Abstract
AIM: There is increasing evidence that alcohol-induced liver damage may be associated with increased oxidative stress. We aimed to investigate free-radical scavenger effect of n-acetylcysteine in rats intragastrically fed with ethanol.
METHODS: Twenty-four rats divided into three groups were fed with ethanol (6 g/kg/d, Group 1), ethanol and n-acetylcysteine (1 g/kg, Group 2), or isocaloric dextrose (control group, Group 3) for 4 wk. Then animals were sacrificed under ether anesthesia, intracardiac blood and liver tissues were obtained. Measurements were performed both in serum and in homogenized liver tissues. Malondialdehyde (MDA) level was measured by TBARS method. Glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) levels were studied by commercial kits. Kruskal-Wallis test was used for statistical analysis.
RESULTS: ALT and AST in Group 1 (154 U/L and 302 U/L, respectively) were higher than those in Group 2 (94 U/L and 155 U/L) and Group 3 (99 U/L and 168 U/L) (P = 0.001 for both). Serum and tissue levels of MDA in Group 1 (1.84 nmol/mL and 96 nmol/100 mg-protein) were higher than Group 2 (0.91 nmol/mL and 64 nmol/100 mg-protein) and Group 3 (0.94 nmol/mL and 49 nmol/100 mg-protein) (P < 0.001 for both). On the other hand, serum GSH-Px level in Group 1 (8.21 U/g-Hb) was lower than Group 2 (16 U/g-Hb) and Group 3 (16 U/g-Hb) (P < 0.001). Serum and liver tissue levels of SOD in Group 1 (11 U/mL and 26 U/100 mg-protein) were lower than Group 2 (18 U/mL and 60 U/100 mg-protein) and Group 3 (20 U/mL and 60 U/100 mg-protein) (P < 0.001 for both).
CONCLUSION: This study demonstrated that ethanol-induced liver damage is associated with oxidative stress, and co-administration of n-acetylcysteine attenuates this damage effectively in rat model.
INTRODUCTION
Reactive oxygen intermediates contributes to the pathogenesis of various hepatic disorders such as paracetamol intoxication, hemochromatosis, toxic hepatitis, and alcoholic liver injury[1-4]. Increased oxygen radical production leads to lipid peroxidation by induced cytochrome P4502E1[5,6]. Enhanced generation of acetaldehyde was shown to cause lipid peroxidation in isolated perfused livers[7]. Oxidative damage correlates with the amount of ethanol consumed[8].
Antioxidants such as vitamin E have been suggested as therapeutic options in acute and chronic liver diseases[9,10]. N-acetylcysteine (NAC) exerts a strong antioxidant activity, and is the treatment of choice in acetaminophen intoxication. NAC provides protection from toxic liver damage by elevating intacellular glutathione concentrations[1,11]. It has also been used in the treatment of CCl4 poisoning[12].
In this study, we tested whether NAC attenuates alcohol-induced free radical damage in the liver in a rat model.
MATERIALS AND METHODS
Male Wistar-Albino rats weighing 220-250 g obtained from University of Istanbul Animal Research Laboratory were kept in the same unit and fed chow (Eris Chow Industry, Istanbul, Turkey) ad libitum. All rats had free access to tap water. All animals received humane care in compliance with the National Institutes of Health criteria for care of laboratory animals.
Twenty-four rats divided into three groups and were given ethanol (Group 1) or both ethanol and NAC (Group 2) or isocaloric dextrose (Group 3). NAC and alcohol were administered respectively 4 h apart. Ethanol and NAC were given intragastrically at doses of 6 g/kg/d and 1 mg/kg/d respectively.
All rats were sacrificed at 1 mo under ether anesthesia. After exploration of the thorax, intracardiac blood and liver samples were quickly obtained. Serum alcohol levels were measured on the day the rats were sacrificed.
Glutathione peroxidase levels in erythrocytes, serum alcohol level and biochemistry were studied immediately. For the remaining studies, serum and liver tissue samples were stored at -70 °C.
Tissue Homogenization: Liver samples were weighed and homogenized in 0.15 M NaCl for lipid peroxidation parameters and for the other studies, and homogenates of 20% were obtained. Tissue homogenates were sonicated two times at 30 sec. intervals. Homogenization and sonication were performed at 4 °C. After sonication, homogenates for lipid peroxidation and biochemical studies were centrifuged at 3000 rpm for 10 min and at 15000 rpm for 15 min respectively. Aliquots of the supernatants were used for both studies[13]. The assayed parameters were expressed per mg protein and protein content of the aliquots was determined by the method of Lowry et al[14].
