooperation of the participants in the HITS cohorts and the ATAHC study is gratefully acknowledged. The authors would also like to thank current and past researchers and staff involved in these studies, in particular Brigid Betz-Stablein, Sammy Chow and Melanie Walker for data and sample retrieval.
Footnotes
Conflict of interest: Dr. Grebely is a consultant/advisor and has received research grants from AbbVie, Bristol Myers Squibb, Gilead Sciences, Merck. Dr. Dore is a consultant/advisor and has received research grants from Abbvie, Bristol Myers Squibb, Gilead, Merck, Janssen and Roche.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitken CK, Lewis J, Tracy SL, Spelman T, Bowden DS, Bharadwaj M, Drummer H, Hetlard M. High Incidence of Hepatitis C Virus Reinfection in a Cohort of Injecting Drug Users. Hepatology. 2008;48(6):1746–1752. doi: 10.1002/hep.22534. [DOI] [PubMed] [Google Scholar]
- Aitken CK, McCaw RF, Bowden DS, Tracy SL, Kelsall JG, Higgs PG, Kerger MJ, Nguyen H, Crofts JN. Molecular epidemiology of hepatitis C virus in a social network of injection drug users. J Infect Dis. 2004;190(9):1586–1595. doi: 10.1086/424678. [DOI] [PubMed] [Google Scholar]
- Bradshaw D, Jacka B, Sacks-Davis R, Lamoury F, Applegate T, Dore G, Down I, Luciani F, Hellard M, Sasadeusz J, Danta M, Matthews G. A novel method comparing sexual networks with the HCV phylogeny in HIV-positive MSM with acute HCV infection identifies two potential intervention targets for permucosally transmitted HCV in Australia. Hiv Medicine. 2014;15:136–136. [Google Scholar]
- Cunningham EB, Jacka B, DeBeck K, Applegate TL, Harrigan PR, Krajden M, Marshall BDL, Montaner J, Lima VD, Olmstead AD, Milloy MJ, Wood E, Grebely J. Methamphetamine injecting is associated with phylogenetic clustering of hepatitis C virus infection among street-involved youth in Vancouver, Canada. Drug and Alcohol Dependence. 2015;152:272–276. doi: 10.1016/j.drugalcdep.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danta M, Brown D, Bhagani S, Pybus OG, Sabin CA, Nelson M, Fisher M, Johnson AM, Dusheiko GM Hiv and H. C. V. g. Acute. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21(8):983–991. doi: 10.1097/QAD.0b013e3281053a0c. [DOI] [PubMed] [Google Scholar]
- Danta M, Rodger AJ. Transmission of HCV in HIV-positive populations. Current Opinion in Hiv and Aids. 2011;6(6):451–458. doi: 10.1097/COH.0b013e32834b4974. [DOI] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 2012;9(8):772–772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira T, Deforche K, Cassol S, Salminen M, Paraskevis D, Seebregts C, Snoeck J, van Rensburg EJ, Wensing AM, van de Vijver DA, Boucher CA, Camacho R, Vandamme AM. An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics. 2005;21(19):3797–3800. doi: 10.1093/bioinformatics/bti607. [DOI] [PubMed] [Google Scholar]
- Dore GJ, Hellard M, Matthews GV, Grebely J, Haber PS, Petoumenos K, Yeung B, Marks P, van Beek I, McCaughan G, White P, French R, Rawlinson W, Lloyd AR, Kaldor JM C. S. G. Australian Trial In Acute Hepatitis. Effective treatment of injecting drug users with recently acquired hepatitis C virus infection. Gastroenterology. 2010;138(1):123–135. e121–122. doi: 10.1053/j.gastro.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore GJ, Law M, MacDonald M, Kaldor JM. Epidemiology of hepatitis C virus infection in Australia. Journal of Clinical Virology. 2003;26(2):171–184. doi: 10.1016/s1386-6532(02)00116-6. [DOI] [PubMed] [Google Scholar]
