Abstract
AIM: To explore the potential carcinogenicity of bile from congenital choledochal cyst (CCC) patients and the mechanism of the carcinogenesis in congenital choledochal cyst patients.
METHODS: 20 bile samples from congenital choledochal cyst patients and 10 normal control bile samples were used for this study. The proliferative effect of bile was measured by using Methabenzthiazuron (MTT) assay; Cell cycle and apoptosis were analyzed by using flow cytometry (FCM), and the PGE2 levels in the supernatant of cultured cholangiocarcinoma cells were quantitated by enzyme-linked immunoabsordent assay (ELISA).
RESULTS: CCC bile could significantly promote the proliferation of human cholangiocarcinoma QBC939 cells compared with normal bile (P = 0.001) and negative control group (P = 0.002), and the proliferative effect of CCC bile could be abolished by addition of cyclooxygenase-2 specific inhibitor celecoxib (20 µM). The QBC939 cells proliferative index was increased significantly after treated with 1% bile from CCC patient (P = 0.008) for 24 h, the percentage of S phase (29.48 ± 3.27)% was increased remarkably (P < 0.001) compared with normal bile (11.72 ± 2.70)%, and the percentage of G0/G1 phase (54.19 ± 9.46)% was decreased remarkably (P = 0.042) compared with normal bile (69.16 ± 10.88)%, however, bile from CCC patient had no significant influence on apoptosis of QBC939 cells (P = 0.719).
CONCLUSION: Bile from congenital choledochal cyst patients can promote the proliferation of human cholangiocarcinoma QBC939 cells via COX-2 and PGE2 pathway.
INTRODUCTION
Congenital choledochal cyst (CCC) is a rare disease in Western countries[1-3]. Most of the reported cases come from Asia, particularly from Japan[4-7]. In recent years, cases of CCC are reported increasingly in China[8-17]. The incidence of carcinoma arising in the wall of a congenital bile duct cyst is high and this lesion is considered as a precancerous state of the biliary tract, but its underlying precise mechanisms remain unclear. For the purpose of resolving these mechanisms we used the whole bile of CCC patients to act directly on the QBC939 cells to determine the effects of CCC bile on the growth of cholangiocacinoma cells.
MATERIALS AND METHODS
Materials
Bile samples collection and treatment: Experiments were classified into CCC bile group, normal control bile group and negative control group. 20 CCC bile samples were obtained from the common bile duct of patients (5 male, 15 female, age range 5-49 years, mean 26.8 years) with CCC underwent operation at the Department of Surgery, Tongji hospital, Wuhan, China. 10 normal control bile samples were obtained from the common bile duct of patients (5 male, 5 female, age range 23-51 years, mean 42.3 years) with a normal hepatobiliary tract underwent surgery at the Department of Surgery, Tongji hospital, Wuhan, China. All patients didn’t take any nonsteroid anti-inflammatory drugs, antibiotics or anti-tumor drugs before operation. Bile samples were filtered (0.22 µm, Millipore) by sterile technique immediately twice and stored at -80 °C. PBS (pH7.2) instead of bile sample was used as negative control. Human extra-hepatic cholangiocarcinoma cell line QBC939 was established and given to us through the courtesy of professor Xu-Guang Wang (Third Military Medical Univesity, China)[18], cells were maintained as mono-layers in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Gibco. USA.), 100 units/mL penicillin and 100 mg/mL streptomycin in a humidified atomosphere of 95% air and 5% CO2 at 37 °C. They were subcultivated every 3-5 days and given fresh medium every other day. 70%-80% subconfluent monolayers of human cholangiocarcinoma cells were employed in all experiments. PGE2 ELISA detection kit was purchased from Jingmei Biotech Co., Wuhan, China. Celecoxib was synthesized and given as a gift by Dr Zhi-Nan Mei (Wuhan University, China)[19]. Stock solution was prepared in dimethylsulfoxide (DMSO) and stored at -20 °C. In all experiments DMSO final concentration in the medium was ≤ 0.1%.
