Abstract
Objectives
Early life environment is essential for lung growth and maximally attained lung function. Whether early life exposures impact on lung function decline in adulthood, an indicator of lung ageing, has scarcely been studied.
Methods
Spirometry data from two time points (follow-up time 9–11 years) and information on early life exposures, health and life-style were available from 12862 persons aged 28–73 years participating in the European population-based cohorts SAPALDIA (n = 5705) and ECRHS (n = 7157). The associations of early life exposures with lung function (FEV1) decline were analysed using mixed-effects linear regression.
Results
Early life exposures were significantly associated with FEV1 decline, with estimates almost as large as personal smoking. FEV1 declined more rapidly among subjects born during the winter season (adjusted difference in FEV1/year of follow-up [95%CI] -2.04ml [-3.29;-0.80]), of older mothers, (-1.82 ml [-3.14;-0.49]) of smoking mothers (-1.82ml [-3.30;-0.34] or with younger siblings (-2.61ml [-3.85;-1.38]). Less rapid FEV1-decline was found in subjects who had attended daycare (3.98ml [2.78;5.18]), and indicated in subjects with pets in childhood (0.97ml [-0.16;2.09]). High maternal age and maternal smoking appeared to potentiate effects of personal smoking. The effects were independent of asthma at any age.
Conclusion
Early life factors predicted lung function decline decades later, suggesting that some mechanisms related lung ageing may be established early in life. Early life programming of susceptibility to adult insults could be a possible pathway that should be explored further.
Introduction
Early childhood is a critical time window for subsequent lung health. Adverse childhood environmental exposures can restrain growth[1], modulate lung function [1, 2] and induce changes to gene-expression, modulating airway pathophysiology.[3, 4] The impact of a range of early life factors have been evidenced such as parental life-style [5, 6], nutrition [7] ambient air pollution [8, 9] or viral infections.[10] Epigenetic programming has been suggested as an underlying mechanism leading to less favourable long term respiratory health outcomes. [7, 11–13]
Emerging research suggests that not only lung growth, but also lung ageing, may be programmed early in life [14, 15]. Lung ageing encompasses the physiological as well as pathological processes which lead to altered lung function and lung diseases with increasing age. [16] Lung function decline is on one hand a normal ageing process, on the other hand it can be potentiated by risk factors, such as smoking [17, 18] or obesity.[17, 19] Early life impact on adult respiratory health is relatively well documented [20–23]. However, the potential impact on lung function decline by early life factors has been scarcely studied and remains inconsistent. Svanes et al., for example, shows that a childhood disadvantage score, including childhood asthma, predicted more rapid lung function decline in middle aged adults [21] and Jackson et al. evidenced earlier and quicker lung function decline for young adults with lower childhood SES [24] whereas Marossy et al. found no significant association between lung function decline in adults age 35–45 and early life respiratory infections.[25]
Smoking is known as the main adult risk factor for accelerated lung function decline; however, causes for varying susceptibility to tobacco exposure between individuals are not well understood. Early life factors, in particular parental smoking, have been hypothesized to play a role for modifying susceptibility.[26–28]. No previous study on early life factors has yet followed participants into old age or had the power to investigate individual early life factors in sufficient detail. We wished to investigate the hypotheses i) that early life factors may predict lung function decline, independent of childhood or parental asthma and ii) that one potential pathway might be through altered susceptibility to adult insults like smoking.
The combined data of the two cohorts, the Swiss Study on Air Pollution And Lung and Heart Disease In Adults (SAPALDIA) and the European Community Respiratory Health Study (ECRHS) offered the opportunity, the necessary population size and age range to study these hypotheses.
Material and Methods
Study population
The study population consists of 12862 subjects of the SAPALDIA [29] and the ECRHS cohort. [30] Both studies have been described in detail elsewhere. In short, the SAPALDIA study population was recruited in 1991 as a population–based, random sample of adults (N = 9651, age 20–60 years) from eight study areas in Switzerland.[29] In the second assessment in 2002/03, lung function measures as well as the questionnaire on socio-demographic characteristics, life-style factors, living, housing and work related characteristics and health status, were repeated. Lung function data from both surveys was available for 5705 participants and mean follow-up time was 10.9 years. In ECRHS I, a random sample of adults aged 20–44 years were recruited in 29 centres (N = 13 359).[30] 7157 participants with lung function measures at two time points (at ECRHS I in1991–1993 and at ECRHS II in 1998–2002), were included into the analyses. The mean follow-up time was 8.8 years. The two cohorts have coordinated study design, questionnaires and common standards for interview and clinical examinations which allows for analyses of the combined data sets. Each study centre received approval by the institutional or regional ethics committee (S1 Text), and all participants signed informed consents.
Because of the large number of study centres, and the large number of study participants from Switzerland, the study centres were categorised into four European regions: Southern (Spain, France-Montpellier, Central (Switzerland, France-Grenoble and Italy-Pavia, -Turin, -Verona), Northern (Iceland, Sweden, Norway, Estonia) and Western Europe (Great-Britain, Belgium, France-Bordeaux and -Paris; S1 Fig).
Early life factors
Information on early life characteristics was collected by an interview-led questionnaire assessing serious respiratory infection <2 years, day care attendance <5 years, bedroom sharing, older and younger siblings, maternal age at delivery, season of birth (winter, defined as born in November to end of January, versus other seasons), paternal and/or maternal smoking during childhood, childhood pet keeping and urban living environment (large town vs. small town or farm/village) (S5 Table).
