Abstract
There is considerable evidence that neuroimaging findings can improve the early diagnosis of Wernicke's encephalopathy (WE) in clinical settings. The most distinctive neuroimaging finding of acute WE are cytotoxic edema and vasogenic edema, which are represented by bilateral symmetric hyperintensity alterations on T2-weighted MR images in the periphery of the third ventricle, periaqueductal area, mammillary bodies and midbrain tectal plate. An initial bout of WE can result in Korsakoff's syndrome (KS), but repeated bouts in conjunction with its typical comorbidity, chronic alcoholism, can result in signs of tissue degeneration in vulnerable brain regions. Chronic abnormalities identified with neuroimaging enable examination of brain damage in living patients with KS and have expanded the understanding of the neuropsychological deficits resulting from thiamine deficiency, alcohol neurotoxicity, and their comorbidity. Brain structure and functional studies indicate that the interactions involving the thalamus, mammillary bodies, hippocampus, frontal lobes, and cerebellum are crucial for memory formation and executive functions, and the interruption of these circuits by WE and chronic alcoholism can contribute substantially to the neuropsychological deficits in KS.
Introduction
Neuroimaging findings associated with Korsakoff's syndrome (KS) derive largely from two different approaches with two different aims. The clinical approach focuses on the acute phase of Wernicke's encephalopathy (WE) in search of acute neuroimaging markers that might support the clinical diagnosis of WE and distinguish it from other neurological disorders, especially in acute confusional patients. Based on these reports, MRI is currently considered a valuable method in clinical settings, where early diagnosis and prompt treatment is critical to prevent the development of KS. The structural and functional neuropsychological approach investigates the sequelae of thiamine deficiency in terms of brain damage and neuropsychological abnormalities, which persist after the acute confusional WE stage resolves. This approach has revealed structural volume deficits and functional impairments by applying statistical analysis with quantitative measures and has expanded our understanding of the neuropsychological deficits observed in KS. Here, we review both approaches.
Clinical Appraoch
WE is an acute neuropsychiatric disorder resulting from thiamine (vitamin B1) deficiency and characterized by a clinical triad of confusion, ocular abnormalities, and ataxia (Victor, Adams et al. 1971). Because the storage capacity for thiamine is limited in humans (Gangolf, Czerniecki et al. 2010), severe thiamine deficiency can result in selective brain lesions, notably those that invade the medial thalamus and periventricular gray matter of the third ventricle, and typically occur within 2 to 3 weeks (Sechi and Serra 2007). Early detection of subclinical thiamine deficiency is challenging, however, because symptoms are usually nonspecific, including loss of appetite, headaches, fatigue, difficult in concentration, irritability, and abdominal discomfort. Although some patients might have an insidious form of subclinical encephalopathy, definite thiamine deficiency more likely develops with an acute onset of encephalopathy.
WE remains a clinical diagnosis, requiring a high index of suspicion in high-risk patients. Unfortunately, it is missed by routine clinical examination in 80% of cases as estimated from a retrospective postmortem analysis of WE (Harper, Giles et al. 1986). The high rate of under-diagnosis of WE is largely due to its relatively variable clinical representations. Many clinicians continue to require presence of the full triad of clinical signs to diagnose WE, even though actually only 16% of the patients exhibit the full clinical triad, and 19% of patients exhibit none of the triad symptoms at the acute onset stage (Harper, Giles et al. 1986). To make matters worse, the symptoms of thiamine deficiency – for instance global confusion and ataxia - are difficult to differentiate from those related to alcohol intoxication or withdrawal. To overcome these problems in diagnosis, there have been consistent efforts in using neuroimaging techniques to support clinical impressions of WE and to distinguish WE from other neurological disorders, especially in acute confusional states.
The Clinical Triad: Signs and Symptoms of WE
Mental status change is the most common symptom of WE and occurs in 34-82% of patients based on postmortem neuropathological studies (Harper, Giles et al. 1986; Thomson, Cook et al. 2008). However the clinical presentations are nonspecific and vary across a spectrum, which comprises confusion, apathy, impaired awareness of an immediate situation, inability to concentrate and, if not treated appropriately, coma and death (Victor, Adams et al. 1989). Ocular abnormality is a sign suggesting WE but occurs in only 15-29% of patients (Harper, Giles et al. 1986; Thomson, Cook et al. 2008). Nystagmus (involuntary eye movements) or ophthalmoplegia (paralyses of conjugate gaze) are common signs, whereas sluggish reactions of the pupils to light or anisocoria (unequal size of pupils) are relatively uncommon (Sechi and Serra 2007). Ataxia (loss of coordinated movement of upper or lower limbs), which usually present as dyscoordinated gait, are observed in 23-25% of patients (Harper, Giles et al. 1986; Thomson, Cook et al. 2008). A quantitative test detected ataxia in 36% of uncomplicated alcoholics as a sign of preclinical WE or residual of subclinical WE, indicative of the sensitivity of vigorous assessment in detecting deficits (Pitel, Zahr et al. 2010).
In addition to or instead of the classic triad symptoms, other presenting symptoms include hypotension, hypothermia, epileptic seizures, and progressive hearing loss (Sechi and Serra 2007). Hearing loss results from the involvement of the quadrigeminal plate (midbrain tectum) of the brainstem, which includes the colliculi (see Figure 3), which also underlies the abnormal occular reflexs. Hallucinations and behavioral disturbances, mimicking an acute psychotic disorder, have been reported in non-alcoholic gastrointestinal surgery cases that led to thiamine deficiency (Jiang, Gagliardi et al. 2006; Worden and Allen 2006). The effects of thiamine deficiency are known to be cumulative and symptoms likely appear sooner in repeated episodes of thiamine deficiency indicating the relevance of medical history, including amount and frequency of alcohol consumption throughout one's lifetime (Thomson and Marshall 2006).
Figure 3.
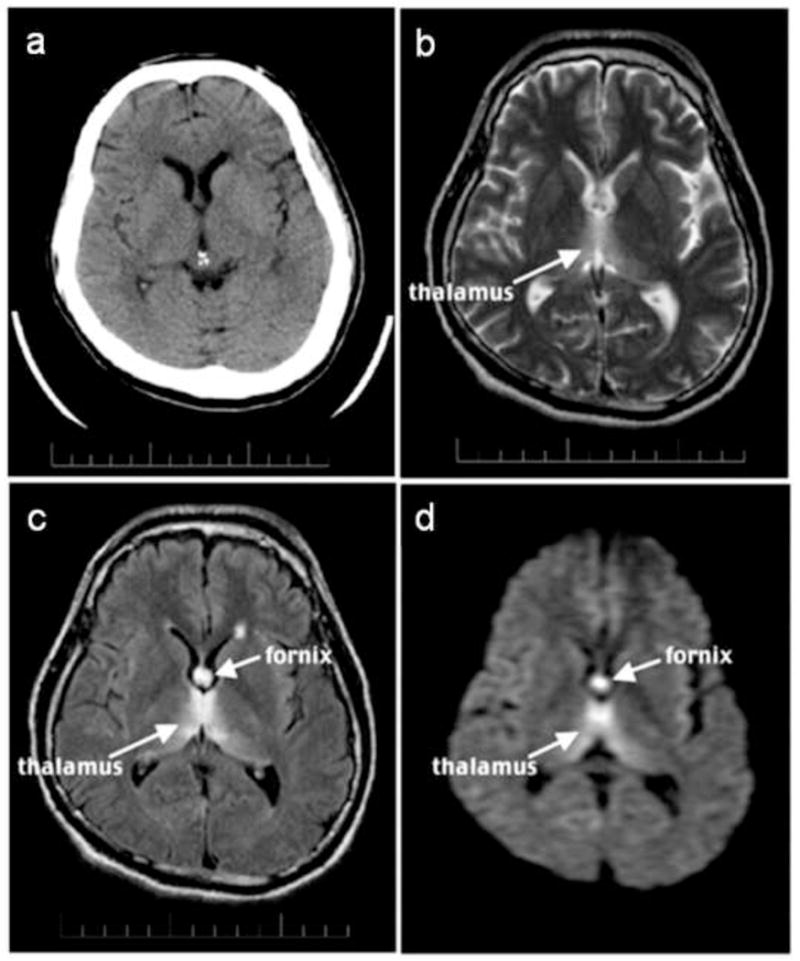
CT and MRI images of 35 year-old man with schizophrenia and acute nutritional deficiency-induced WE.