- Grebely J, Dore GJ. Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral Res. 2014;104:62–72. doi: 10.1016/j.antiviral.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Grebely J, Matthews GV, Lloyd AR, Dore GJ. Elimination of hepatitis C virus infection among people who inject drugs through treatment as prevention: feasibility and future requirements. Clin Infect Dis. 2013;57(7):1014–1020. doi: 10.1093/cid/cit377. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology. 2003;52(5):696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Hellard M, McBryde E, Sacks Davis R, Rolls DA, Higgs P, Aitken C, Thompson A, Doyle J, Pattison P, Robins G. Hepatitis C transmission and treatment as prevention - The role of the injecting network. Int J Drug Policy. 2015 doi: 10.1016/j.drugpo.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Hellard ME, Hocking JS, Crofts N. The prevalence and the risk behaviours associated with the transmission of hepatitis C virus in Australian correctional facilities. Epidemiology and Infection. 2004;132(3):409–415. doi: 10.1017/s0950268803001882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hué S, Pillay D, Clewley JP, Pybus OG. Genetic analysis reveals the complex structure of HIV-1 transmission within defined risk groups. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(12):4425–4429. doi: 10.1073/pnas.0407534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka B, Applegate T, Krajden M, Olmstead A, Harrigan PR, Marshall BD, DeBeck K, Milloy MJ, Lamoury F, Pybus OG, Lima VD, Magiorkinis G, Montoya V, Montaner J, Joy J, Woods C, Dobrer S, Dore GJ, Poon AF, Grebely J. Phylogenetic clustering of hepatitis C virus among people who inject drugs in Vancouver, Canada. Hepatology. 2014;60(5):1571–1580. doi: 10.1002/hep.27310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken C, Richardson R, Yusim K. The Los Alamos Hepatitis C sequence database. Gastroenterology. 2004;126(4):A760–A760. doi: 10.1093/bioinformatics/bth485. [DOI] [PubMed] [Google Scholar]
- Lamoury FM, Jacka B, Bartlett S, Bull RA, Wong A, Amin J, Schinkel J, Poon AF, Matthews GV, Grebely J, Dore GJ, Applegate TL. The Influence of Hepatitis C Virus Genetic Region on Phylogenetic Clustering Analysis. PLoS One. 2015;10(7):e0131437. doi: 10.1371/journal.pone.0131437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lewis F, Hughes GJ, Rambaut A, Pozniak A, Leigh Brown AJ. Episodic sexual transmission of HIV revealed by molecular phylodynamics. PLoS Med. 2008;5(3):e50. doi: 10.1371/journal.pmed.0050050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher L, Li JO, Jalaludin B, Chant KG, Kaldor JM. High hepatitis C incidence in new injecting drug users: a policy failure? Australian and New Zealand Journal of Public Health. 2007;31(1):30–35. doi: 10.1111/j.1753-6405.2007.00007.x. [DOI] [PubMed] [Google Scholar]
- Mahony AA, Donnan EJ, Lester RA, Doyle JS, Knox J, Tracy SL, Bowden S, Sasadeusz JJ. Beyond injecting drug use: investigation of a Victorian cluster of hepatitis C among HIV-infected men who have sex with men. Medical Journal of Australia. 2013;198(4):210–214. doi: 10.5694/mja12.10556. [DOI] [PubMed] [Google Scholar]
- Martin NK, Vickerman P, Dore GJ, Hickman M. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Curr Opin HIV AIDS. 2015;10(5):374–380. doi: 10.1097/COH.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, Foster GR, Dillon JF, Goldberg DJ, Dore GJ, Hickman M. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–1609. doi: 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews GV, Pham ST, Hellard M, Grebely J, Zhang L, Oon A, Marks P, van Beek I, Rawlinson W, Kaldor JM, Lloyd A, Dore GJ, White PA, Group AS. Patterns and characteristics of hepatitis C transmission clusters among HIV-positive and HIV-negative individuals in the Australian trial in acute hepatitis C. Clin Infect Dis. 2011;52(6):803–811. doi: 10.1093/cid/ciq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Gateway Computing Environments Workshop (GCE); 2010.2010. [Google Scholar]
- Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, Tobler L, Andrews W, Avanesyan L, Cooper S, Busch MP. Acute Hepatitis C Virus Infection in Young Adult Injection Drug Users: A Prospective Study of Incident Infection, Resolution, and Reinfection. Journal of Infectious Diseases. 2009;200(8):1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K, Morris MD, Hahn JA, Maher L, Prins M. Injection Drug Use and Hepatitis C Virus Infection in Young Adult Injectors: Using Evidence to Inform Comprehensive Prevention. Clinical Infectious Diseases. 2013;57:S32–S38. doi: 10.1093/cid/cit300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay D, Rambaut A, Geretti AM, Brown AJ. HIV phylogenetics. BMJ. 2007;335(7618):460–461. doi: 10.1136/bmj.39315.398843.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pybus OG, Cochrane A, Holmes EC, Simmonds P. The hepatitis C virus epidemic among injecting drug users. Infect Genet Evol. 2005;5(2):131–139. doi: 10.1016/j.meegid.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Ragonnet-Cronin M, Hodcroft E, Hue S, Fearnhill E, Delpech V, Brown AJL, Lycett S U. H. D. R. Database. Automated analysis of phylogenetic clusters. Bmc Bioinformatics. 2013;14 doi: 10.1186/1471-2105-14-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks-Davis R, Daraganova G, Aitken C, Higgs P, Tracy L, Bowden S, Jenkinson R, Rolls D, Pattison P, Robins G, Grebely J, Barry A, Hellard M. Hepatitis C virus phylogenetic clustering is associated with the social-injecting network in a cohort of people who inject drugs. PLoS One. 2012;7(10):e47335. doi: 10.1371/journal.pone.0047335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks-Davis R, Horyniak D, Grebely J, Hellard M. Behavioural interventions for preventing hepatitis C infection in people who inject drugs: A global systematic review. International Journal of Drug Policy. 2012;23(3):176–184. doi: 10.1016/j.drugpo.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59(1):318–327. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Ludwig T, Meier H. RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics. 2005;21(4):456–463. doi: 10.1093/bioinformatics/bti191. [DOI] [PubMed] [Google Scholar]
- Teutsch S, Luciani F, Scheuer N, McCredie L, Hosseiny P, Rawlinson W, Kaldor J, Dore GJ, Dolan K, Ffrench R, Lloyd A, Haber P, Levy M. Incidence of primary hepatitis C infection and risk factors for transmission in an Australian prisoner cohort. BMC Public Health. 2010;10:633. doi: 10.1186/1471-2458-10-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar C, Rance J, Dore GJ, Grebely J, Grp ES. Barriers and facilitators for assessment and treatment of hepatitis C virus infection in the opioid substitution treatment setting: insights from the ETHOS study. Journal of Viral Hepatitis. 2014;21(8):560–567. doi: 10.1111/jvh.12183. [DOI] [PubMed] [Google Scholar]
- Urbanus AT, van de Laar TJ, Stolte IG, Schinkel J, Heijman T, Coutinho RA, Prins M. Hepatitis C virus infections among HIV-infected men who have sex with men: an expanding epidemic. Aids. 2009;23(12):F1–F7. doi: 10.1097/QAD.0b013e32832e5631. [DOI] [PubMed] [Google Scholar]
- van de Laar T, Pybus O, Bruisten S, Brown D, Nelson M, Bhagani S, Vogel M, Baumgarten A, Chaix ML, Fisher M, Gotz H, Matthews GV, Neifer S, White P, Rawlinson W, Pol S, Rockstroh J, Coutinho R, Dore GJ, Dusheiko GM, Danta M. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136(5):1609–1617. doi: 10.1053/j.gastro.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B, Dore GJ, Lloyd AR, Rawlinson WD, Maher L. Opioid substitution therapy protects against hepatitis C virus acquisition in people who inject drugs: the HITS-c study. Medical Journal of Australia. 2014;201(6):326–329. doi: 10.5694/mja13.00153. [DOI] [PubMed] [Google Scholar]
- Woods CK, Brumme CJ, Liu TF, Chui CKS, Chu AL, Wynhoven B, Hall TA, Trevino C, Shafer RW, Harrigan PR. Automating HIV Drug Resistance Genotyping with RECall, a Freely Accessible Sequence Analysis Tool. Journal of Clinical Microbiology. 2012;50(6):1936–1942. doi: 10.1128/JCM.06689-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.