Lung function measurements
Spirometry testing in both studies was performed according to the ECRHS protocol following the American Thoracic Society guidelines.[31] The maximum FEV1 and maximum forced vital capacity (FVC) of up to five technically acceptable manoeuvres were determined. Annual decline in FEV1 (ΔFEV1/yr.) was calculated by subtracting the baseline from the follow-up value and dividing the difference by the individual time of follow-up in years, a negative value representing a decline. SAPALDIA used identical spirometry devices (model 2200, SensorMedics Corp., Yorba Linda, CA, USA) and protocols in both examinations,[32] as did ECRHS centres using same or comparable spirometers (Spiro Medics; Biomedin).[21]
Covariates
Asthma status: Participants reported doctor diagnosed asthma and age of onset. Childhood and teenage asthma was defined based on the age of reported first asthma attack (<10 yrs. of age, respectively <20 yrs.). Adult asthma was defined as having the first asthma attack after the age of 20 yrs.
Other: Socio-demographic, individual, lifestyle factors associated with respiratory health and family predisposition, such as parental and sibling asthma, were considered as confounders. Smoking status was defined on self-reported smoking history. Pack years at the 2nd survey were calculated based on an estimation of the average number of cigarettes smoked per year. Missing data for pack years (N = 225) and maternal age at birth (N = 237) were imputed by a “simple” imputation technique based on multivariate regression models to predict the missing information. Imputed data were only used as confounders in multivariate analyses.
Statistical methods
Descriptive analyses, including comparative lung function (FEV1) at first survey and lung function decline (ΔFEV1/yr.), were ran by European region and by smoking status. We studied the impact of early life factors on lung function decline in a mixed-effects linear regression model; in a first step, for single early life factors (S1 Table), and secondly, adjusting mutually for all early life exposures. Model adjustments were made for mid age, mid age square, mid BMI (mid = [survey 1 + survey 2]/2), change in BMI (between survey 1 and 2), height, pack years smoked, age at highest education, based on the significance level of p≤0.2. Different regional adjustments, including the four European regions (main analyses), the cohorts (ECRHS, SAPALDIA) or study centre as random factors, were performed. To investigate independency of the investigated early life factors from childhood & teenage and adult asthma, we ran additional models adjusting for the reported asthma status, as well as sensitivity analyses excluding subjects who reported 1.) asthma as a child (<10 yrs.) 2.) ever having had asthma, 3.) COPD, defined as pre-bronchodilator FEV1/FVC <0.70 [33]. The potentially increased susceptibility to adult smoking was investigated by interaction terms between significant adverse early life factors and participants’ current smoking exposure (e.g. smoking*maternal smoking), and by stratified analyses by smoking status and sex. Significance of interaction was assumed at a p-value of ≥0.1. Furthermore, we ran a sensitivity analysis excluding participants under 25 yrs. at the first survey and stratified analyses by gender, smoking status and European region. To take regional differences in population size into account we performed meta-analyses by European region. All analyses were conducted using STATA 12 (www.stata.com/stata12). The final significance level was p = 0.05 for all results.
Results
Characteristics of the 12862 study participants by European region are provided in table 1. The early life factors, except season of birth (p = 0.7), differed significantly between the regions (p<0.001, Table 1). Age-and height-adjusted lung function and lung function decline is presented in table 2. In both men and women FEV1 decline was higher in the Northern European region and among current smokers (Table 2).
Table 1. Characteristics of the study population of the ECRHS and SAPALDIA cohorts, by European region.
| European Regions | All | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Southern | Central | Northern | Western | |||||||
| N = 1517 | N = 6530 | N = 3089 | N = 1726 | N = 12862 | ||||||
| Socio-demographic factors† | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| Age, 2nd survey (yrs.) | 42 | 7 | 51 | 11 | 43 | 7 | 44 | 7 | 47 | 10 |
| BMI, 2nd survey (kg/m2) | 27 | 5 | 26 | 4 | 26 | 4 | 26 | 5 | 26 | 4 |
| Age at highest (yrs.) educational degree | 19 | 5 | 20 | 4 | 23 | 6 | 19 | 4 | 20 | 5 |
| Pack years, 2nd survey | ||||||||||
| in all | 15 | 20 | 11 | 19 | 9 | 14 | 10 | 17 | 11 | 18 |
| in current smokers | 25 | 20 | 23 | 21 | 19 | 16 | 24 | 20 | 22 | 20 |
| Early life factors† | no. | % | no. | % | no. | % | no. | % | no. | % |
| Season of birth (winter) | 395 | 26 | 1624 | 25 | 777 | 25 | 422 | 24 | 3218 | 25 |
| Maternal age (>31 yrs.) | 546 | 36 | 2023 | 31 | 871 | 28 | 474 | 27 | 3914 | 30 |
| Maternal smoking | 60 | 4 | 833 | 13 | 982 | 32 | 511 | 30 | 2386 | 19 |
| Paternal smoking | 1032 | 68 | 3716 | 57 | 1835 | 59 | 1158 | 67 | 7741 | 60 |
| Severe respiratory infection | 124 | 8 | 560 | 9 | 364 | 12 | 156 | 9 | 1204 | 9 |
| Urban living environment | 488 | 32 | 1972 | 30 | 1550 | 50 | 694 | 40 | 4704 | 37 |
| Sharing bedroom | 784 | 52 | 1728 | 27 | 917 | 30 | 950 | 55 | 4379 | 34 |
| Daycare attendance | 730 | 48 | 3029 | 46 | 1422 | 46 | 692 | 40 | 5873 | 46 |
| Family pet (< 5 yrs.) | 916 | 60 | 2880 | 44 | 1134 | 37 | 592 | 34 | 5522 | 43 |
| Older siblings | 993 | 65.5 | 3942 | 61 | 1747 | 57 | 1040 | 60 | 7722 | 60 |
| Younger siblings | 987 | 65 | 4012 | 62 | 1769 | 57 | 1035 | 60 | 7803 | 61 |
| Asthma† | ||||||||||
| Childhood asthma | 49 | 3 | 190 | 3 | 155 | 5 | 107 | 6 | 501 | 4 |
| Adult asthma | 116 | 8 | 213 | 3 | 258 | 9 | 150 | 10 | 737 | 6 |
| Paternal asthma | 117 | 8 | 409 | 6 | 181 | 6 | 88 | 5 | 795 | 6 |
| Maternal asthma | 93 | 6 | 310 | 5 | 235 | 8 | 107 | 6 | 745 | 6 |
| Sibling asthma | 199 | 13 | 760 | 12 | 263 | 9 | 186 | 11 | 1408 | 11 |
† Differences across European regions tested by ANOVA or Chi2 as appropriate, all p<0.001 with exception of season of birth (p-value = 0.7)
Table 2. Age and height adjusted FEV1 and FEV1 decline per follow-up year (ΔFEV1/yr.) in women and men, by European region and by smoking status.