Lipid peroxidation
Lipid peroxidation was measured by thiobarbituric acid method, a modified form of the procedure described by Beuge and Aust[15]. This method measures several aldehydes derived from lipid hydroperoxides and also known as TBARS (thiobarbituric acid reactive substances) method.
Superoxide dismutase (SOD) level
Serum and homogenized liver tissue SOD levels were measured by using commercial kits. (Randox-Ransod, Cat No:SD 125).
Glutathione level
Glutathione concentration was determined according to the method of Beutler et al[16] using metaphosphoric acid for protein precipitation and 5'5-dithiobis-2-nitrobenzoic acid for colour development. Erythrocyte and tissue glutathione concentrations were expressed as mg/grHb, mg/gr wet weight respectively.
Glutathione peroxidase level
Serum and homogenized liver tissue glutathione peroxidase levels were also measured by using commercial kits. (Randox-Ransel, Cat No: RS 505).
Biochemical studies were performed by using autoanalyser (Hitachi 717). Hemoglobin level was measured manually by cyanmethemoglobin method.
Alcohol level
Serum alcohol level was measured by fluorescent polarizing immunoassay using commercial kits (Abbot TDx, Cat No: 378190100).
Statistical analysis
All results are expressed as mean ± standard deviation. Comparisons between the groups were performed by Kruskal-Wallis variance analysis and a P value < 0.05 was accepted as statistically significant.
RESULTS
Blood alcohol levels of Group 1 and 2 were comparable (207 ± 33 mg/dL vs 182 ± 27 mg/dL).
AST level in Group 1 (302.00 ± 70.68 U/L), was higher than those in Group 2 (155.25 ± 24.07 U/L), and in Group 3 (168.25 ± 32.08 U/L) (P = 0.001). ALT level also in Group 1 (154.13 ± 33.59 U/L), was higher than those in Group 2 (94.25 ± 16.02 U/L), and in Group 3 (99.00 ± 19.86 U/L) (P = 0.001) (Figure 1).
Figure 1.
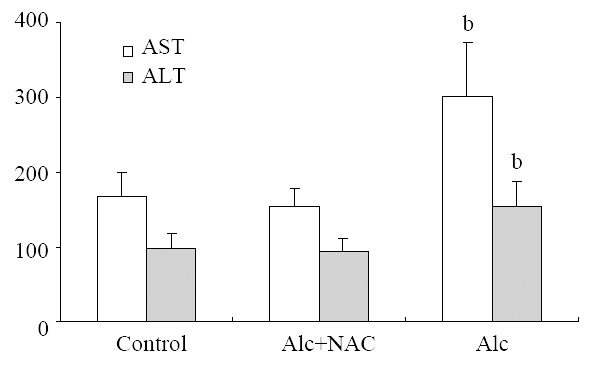
Serum AST and ALT levels (U/L). Alc: Alcohol, NAC: N-acetylcysteine, bP = 0.001 vs control and Alc + NAC group.
Although serum GGT level in Group 1 (8.50 ± 3.16 U/L) tend to be higher than those in Group 2 (8.25 ± 3.92 U/L) and Group 3 (6.88 ± 3.76 U/L), the difference was not significant. However tissue GGT level in Group 1 (18.75 ± 5.90 U/g-protein) was significantly higher than those in Group 2 (7.23 ± 6.09 U/g-protein) and Group 3 (6.25 ± 3.33 U/g-protein) (P < 0.001) (Figure 2).
Figure 2.
