Abstract
Extracellular vesicles (EV) awakened interest in the research on mesenchymal stromal cells by exploring their paracrine effects. However, many isolation protocols use bovine serum albumin (BSA) during the preparation of the EV. Therefore, we produced ‘sham’ BSA-EV and tested them in comparison to EV derived from mesenchymal stromal cells (MSC-EV). We found that BSA-EV did not express MSC-specific surface markers like CD29 and CD90. However, they were capable of reducing serum-starvation induced apoptosis in vitro in a kidney epithelial cell line to a similar extent as MSC-EV as measured by Annexin V/7-Aminoactinomycin D labeling and flow cytometry.
Keywords: Mesenchymal stromal cells, Exosomes, Serum albumin
Research on extracellular vesicles (EV), including microvesicles and exosomes, has expanded widely in the last several years because of their prominent diagnostic and therapeutic potential in diverse fields of medicine[1]. Many cell types were found to release EVs, including tumor cells, dendritic cells, stem cells and progenitor cells. Especially for mesenchymal stromal cells, the EVs were investigated to provide answers regarding the mechanisms of how the cells have mediated functional effects in tissue repair and immuno-modulation[2,3].
Many of the EV isolation protocols involve the use of 0.5% bovine serum albumin (BSA)[4] or 1% human serum albumin (HSA)[5] during the time when otherwise serum-free media is applied to the cells to encourage the release of vesicles. The rationale behind this step is to prevent cell death and thereby reduce the amount of cell debris and apoptotic bodies that might be released into the conditioned media. However, it is known that bovine serum from which albumin is derived contains secreted vesicles under basal conditions that can have functional effects[6,7] and that increased concentrations of EVs have been measured under stressful conditions[8]. Other groups have shown functional results using EVs isolated from culture media that contained 10% fetal calf serum (FCS) where the serum was not sufficiently treated to ensure exosome clearance[9].
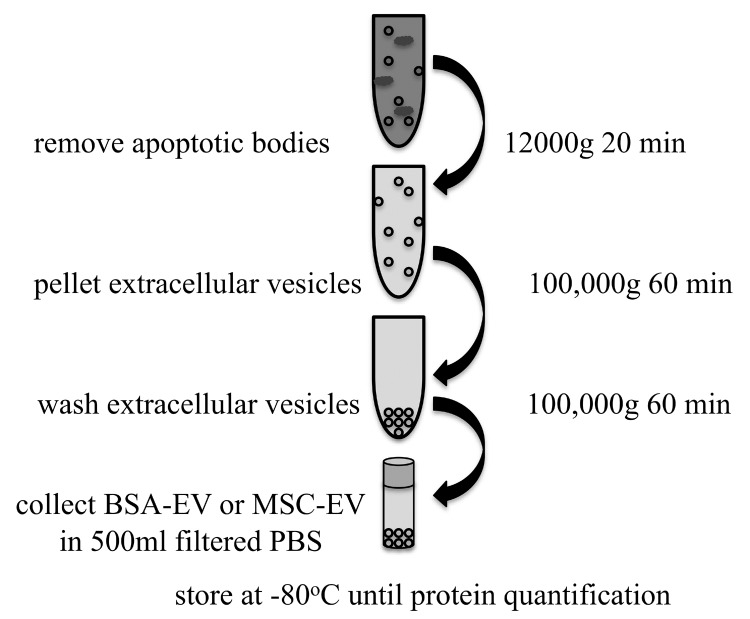
The addition of serum albumin, therefore, introduces two complications to the study of MSC vesicles. One, that the albumin may still contain native vesicles, and the second, that the protein quantification of vesicles must account for the added protein of the albumin. We tried to address this critical issue by producing “sham” vesicles made by applying the isolation procedure for EVs from MSCs to the BSA that would normally be added to the medium and called these “sham produced vesicles” BSA-EV. The complete procedure is shown in Figure 1. Briefly, a 4-hour ultracentrifugation of 7.5% BSA (Sigma A8412) at 110,000g at 4°C was performed to reduce native vesicles from the commercially prepared BSA. To mimic the amount of BSA that would be present in 200 ml conditioned media with 0.5% BSA, 13.2 ml of this ultra-centrifuged 7.5% BSA was then diluted with phosphate-buffered saline (PBS) (previously filtered through 0.1 µm pores) filling one ultracentrifuge tube to approximately 36 ml. The other 5 tubes were used to isolate vesicles from serum-free media with 0.5% ultra-centrifuged BSA added which was conditioned by 80% confluent umbilical cord MSC for 20 hours (MSC-EV). The conditioned media was already centrifuged at 2000g for 20 min at 4°C to remove cell debris before ultracentrifugation. After a centrifugation step at 12000g for 20 min at 4°C to remove apoptotic bodies, MSC-EV or BSA-EV were pelleted using centrifugation at 100,000g for 1 hour. The supernatant was removed down to 1 ml, pellets washed with 35 ml of 0.1 µm filtered PBS, and the 5 MSC-EV pellets were combined into one tube. Following another centrifugation at 100,000g for 1 hour at 4°C, the pellet of either MSC-EV or BSA-EV was resuspended in approximately 500 µl filtered PBS, aliquoted into cryotubes and frozen at -80°C until use. Protein quantification was performed using a bicinchoninic acid (BCA) protein assay kit applying the microplate protocol provided.