| Adjusted FEV1† (ml) | s.e. | Adjusted FEV1 decline/year‡ (ml) | s.e. | |
|---|---|---|---|---|
| Women | ||||
| Southern European Region (N = 750) | 2886 | 15 | -25.2 | -1.1 |
| Central European Region (N = 3407) | 2848 | 7 | -27.4 | -0.6 |
| Northern European Region (N = 1578) | 2871 | 10 | -30.5 | -0.8 |
| Western European Region (N = 914) | 2856 | 13 | -26.9 | -1.0 |
| Never smoker (N = 4959) § | 2874 | 6 | -26.9 | -0.4 |
| Current smoker (N = 1690) § | 2816 | 11 | -30.4 | -0.7 |
| Men | ||||
| Southern European Region (N = 767) | 3886 | 22 | -34.2 | -1.4 |
| Central European Region (N = 3123) | 3876 | 11 | -34.7 | -0.8 |
| Northern European Region (N = 1511) | 3872 | 15 | -39.6 | -1.1 |
| Western European Region (N = 812) | 3867 | 20 | -35.8 | -1.3 |
| Never smoker (N = 4341) § | 3916 | 9 | -34.9 | -0.5 |
| Current smoker (N = 1872) § | 3781 | 14 | -38.3 | -0.8 |
s.e. standard error; † age and height adjusted FEV1 at 2nd survey;
‡ age and height adjusted difference in FEV1 (ml) per year of follow up;
§ additionally adjusted for European regions (fixed effect)
The associations of early life factors with adult FEV1 decline, analysed with mixed-effects linear regression analyses and mutual adjustment for all the early life factors, are presented in Table 3. The lung function decline was significantly more rapid in participants who were born during the winter season, to mothers >31 years at delivery, with ≥2 younger siblings or exposed to maternal smoking. Significantly less rapid lung function decline was observed in persons who had attended day care in childhood and was indicated for early life pet exposure (Table 3).
Table 3. The associations of early life factors with lung function decline (mutually adjusted models).
| Early life factors | Adjusted difference inFEV1 decline † | ||||
|---|---|---|---|---|---|
| in ml ‡ | 95% CI | p-value | |||
| Season of birth | (winter vs. other) | -2.04 | -3.29 | -0.80 | 0.001 |
| Maternal age | (>31 vs <31 yrs.) | -1.82 | -3.14 | -0.49 | 0.007 |
| Maternal smoking | (yes vs. no) | -1.82 | -3.30 | -0.34 | 0.016 |
| Paternal smoking | (yes vs. no) | 0.56 | -0.57 | 1.69 | 0.332 |
| Severe respiratory infection | (yes vs. no) | -0.57 | -2.42 | 1.29 | 0.549 |
| Urban living environment | (urban vs. rural) | 0.62 | -0.84 | 2.08 | 0.408 |
| Daycare attendance | (yes vs. no) | 3.98 | 2.78 | 5.18 | 0.000 |
| Sharing bedroom | (yes vs. no) | -0.42 | -1.57 | 0.74 | 0.481 |
| Family pet (<5 years) | (yes vs. no) | 0.97 | -0.16 | 2.09 | 0.091 |
| Older siblings ≥2 | (≥2 vs. <2) | 0.56 | -1.00 | 2.12 | 0.479 |
| Younger siblings <2 | (<2 vs. ≥2) | -2.61 | -3.85 | -1.38 | 0.000 |
† Change in FEV1 (ml) by follow up year—a negative coefficient implies more rapid FEV1 decline and a positive coefficient implies less rapid decline.
‡ Estimates from mixed-effects linear regression, mutually adjusted for all other early life factors investigated, and for sex, mid age, mid age square, mid BMI, change in BMI (between survey 1 and 2), height, pack years smoked, age at highest education, European region (random effect)
CI = Confidence Interval
Sensitivity analyses excluding asthmatic participants (S4 Table) and adjusting for reported asthma at any age (S5 Table) showed consistent results.
