Abstract
We investigated the expression pattern of Dkk-3, a secreted Wnt pathway inhibitor, in mouse intestinal tissue and three-dimensional cultured Caco-2 spheroids. Dkk-3 was expressed at the bottom of crypts from the mouse small intestine. Human colon adenocarcinoma Caco-2 cells expressed Dkk-3 under a semi-confluent condition, but Dkk-3 expression was seen in only some of the Caco-2 cells when the cells were sparse. Caco-2 cells formed hollow spheroids in Matrigel, and the layer-forming cells in the hollow spheroids expressed Dkk-3. Our results demonstrated that Dkk-3 might affect intestinal cells when the fate of stem cells changes.
Keywords: Dkk-3, intestinal tissue, Caco-2 cells
Introduction
Dkk-3, a secreted Wnt pathway inhibitor, was down-regulated in a number of human cancer cell lines and clinical cancer tissues and thus Dkk-3 is known as REIC because of its reduced expression in immortalized cells[1]. Overexpression of Dkk-3 using an adenovirus vector induced apoptosis in a variety of cancer cells and this anti- tumor effect was triggered by ER stress caused by overexpression of Dkk-3[2]. Expression of Dkk-3 was observed in many tissues including the small intestine and colon[3]. Furthermore, we previously revealed that Dkk-3 expression was reduced in normal skin cells in inflammatory conditions and also in skin cancer cells[4]. However, the physiological function of Dkk-3 is still unclear. In the present study, we investigated the expression of Dkk- 3 in mouse intestinal tissue and three-dimensional cultured Caco-2 spheroids.
Results
To screen for Dkk-3 expression in mouse normal tissues, we performed fluorescent immunohistochemistry of Dkk-3 protein using adult mouse tissues. The mouse small intestine showed strong and localized expression (Figure 1A). Interestingly, Dkk-3 expression was seen at the bottom of intestinal crypts (Figure 1B).
Figure 1.
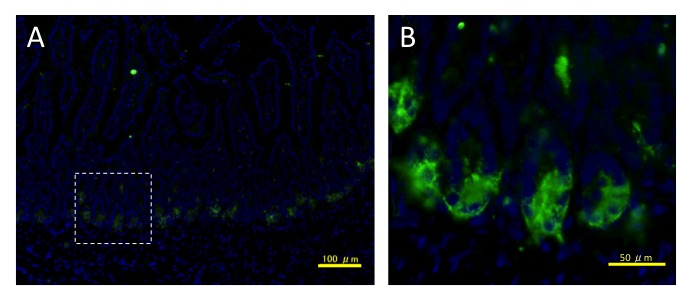
Expression of Dkk-3 in the mouse small intestine. Sections of small intestine tissue were stained for Dkk-3 (in green). Nuclei were stained with DAPI (in blue). Stained sections were observed by a fluorescent microscope at a low magnification (A) or a high magnification (B). The dashed square in A delineates the magnified area in B. Scale bars: 100 µm (A), 50 µm (B).
Next we analyzed the expression pattern of Dkk-3 in human colon adenocarcinoma Caco-2 cells in a monolayer culture condition. Almost all of the Caco-2 cells expressed Dkk-3 under a semi- confluent condition, and the cell membrane showed strong expression (Figure 2A). However, Dkk-3 expression was seen in only some of the Caco-2 cells when the cells were sparse (Figure 2B). Interestingly, Dkk-3 and an intestinal transcription factor, Caudal-related homeobox transcription factor 2 (CDX2), showed reciprocal expression patterns in sparse Caco-2 colonies.
Figure 2.
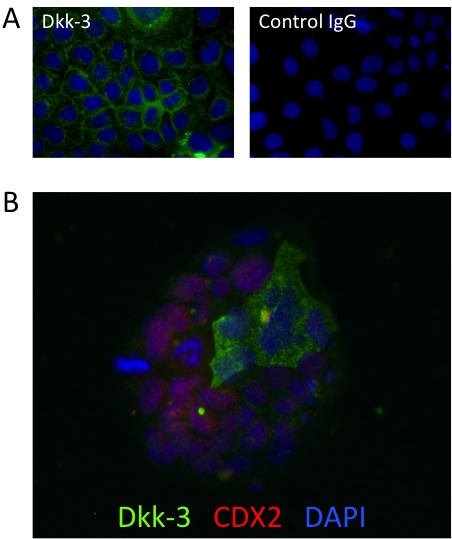
Expression of Dkk-3 in monolayer cultured Caco-2 cells.