Figure 1.
Schematic of extracellular vesicle (EV) production. Eight T175 culture flasks containing human MSC grown to 80% confluence were given serum-free media with 0.5% ultra-centrifuged BSA for 20 hours and this conditioned media was centrifuged to remove cell debris. An equivalent amount of ultra-centrifuged BSA was diluted with filtered PBS. Both were then separately centrifuged to remove apoptotic bodies before isolating a pellet of extracellular vesicles. The pellet was washed and resuspended in filtered PBS and frozen at -80°C until quantification of total protein content by bicinchoninic acid (BCA) assay.
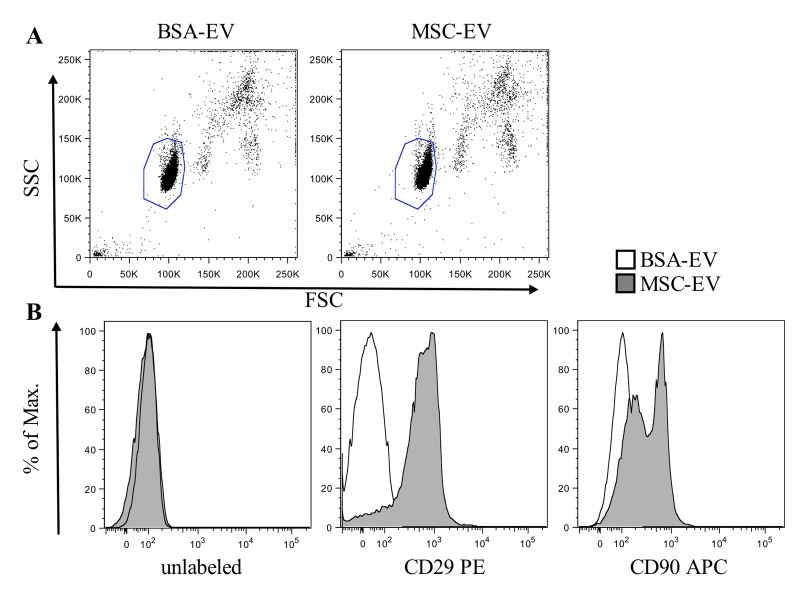
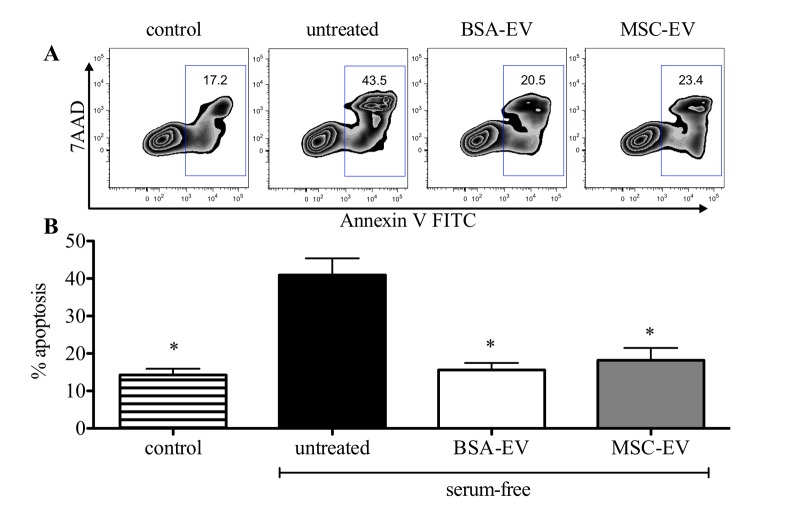
We found that BSA-EV showed a strong protein signal, with 3 different preparations showing a mean protein concentration of 203 ± 40 µg/ml, while MSC-EV contained 319 ± 177 µg/ml. Other investigators have noted difficulty in accurately accessing exosome protein quantities from culture media when control media is not used to account for bovine derived exosomes[10]. Consequently, quantification by total protein measurement, though frequently used, is neither meaningful nor accurate. However, by analyzing MSC-EVs and sham BSA-EVs coupled to 4 µm aldehyde/sulfate latex beads (Figure 2A) for expression of typical MSC surface markers, only MSC derived vesicles showed clear expression of CD90 and CD29 when analysis was performed with a MACSQuant® flow cytometer and FlowJo software (Figure 2B). Despite lacking MSC-EV characteristics, BSA-EVs showed clear protective effects against starvation-induced apoptosis of the rat kidney epithelial cell line NRK-52E in vitro when evaluated with Annexin V/7-Aminoactinomycin D labeling by flow cytometry on a FACSCanto™ II analyzer (Figure 3A). The addition of 20 µg protein from either BSA-EV or MSC-EV led to a statistically significant reduction in Annexin V staining (Figure 3B). Other groups have shown an effect on the degree of apoptosis of a cardiomyocytic cell line under serum-free conditions when conditioned medium containing 1% HSA was used to isolate exosomes from human cardiac progenitor cells[5].