Analyses stratified by sex yielded consistent results in men and women, with the exception of parental smoking and respiratory infections (S2 Table, S2 Fig): Differential effects were observed for maternal smoking by sex, with higher effect estimates in men (ΔFEV1/yr. -2.23 ml [-5.11 to -0.33]) than in women (-0.57 ml [-2.34 to 1.20], pheterogeity = 0.08). In non-smokers, respiratory infections were more strongly associated with FEV1 decline in men (pheterogeity = 0.053). Separate analysis for synergistic effects between adverse early life factors and adult smoking showed that maternal smoking and higher maternal age were more strongly associated with FEV1 decline among current smokers than among never-smokers (S3 Table). In participants exposed to both early life factors we observed a significantly larger effect estimates compared to a single exposure (Table 4). The synergistic effects were larger in men than in women (higher maternal age & smoking -4.45 ml [-7.04 to -1.85] vs. (-3.98 ml [-6.80 to -1.14]; maternal smoking& smoking -5.87 [-10.01 to -1.7] vs. -3.17 [-6.39 to -0.08]). Further stratified analyses by sex yielded a significant interaction between maternal age and current smoking status among men (pinteraction = 0.023) but not in women. Synergistic effects were not observed for season of birth or younger siblings.
Table 4. Synergistic effects of early life factors and participants’ current smoking status with regard to lung function decline.
| Adjusted difference in FEV1 decline† | |||||
|---|---|---|---|---|---|
| Early life exposure | in ml ‡ | CI 95% | p-value | ||
| maternal age >31 yrs. | participant smoking | ||||
| No | No | Ref. | |||
| Yes | No | -0.44 | -1.92 | 1.04 | 0.559 |
| No | Yes | -1.21 | -2.87 | 0.44 | 0.150 |
| Yes | Yes | -4.20 | -6.44 | -1.97 | 0.000 |
| maternal smoking | participant smoking | ||||
| No | No | Ref. | |||
| Yes | No | -1.75 | -3.55 | 0.04 | 0.056 |
| No | Yes | -1.82 | -3.38 | -0.25 | 0.023 |
| Yes | Yes | -4.44 | -7.04 | -1.85 | 0.001 |
† Change in FEV1 (ml) by follow up year—a negative coefficient implies more rapid FEV1 decline and a positive coefficient implies less rapid decline.
‡ Estimates from mixed-effects linear regression, mutually adjusted for all other early life factors and for sex, mid age, mid age square, mid BMI, change in BMI (between survey 1 and 2), height, age at highest education, pack-years, European region (random effect).
CI = Confidence Interval
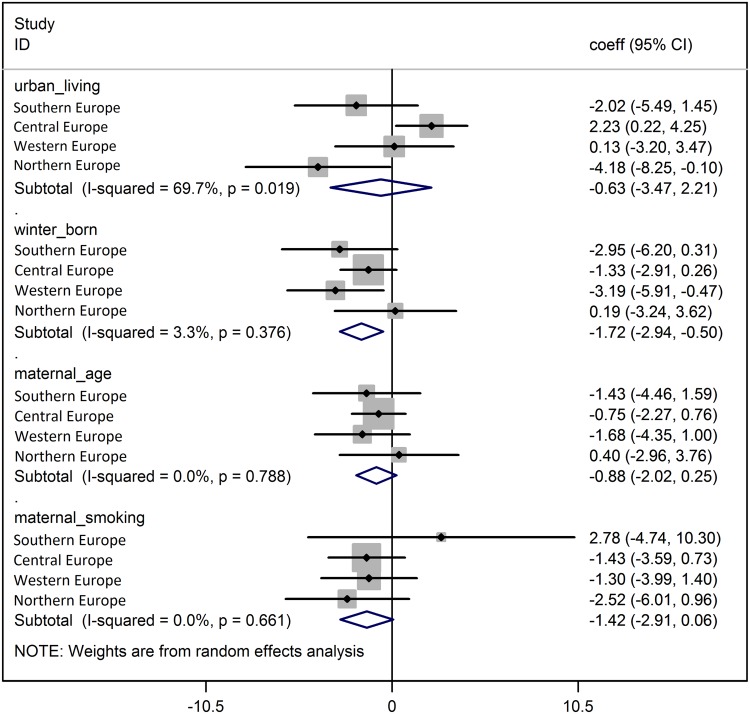
Meta-analyses showed that the associations of early life factors with FEV1 decline were generally consistent across European regions (adjusted for study centres within each region) (Fig 1, S2 Fig). Only the association with urban living environment showed a significant heterogeneity, with protective effects in Central Europe whereas adverse effects were present in Western and Southern Europe (heterogeneity p-value 0.016, Fig 1).
Fig 1. Meta-analyses across European regions: selected early life factors and FEV1 decline.
ΔFEV1/yr. corresponds to change in FEV1 (ml) per year of follow-up—a negative coefficient implies more rapid FEV1 decline and a positive coefficient implies less rapid decline Meta-Analyses by European region, mutually adjusted for all other early life factors investigated and for sex, mid age, mid age square, mid BMI, change in BMI (between survey 1 and 2), height, lifetime pack years smoked, age at highest education, study area (random effect).
Discussion
In two European multi-centre cohort studies, including 12862 persons aged 28–73 at follow-up and relying on comparable methodology, we found that early life factors predicted lung function decline into old age, suggesting that lung ageing is programmed early in life. Our analyses show a substantial impact on lung function decline by early life factors, ranging from adverse effects almost as large as the effects of personal smoking, to “protective” effects of similar magnitude for having older siblings. The early life impact was independent of participant’s lifetime asthma status. A more rapid lung function decline was associated with season of birth, higher maternal age at delivery, maternal smoking and the presence of younger siblings, whereas early day-care attendance, older siblings and childhood pet keeping appeared to be associated with a less rapid decline. One must consider, that these early life factors may reflect underlying mechanisms, [34] having led to or potentiated the observed lung function decline. The main findings were consistent across European regions with the exception of urban living environment. There was some evidence of a differential impact of early life factors by sex, suggesting that early life programming may be related to gender. Accelerated decline related to adverse early life factors was more pronounced among current smokers than never-smokers, possibly pointing to an increased susceptibility to smoking in subjects exposed to higher maternal age and maternal smoking.