Methods
Cytotoxicity pretesting Cytotoxicity pretesting was taken with each of the gradient diluted bile sample to determine the concentration of experimental bile samples. Our results showed that 1% bile (10 µL bile/mL medium) had no significant cytotoxic influence on QBC939 cells.
MTT assay The human cholangiocarcinoma cells QBC939 in proliferating status was determined by using MTT assay. Cholangiocarcinoma cells were seeded at a density of 1 × 104 cells per well in flat-bottomed 96-well microplates. 12 h after incubation, cells were treated with 1% bile samples with or without 20 µM celecoxib. After 24 h incubation, 20 µL MTT (5 g/L) was added to each well, cultured for 4 h. After removed of supernatant, 150 µL DMSO was added and shaken for 5 min untill the crystal was dissolved. OD490nm value was measured by using an enzyme-linked immunoabsorbent assay reader. The negative control well had no cells and was used as zero point of absorbance. Each assay was performed three times in triplicate.
ELISA The PGE2 levels in the supernatant of cultured human cholangiocarcinoma cells QBC939 were quantitated by ELISA: Cells were seeded into 24-well microplates (4.0 × 105/well) and allowed to adhere overnight. The cells were then incubated in the presence 1% bile samples with or without 20 µM celecoxib for 24 h. The supernatants were aspirated and centrifuged to prepare for the detection of PGE2. 0.5 mL supernatant was added into 0.1 mL 1N HCl and centrifuged for 10 min at room temperature; then 0.1 mL 1.2N NaOH was used to neutralize the acidified samples. Standard solution (200 µL per well) or activated samples were added into the microplates. Then the steps were proceeded as instructed. The value of OD of each well was determined at 450 nm. The supernatants were harvested in triplicate and the experiment was performed three times.
Flow cytometric analysis Cholangiocarcinoma cells QBC939 were trypsinized and plated in 6-well culture dishes in the presence of 1% CCC bile or 1% normal bile. After 24 h, cells were harvested, centrifuged at low speed and fixed in 70% ethanol. After overnight incubation at 4 °C, cells were stained with 50 µg/mL propidium iodide in the presence of Rnase A (10 µg/mL) and 0.1% Triton X-100 and determined by using a flow cytometer.
Statistical analysis
Data were expressed as x ± s. Student’s t-test was used for statistical analysis. P < 0.05 indicates significant difference.
RESULTS
Assay of COX-2 activity
The COX-2 activity was determined by PGE2 levels in the supernatant released by the cultured human cholangiocarcinoma cells QBC939. The concentrations of PGE2 in culture medium of QBC939 cells treated with 1% CCC bile, 1% normal bile or 1% PBS (pH7.2) with or without 20 µM celecoxib for 24 h were quantitated by ELISA (Table 1). CCC bile could induce the release of PGE2 in QBC939 cells: the PGE2 level was higher significantly (P < 0.001) in CCC bile group (184.0 ± 9.7 ng/well) compared with that in normal control bile group (131.0 ± 7.3 ng/well) and negative control group (123.3 ± 8.4). Celecoxib could suppress QBC939 cells PGE2 production, the PGE2 concentration was 80.3 ± 7.1 ng/well, 74.1 ± 9.7 ng/well and 72.4 ± 10.8 ng/well in CCC bile group, normal control bile group and negative control bile group respectively, when pre-treated with 20 µM celecoxib, there was no statistical difference (P > 0.05).
Table 1.
PGE2 level released by QBC939 in the existence of bile with or without celecoxib
| Group | n | PGE2 | P | +CE PGE2 | P |
| A | 20 | 184.0 ± 9.7 | bP < 0.001, dP < 0.001 | 80.3 ± 7.1 | bP = 0.057, dP = 0.055 |
| B | 10 | 131.0 ± 7.3 | bP = 0.087 | 74.1 ± 9.7 | bP = 0.771 |
| C | 5 | 123.3 ± 8.4 | 72.4 ± 10.8 |
The concentrations of PGE2 (ng/well) in culture medium of QBC939 cells treated with 1% CCC bile (A), 1% normal bile (B) or 1% PBS (C) with or without 20 µM celecoxib (+CE) for 24 h were quantitated by ELISA. Data were expressed as x ± s, b vs C, d vs B.