Figure 2.
Only MSC-EV and not BSA-EV coupled to beads show MSC surface markers. Five micrograms of BSA-EV or MSC-EV protein per staining was incubated with 4 µm aldehyde/sulfate latex beads for one hour, then washed and labeled by a 30 minute incubation with mouse anti-human antibodies against CD29 or CD90 and analyzed with a MACSQuant® flow cytometer and FlowJo software. (A) Representative plots of forward against side scatter are shown. (B) Histograms of bead coupled BSA-EV (white) and MSC-EV (grey) are shown unlabeled (left) or when labeled with CD29-PE (middle) or CD90-APC (right) anti-human antibodies.
Figure 3.
MSC-EV but also BSA-EV reduce starvation-induced apoptosis. (A) Apoptosis of NRK-52E cells was evaluated by labeling with Annexin V FITC and 7-Aminoactinomycin D (7AAD) and performing flow cytometry on a FACSC™ anto™ II analyzer. Representative FlowJo FACS plots are shown without induction (control), or when apoptosis was induced with serum-free conditions overnight in the absence (untreated) or presence of 20 µg of extracellular vesicles produced from BSA (BSA-EV) or MSC (MSC-EV). (B) The total percentage of all AnnexinV+ cells was evaluated in 5 experiments with at least duplicate samples. *P<0.05 by ANOVA with Bonferroni post-test following confirmation of normal distribution by the D’Agostino and Pearson omnibus normality test performed using GraphPad Prism software.
Therefore, assays based on serum starvation must account for the effect of serum reconstitution from this added protein.
Our data conclude that the analysis of both quantity and functional effect of MSC-EVs produced by starvation medium containing serum albumin must be tested in comparison to appropriate “sham” vesicle controls to ensure they are based on cell-specific EVs rather than protein contamination. We recommend against using BSA during the isolation procedure to avoid inaccurate results for both the protein quantification and characterization of the physical properties and functional effects of the cell specific EV.
Acknowledgments
We would like to thank the FACS core facility of the BCRT for technical support. Funding
Abbreviations
- 7-AAD:
7-Aminoactinomycin D
- BCA:
Bicinchoninic Acid
- BSA:
Bovine Serum Albumin
- EV:
Extracellular Vesicles
- FCS:
Fetal Calf Serum
- HSA:
Human Serum Albumin
- MSC:
Mesenchymal Stromal Cell
- PBS:
Phosphate-Buffered Saline
Potential Conflicts of Interests
None
Funding Statement
The project was funded by the Bundesministerium für Bildung und Forschung (BMBF) grant 1315848A.
References
- 1.De Jong OG, Van Balkom BW, Schiffelers RM, Bouten CV, Verhaar MC. Extracellular vesicles: potential roles in regenerative medicine. Front Immunol. 2014;5(608) doi: 10.3389/fimmu.2014.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tetta C, Bruno S, Fonsato V, Deregibus MC, Camussi G. The role of microvesicles in tissue repair. Organogenesis. 2011;7(2):105–115. doi: 10.4161/org.7.2.15782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233–1244. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 4.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7(3) doi: 10.1371/journal.pone. e33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103(4):530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 6.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-'t Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shelke GV, Lässer C, Gho YS, Lötvall J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang HC, Liu XB, Huang S, Bi XY, Wang HX, Xie LX, Wang YQ, Cao XF, Lv J, Xiao FJ, Yang Y, Guo ZK. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012;21(18):3289–3297. doi: 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med. 2014;92(4):387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 10.Franquesa M, Hoogduijn MJ, Ripoll E, Luk F, Salih M, Betjes MG, Torras J, Baan CC, Grinyó JM, Merino AM. Update on controls for isolation and quantification methodology of extracellular vesicles derived from adipose tissue mesenchymal stem cells. Front Immunol. 2014;5(525) doi: 10.3389/fimmu.2014.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]