This is the first study to investigate early life impact on lung function decline into old age, and to address the hypothesis on interaction with adult insults like smoking. Our findings agree with some of the earlier studies showing association between early life environment, e.g. maternal smoking, and lung function decline in younger adults.[6, 21, 26, 27]. We did not observe a significant effect for respiratory disease on lung function decline, neither did Svanes et al.[21] or Marossy et al.,[25] although early infections have been associated with lung growth and function.[15] In our study, non-differential misclassification by retrospective recall or different severity of childhood disease may explain the null-finding. Such imprecision would most likely attenuate the true association.
Among the novel findings is the association of lung function decline with season of birth. This association was strong and consistent between European regions. Participants born in winter months had a more rapid decline in lung function. Being born in winter has been related to in-utero exposures to viral infections or allergens and to a higher frequency of respiratory infections in the first months of life,[35] both likely major influences on the subsequent establishment of immune response.[36] Low maternal vitamin D levels, more frequent in winter season, have also been discussed as possible factor influencing childhood wheeze and asthma.[37] Season of birth cannot be separated, however, from season of conception and pregnancy. Our finding that season of birth is somehow related to early life programming of lung ageing should fuel further research into underlying mechanisms.
We found a more pronounced lung function decline in participants born to older mothers. Maternal age at delivery reflects biological ageing of the mother as well as sociocultural characteristics. Pregnancy complications [38] and Caesarean section are more common in older mothers, and may be of importance for immunological functions in infants [39] and beyond [40]. A birth cohort effect may also play a role, as participants born between 1930 and 1950 were more often born to older mothers than younger participants (data not shown). Our result conflict with earlier publications,[41, 42] but are in line with a recent study by Caudri et al. observing a differential impact of maternal age depending on the wheeze phenotypes, implying older age to be associated with late onset wheeze. [43] We also observed that maternal smoking is a significant predictor for accelerated lung function decline even in older age. Long-term impact of maternal smoking have previously been shown with regard to asthma [5] and lung function in young to mid-age adults.[21, 26, 27] Inflammatory pathways are believed to be central; however, many open questions remain regarding the underlying mechanisms.[26, 34] In our cohorts lung function decline was more pronounced in smokers who in addition were exposed to either maternal smoking or higher maternal age. This indicates that susceptibility to later adult insults might be programmed early in life. A synergistic effect of parental smoking has been described by Guerra et al. [26] who found a steeper decline of lung function in young adults exposed to both parental and individual smoking. Upton et al. showed that the effect on airflow limitation by 10 cigarettes/day maternal smoking was numerically equivalent to 10 years active smoking. [27] The importance of the early life environment for susceptibility to adult insults has already been discussed by Ramsey et al., who found an additive influence on FEV1 decline by parental and individual socio-economic position. Previous findings on synergistic effects support our novel finding on an increased susceptibility to smoking in subjects born to older mothers. Our data further support some heterogeneity by gender: we found a stronger overall impact of maternal age and current smoking in men as compared to women, even after adjustment for pack years. There are several known mechanisms which may explain different susceptibility by sex to life-time exposures, [13, 44, 45] and several studies have shown a higher respiratory vulnerability in men. [5, 46–48] The concept of increased vulnerability at certain time-points in life is also underlying the analyses of both older and younger siblings, representing an early and a later prolonged exposure to a variety of microbes. While the effect of older siblings was non-significant, we found an independent and adverse effect of the presence of younger siblings. The analytic approach and the result is supported by a publication by Svanes et al. that found a U-shaped association between number of siblings and adult asthma,[23] and a recent publication by Grabenhenrich et al. also observing a U-form association between asthma and age at entering day care.[42]
Day care attendance was related to less rapid lung function decline, independent of gender or smoking status, and similar “protective effects” were indicated for childhood pet keeping and having older siblings. This is the first analysis of lung function decline in relation to these early life exposures. Our findings are in agreement with previous research in younger populations and other respiratory health outcomes, in particular allergic diseases.[49] The data imply that these factors, generally discussed in view of the hygiene hypothesis,[50] can induce long-term modifications of the immune system.
While a study within ECRHS showed that some childhood exposures are reported with high consistency, irrespective of respiratory health status,[51] residual confounding due to recall bias cannot be excluded. All factors except maternal age were recorded at baseline, prior to measurement of lung function at baseline and at follow-up. The retrospective reporting of these factors might have led to imprecise recall, which is likely to have attenuated true associations. It is, however, unlikely that recall would be differential with regard to the outcome of the present analysis—the difference between two objectively measured lung function values. It is also unlikely that recall bias should be uniform across different cohorts and European countries.
The consistent results across European regions suggest that the associations may be due to homogeneous biological mechanisms rather than heterogeneous socio-cultural differences. Heterogeneity between regions was only found for the impact of urban living environment. We observed a significant protective effect on lung function decline by urbanity in Central Europe, while the opposite was found in Western and Southern Europe. Urbanity can be considered a proxy for opposite health determinants, e.g. higher pollutant exposure versus better access to health care. Our data did not allow investigating the latent construct urbanity any further.