- CT.
- T2- weighted late-echo fast spine echo image.
- Fluid-attenuated inversion recovery image.
- Diffusion-weighted image. Reprinted from (Sullivan and Pfefferbaum 2009).
Cytotoxic and Vasogenic Edema
Cytotoxic edema and vasogenic edema (see Figure1; also see Kril and Harper, 2012, this issue) are the most typical neuroimaging findings of WE, presenting as bilateral symmetrical hyperintense signals on T2-weighted MR images (see Box 1). At the acute symptomatic stage of WE, thiamine-related glucose and oxidative cellular energy metabolism are disturbed, which results in imbalance of ionic gradients across the cell membrane, which leads to intracellular water-shift and cell injury (cytotoxic edema), and the breakdown of the blood-brain barrier permeability, which allows intravascular fluid to penetrate into cerebral parenchymal extracellular space (vasogenic edema) (Hazell, Todd et al. 1998; Sechi and Serra 2007). The most frequently affected regions are the medial thalamus and periventricular region of the third ventricle (80-85%) periaqueductal area (59-65%), mammillary bodies (38-45%), and midbrain tectum (superior and inferior colliculi) (36-38%) (Zuccoli, Gallucci et al. 2007; Zuccoli and Pipitone 2009) (see Figure 2). These regions are proposed to be more sensitive to thiamine deficiency due to their high rate of thiamine-related glucose and oxidative metabolism (Butterworth, Kril et al. 1993).
Figure1. Cytotoxic and Vasogenic Edema.
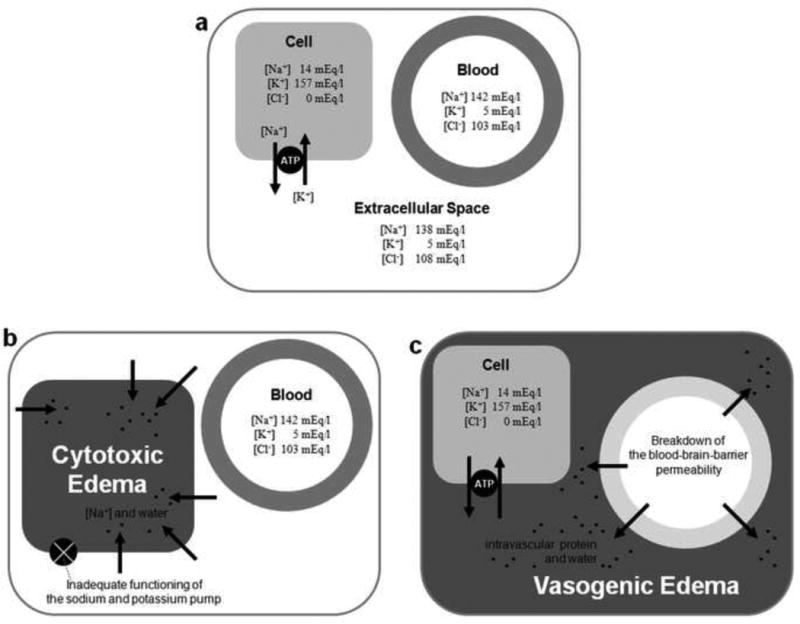
(a) Concentration distributions of organic and inorganic osmoles in intracellular space, extracellular space, and blood plasma in the normal brain. (b) Inadequate functioning of the ionic pump results in imbalance of ionic gradients across the cell membrane and intracellular water-shift (Cytotoxic edema). (c) Breakdown of the blood-brain barrier permeability allows intravascular fluid to penetrate into cerebral parenchymal extracellular space (Vascular Edema). Modified from (Kawamata, Mori et al. 2007).
[Box1] Structural Magnetic Resonance Imaging (MRI).
Conventional structural magnetic resonance (MR) imaging is based on the fact that different tissue types in the brain contain different proportions of water. The gray matter (neurons and glial cells) and white matter (axons) are about 80 and 70 percent water, while the cerebrospinal fluid (CSF) is nearly 100 percent water. MR images are constructed by manipulating the way in which water protons are excited to a non-equilibrium state, yielding intensity differences between tissue types according to how fast they return the equilibrium state (relaxation rate). For instance, water is not as efficient as fat in T1 relaxation due to the high mobility of water molecules. Therefore on T1-weighted images, fat-containing tissues appear bright and water-containing tissues appear dark. The reverse is true for T2-weighted images. Generally speaking, T1-weighted images provide good contrast between the gray and white matter structure, whereas T-2 weighted images have better sensitivity to detect subtle increases in tissue water content that can occur in conditions like edema, ischemia, demyelination, and other tissue abnormalities.
Figure 2. Typical MR findings of acute Wernicke Encephalopathy.
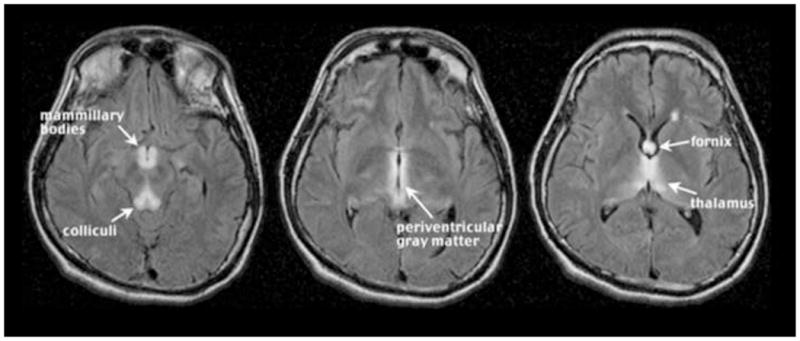
Fluid-attenuated inversion recovery images presenting symmetric high-signal intensity alterations in the mammillary bodies and colliculi (a), periventricular gray matter (b), and fornix and thalamus (c). Reprinted from (Sullivan and Pfefferbaum 2009).
Additional regions, less typically affected, also appear as symmetrical signal hyperintensities. These atypical regions are the caudate nucleus, cranial nerve nuclei, red nuclei, corpus callosum, dentate nuclei, cerebellum, and frontal and parietal cerebral cortices (Zuccoli and Pipitone 2009). In most cases, these atypical MR findings accompany typical findings. The atypical findings are more frequently observed in non-alcoholic WE patients (Zuccoli, Santa Cruz et al. 2009), whereas the mammillary bodies are rarely affected in non-alcoholic WE patients (Zhong, Jin et al. 2005; Fei, Zhong et al. 2008). The cortical involvement has been proposed to indicate poor prognosis (Zhong, Jin et al. 2005; Fei, Zhong et al. 2008).