Semi-confluent (A) and sparse colony-forming (B) Caco-2 cells were stained for Dkk-3 (in green) and CDX2 (in red). Nuclei were stained with DAPI (in blue). Control IgG was used as a negative control.
We then cultured Caco-2 cells in a three-dimensional condition. Single-cell suspensions of Caco-2 cells were re-suspended in a basement membrane matrix (Matrigel) and cultured in 24-well plates. After cultivation for five days, Caco-2 cells had formed small solid spheres. Then their morphology changed to hollow spheroids after another five days (Figure 3A). Immunocytochemistry experiments demonstrated that Caco-2 cells in the hollow spheroids expressed Dkk-3.
Figure 3.
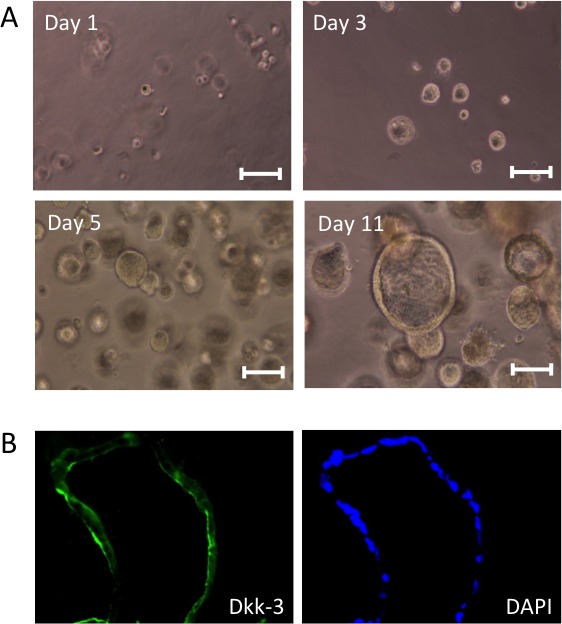
Formation of Caco-2 spheroids and their Dkk-3 expression. (A)
Spheroids formed by Caco-2 cells in Matrigel were observed by a bright field. (B) Sections of the spheroids were stained for Dkk-3 (in green). Nuclei were stained with DAPI (in blue). Scale bars: 100 µm.
Discussion
Based on our previous studies using skin tissue, Dkk-3 expression was localized at the stem cell niche and/or the neighboring cells[5]. Dkk-3 expression was detected in many epithelial tissues including small intestine and colon[3].
In this study, we analyzed Dkk-3 expression in the mouse small intestine and 3D culture Caco-2 spheroids. Expression of Dkk-3 in the small intestine was restricted to the bottom of crypts (Figure 1B). Stem cells of intestinal tissue are located at the bottom of crypts[6]. Dkk family proteins are known as Wnt pathway inhibitors, and Wnt5a signaling was reported to regulate crypt regeneration after tissue injury[7]. The expression of Dkk-3 in the crypts was not in the precise position where the intestinal stem cells have located. However the Dkk-3 expressing cells reside next to the intestinal stem cell niche therefore the results indicate a possible role of Dkk-3 in stem cell function. Interestingly, Dkk-3 was shown to be expressed at the stem cell niche not only in intestinal tissue but also in the skin hair bulge region[5].