With respect to parental smoking, we could not assess how much parents have smoked and for how long. This limitation possibly reduced the power of our study, since one would assume that the synergy would increase with higher doses of exposure in childhood.[27] Residual confounding is also possible due to a lack of information on further potentially influencing factors e.g. nutrition, air pollution exposure in childhood or birth characteristics. Individuals with particularly poor early life development could possibly be more or less likely to participate in the cohort, thus, the prevalence of early life factors might not reflect the true population prevalence. In view of our study hypothesis, this bias would limit the generalizability of our study, but not produce spurious associations. As some study participants were younger than 25 years at the first survey, late lung growth may have confounded the analyses. However, excluding these subjects resulted in consistent associations. The analytical strategy, including all factors in mutually adjusted analyses, may have introduced over-adjustment, potentially reducing the power to identify less strong direct associations.
Investigating early life factors and potential interactions demands large study populations, which was achieved by combining the cohorts SAPALDIA and ECRHS. We had the power to study even potentially small effects of early life factors and to identify sub-groups at risk, given the inclusion of persons in the 7th decade with the advantage of rich information about declining lung function in this aging cohort. This multinational study population constitutes both strengths and limitations. On one hand, our homogenous results across the European regions underline the importance of early life environment in Europe. On the other hand regional differences in the effects of childhood factors may attenuate the overall effects when adjusted for regions and regional specific risks may not have been picked up. The results cannot be generalized to countries where the investigated childhood factors, such as day care, must be considered proxies of considerably different exposures.
In conclusion, our results showed that lung function decline into old age were predicted by early life factors. This novel finding supports the hypothesis that lung ageing, which is captured by lung function decline, is programmed early in life. We further found that lung function decline was more rapid in those with early life disadvantages who subsequently smoked, suggesting that altered individual susceptibility to adult insults could be one possible pathway for persisting effects of early life factors decades later. Interestingly, lung function was found to decline more rapidly in people born during the winter season, consistently across European regions; this effect merits further investigation. From a public health point of view, early life impact on lung function decline and on susceptibility to adult exposures offers possibilities for intervention, and from a clinical point of view, these findings identify a population at risk for accelerated lung function decline.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
(DOC)
Data Availability
ECRHS and SAPALDIA cohort studies are bound to the local ethical and legal restrictions with respect to study data. At the time of SAPALDIA 1 and 2, and ECRHS I and II providing data publically was not considered and participants were not asked for consent to provide individual data to the public. The data are available on request to scientists. Requests are to be addressed to the study primary investigators (www.ecrhs.org/; www.sapaldia.ch) or via the first and/or last authors of the manuscript.
Funding Statement
The first author was supported by a Marie Heim-Vögtlin grant from the Swiss National Science Foundation (grant # PMPDP3_129021/1; # PMPDP3_141671/1), the Lung league Beider Basel, Lung league Graubünden, the Stiftung ehemals Bündner Heilstätten and the COST action BM1201. Research support received by the SAPALDIA and ECRHS cohorts can be found in the online supplement (e-supplement). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Apostol GG, Jacobs DR Jr., Tsai AW, Crow RS, Williams OD, Townsend MC, et al. Early Life Factors Contribute to the Decrease in Lung Function between Ages 18 and 40: The Coronary Artery Risk Development in Young Adults Study. Am J Respir Crit Care Med. 2002;166(2):166–72. 10.1164/rccm.2007035 [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. Bmj. 1991;303(6804):671–5. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.London SJ, James Gauderman W, Avol E, Rappaport EB, Peters JM. Family history and the risk of early-onset persistent, early-onset transient, and late-onset asthma. Epidemiology. 2001;12(5):577–83. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez FD. The Origins of Asthma and Chronic Obstructive Pulmonary Disease in Early Life. Proceedings of the American Thoracic Society. 2009;6(3):272–7. 10.1513/pats.200808-092RM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svanes C, Omenaas E, Jarvis D, Chinn S, Gulsvik A, Burney P. Parental smoking in childhood and adult obstructive lung disease: results from the European Community Respiratory Health Survey. Thorax. 2004;59(4):295–302. 10.1136/thx.2003.009746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramsay SE, Whincup PH, Lennon LT, Morris RW, Wannamethee SG. Longitudinal associations of socioeconomic position in childhood and adulthood with decline in lung function over 20 years: results from a population-based cohort of British men. Thorax. 2011;66(12):1058–64. 10.1136/thoraxjnl-2011-200621 [DOI] [PubMed] [Google Scholar]
- 7.Palmer DJ, Huang R-C, Craig JM, Prescott SL. Nutritional Influences on Epigenetic Programming: Asthma, Allergy, and Obesity. Immunology and Allergy Clinics of North America. 2014;34(4):825–37. 10.1016/j.iac.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 8.Carlsen K-Hk, Carlsen KCLd. Respiratory effects of tobacco smoking on infants and young children. Paediatric Respiratory Reviews. 2008;9(1):11–20. 10.1016/j.prrv.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 9.Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015;70(3):245–56. 10.1111/all.12561 . [DOI] [PubMed] [Google Scholar]