MRI versus CT
Although CT can detect edematous lesions as low-density abnormalities, MR imaging has an advantage over CT of visualizing and quantifying such lesions, which appear as signal hyperintensities on T2-weighted images because of their high water content (compare Figure 3a and 3b). When directly comparing CT and MRI in the detection of WE-related neuropathology, CT identified low-density signal abnormalities in paraventricular regions of the thalamus in 13% of WE patients, whereas MRI identified T2 signal hyperintensities in the same region in 47%. In addition, MRI could detect high-signal intensities in the periaqueductal grey matter in 40% of WE patients. CT could detect volume deficits in the cerebral cortex, frontal lobe, cerebellum, and vermis in both WE patients (53-86%) and uncomplicated alcoholics (12-53%); however, MRI also proved to be a better method for the detection of volume deficits in the cerebral cortex, frontal lobe, cerebellum and vermis (93-100% in WE, 33-86% in uncomplicated alcoholics) (Antunez, Estruch et al. 1998). Overall, the sensitivity of MRI in revealing evidence of WE was 53% and the specificity was 93% (Antunez, Estruch et al. 1998).
MR Fluid-Attenuate Inversion Recovery Sequences
To lower the false negative rate and increase the sensitivity of MR imaging in clinical settings, new acquisition sequences have been applied to detect acute signs of WE. With an additional additional radio frequency pulse and additional manipulation of the magnetic gradients, a T2-weighted sequence can be converted to fluid attenuated inversion recovery (FLAIR) sequences, in which the CSF appears dark by eliminating the normal high-intensity of free water, but edematous tissues remain bright. The FLAIR sequence is particularly sensitive to detect edematous lesions near the ventricles. When compared with conventional T2 weighted spin-echo sequences (compare Figure 3b and 3c), the affected sites of WE were better detected by FLAIR sequences (Ashikaga, Araki et al. 1997).
Contrast Enhancement
Signal abnormalities in affected regions can be characterized by T1-reducing contrast enhancement with intravenous injection of contrast agents, such as gadolinium. This agent cannot cross a healthy blood-brain barrier (BBB); however, if the BBB is disrupted, gadolinium diffuses into the interstitial space of BBB breaches. Contrast enhancement of the mammillary bodies correlated with chronic alcohol consumption in alcoholic subjects without detected WE (Zuccoli, Gallucci et al. 2007). One case report noted that the contrast enhancement of the mammillary bodies were the only sign of acute WE in the absence of T2 abnormalities in the thalamus and midbrain (Shogry and Curnes 1994). Another study reported that gadolinium contrast enhancement, however, can be absent in acute WE, when early cytotoxic edema precedes the disruption of the BBB and vasogenic edema (Mascalchi, Simonelli et al. 1999). Therefore T2-weighted and FLAIR sequences to identify cytotoxic edematous tissues appear to be more reliable than gadolinium enhancement for detecting BBB impairment.
MR Diffusion-Weighted Imaging
MR diffusion-weighted imaging (DWI, see Box 2), accompanied by quantative measurement of the apparent diffusion coefficient (ADC), can help detection of edematous tissue (see Figure 3d). DWI is a sensitive method for identification of edematous lesions early in the course of WE and has the advantage of distinguishing between cytotoxic and vasogenic edema. Cytotoxic edematous lesions show hyperintensity on DWI images with low ADC values (restricted diffusion of water molecules), whereas vasogenic edematous lesions show hyperintensity on DWI images with high ADC values (unrestricted or high diffusion) in the affected regions (Chung, Kim et al. 2003; Halavaara, Brander et al. 2003; Lapergue, Klein et al. 2006; Unlu, Cakir et al. 2006).
[Box2] MR-diffusion-weighted Imaging (DWI).
The passive movement (diffusion) of water molecules in tissue with unconstrained microstructure, such as the CSF, is “isotropic,” which means the molecules move equally in all directions. In tissue with an orderly microstructure, such as the axons in the white matter, the diffusion is constrained and the water molecules tend to move in a given orientation (“anisotropic”). MR imaging is sensitive to diffusion, because freely diffusing water molecules suppresses the MR signal. Brain pathologic lesions, for example demyelination and cytotoxic edema, are known to restrict diffusion and appear as hyperintense signal on MR diffusion-weighted imaging. By contrast, vasogenic edema lesions, i.e., freely diffusing water into the extracellular space, have high level of diffusivity and show hypointense signal alterations.
[Suggestion for further reading: Jones, D.K. (2011). Diffusion MRI, Theory, Methods, and Applications. New York: Oxford University Press.]
MR Spectroscopy
MR spectroscopy (MRS, see Box 3 for description of MRS and major metabolites that are spectroscopically visible) studies have reported low N-acetylaspartate/creatine ratio (NAA/Cr), suggestive of neuronal compromise, and abnormally high lactate peak, suggestive of cell compromise, in the thalamus and cerebellum of KS patients (Murata, Fujito et al. 2001; Mascalchi, Belli et al. 2002; Rugilo, Uribe Roca et al. 2003). The low NAA/Cr has been reported to improve in parallel with clinical improvement following thiamine therapy in some cases (Murata, Fujito et al. 2001). Animal models of WE (see Box 4) have indicated that serial combination of MRI and MRS might be helpful in tracking the brain damage and treatment responses (Pfefferbaum, Adalsteinsson et al. 2007).
[Box3] MR spectroscopy (MRS).
Atomic nuclei are surrounded by a cloud of electrons, which generate a small magnetic field shift specific to each molecule or compound. MR spectroscopy (MRS) detects this slight magnetic shift derived from atomic nuclei of molecules and provides information about the chemical composition of the tissue. MRS-visible metabolites are N-acetylaspartate (which is considered as a marker of neuronal integrity), choline containing compounds (which is related to cell membrane synthesis), creatine (which is involved in high-energy phosphate metabolism), and glutamate (which is principal excitatory neurotransmitter).
[Suggestion for further reading: Gillard, J., Waldman, A., & Barker, P. (2005). Clinical MR Neuroimaging: Diffusion, Perfusion and Spectroscopy. Cambridge: Cambridge University Press.]
[Box4] Rodent models of WKS.
Many of the complexities of studying the effects of thiamine deficiency and chronic alcohol consumption on the human brain can be controlled, to some extent, by using laboratory animal models of WKS. The rodent model that has been studied in detail is the pyrithiamine-induced thiamine deficiency model, which uses a thiamine antagonist (pyrithiamine) to inhibit metabolism of thiamine (Langlais and Savage 1995; Vetreno, Ramos et al. 2012). A longitudinal MRI-MRS study of pyrithiamine-induced thiamine deficient rats demonstrated symmetrical hyperintense signals in the thalamus and mammillary bodies. Comparing the MR images taken before and after thiamine treatment, signal normalization was demonstrated in the thalamus but not in the mammillary bodies. The MRS findings showed a significant decline and then partial recovery in thalamic NAA. Thiamine-deficient rats with prior alcohol exposure were especially prone to developing brain lesions and showed attenuated recovery in the thalamus (Pfefferbaum, Adalsteinsson et al. 2007).
Neuropsychological Approach
KS is characterized by a disproportionate impairment in declarative memory relative to other components of cognitive functioning and results from thiamine deficiency (Kopelman 1995). KS usually follows or accompanies WE. Acute confusion usually improves within 1 to 2 days, but an attenuated state of global confusion might take 2 to 3 weeks or longer to clear. The memory deficits of KS become more obvious as the confusional stage of WE resolves. Given such a pathophysiological continuum, these two conditions are often coupled as the Wernicke-Korsakoff syndrome (WKS).