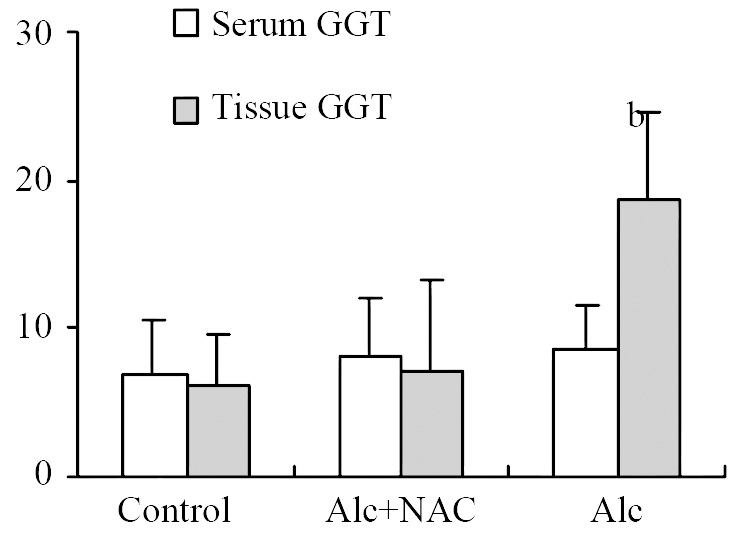
Serum (U/L) and tissue (U/g-protein) GGT levels. Alc: Alcohol, NAC: N-acetylcysteine, bP < 0.001 vs control and Alc + NAC group.
Although serum ALP level in Group 1 (131.38 ± 33.84 U/L) tend to be higher than those in Group 2 (109.50 ± 49.75 U/L) and Group 3 (93.13 ± 32.42 U/L), the difference was not significant either. But tissue ALP level in Group 1 (26.88 ± 3.31 U/g-protein) was significantly higher than those in Group 2 (12.63 ± 2.67 U/g-protein) and Group 3 (11.25 ± 2.49 U/g-protein) (P < 0.001) (Figure 3).
Figure 3.
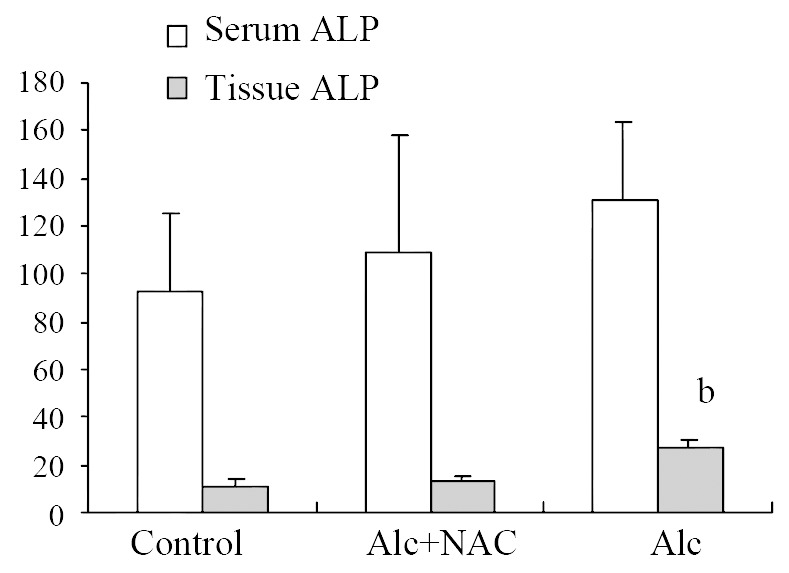
Serum (U/L) and Tissue (U/g-protein) ALP levels Alc: Alcohol, NAC: N-acetylcysteine, bP < 0.001 vs control and Alc + NAC group.
Erythrocyte glutathione level was lower in Group 1 (356.2 ± 18.3 mg/g-Hb) when compared to Group 2 (387.8 ± 13.1 mg/g-Hb) and Group 3 (398.0 ± 18.0 mg/g-Hb) (P < 0.01) (Figure 4).
Figure 4.
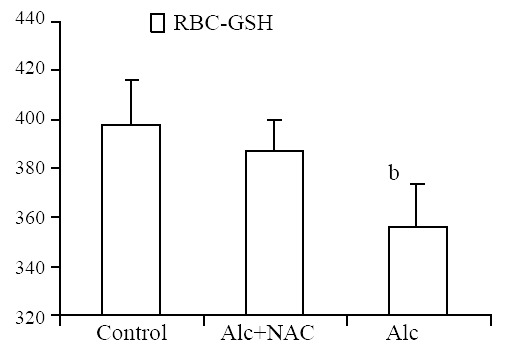
Erythrocyte glutathione levels (U/g-Hb). Alc: Alcohol, NAC: N-acetylcysteine, bP < 0.01 vs control and Alc + NAC group.