Effects of bile on the growth of QBC939
QBC939 cells were incubated in 1% bile samples with or without 20 µM celecoxib, and the cell density was measured by using MTT assay (Table 2). CCC bile co uld significantly promote the proliferation of human cholangiocarcinoma QBC939 cells compared with normal bile (P = 0.001) and negative control group (P = 0.002), and the proliferative effect of CCC bile could be abolished by addition of cyclooxygenase-2 specific inhibitor celecoxib.
Table 2.
Effects of bile on the growth of QBC939 with or with-out celecoxib
| Group | n | OD490 | P | +CE OD490 | P |
| A | 20 | 0.59 ± 0.17 | bP = 0.002, dP = 0.001 | 0.29 ± 0.09 | bP = 0.089, dP = 0.190 |
| B | 10 | 0.47 ± 0.14 | bP = 0.398 | 0.26 ± 0.07 | bP = 0.052 |
| C | 5 | 0.43 ± 0.10 | 0.24 ± 0.09 |
QBC939 cells were incubated in 1% CCC bile (A), 1% normal bile (B) or 1% PBS (C) with or without 20 µM celecoxib (+CE), and the cells density (OD490nm) was measured by using MTT assay. Data were expressed as -x ± s, b vs C, d vs B.
Flow cytometric analysis of cell cycle and apoptosis
The QBC939 cells proliferative index (PI) increased significantly (P = 0.008) after treated with 1% CCC bile (41.53 ± 5.68) compared with normal bile (25.46 ± 4.41), PI = (S + G2/M)% × 100. The percentage of S phase cells was increased remarkably (P < 0.001) in CCC bile group (29.48 ± 3.27)% compared with that in normal bile group (11.72 ± 2.70)%, and the percentage of G0/G1 phase cells was decreased remarkably (P = 0.002) in CCC group (54.19 ± 9.46)% compared with that in normal group (69.16 ± 10.88)%, CCC bile had no significant influence on the apoptosis of QBC939 cells compared with normal bile (P = 0.719). The percentage of apoptotic cells in CCC bile group and normal bile group were (2.38 ± 0.41)% and (2.09 ± 0.36)% respectively (Figure 1).
Figure 1.
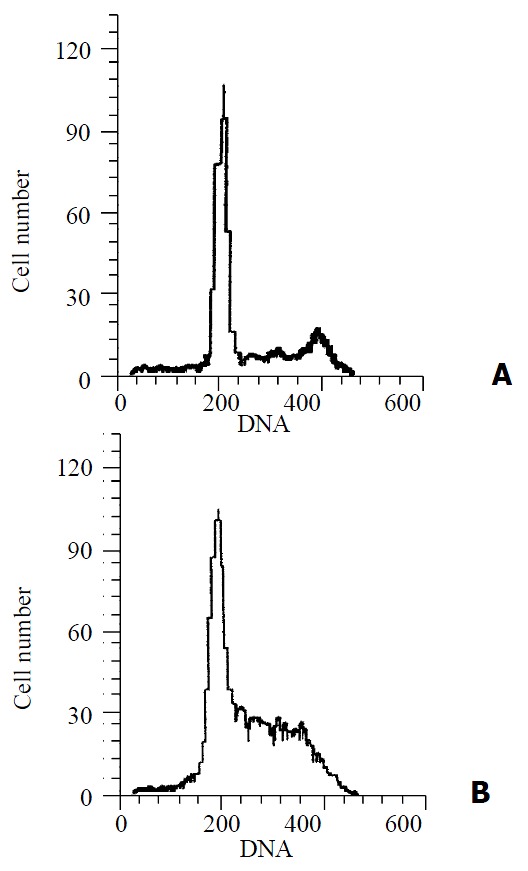
Representative data of cell cycle and apoptosis from QBC939 cells in the presence of 1% CCC bile (B) or 1% normal bile (A) for 24 h was analyzed by using flow cytometry.