- 10.Tennant PW, Gibson GJ, Parker L, Pearce MS. Childhood respiratory illness and lung function at ages 14 and 50 years: childhood respiratory illness and lung function. Chest. 2009;137(1):146–55. 10.1378/chest.09-0352 [DOI] [PubMed] [Google Scholar]
- 11.von Mutius E. Gene-environment interactions in asthma. The Journal of allergy and clinical immunology. 2009;123(1):3–11; quiz 2–3. 10.1016/j.jaci.2008.10.046 [DOI] [PubMed] [Google Scholar]
- 12.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li ZW, Tomfohr J, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118(10):3462–9. 10.1172/Jci34378 WOS:000259828600027. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Krauss-Etschmann S, Bush A, Bellusci S, Brusselle GG, Dahlen SE, Dehmel S, et al. Of flies, mice and men: a systematic approach to understanding the early life origins of chronic lung disease. Thorax. 2013;68(4):380–4. 10.1136/thoraxjnl-2012-201902 . [DOI] [PubMed] [Google Scholar]
- 14.Krauss-Etschmann S, Bush A, Bellusci S, Brusselle GG, Dahlen SE, Dehmel S, et al. Of flies, mice and men: a systematic approach to understanding the early life origins of chronic lung disease. Thorax. 2012. 10.1136/thoraxjnl-2012-201902 [DOI] [PubMed] [Google Scholar]
- 15.Stocks J, Sonnappa S. Early life influences on the development of chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2013;7(3):161–73. 10.1177/1753465813479428 WOS:000336023700004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thannickal VJ, Murthy M, Balch WE, Chandel NS, Meiners S, Eickelberg O, et al. Blue Journal Conference. Aging and Susceptibility to Lung Disease. American Journal of Respiratory and Critical Care Medicine. 2015;191(3):261–9. 10.1164/rccm.201410-1876PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinn S, Jarvis D, Melotti R, Luczynska C, Ackermann-Liebrich U, Anto JM. Smoking cessation, lung function, and weight gain: a follow-up study. Lancet. 2005;365:1629–35. 10.1016/S0140-6736(05)66511-7 [DOI] [PubMed] [Google Scholar]
- 18.Petersen H, Sood A, Meek PM, Shen X, Cheng Y, Belinsky SA, et al. RApid lung function decline in smokers is a risk factor for copd and is attenuated by angiotensin-converting enzyme inhibitor use. CHEST Journal. 2014;145(4):695–703. 10.1378/chest.13-0799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pistelli F, Bottai M, Carrozzi L, Pede FD, Baldacci S, Maio S, et al. Changes in obesity status and lung function decline in a general population sample. Respiratory Medicine. 2008;102(5):674–80. 10.1016/j.rmed.2007.12.022 [DOI] [PubMed] [Google Scholar]
- 20.de Marco R, Accordini S, Marcon A, Cerveri I, Anto JM, Gislason T, et al. Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med. 2013;183(7):891–7. . [DOI] [PubMed] [Google Scholar]
- 21.Svanes C, Sunyer J, Plana E, Dharmage S, Heinrich J, Jarvis D, et al. Early life origins of chronic obstructive pulmonary disease. Thorax. 2009:thx.2008.112136. 10.1136/thx.2008.112136 [DOI] [PubMed] [Google Scholar]
- 22.Svanes C, Heinrich J, Jarvis D, Chinn S, Omenaas E, Gulsvik A, et al. Pet-keeping in childhood and adult asthma and hay fever: European community respiratory health survey. The Journal of allergy and clinical immunology. 2003;112(2):289–300. . [DOI] [PubMed] [Google Scholar]
- 23.Svanes C, Jarvis D, Chinn S, Omenaas E, Gulsvik A, Burney P, et al. Early exposure to children in family and day care as related to adult asthma and hay fever: results from the European Community Respiratory Health Survey. Thorax. 2002;57(11):945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson B, Kubzansky LD, Cohen S, Weiss S, Wright RJ. A matter of life and breath: childhood socioeconomic status is related to young adult pulmonary function in the CARDIA study. International journal of epidemiology. 2004;33(2):271–8. 10.1093/ije/dyh003 [DOI] [PubMed] [Google Scholar]
- 25.Marossy AE, Strachan DP, Rudnicka AR, Anderson HR. Childhood chest illness and the rate of decline of adult lung function between ages 35 and 45 years. Am J Respir Crit Care Med. 2007;175(4):355–9. 10.1164/rccm.200607-1023OC . [DOI] [PubMed] [Google Scholar]
- 26.Guerra S, Stern DA, Zhou M, Sherrill DL, Wright AL, Morgan WJ, et al. Combined effects of parental and active smoking on early lung function deficits: a prospective study from birth to age 26 years. Thorax. 2013;68(11):1021–8. 10.1136/thoraxjnl-2013-203538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upton MN, Smith GD, McConnachie A, Hart CL, Watt GCM. Maternal and Personal Cigarette Smoking Synergize to Increase Airflow Limitation in Adults. American Journal of Respiratory and Critical Care Medicine. 2004;169(4):479–87. 10.1164/rccm.200211-1357OC [DOI] [PubMed] [Google Scholar]
- 28.Svanes O, Skorge TD, Johannessen A, Bertelsen RJ, Bratveit M, Forsberg B, et al. Respiratory Health in Cleaners in Northern Europe: Is Susceptibility Established in Early Life? PloS one. 2015;10(7):e0131959 10.1371/journal.pone.0131959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ackermann-Liebrich U, Kuna-Dibbert B, Probst-Hensch NM, Schindler C, Felber Dietrich D, Stutz EZ, et al. Follow-up of the Swiss Cohort Study on Air Pollution and Lung Diseases in Adults (SAPALDIA 2) 1991–2003: methods and characterization of participants. Soz Praventivmed. 2005;50(4):245–63. . [DOI] [PubMed] [Google Scholar]
- 30.Burney P, Luczynska C, Chinn S, Jarvis D. The European Community Respiratory Health Survey. Eur Respir J. 1994;7(5):954–60. [DOI] [PubMed] [Google Scholar]
- 31.American Thoracic Society Nal. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–36. 10.1164/ajrccm.152.3.7663792 . [DOI] [PubMed] [Google Scholar]
- 32.Kunzli N, Kuna-Dibbert B, Keidel D, Keller R, Brandli O, Schindler C, et al. Longitudinal validity of spirometers—a challenge in longitudinal studies. Swiss Med Wkly. 2005;135(33–34):503–8. . [DOI] [PubMed] [Google Scholar]
- 33.Global Initiatve for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD 2013 update, accessed May 2013.