Blood glutathione peroxidase level was lower in Group 1 (8.21 ± 1.15 U/g-Hb) when compared to Group 2 (16.04 ± 2.38 U/g-Hb) and Group 3 (16.84 ± 2.68 U/g-Hb) (P < 0.001) (Figure 5). Also serum SOD level was lower in Group 1 (11.08 ± 1.13 U/mL) when compared to Group 2 (17.92 ± 0.81 U/mL) and Group 3 (19.68 ± 1.76 U/mL) (P < 0.001). The same was true for the tissue SOD levels: it was lower in Group 1 (26.04 ± 8.49 U/100 mg-protein) when compared to Group 2 (59.96 ± 10.23 U/100 mg-protein) and Group 3 (60.34 ± 8.24 U/100 mg-protein.) (P < 0.001) (Figure 6).
Figure 5.
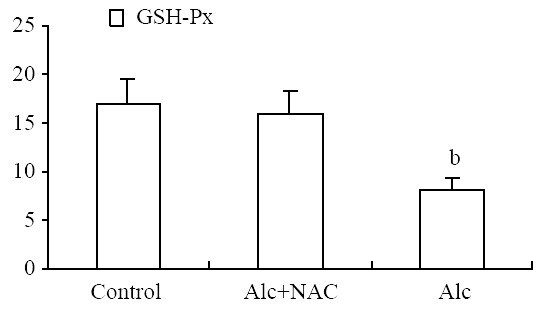
Blood glutathione peroxidase levels (U/g-Hb). Alc: Alcohol, NAC: N-acetylcysteine, aP < 0.001 vs control and Alc + NAC group.
Figure 6.
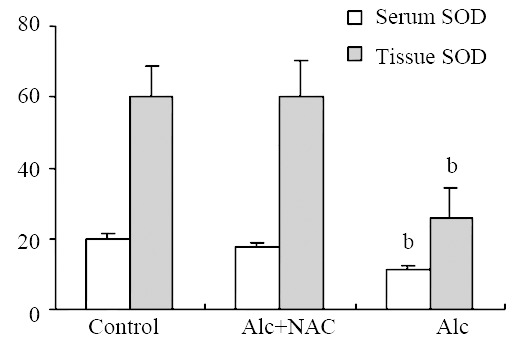
Serum (U/mL) and tissue (U/100 mg-protein) superoxide dismutase (SOD) levels. Alc: Alcohol, NAC: N-acetylcysteine, bP < 0.001 vs control and Alc + NAC group.
Serum MDA level was significantly higher in Group 1(1.84 ± 0.14 nmol/mL) than those in Group 2 (0.91 ± 0.14 nmol/mL) and Group 3 (0.94 ± 0.11 nmol/mL) (P < 0.001) (Figure 7). For the tissue levels, it was higher also in Group 1 (96.00 ± 18.20 nmol/100 mg-protein) than those in Group 2 (64.00 ± 11.63 nmol/100 mg-protein) and Group 3 (49.63 ± 12.11 nmol/100 mg-protein) (P < 0.001) (Figure 8).
Figure 7.
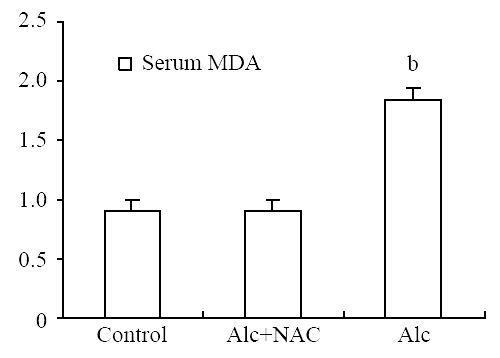
Serum MDA level (nmol/mL). Alc: Alcohol, NAC: N-acetylcysteine, bP < 0.001 vs control and Alc + NAC group.
Figure 8.
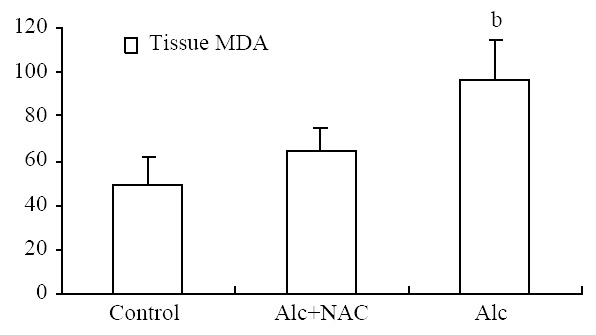
Tissue MDA level (nmol/100 mg-protein). Alc: Alcohol, NAC: N-acetylcysteine, bP < 0.001 vs control and Alc + NAC group.