Neuropsychological tests assessing multiple cognitive domains have revealed that patients with KS have severe impairments in explicit memory, both retrograde and anterograde (Kopelman 1995; Fama, Marsh et al. 2004). By contrast, components of implicit memory, including priming and visuoperceptual learning, largely remain intact (Verfaellie, Cermak et al. 1990; Fama, Pfefferbaum et al. 2006; d'Ydewalle and Van Damme 2007). There have been extensive studies and debate on the neural basis for the characteristic memory and executive deficits in KS (see Figure 4), and other papers in this issue address these differences and controversies (Fama and Pitel 2012; Hayes, Fortier et al. 2012; Kopelman and Kessels 2012; Oscar-Berman 2012; Race and Verfaellie 2012).
Figure 4. Functions and associated brain regions targeted by chronic alcohol consumption and thiamine deficiency.
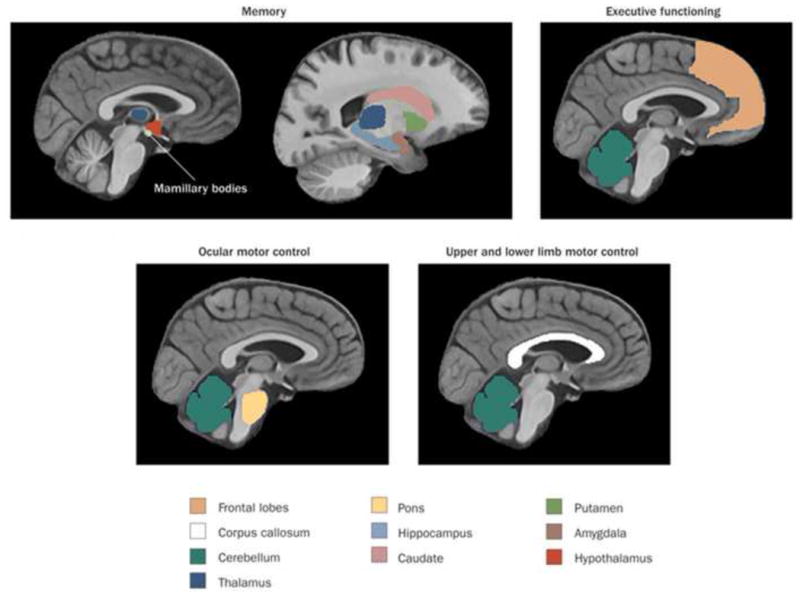
Reprinted from (Zahr, Kaufman et al. 2011).
Uncomplicated Alcoholism and Alcoholic KS
Because KS occurs most commonly in nutritionally compromised alcoholics, the neuroimaging findings of KS should be considered in the context of the brain insult related to alcoholism itself (Sullivan and Pfefferbaum 2009). In addition to chronic alcoholism, a number of medical conditions can result in thiamine deficiency, including gastrointestinal surgeries, recurrent vomiting as accompany pregnancy and bulimia, cancer and chemotherapeutic treatments, and systemic diseases such as AIDS (Sechi and Serra 2007). Alcoholism, however, still remains the most common cause of thiamine deficiency in industrialized countries (Thomson 2000). Alcoholics are at special risk for thiamine deficiency because of poor nutritional intake, compromised absorption from the gastrointestinal tract, reduced thiamine storage and impaired thiamine utilization (Thomson 2000).
There is evidence of a synergic effect between thiamine deficiency and chronic alcohol consumption on brain damage (Ciccia and Langlais 2000; Crowe and El-Hadj 2002; He, Sullivan et al. 2007). These interactions may well contribute to the heterogeneity of brain damage in KS, according to the history and severity of alcohol consumption. Similarly, the neuropsychological deficits of KS are also proposed to lie along a continuum between uncomplicated alcoholism and alcoholism with KS and may be the functional sequelae of the dietary and alcoholism liabilities (Butters and Brandt 1985; Pitel, Beaunieux et al. 2008; Sullivan and Pfefferbaum 2009).
Atrophy and Tissue Degeneration
A gross morphological view of the brains of WKS patients reveals cortical thinning, sulcal widening, and ventricular enlargement. Apparent atrophy of affected regions is observable at the acute phase of WE, which might reflect rapid tissue degeneration or atrophy from earlier unrecognized episodes. In support of this possibility, postmortem neuropathology studies have identified acute WE lesions superimposed on older ones (Torvik, Lindboe et al. 1982). Following the resolution of edema and inflammation of acute WE, typical neuroimaging findings of KS are presented as volume deficits in affected brain regions (Sullivan and Pfefferbaum 2009). Accordingly, signs of tissue degeneration, which were observed as hyperintensities in the periventricular and periaqueductal areas at the acute WE stage, were then replaced by ventricle enlargement and aqueductal dilatation when re-examined 6-12 months later in the chronic KS stage (Gallucci, Bozzao et al. 1990).
MR studies using quantitative examinations have revealed graded regional volume shrinkage when directly comparing uncomplicated alcoholics and alcoholics with KS (mild deficits in uncomplicated alcoholics and severe deficits in KS; see Figure 5). The mammillary bodies, thalamus, pons, hippocampus, cerebellar hemispheres and anterior superior vermis, after adjusted for age and intracranial volume, demonstrated significant volume deficits in uncomplicated alcoholics, which were 0.5-1.0 standard deviation below healthy controls, yet less severe than those in alcoholics with KS, which were 1.0-2.0 standard deviations below (Sullivan and Pfefferbaum 2009). Graded volume deficits in the mammillary bodies, medial thalamus, left insula and genu of the corpus callosum of KS and non-KS alcoholic patients were confirmed in a recent study applying a voxel-based approach (Pitel, Chetelat et al. 2012). Besides these regions of graded abnormality, the distribution and severity of brain damage was strikingly similar between the two groups, which suggest that a continuum from uncomplicated alcoholism to KS is likely selective to structures that are vulnerable to thiamine deficiency. Although volume deficits detected in alcoholics with KS are traditionally believed to be irreversible, MR studies have demonstrated that some structural brain changes - for instance the thalamus, temporal lobe, brainstem and cerebellum - are at least partially reversible with prolonged alcohol abstinence in “uncomplicated” alcoholics (Cardenas, Studholme et al. 2007).
Figure 5. Graded brain volume deficits in uncomplicated alcoholism and Korsakoff's syndrome.