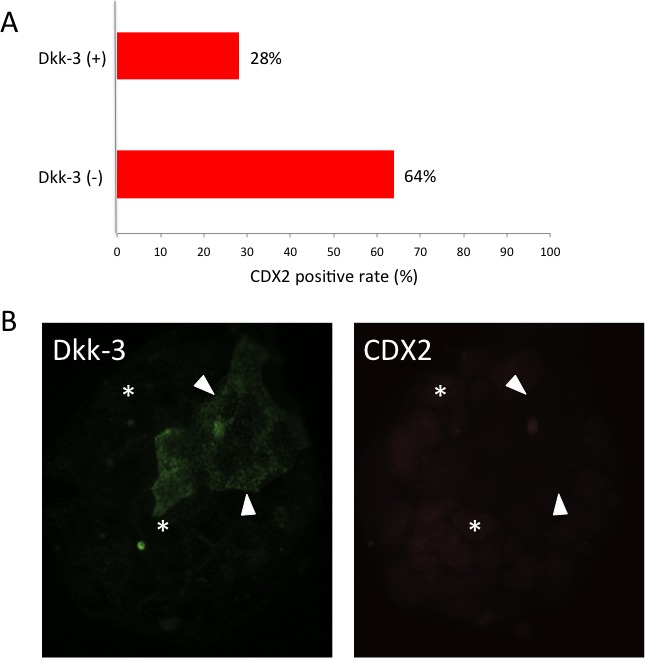
Caco-2 cells were initially established from human colon adenocarcinoma tissue and they differentiate in appropriate culture to express intestinal epithelium markers. Since the cell-cell junction of well-differentiated Caco-2 cells is similar to that of small intestine tissue, Caco-2 cells have been used for drug metabolism assays[8]. In a monolayer culture condition, semi-confluent Caco-2 cells expressed Dkk-3 and the cell membrane showed strong expression (Figure 2A). Conversely, only some of the Caco-2 cells in sparse small colonies expressed Dkk-3 (Figure 3). Our results also showed that Dkk-3 and CDX2 have reciprocal expression patterns in these sparse colonies. Additionally, we have compared the number of CDX2-positive cells in sparse small colonies and calculated the percentage of co- expression. CDX2 positive rate in Dkk3-positive cells was 28% and that in Dkk3-negative cells was 64% (Supplementary Figure 1). Localization of Dkk-3 was changed during Caco-2 status, however further analysis will be needed. The homeobox gene CDX2 is a widely used marker of intestinal differentiation[9]. These data demonstrated that Dkk-3 might affect stem cell fate during intestinal differentiation.
It was reported that Caco-2 cells could form spheroids in several types of hydrogel. Elamin and co-workers reported that Caco-2 cells formed hollow spheroids consisting of a single cell layer in Matrigel and that the cells forming a layer of the spheroids were differentiated [10]. In our experiments, Caco-2 cells formed hollow spheroids after 5-11 days in Matrigel (Figure 3A). Immunocytochemistry of the spheroids demonstrated that the layer- forming Caco-2 cells in the hollow spheroids expressed Dkk-3 (Figure 3B). Thus, differentiated Caco-2 cells expressed Dkk-3 in a three-dimensional culture system.
The actual functions of Dkk-3 in intestinal tissue and Caco-2 spheroids were still unclear, but our data indicated that Dkk-3 might affect intestinal cells when the fate of stem cells changes. Further analysis of the stem cell biology of intestinal tissues is needed.
Supplementary Information
Supplementary Materials and Methods
Culture Cells
Caco-2 cells, human colon adenocarcinoma cells, were initially grown as standard monolayers in Dulbecco’s modified MEM supplemented with 10% fetal bovine serum (Thermo Scientific, Waltham, MA, USA). The cells were passaged using 0.2% trypsin with 0.02% EDTA in phosphate-buffered saline.
Three-dimensional Cell Culture
Single-cell suspensions of Caco-2 cells were re-suspended in a serum-free medium, mixed with Matrigel (Corning), and plated on 24-well plastic plates. Then complete growth medium was added and spheroids were allowed to form over a period of 5-11 days.
Immunostaining
Frozen small intestine tissues from female ICR mice aged 8 weeks (CREA Japan) were sliced into sections of 6 μm in thickness on a cryostat. Caco-2 cells in a monolayer culture condition were inoculated in glass slide culture vessels (Thermo Scientific). Caco-2 spheroids in Matrigel were collected and processed for 6 μm thick frozen sections. All of the samples for immunostaining were fixed with acetone and blocked with 10% skim milk. The primary antibodies used in the study were antibodies for Dkk-3 (R&D Systems) and CDX2 (DAKO). The secondary antibodies were Alexa Fluor 488 donkey anti-goat IgG (H + L) antibody and Alexa 594 donkey anti-mouse IgG (Thermo Scientific). No signal was seen when isotype control IgG was used as the first antibody.