DISCUSSION
Ethanol is capable of generating oxygen radicals, inhibiting glutathione synthesis, producing glutathione loss from the tissue, increasing malonyldialdehyde levels and impairing antioxidant defense systems in humans and experimental animals. Lipid peroxidation results from the increased oxygen radical production by the induced 2E1[6]. Enhanced generation of acetaldehyde was also shown to be capable of causing lipid peroxidation in isolated perfused livers[7]. Lipid peroxidation is not only a reflection of tissue damage, it may also play a pathogenic role, for instance by promoting collagen production[17]. The removal of the toxic metabolites is believed to be the vital initial step in providing cell survival during ethanol intoxication[18].
Genc et al[19] used melatonin in preventing lipid peroxidation due to acute alcohol intoxication. They found that melatonin administration prior to alcohol did not alter MDA and GSH levels of the tissue but an antioxidant enzyme (CuZn-SOD) was higher in animals receiving alcohol + melatonin. However since absorption and kinetics of this hormone are not widely known, and its antioxidant effect depend on both the tissue studied and the dose applied, the results of a melatonin study may not reflect the effects of other antioxidants.
Nanji et al[20] used thromboxane inhibitors in alcoholic liver disease in rats. They found that treatment with thromboxane inhibitors prevented necrosis and inflammation, and suggested a role for the use of thromboxane inhibitors in the treatment of alcoholic liver disease. Flora et al[21] tested NAC and a chelator agent meso 2,3-dimercaptosuccinic acid combination in the treatment of arsenic-induced oxidative stress, and found that these agents had a capability of reversing the toxic effects.
Bruck et al[1] have used NAC in the treatment of thioacetamide-induced fulminant hepatic failure in the rat model. They found no protective effect of NAC. In this model, total hepatic glutathione content is not affected. Instead, other free radical scavengers, dimethylsulfoxide and dimethylthiourea having additional modes of action such as inhibition of nitric oxide formation prevented liver injury.
The results of our study show that co-administration of NAC diminishes oxidative stress, by increasing antioxidant enzymes. This restoration of oxidant/antioxidant balance is reflected by lower levels of transaminases, ALP, and GGT. Although the decrease in serum level of the latter two enzymes was not significant, tissue levels were lower. NAC has been used in acetaminophen intoxication. It reduces the incidence of organ failure and enhances survival[22]. It acts by reducing tissue hypoxia, mediated by the activity of the nitric oxide/soluble guanylate cyclase system[1]. Cysteine derived from NAC also serves as a precursor of glutathione which forms conjugates with carbon tetrachloride metabolites[23] and increases intracellular glutathione concentrations[1]. In a recent study, NAC - in a lower dose than used in the current study - has been shown to prevent the fatty acid changes produced by ethanol and also reduce inflammatory response by reducing the level of prostaglandin[24].
Antioxidant protective mechanisms are both enzymatic and nonenzymatic. Impairments in these defense systems have been shown in alcoholics: alterations in ascorbic acid levels, glutathione, selenium, and vitamin E have been observed[25,26]. Reduced hepatic alpha-tocopherol content after long-term ethanol feeding in rats under adequate intake of vitamin E[27] and also in alcoholics[28] has been reported. Alpha-tocopherol level was found to be reduced in the blood of the alcoholics[29]. In addition to acetaldehyde and free radical generation by the ethanol-induced microsomes, these deficient defense systems were suggested to contribute to liver damage via lipid peroxidation[17]. Lipid peroxidation is a reflection of tissue damage and plays a pathogenic role by promoting collagen production[30].
A growing body of experimental and clinical experience shows the importance of free radicals in ethanol-induced liver damage. Free radical scavenging property may be beneficial as ascertained by previous studies of NAC in both acetaminophen and carbon tetrachloride intoxication. In this rat model, we have used NAC to attenuate ethanol-induced free radical damage. In both serum and tissue levels, we observed a favorable result. The results of this study suggest a role for NAC in the management of ethanol-induced liver damage as a safe, cheap, and effective option.