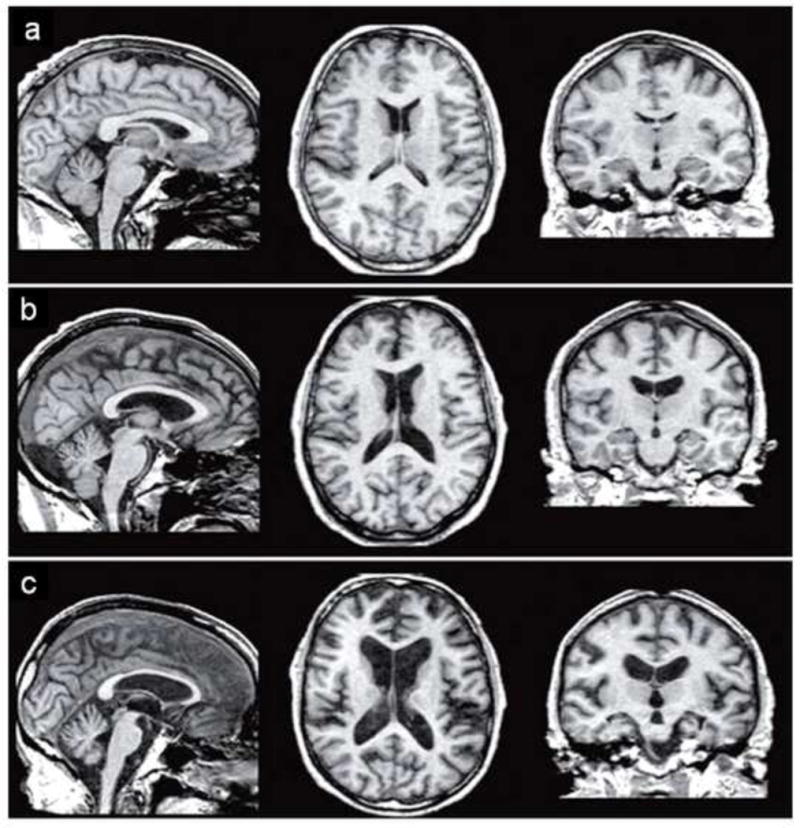
T1 weighted MR images show a graded enlargement of the ventricle (indicating shrinkage of the surrounding tissue) in a (63-year old healthy control male), b (59 year old man with alcoholism), and c (63-year old man with WKS). Reprinted from (Zahr, Kaufman et al. 2011).
Thalamus and Mammillary Bodies
The dorsomedial thalamus was first proposed as a critical locus for persistent memory impairment in patients with KS (Victor, Adams et al. 1971). In contrast to this position, neuropathological studies consistently reported that anterograde amnesia is associated with atrophy of the midline thalamic nuclei, not the mammillary bodies (Mair, Warrington et al. 1979; Mayes, Meudell et al. 1988; Visser, Krabbendam et al. 1999). A neuropathology study (Harding, Halliday et al. 2000) reported that neuronal loss in the anterior thalamic nuclei was the best predictor of memory impairment in KS. The primary role of the anterior thalamus in KS is supported by neuroimaging studies, which report that structural lesion in the mammillo-thalamic tract – white matter projections from the mammillary body to the anterior thalamus – results in KS (Yoneoka, Takeda et al. 2004; Josseaume, Auffray Calvier et al. 2007). PET studies also have reported that permanent amnesia was associated with hypometabolism in the thalamus (Aupee, Desgranges et al. 2001; Reed, Lasserson et al. 2003).
Mammillary body shrinkage, which is observed upwards of 60-80% in postmortem neuropathology studies (Harper 1983; Sheedy, Lara et al. 1999), has been proposed as a specific macroscopic lesion of chronic WE and KS. Using MR imaging, mammillary body shrinkage was observed in 78% of the WE patients when compared to patients with Alzheimer's disease (Charness and DeLaPaz 1987). Another MR study reported mammillary body shrinkage in 40% of WE patients and 27% in asymptomatic alcoholics (Antunez, Estruch et al. 1998). However, mammillary body shrinkage has also been observed in cases without amnesia, and the correlation between mammillary body volume and memory impairment are inconsistent (Shear, Sullivan et al. 1996; Sullivan, Lane et al. 1999; Harding, Halliday et al. 2000). These findings indicate that mammillary body degeneration might participate in, but is not sufficient to account for, the memory impairment of KS.
Functional MR imaging (fMRI, see Box 5) has an advantage over static structural imaging because behavioral experiments can be conducted concurrently with MR imaging to investigate brain structure-functional relationships (Zahr, Kaufman et al. 2011). A disadvantage of functional imaging is that KS patients are often too cognitively impaired to follow directions adequately to produce valid data in the fMRI environment. To overcome this limitation, a series of studies employed a resting state functional connectivity MR paradigm (see Box 6), which measured the temporal correlations of the blood-oxygen-dependent signals while the subjects were instructed to do nothing but rest for several minutes while undergoing MR imaging. The initial study reported that the functional connectivity of the mammillo-thalamic tract correlated with verbal memory scores on tests taken outside of the scanner by alcoholics recovering from KS (Kim, Ku et al. 2009). In a follow-up study, the time-series changes of the mammillo-thalamic functional connectivity generally paralleled to the changes of memory scores in a KS patient who experienced two WE episodes over a 20-month span. The mammillo-thalamic functional connectivity which was similar to healthy controls after recovering from the first WE episode, was disrupted during the acute stage of the recurrent WE episode but improved in parallel with the performance on verbal memory test, following thiamine treatment (Kim, Ku et al. 2010).
[Box5] Functional Magnetic Resonance Imaging (fMRI).
Functional Magnetic Resonance Imaging takes advantage of the blood oxygen level dependent (BOLD) contrast mechanism (Ogawa, Lee et al. 1990; Kwong, Belliveau et al. 1992). This fMRI activation is not a direct measure of neuron activity but is a measure derived from the difference between two active conditions, that is, it reflects a difference in hemodynamic response. When neurons become active while performing a task, the increased neural activity causes an increased demand for oxygen, and the local blood flow to those brain regions increases the amount of oxygenated hemoglobin relative to deoxygenated hemoglobin. Deoxygenated hemoglobin is more magnetic (paramagnetic) and attenuates the MR signal intensity, whereas oxygenated hemoglobin is virtually nonmagnetic (diamagnetic) and has little effect on the magnetic field itself. The brain regions showing the greatest BOLD contrasts are assumed to be the regions most involved in performing the task.
[Box6] Resting-state Functional Connectivity.
Functional connectivity is defined as the temporal correlation of neurophysiological events measured in different brain regions (Friston, Frith et al. 1993). Analyses of fMRI data have shown significant temporal correlations in low frequency BOLD signal fluctuations of brain regions, even when the subject is not performing a task (resting-state) (Biswal, Yetkin et al. 1995). These temporal correlations are presumed to reflect co-activation of intrinsic functionally linked regions and have been demonstrated across several distinct networks related to sensory, motor, and cognitive functions (Kim, Ku et al. 2009; Chanraud, Pitel et al. 2011).
Hippocampus
The hippocampus also shows graded volume deficits in KS alcoholics relative to uncomplicated alcoholics (Sullivan and Pfefferbaum 2009). Hippocampal volume deficits of KS alcoholics were of similar extent to those observed in patients with Alzheimer' disease with known hippocampal volume deficits (Sullivan and Marsh 2003). In addition, hippocampal volume deficits have been reported to correlate with declarative memory impairment in KS alcoholics (Sullivan and Marsh 2003). An fMRI study that compared a KS subject with healthy controls reported that unlike controls the KS subject demonstrated no hippocampal activations and poor performance on recognition memory tasks in the MR scanner (Caulo, Van Hecke et al. 2005). The anterior thalamus has multiple direct and indirect interactions with the hippocampus, which are critical for episodic memory. Accordingly, it has been suggested that the memory impairment is probably caused by interruption of the diencephalic-hippocampal circuitry, including the thalamic nuclei and mamillary bodies, rather than arising from discrete lesions in the anterior thalamic nuclei (Kopelman 2002; Sullivan and Marsh 2003; Harper 2009; Aggleton, O'Mara et al. 2010).