DISCUSSION
As one of the high risk factors of cholangiocarcinoma, CCC is a common disease in eastern countries. Choledochal cysts are associated with a 10% overall incidence of cholangiocarcinoma: there is a 1% cumulative risk which plateaus after 15-20 years[20]. In almost all cases congenital bile duct cysts are related to anomalous arrangements of the pancreaticobiliary duct system (APBDU) which seems to play a crucial role in the development of cystic bile ducts and biliary carcinogenesis. Bile stasis together with reflux of pancreatic juice causing longstanding inflammation and activation of bile acids might be the factors in carcinogenesis of the exposed bile duct epithelium in the cystic wall[21]. Ohtsuka[22] have reportd that bile presented one possible explanation for the predisposition to carcinoma in choledochocele as bile containing amylase may stagnate in the choledochocele and then carcinoma may develop in the inner epithelium of the choledochocele by the same mechanism as that leading to carcinogenesis in patients with APBDU.
Elevation of the secondary and free bile acid concentrations is considered as a risk factor for biliary carcinogenesis in CCC patients. Yuzuru et al[23] have suggested that elevation of the lysolecithin (LL) in the bile is one of the factors for development of biliary tract carcinoma in patients with CCC: the LL in the phospholipid, which is produced from lecithin by activated phospholipase A2 in refluxed pancreatic juice, was significantly elevated in the CCC group. Yoon et al[24] have indicated that bile acids both induced EGFR phosphorylation and enhanced COX-2 protein expression. EGFR was activated by bile acids and functioned to induce COX-2 expression by a MAPK cascade. The induction of COX-2 might participate in the genesis and progression of cholangiocarcinomas.
In an effort to delineate the underlying mechanisms of the carcinogenesis in CCC patients, we used the whole bile from CCC patients for the first time to act directly on the human cholangiocacinoma QBC939 cells in vitro to determine the effects of CCC bile on the growth of human cholangiocacinoma cells. Our data showed that CCC bile could significantly promote the proliferation of human cholangiocarcinoma QBC939 cells compared with normal bile, and the proliferative effect of CCC bile could be abolished by addition of cyclooxygenase-2 specific inhibitor celecoxib. Our research indicated that CCC bile promoted the proliferation of human cholangiocarcinoma QBC939 cells via COX-2 and PGE2 pathway.
Substantial evidences have shown that COX-2 is important in carcinogenesis[25-33]. Celecoxib as a new COX-2 selective inhibitor has shown its safety and efficiency in human and animal. Several studies have demonstrated that celecoxib has significant efficacy in animal models: Celecoxib inhibited intestinal tumor multiplicity up to 71% compared with controls in the Min mouse model, inhibited colorectal tumor burden in the rat azoxymethane (AOM) model[34-36]. Recently celecoxib has been approved by FDA to reduce the number of adenomatous colorectal polyps in patients with familial adenomatous polyposis (FAP). Our data suggested that celecoxib as a chemopreventive and chemotherapeutic agent might be effective in cholangiocarcinomas and could be used as a chemopreventive strategy in the people of high-risk conditions for the development of cholangiocarcinoma such as CCC patients. Our research demonstated that the QBC939 cells proliferative index increased significantly after treated with CCC bile for 24 h, the percentage of S phase was increased remarkably compared with normal bile, and the percentage of G0/G1 phase was decreased remarkably compared with normal bile, however, CCC bile had no significant influence on apoptosis of QBC939 cells. These data suggested that CCC bile could effect on the QBC939 cell proliferation cycle and the proliferative activity of CCC bile was on cell cycle but not on apoptosis.
In conclusion, CCC bile can promote the proliferation of human cholangiocarcinoma QBC939 cells and these effects are via COX-2 and PGE2 pathway.