Footnotes
This study was presented in Digestive Disease Week, 20-23rd May, 2001, Atlanta, Georgia as an oral presentation
Supported by the research Fund of the University of Istanbul. No: T-589/240698
Edited by Xu JY and Xu XQ
References
- 1.Bruck R, Aeed H, Shirin H, Matas Z, Zaidel L, Avni Y, Halpern Z. The hydroxyl radical scavengers dimethylsulfoxide and dimethylthiourea protect rats against thioacetamide-induced fulminant hepatic failure. J Hepatol. 1999;31:27–38. doi: 10.1016/s0168-8278(99)80160-3. [DOI] [PubMed] [Google Scholar]
- 2.Bacon BR, Tavill AS, Brittenham GM, Park CH, Recknagel RO. Hepatic lipid peroxidation in vivo in rats with chronic iron overload. J Clin Invest. 1983;71:429–439. doi: 10.1172/JCI110787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle ME, Miccadei S, Nakae D, Farber JL. Superoxide dismutase and catalase protect cultured hepatocytes from the cytotoxicity of acetaminophen. Biochem Biophys Res Commun. 1987;149:889–896. doi: 10.1016/0006-291x(87)90491-8. [DOI] [PubMed] [Google Scholar]
- 4.Shaw S, Jayatilleke E, Ross WA, Gordon ER, Leiber CS. Ethanol-induced lipid peroxidation: potentiation by long-term alcohol feeding and attenuation by methionine. J Lab Clin Med. 1981;98:417–424. [PubMed] [Google Scholar]
- 5.Dai Y, Rashba-Step J, Cederbaum AI. Stable expression of human cytochrome P4502E1 in HepG2 cells: characterization of catalytic activities and production of reactive oxygen intermediates. Biochemistry. 1993;32:6928–6937. doi: 10.1021/bi00078a017. [DOI] [PubMed] [Google Scholar]
- 6.Castillo T, Koop DR, Kamimura S, Triadafilopoulos G, Tsukamoto H. Role of cytochrome P-450 2E1 in ethanol-, carbon tetrachloride- and iron-dependent microsomal lipid peroxidation. Hepatology. 1992;16:992–996. doi: 10.1002/hep.1840160423. [DOI] [PubMed] [Google Scholar]
- 7.Müller A, Sies H. Role of alcohol dehydrogenase activity and the acetaldehyde in ethanol- induced ethane and pentane production by isolated perfused rat liver. Biochem J. 1982;206:153–156. doi: 10.1042/bj2060153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clot P, Tabone M, Aricò S, Albano E. Monitoring oxidative damage in patients with liver cirrhosis and different daily alcohol intake. Gut. 1994;35:1637–1643. doi: 10.1136/gut.35.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu SL, Degli Esposti S, Yao T, Diehl AM, Zern MA. Vitamin E therapy of acute CCl4-induced hepatic injury in mice is associated with inhibition of nuclear factor kappa B binding. Hepatology. 1995;22:1474–1481. [PubMed] [Google Scholar]
- 10.Brown KE, Poulos JE, Li L, Soweid AM, Ramm GA, O'Neill R, Britton RS, Bacon BR. Effect of vitamin E supplementation on hepatic fibrogenesis in chronic dietary iron overload. Am J Physiol. 1997;272:G116–G123. doi: 10.1152/ajpgi.1997.272.1.G116. [DOI] [PubMed] [Google Scholar]
- 11.Lauterburg BH, Corcoran GB, Mitchell JR. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J Clin Invest. 1983;71:980–991. doi: 10.1172/JCI110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard RJ, Blake DR, Pall H, Williams A, Green ID. Allopurinol/N-acetylcysteine for carbon monoxide poisoning. Lancet. 1987;2:628–629. doi: 10.1016/s0140-6736(87)93018-2. [DOI] [PubMed] [Google Scholar]
- 13.Brown KE, Kinter MT, Oberley TD, Freeman ML, Frierson HF, Ridnour LA, Tao Y, Oberley LW, Spitz DR. Enhanced gamma-glutamyl transpeptidase expression and selective loss of CuZn superoxide dismutase in hepatic iron overload. Free Radic Biol Med. 1998;24:545–555. doi: 10.1016/s0891-5849(97)00284-0. [DOI] [PubMed] [Google Scholar]