Frontal Lobe
There is clear evidence that alcoholic KS patients have executive dysfunctions (Janowsky, Shimamura et al. 1989; Kopelman 1991; Oscar-Berman, Kirkley et al. 2004). The vulnerability of the frontal lobe to chronic alcohol consumption with or without thiamine deficiency is widely accepted based on neuropathological (Torvik 1987; Harper, Gold et al. 1989) and neuroimaging studies (Pfefferbaum, Sullivan et al. 1997; Cardenas, Studholme et al. 2007; Zahr, Kaufman et al. 2011). Behavioral studies that have directly compared KS alcoholics with non-KS alcoholics showed that executive and memory deficits were most apparent in KS alcoholics, whereas the deficits in non-KS alcoholics were less conclusive (Krabbendam, Visser et al. 2000; Brokate, Hildebrandt et al. 2003; Oscar-Berman, Kirkley et al. 2004).
Although KS patients show impairment in both anterograde and retrograde memory, the severity of impairment in these two memory systems are poorly correlated (Kopelman 1991; Fama, Marsh et al. 2004), indicating that the two memory processes are subserved by different brain systems. Whereas the anterograde memory deficits of KS has been attributed to the dysfunction of the thalamus and hippocampus, working memory and retrograde memory deficits have been proposed to be related to frontal lobe dysfunction (Kopelman 1991; Fama, Marsh et al. 2004). This dissociation is in line with neuroimaging studies that reported abnormal low frontal cerebral blood flow and impaired performance on memory tests in KS patients (Hunter, McLuskie et al. 1989; Reed, Lasserson et al. 2003).
Cerebellum
The cerebellum is vulnerable to damage from thiamine deficiency (Mulholland 2006). Graded regional volume shrinkage (healthy control > uncomplicated alcoholic > KS) in the cerebellar hemispheres and anterior superior vermis occur (Sullivan, Deshmukh et al. 2000). As the networks of the cerebellum are now known to extend to the frontal lobe, the cerebellum volume deficits have the potential to underlie motor symptoms traditionally attributed to the cerebellum, but also deficits of executive functions that were previously related only to the frontal lobe (Sullivan, Deshmukh et al. 2000; Sullivan, Harding et al. 2003). Impairment of working memory, which is not specific to, yet present in KS, is proposed to represent the impairment of the frontocerebellar circuitry due to chronic alcohol consumption (Sullivan, Harding et al. 2003; Pitel, Beaunieux et al. 2008).
Conclusion
This review has covered the major neuroimaging findings in KS and its antecedent, WE, in the context of two different approaches. Based on studies from the clinical approach, typical MRI findings of cytotoxic or vasogenic edema are now considered as a valuable tool to improve early detection of WE in clinical settings. Presence of bilateral symmetrical signal hyperintensities in the periventricular region of the third ventricle, periaqueductal area and mammillary bodies strongly supports clinical impressions of WE. Neuroimaging studies from the neuropsychological approach have corroborated postmortem neuropathological studies and have expanded the understanding of the neuropsychological deficits resulting from thiamine deficiency, alcohol neurotoxicity, and their combined effect. Growing evidence of neuroimaging studies indicates that the interactions among the thalamus, hippocampus, frontal lobe, and cerebellum are crucial for memory formation and executive functions, and that the interruption of these circuits, interconnecting these regions and connecting these regions to neural substrates of other relevant mnemonic system, underlies the neuropsychological deficits in KS. Given that this characterization of the neural substrates of WE and KS derives from a composite of findings largely from group studies, application to clinical decisions relevant to the individual patient requires convergence of positive diagnostic signs determined from multiple venues, including neuroimaging, neuropsychological, and blood chemistry assessment together with discerning historical examination and perceptive review of systems.
Acknowledgments
Support for this work was provided by the US National Institutes of Health grants AA010721, AA017168 and AA017923.
Footnotes
The authors declare no conflict of interest.
References
- Aggleton JP, O'Mara SM, et al. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci. 2010;31(12):2292–307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunez E, Estruch R, et al. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke's encephalopathy. AJR Am J Roentgenol. 1998;171(4):1131–7. doi: 10.2214/ajr.171.4.9763009. [DOI] [PubMed] [Google Scholar]
- Ashikaga R, Araki Y, et al. FLAIR appearance of Wernicke encephalopathy. Radiat Med. 1997;15(4):251–3. [PubMed] [Google Scholar]
- Aupee AM, Desgranges B, et al. Voxel-based mapping of brain hypometabolism in permanent amnesia with PET. Neuroimage. 2001;13(6 Pt 1):1164–73. doi: 10.1006/nimg.2001.0762. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brokate B, Hildebrandt H, et al. Frontal lobe dysfunctions in Korsakoff's syndrome and chronic alcoholism: continuity or discontinuity? Neuropsychology. 2003;17(3):420–8. doi: 10.1037/0894-4105.17.3.420. [DOI] [PubMed] [Google Scholar]
- Butters N, Brandt J. The continuity hypothesis: the relationship of long-term alcoholism to the Wernicke-Korsakoff syndrome. Recent Dev Alcohol. 1985;3:207–26. doi: 10.1007/978-1-4615-7715-7_17. [DOI] [PubMed] [Google Scholar]
- Butterworth RF, Kril JJ, et al. Thiamine-dependent enzyme changes in the brains of alcoholics: relationship to the Wernicke-Korsakoff syndrome. Alcohol Clin Exp Res. 1993;17(5):1084–8. doi: 10.1111/j.1530-0277.1993.tb05668.x. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, et al. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34(3):879–87. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulo M, Van Hecke J, et al. Functional MRI study of diencephalic amnesia in Wernicke-Korsakoff syndrome. Brain. 2005;128(Pt 7):1584–94. doi: 10.1093/brain/awh496. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, et al. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011;21(10):2272–81. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charness ME, DeLaPaz RL. Mamillary body atrophy in Wernicke's encephalopathy: antemortem identification using magnetic resonance imaging. Ann Neurol. 1987;22(5):595–600. doi: 10.1002/ana.410220506. [DOI] [PubMed] [Google Scholar]
- Chung TI, Kim JS, et al. Diffusion weighted MR imaging of acute Wernicke's encephalopathy. Eur J Radiol. 2003;45(3):256–8. doi: 10.1016/s0720-048x(02)00009-8. [DOI] [PubMed] [Google Scholar]
- Ciccia RM, Langlais PJ. An examination of the synergistic interaction of ethanol and thiamine deficiency in the development of neurological signs and long-term cognitive and memory impairments. Alcohol Clin Exp Res. 2000;24(5):622–34. [PubMed] [Google Scholar]
- Crowe SF, El-Hadj D. Phenytoin ameliorates the memory deficit induced in the young chick by ethanol toxicity in association with thiamine deficiency. Pharmacol Biochem Behav. 2002;71(1-2):215–21. doi: 10.1016/s0091-3057(01)00650-5. [DOI] [PubMed] [Google Scholar]