Supplementary Figure 1: Reciprocal expression patterns of Dkk-3 and CDX2 in Caco-2 cells. (A) CDX2 positive rate in Dkk3-positive cells was 28% and that in Dkk3-negative cells was 64% in sparse small colonies . (B) Arrowheads and asterisks indicate representative Dkk-3 (+) CDX2 (-) cells and representative Dkk-3 (-) CDX2 (+) cells respectively.
Acknowledgments
This work was supported in part by JSPS Grants-in-Aid for Scientific Research KAKENHI (Grant Number 24591943, 26106725, 15K01303 to K. Kataoka).
Abbreviations
- Dkk:
Dickkopf
- REIC:
Reduced expression in immortalized cells CDX : Caudal-related homeobox transcription factor DAPI : 4',6-diamidino-2-phenylindole EDTA : Ethylenediaminetetraacetic acid
Potential Conflicts of Interests
None
References
- 1.Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y, Namba M. A REIC gene shows down-regulation in human immortalized cells and human tumor-derived cell lines. Biochem Biophys Res Commun. 2000;268(1):20–24. doi: 10.1006/bbrc.1999.2067. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe M, Nasu Y, Kumon H. Adenovirus-mediated REIC/Dkk-3 gene therapy: Development of an autologous cancer vaccination therapy (Review). Oncol Lett. 2014;7(3):595–601. doi: 10.3892/ol.2013.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang K, Watanabe M, Kashiwakura Y, Li SA, Edamura K, Huang P, Yamaguchi K, Nasu Y, Kobayashi Y, Sakaguchi M, Ochiai K, Yamada H, Takei K, Ueki H, Huh NH, Li M, Kaku H, Na Y, Kumon H. Expression pattern of REIC/Dkk-3 in various cell types and the implications of the soluble form in prostatic acinar development. Int J Oncol. 2010;37(6):1495–501. doi: 10.3892/ijo_00000802. [DOI] [PubMed] [Google Scholar]
- 4.Du G, Kataoka K, Sakaguchi M, Abarzua F, Than SS, Sonegawa H, Makino T, Shimizu T, Huh NH. Expression of REIC/Dkk-3 in normal and hyperproliferative epidermis. Exp Dermatol. 2011;20(3):273–277. doi: 10.1111/j.1600-0625.2010.01244.x. [DOI] [PubMed] [Google Scholar]
- 5.Kataoka K, Du G, Maehara N, Murata H, Sakaguchi M, Huh N. Expression pattern of REIC/Dkk-3 in mouse squamous epithelia. Clin Exp Dermatol. 2012;37(4):428–431. doi: 10.1111/j.1365-2230.2011.04301.x. [DOI] [PubMed] [Google Scholar]
- 6.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154(2):274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-P signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338(6103):108–113. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awortwe C, Fasinu PS, Rosenkranz B. Application of Caco-2 cell line in herb-drug interaction studies: current approaches and challenges. J Pharm Pharm Sci. 2014;17(1):1–19. doi: 10.18433/j30k63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saad RS, Ghorab Z, Khalifa MA, Xu M. CDX2 as a marker for intestinal differentiation: Its utility and limitations. World J Gastrointest Surg. 2011;3(11):159–166. doi: 10.4240/wjgs.v3.i11.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elamin E, Jonkers D, Juuti-Uusitalo K, van Ijzendoorn S, Troost F, Duimel H, Broers J, Verheyen F, Dekker J, Masclee A. Effects of ethanol and acetaldehyde on tight junction integrity: in vitro study in a three dimensional intestinal epithelial cell culture model. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035008. e35008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Reciprocal expression patterns of Dkk-3 and CDX2 in Caco-2 cells. (A) CDX2 positive rate in Dkk3-positive cells was 28% and that in Dkk3-negative cells was 64% in sparse small colonies . (B) Arrowheads and asterisks indicate representative Dkk-3 (+) CDX2 (-) cells and representative Dkk-3 (-) CDX2 (+) cells respectively.