- 14.LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 16.BEUTLER E, DURON O, KELLY BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 17.Lieber CS. Alcohol and the liver: 1994 update. Gastroenterology. 1994;106:1085–1105. doi: 10.1016/0016-5085(94)90772-2. [DOI] [PubMed] [Google Scholar]
- 18.Nordmann R, Ribière C, Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic Biol Med. 1992;12:219–240. doi: 10.1016/0891-5849(92)90030-k. [DOI] [PubMed] [Google Scholar]
- 19.Genç S, Gürdöl F, Oner-Iyidoğan Y, Onaran I. The effect of melatonin administration on ethanol-induced lipid peroxidation in rats. Pharmacol Res. 1998;37:37–40. doi: 10.1006/phrs.1997.0263. [DOI] [PubMed] [Google Scholar]
- 20.Nanji AA, Khwaja S, Rahemtulla A, Miao L, Zhao S, Tahan SR. Thromboxane inhibitors attenuate pathological changes in alcoholic liver disease in the rat. Gastroenterology. 1997;112:200–207. doi: 10.1016/s0016-5085(97)70236-1. [DOI] [PubMed] [Google Scholar]
- 21.Flora SJ. Arsenic-induced oxidative stress and its reversibility following combined administration of N-acetylcysteine and meso 2,3-dimercaptosuccinic acid in rats. Clin Exp Pharmacol Physiol. 1999;26:865–869. doi: 10.1046/j.1440-1681.1999.03157.x. [DOI] [PubMed] [Google Scholar]
- 22.Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, Proudfoot AT. Intravenous N-acetylcystine: the treatment of choice for paracetamol poisoning. Br Med J. 1979;2:1097–1100. doi: 10.1136/bmj.2.6198.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathieson PW, Williams G, MacSweeney JE. Survival after massive ingestion of carbon tetrachloride treated by intravenous infusion of acetylcysteine. Hum Toxicol. 1985;4:627–631. doi: 10.1177/096032718500400610. [DOI] [PubMed] [Google Scholar]
- 24.Akrishnan VR, Menon VP. Potential role of antioxidants during ethanol-induced changes in the fatty acid composition and arachidonic acid metabolites in male Wistar rats. Cell Biol Toxicol. 2001;17:11–22. doi: 10.1023/a:1010998929785. [DOI] [PubMed] [Google Scholar]
- 25.Tanner AR, Bantock I, Hinks L, Lloyd B, Turner NR, Wright R. Depressed selenium and vitamin E levels in an alcoholic population. Possible relationship to hepatic injury through increased lipid peroxidation. Dig Dis Sci. 1986;31:1307–1312. doi: 10.1007/BF01299808. [DOI] [PubMed] [Google Scholar]
- 26.Bonjour JP. Vitamins and alcoholism. I. Ascorbic acid. Int J Vitam Nutr Res. 1979;49:434–441. [PubMed] [Google Scholar]
- 27.Bjørneboe GE, Bjørneboe A, Hagen BF, Mørland J, Drevon CA. Reduced hepatic alpha-tocopherol content after long-term administration of ethanol to rats. Biochim Biophys Acta. 1987;918:236–241. doi: 10.1016/0005-2760(87)90226-8. [DOI] [PubMed] [Google Scholar]
- 28.Leo MA, Rosman AS, Lieber CS. Differential depletion of carotenoids and tocopherol in liver disease. Hepatology. 1993;17:977–986. [PubMed] [Google Scholar]
- 29.Bjørneboe GE, Johnsen J, Bjørneboe A, Marklund SL, Skylv N, Høiseth A, Bache-Wiig JE, Mørland J, Drevon CA. Some aspects of antioxidant status in blood from alcoholics. Alcohol Clin Exp Res. 1988;12:806–810. doi: 10.1111/j.1530-0277.1988.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 30.Geesin JC, Hendricks LJ, Falkenstein PA, Gordon JS, Berg RA. Regulation of collagen synthesis by ascorbic acid: characterization of the role of ascorbate-stimulated lipid peroxidation. Arch Biochem Biophys. 1991;290:127–132. doi: 10.1016/0003-9861(91)90598-d. [DOI] [PubMed] [Google Scholar]