- d'Ydewalle G, Van Damme I. Memory and the Korsakoff syndrome: not remembering what is remembered. Neuropsychologia. 2007;45(5):905–20. doi: 10.1016/j.neuropsychologia.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Fama R, Marsh L, et al. Dissociation of remote and anterograde memory impairment and neural correlates in alcoholic Korsakoff syndrome. J Int Neuropsychol Soc. 2004;10(3):427–41. doi: 10.1017/S135561770410310X. [DOI] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, et al. Visuoperceptual learning in alcoholic Korsakoff syndrome. Alcohol Clin Exp Res. 2006;30(4):680–7. doi: 10.1111/j.1530-0277.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- Fama R, Pitel AL. Neuropsychol Rev. 2012 doi: 10.1007/s11065-012-9207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei GQ, Zhong C, et al. Clinical characteristics and MR imaging features of nonalcoholic Wernicke encephalopathy. AJNR Am J Neuroradiol. 2008;29(1):164–9. doi: 10.3174/ajnr.A0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, et al. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13(1):5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Gallucci M, Bozzao A, et al. Wernicke encephalopathy: MR findings in five patients. AJR Am J Roentgenol. 1990;155(6):1309–14. doi: 10.2214/ajr.155.6.2122685. [DOI] [PubMed] [Google Scholar]
- Gangolf M, Czerniecki J, et al. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS One. 2010;5(10):e13616. doi: 10.1371/journal.pone.0013616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halavaara J, Brander A, et al. Wernicke's encephalopathy: is diffusion-weighted MRI useful? Neuroradiology. 2003;45(8):519–23. doi: 10.1007/s00234-003-1043-8. [DOI] [PubMed] [Google Scholar]
- Harding A, Halliday G, et al. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 123 ( Pt 1): 141-54. 2000 doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Harper C. The incidence of Wernicke's encephalopathy in Australia--a neuropathological study of 131 cases. J Neurol Neurosurg Psychiatry. 1983;46(7):593–8. doi: 10.1136/jnnp.46.7.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009;44(2):136–40. doi: 10.1093/alcalc/agn102. [DOI] [PubMed] [Google Scholar]
- Harper C, Gold J, et al. The prevalence of the Wernicke-Korsakoff syndrome in Sydney, Australia: a prospective necropsy study. J Neurol Neurosurg Psychiatry. 1989;52(2):282–5. doi: 10.1136/jnnp.52.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Giles M, et al. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49(4):341–5. doi: 10.1136/jnnp.49.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Fortier CB, et al. Implicite Memroy in Korsakoff's Syndrome: A Review of Procedural Learning and Priming Studies “ Neuropsychol Rev. 2012 doi: 10.1007/s11065-012-9204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell AS, Todd KG, et al. Mechanisms of neuronal cell death in Wernicke's encephalopathy. Metab Brain Dis. 1998;13(2):97–122. doi: 10.1023/a:1020657129593. [DOI] [PubMed] [Google Scholar]
- He X, Sullivan EV, et al. Interaction of thiamine deficiency and voluntary alcohol consumption disrupts rat corpus callosum ultrastructure. Neuropsychopharmacology. 2007;32(10):2207–16. doi: 10.1038/sj.npp.1301332. [DOI] [PubMed] [Google Scholar]
- Hunter R, McLuskie R, et al. The pattern of function-related regional cerebral blood flow investigated by single photon emission tomography with 99mTc-HMPAO in patients with presenile Alzheimer's disease and Korsakoff's psychosis. Psychol Med. 1989;19(4):847–55. doi: 10.1017/s0033291700005560. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, et al. Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behav Neurosci. 1989;103(3):548–60. doi: 10.1037//0735-7044.103.3.548. [DOI] [PubMed] [Google Scholar]
- Jiang W, Gagliardi JP, et al. Acute psychotic disorder after gastric bypass surgery: differential diagnosis and treatment. Am J Psychiatry. 2006;163(1):15–9. doi: 10.1176/appi.ajp.163.1.15. [DOI] [PubMed] [Google Scholar]
- Josseaume T, Auffray Calvier E, et al. [Acute anterograde amnesia by infarction of the mamillothalamic tracts] J Neuroradiol. 2007;34(1):59–62. doi: 10.1016/j.neurad.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Kawamata T, Mori T, et al. Tissue hyperosmolality and brain edema in cerebral contusion. Neurosurg Focus. 2007;22(5):E5. doi: 10.3171/foc.2007.22.5.6. [DOI] [PubMed] [Google Scholar]
- Kim E, Ku J, et al. Restoration of mammillothalamic functional connectivity through thiamine replacement therapy in Wernicke's encephalopathy. Neurosci Lett. 2010;479(3):257–61. doi: 10.1016/j.neulet.2010.05.074. [DOI] [PubMed] [Google Scholar]
- Kim E, Ku J, et al. Mammillothalamic functional connectivity and memory function in Wernicke's encephalopathy. Brain. 2009;132(Pt 2):369–76. doi: 10.1093/brain/awn311. [DOI] [PubMed] [Google Scholar]
- Kopelman M, Kessels R. Context Memory in Korsakoff's Syndrome. Neuropsychol Rev. 2012 doi: 10.1007/s11065-012-9202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD. Frontal dysfunction and memory deficits in the alcoholic Korsakoff syndrome and Alzheimer-type dementia. Brain. 1991;114(Pt 1A):117–37. [PubMed] [Google Scholar]
- Kopelman MD. The Korsakoff syndrome. Br J Psychiatry. 1995;166(2):154–73. doi: 10.1192/bjp.166.2.154. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Disorders of memory. Brain. 2002;125(Pt 10):2152–90. doi: 10.1093/brain/awf229. [DOI] [PubMed] [Google Scholar]
- Krabbendam L, Visser PJ, et al. Normal cognitive performance in patients with chronic alcoholism in contrast to patients with Korsakoff's syndrome. J Neuropsychiatry Clin Neurosci. 2000;12(1):44–50. doi: 10.1176/jnp.12.1.44. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89(12):5675–9. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlais PJ, Savage LM. Thiamine deficiency in rats produces cognitive and memory deficits on spatial tasks that correlate with tissue loss in diencephalon, cortex and white matter. Behav Brain Res. 1995;68(1):75–89. doi: 10.1016/0166-4328(94)00162-9. [DOI] [PubMed] [Google Scholar]
- Lapergue B, Klein I, et al. Diffusion weighted imaging of cerebellar lesions in Wernicke's encephalopathy. J Neuroradiol. 2006;33(2):126–8. doi: 10.1016/s0150-9861(06)77243-1. [DOI] [PubMed] [Google Scholar]
- Mair WG, Warrington EK, et al. Memory disorder in Korsakoff's psychosis: a neuropathological and neuropsychological investigation of two cases. Brain. 1979;102(4):749–83. doi: 10.1093/brain/102.4.749. [DOI] [PubMed] [Google Scholar]
- Mascalchi M, Belli G, et al. Proton MR spectroscopy of Wernicke encephalopathy. AJNR Am J Neuroradiol. 2002;23(10):1803–6. [PMC free article] [PubMed] [Google Scholar]
- Mascalchi M, Simonelli P, et al. Do acute lesions of Wernicke's encephalopathy show contrast enhancement? Report of three cases and review of the literature. Neuroradiology. 1999;41(4):249–54. doi: 10.1007/s002340050741. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Meudell PR, et al. Location of lesions in Korsakoff's syndrome: neuropsychological and neuropathological data on two patients. Cortex. 1988;24(3):367–88. doi: 10.1016/s0010-9452(88)80001-7. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ. Susceptibility of the cerebellum to thiamine deficiency. Cerebellum. 2006;5(1):55–63. doi: 10.1080/14734220600551707. [DOI] [PubMed] [Google Scholar]