- 34.Carraro S, Scheltema N, Bont L, Baraldi E. Early-life origins of chronic respiratory diseases: understanding and promoting healthy ageing. The European respiratory journal. 2014;44(6):1682–96. 10.1183/09031936.00084114 . [DOI] [PubMed] [Google Scholar]
- 35.Gazala E, Ron-Feldman V, Alterman M, Kama S, Novack L. The association between birth season and future development of childhood asthma. Pediatric Pulmonology. 2006;41(12):1125–8. [DOI] [PubMed] [Google Scholar]
- 36.Gold DR, Bloomberg GR, Cruikshank WW, Visness CM, Schwarz J, Kattan M, et al. Parental characteristics, somatic fetal growth, and season of birth influence innate and adaptive cord blood cytokine responses. Journal of Allergy and Clinical Immunology. 2009;124(5):1078–87. 10.1016/j.jaci.2009.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ginde AA, Sutherland ER. Vitamin D in asthma: Panacea or true promise? Journal of Allergy and Clinical Immunology. 2010;126(1):59–60. 10.1016/j.jaci.2010.05.030 [DOI] [PubMed] [Google Scholar]
- 38.Cleary-Goldman J, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, et al. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 Pt 1):983–90. . [DOI] [PubMed] [Google Scholar]
- 39.Magnus MC, Håberg SE, Stigum H, Nafstad P, London SJ, Vangen S, et al. Delivery by Cesarean Section and Early Childhood Respiratory Symptoms and Disorders. American Journal of Epidemiology. 174(11):1275–85. 10.1093/aje/kwr242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salminen S, Gibson GR, McCartney AL, Isolauri E. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut. 2004;53(9):1388–9. 10.1136/gut.2004.041640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherriff A, Peters TJ, Henderson J, Strachan D, Team aTAS. Risk factor associations with wheezing patterns in children followed longitudinally from birth to 3 ½ years. International journal of epidemiology. 2001;30(6):1473–84. 10.1093/ije/30.6.1473 [DOI] [PubMed] [Google Scholar]
- 42.Grabenhenrich LB, Gough H, Reich A, Eckers N, Zepp F, Nitsche O, et al. Early-life determinants of asthma from birth to age 20 years: A German birth cohort study. Journal of Allergy and Clinical Immunology. (0). 10.1016/j.jaci.2013.11.035 [DOI] [PubMed] [Google Scholar]
- 43.Caudri D, Savenije OEM, Smit HA, Postma DS, Koppelman GH, Wijga AH, et al. Perinatal risk factors for wheezing phenotypes in the first 8 years of life. Clinical & Experimental Allergy. 2013;43(12):1395–405. 10.1111/cea.12173 [DOI] [PubMed] [Google Scholar]
- 44.Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. 1999;54(12):1119–38. 10.1136/thx.54.12.1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harding R, Maritz G. Maternal and fetal origins of lung disease in adulthood. Semin Fetal Neonatal Med. 2012;17(2):67–72. 10.1016/j.siny.2012.01.005 . [DOI] [PubMed] [Google Scholar]
- 46.Hayatbakhsh MR, Sadasivam S, Mamun AA, Najman JM, Williams GM, O’Callaghan MJ. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax. 2009;64(9):810–4. 10.1136/thx.2009.116301 [DOI] [PubMed] [Google Scholar]
- 47.Dong GH, Chen T, Liu MM, Wang D, Ma YN, Ren WH, et al. Gender Differences and Effect of Air Pollution on Asthma in Children with and without Allergic Predisposition: Northeast Chinese Children Health Study. PloS one. 2011;6(7). ARTN e22470 10.1371/journal.pone.0022470 WOS:000292929500069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong G-H, Chen T, Liu M-M, Wang D, Ma Y-N, Ren W-H, et al. Gender Differences and Effect of Air Pollution on Asthma in Children with and without Allergic Predisposition: Northeast Chinese Children Health Study. PLoS ONE. 2011;6(7):e22470 10.1371/journal.pone.0022470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kozyrskyj AL, Bahreinian S, Azad MB. Early life exposures: impact on asthma and allergic disease. Curr Opin Allergy Clin Immunol. 2011;11(5):400–6. 10.1097/ACI.0b013e328349b166 . [DOI] [PubMed] [Google Scholar]
- 50.Ege MJ, Mayer M, Normand A-Cc, Genuneit J, Cookson WOCM, Braun-Fahrländer C, et al. Exposure to Environmental Microorganisms and Childhood Asthma. New England Journal of Medicine. 2011;364(8):701–9. 10.1056/NEJMoa1007302 [DOI] [PubMed] [Google Scholar]
- 51.Svanes C, Dharmage S, Sunyer J, Zock JP, Norback D, Wjst M, et al. Long-term reliability in reporting of childhood pets by adults interviewed twice, 9 years apart. Results from the European Community Respiratory Health Survey I and II. Indoor Air. 2008;18(2):84–92. 10.1111/j.1600-0668.2008.00523.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
(DOC)
Data Availability Statement
ECRHS and SAPALDIA cohort studies are bound to the local ethical and legal restrictions with respect to study data. At the time of SAPALDIA 1 and 2, and ECRHS I and II providing data publically was not considered and participants were not asked for consent to provide individual data to the public. The data are available on request to scientists. Requests are to be addressed to the study primary investigators (www.ecrhs.org/; www.sapaldia.ch) or via the first and/or last authors of the manuscript.