Footnotes
Edited by Xu JY
References
- 1.Cucinotta E, Palmeri R, Lazzara S, Salamone I, Melita G, Melita P. [Diagnostic problems of choledochal cysts in the adult] Chir Ital. 2002;54:245–248. [PubMed] [Google Scholar]
- 2.Nassar AH, Chakhtoura N, Martin D, Parra-Davila E, Sleeman D. Choledochal cysts diagnosed in pregnancy: a case report and review of treatment options. J Matern Fetal Med. 2001;10:363–365. doi: 10.1080/714052763. [DOI] [PubMed] [Google Scholar]
- 3.Wienke J, Falen S, McCartney W. Hepatobiliary scan showing type II choledochal cyst. Clin Nucl Med. 2001;26:1010–1012. doi: 10.1097/00003072-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Komuro H, Makino SI, Yasuda Y, Ishibashi T, Tahara K, Nagai H. Pancreatic complications in choledochal cyst and their surgical outcomes. World J Surg. 2001;25:1519–1523. doi: 10.1007/s00268-001-0171-8. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Yamataka A, Yian-Xia W, Da-Yong W, Segawa O, Lane GJ, Kun W, Jin-Zhe Z, Miyano T. Ectopic distal location of the papilla of vater in congenital biliary dilatation: Implications for pathogenesis. J Pediatr Surg. 2001;36:1617–1622. doi: 10.1053/jpsu.2001.27932. [DOI] [PubMed] [Google Scholar]
- 6.O'Neill JA. Choledochal cyst. Curr Probl Surg. 1992;29:361–410. doi: 10.1016/0011-3840(92)90025-x. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchida Y, Takahashi A, Suzuki N, Kuroiwa M, Murai H, Toki F, Kawarasaki H, Hashizume K, Honna T. Development of intrahepatic biliary stones after excision of choledochal cysts. J Pediatr Surg. 2002;37:165–167. doi: 10.1053/jpsu.2002.30243. [DOI] [PubMed] [Google Scholar]
- 8.Shi LB, Peng SY, Meng XK, Peng CH, Liu YB, Chen XP, Ji ZL, Yang DT, Chen HR. Diagnosis and treatment of congenital choledochal cyst: 20 years' experience in China. World J Gastroenterol. 2001;7:732–734. doi: 10.3748/wjg.v7.i5.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Lu XH. The diagnosis of choledochal cyst (A report of 50 cases) Xin Xiaohuabingxue Zazhi. 1996;4:259. [Google Scholar]
- 10.Zhang Z, Wei HL. Congenital choledochal dilatation in adult (A report of 3 cases) Xin Xiaohuabingxue Zazhi. 1996;4(Suppl 5):32. [Google Scholar]
- 11.Qiao Q, Sun Z, Huang Y. Diagnosis and treatment of congeni-tal choledochal cysts in adults. Zhonghua Waike Zazhi. 1997;35:610–612. [PubMed] [Google Scholar]
- 12.Wang L, Wang SF, Li YG. Choledochal cyst (A report of 2 cases) Xin Xiaohua bingxue Zazhi. 1996;4(Suppl 5):210. [Google Scholar]
- 13.Tao K, Li K, Dou K, Fu Y. [Analysis and prevention of reoperation on congenital choledochal cyst] Zhonghua Waike Zazhi. 1999;37:344–346. [PubMed] [Google Scholar]
- 14.Dou K, Guan W, Li K, Gao Z, Fu Y, Zhang X, Cao Y, Zhao Q, Shi X, Yue S, et al. [Living related liver transplantation: a case report] Zhonghua Waike Zazhi. 1998;36:203–205. [PubMed] [Google Scholar]
- 15.Lin JTH YH, Ni YH, Lai HS, Peng SS. Magnetic resonance cholangiopancreatography diagnosed pancreatitis associated choledochal cyst: report of one case. Acta Paediatr Taiwan. 2001;42:363–366. [PubMed] [Google Scholar]
- 16.Li M, Jin Q, Feng J. [Early postoperative complications of choledochal cyst excision and reconstruction of biliary tract] Zhonghua Waike Zazhi. 2001;39:686–689. [PubMed] [Google Scholar]
- 17.Zhao L, Li Z, Ma H, Zhang X, Mou X, Zhang D, Lin W, Niu A. Congenital choledochal cyst with pancreatitis. Chin Med J (Engl) 1999;112:637–640. [PubMed] [Google Scholar]