- Murata T, Fujito T, et al. Serial MRI and (1)H-MRS of Wernicke's encephalopathy: report of a case with remarkable cerebellar lesions on MRI. Psychiatry Res. 2001;108(1):49–55. doi: 10.1016/s0925-4927(01)00304-3. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, et al. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14(1):68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Function and Dysfunction of Prefrontal Brain Circuit in Alcoholic Korsakoff's Syndrome. Neuropsychol Rev. 2012 doi: 10.1007/s11065-012-9198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Kirkley SM, et al. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcohol Clin Exp Res. 2004;28(4):667–75. doi: 10.1097/01.alc.0000122761.09179.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, et al. Development and resolution of brain lesions caused by pyrithiamine- and dietary-induced thiamine deficiency and alcohol exposure in the alcohol-preferring rat: a longitudinal magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2007;32(5):1159–77. doi: 10.1038/sj.npp.1301107. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, et al. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21(3):521–9. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, et al. Episodic and working memory deficits in alcoholic Korsakoff patients: the continuity theory revisited. Alcohol Clin Exp Res. 2008;32(7):1229–41. doi: 10.1111/j.1530-0277.2008.00677.x. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Chetelat G, et al. Macrostructural abnormalities in Korsakoff syndrome compared with uncomplicated alcoholism. Neurology. 2012 doi: 10.1212/WNL.0b013e318251834e. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Zahr NM, et al. Signs of preclinical Wernicke's encephalopathy and thiamine levels as predictors of neuropsychological deficits in alcoholism without Korsakoff's syndrome. Neuropsychopharmacology. 2010;36(3):580–8. doi: 10.1038/npp.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race E, Verfaellie M. Remote Memory Functiona and Dysfuncitona in Korsakoff's Syndrome. Neuropsychol Rev. 2012 doi: 10.1007/s11065-012-9197-y. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Lasserson D, et al. FDG-PET findings in the Wernicke-Korsakoff syndrome. Cortex. 2003;39(4-5):1027–45. doi: 10.1016/s0010-9452(08)70876-1. [DOI] [PubMed] [Google Scholar]
- Rugilo CA, Uribe Roca MC, et al. Proton MR spectroscopy in Wernicke encephalopathy. AJNR Am J Neuroradiol. 2003;24(5):952–5. [PMC free article] [PubMed] [Google Scholar]
- Sechi G, Serra A. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6(5):442–55. doi: 10.1016/S1474-4422(07)70104-7. [DOI] [PubMed] [Google Scholar]
- Shear PK, Sullivan EV, et al. Mammillary body and cerebellar shrinkage in chronic alcoholics with and without amnesia. Alcohol Clin Exp Res. 1996;20(8):1489–95. doi: 10.1111/j.1530-0277.1996.tb01153.x. [DOI] [PubMed] [Google Scholar]
- Sheedy D, Lara A, et al. Size of mamillary bodies in health and disease: useful measurements in neuroradiological diagnosis of Wernicke's encephalopathy. Alcohol Clin Exp Res. 1999;23(10):1624–8. [PMC free article] [PubMed] [Google Scholar]
- Shogry ME, Curnes JT. Mamillary body enhancement on MR as the only sign of acute Wernicke encephalopathy. AJNR Am J Neuroradiol. 1994;15(1):172–4. [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, et al. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: relation to ataxia. Neuropsychology. 2000;14(3):341–52. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, et al. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res. 2003;27(2):301–9. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Lane B, et al. In vivo mammillary body volume deficits in amnesic and nonamnesic alcoholics. Alcohol Clin Exp Res. 1999;23(10):1629–36. [PubMed] [Google Scholar]
- Sullivan EV, Marsh L. Hippocampal volume deficits in alcoholic Korsakoff's syndrome. Neurology. 2003;61(12):1716–9. doi: 10.1212/01.wnl.0000098940.31882.bb. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol. 2009;44(2):155–65. doi: 10.1093/alcalc/agn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AD. Mechanisms of vitamin deficiency in chronic alcohol misusers and the development of the Wernicke-Korsakoff syndrome. Alcohol Alcohol Suppl. 2000;35(1):2–7. doi: 10.1093/alcalc/35.supplement_1.2. [DOI] [PubMed] [Google Scholar]
- Thomson AD, Cook CC, et al. Wernicke's encephalopathy: ‘Plus ca change, plus c'est la meme chose'.”Alcohol Alcohol. 2008;43(2):180–6. doi: 10.1093/alcalc/agm149. [DOI] [PubMed] [Google Scholar]
- Thomson AD, Marshall EJ. The natural history and pathophysiology of Wernicke's Encephalopathy and Korsakoff's Psychosis. Alcohol Alcohol. 2006;41(2):151–8. doi: 10.1093/alcalc/agh249. [DOI] [PubMed] [Google Scholar]
- Torvik A. Brain lesions in alcoholics: neuropathological observations. Acta Med Scand Suppl. 1987;717:47–54. doi: 10.1111/j.0954-6820.1987.tb13041.x. [DOI] [PubMed] [Google Scholar]
- Torvik A, Lindboe CF, et al. Brain lesions in alcoholics. A neuropathological study with clinical correlations. J Neurol Sci. 1982;56(2-3):233–48. doi: 10.1016/0022-510x(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Unlu E, Cakir B, et al. MRI findings of Wernicke encephalopathy revisited due to hunger strike. Eur J Radiol. 2006;57(1):43–53. doi: 10.1016/j.ejrad.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Cermak LS, et al. Strategic and automatic priming of semantic memory in alcoholic Korsakoff patients. Brain Cogn. 1990;13(2):178–92. doi: 10.1016/0278-2626(90)90049-t. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Ramos RL, et al. Brain and behavioral pathology in an animal model of Wernicke's encephalopathy and Wernicke-Korsakoff Syndrome. Brain Res. 2012;1436:178–92. doi: 10.1016/j.brainres.2011.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor M, Adams RD, et al. The Wernicke-Korsakoff syndrome; a clinical and pathological study of 245 patients, 82 with post-mortem examinations. Philadelphia,, F.A. Davis. 1971 [PubMed] [Google Scholar]
- Victor M, Adams RD, et al. The Wernicke-Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition. Philadelphia, PA, F.A. Davis Co 1989 [Google Scholar]
- Visser PJ, Krabbendam L, et al. Brain correlates of memory dysfunction in alcoholic Korsakoff's syndrome. J Neurol Neurosurg Psychiatry. 1999;67(6):774–8. doi: 10.1136/jnnp.67.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden RW, Allen HM. Wernicke's encephalopathy after gastric bypass that masqueraded as acute psychosis: a case report. Curr Surg. 2006;63(2):114–6. doi: 10.1016/j.cursur.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yoneoka Y, Takeda N, et al. Acute Korsakoff syndrome following mammillothalamic tract infarction. AJNR Am J Neuroradiol. 2004;25(6):964–8. [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Kaufman KL, et al. Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol. 2011;7(5):284–94. doi: 10.1038/nrneurol.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Jin L, et al. MR Imaging of nonalcoholic Wernicke encephalopathy: a follow-up study. AJNR Am J Neuroradiol. 2005;26(9):2301–5. [PMC free article] [PubMed] [Google Scholar]
- Zuccoli G, Gallucci M, et al. Wernicke encephalopathy: MR findings at clinical presentation in twenty-six alcoholic and nonalcoholic patients. AJNR Am J Neuroradiol. 2007;28(7):1328–31. doi: 10.3174/ajnr.A0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccoli G, Pipitone N. Neuroimaging findings in acute Wernicke's encephalopathy: review of the literature. AJR Am J Roentgenol. 2009;192(2):501–8. doi: 10.2214/AJR.07.3959. [DOI] [PubMed] [Google Scholar]
- Zuccoli G, Santa Cruz D, et al. MR imaging findings in 56 patients with Wernicke encephalopathy: nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol. 2009;30(1):171–6. doi: 10.3174/ajnr.A1280. [DOI] [PMC free article] [PubMed] [Google Scholar]