- 18.Wang SG, Han BL, Duan HC, Chen YS, Peng ZM. Stablishment of the extrahepatic cholangiocarcinoma cell line. Zhonghua Shiyan Waike Zazhi. 1997;14:67–68. [Google Scholar]
- 19.Mei ZY, Shi Z, Wang XH, Luo XD. Synthesis of COX-2 Inhibitor Celecoxib. Zhongguo Yiyao Gongye Zazhi. 2000;31:433–434. [Google Scholar]
- 20.Chapman RW. Risk factors for biliary tract carcinogenesis. Ann Oncol. 1999;10 Suppl 4:308–311. [PubMed] [Google Scholar]
- 21.Holzinger F, Baer HU, Schilling M, Stauffer EJ, Büchler MW. Congenital bile duct cyst: a premalignant lesion of the biliary tract associated with adenocarcinoma--a case report. Z Gastroenterol. 1996;34:382–385. [PubMed] [Google Scholar]
- 22.Ohtsuka T, Inoue K, Ohuchida J, Nabae T, Takahata S, Niiyama H, Yokohata K, Ogawa Y, Yamaguchi K, Chijiiwa K, et al. Carcinoma arising in choledochocele. Endoscopy. 2001;33:614–619. doi: 10.1055/s-2001-15324. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama Y, Kobori H, Hakamada K, Seito D, Sasaki M. Altered bile composition in the gallbladder and common bile duct of patients with anomalous pancreaticobiliary ductal junction. World J Surg. 2000;24:17–20; discussion 21. doi: 10.1007/s002689910004. [DOI] [PubMed] [Google Scholar]
- 24.Yoon JH, Higuchi H, Werneburg NW, Kaufmann SH, Gores GJ. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology. 2002;122:985–993. doi: 10.1053/gast.2002.32410. [DOI] [PubMed] [Google Scholar]
- 25.Gao HJ, Yu LZ, Sun G, Miao K, Bai JF, Zhang XY, Lu XZ, Zhao ZQ. The expression of Cox-2 Proteins in gastric cancer tissue and accompanying tissue. Shijie Huaren Xiaohua Zazhi. 2000;8:578–579. [Google Scholar]
- 26.Zhuang ZH, Wang LD. Non-steroid anti-inflammatory drug and digestive tract tumors. Shijie Huaren Xiaohua Zazhi. 2001;9:1050–1053. [Google Scholar]
- 27.Tian G, Yu JP, Luo HS, Yu BP, Yue H, Li JY, Mei Q. Effect of nimesulide on proliferation and apoptosis of human hepatoma SMMC-7721 cells. World J Gastroenterol. 2002;8:483–487. doi: 10.3748/wjg.v8.i3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun B, Wu YL, Zhang XJ, Wang SN, He HY, Qiao MM, Zhang YP, Zhong J. Effects of Sulindac on growth inhibition and apoptosis induction in human gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 2001;9:997–1002. [Google Scholar]
- 29.Tian G, Yu TP, Luo HS, Yu BP, Li JY. The expression and effect of cyclooxygenase-2 in acute hepatic injury. Shijie Huaren Xiaohua Zazhi. 2002;10:24–27. [Google Scholar]
- 30.Li JY, Yu JP, Luo HS, Yu BP, Huang JA. Effects of nonsteroidal anti-inflammatory drugs on the proliferation and cyclooxygenase activity of gastric cancer cell line SGC7901. Shijie Huaren Xiaohua Zazhi. 2002;10:262–265. [Google Scholar]
- 31.Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796–800. doi: 10.3748/wjg.v7.i6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sirica AE, Lai GH, Zhang Z. Biliary cancer growth factor pathways, cyclo-oxygenase-2 and potential therapeutic strategies. J Gastroenterol Hepatol. 2001;16:363–372. doi: 10.1046/j.1440-1746.2001.02438.x. [DOI] [PubMed] [Google Scholar]
- 33.Chariyalertsak S, Sirikulchayanonta V, Mayer D, Kopp-Schneider A, Fürstenberger G, Marks F, Müller-Decker K. Aberrant cyclooxygenase isozyme expression in human intrahepatic cholangiocarcinoma. Gut. 2001;48:80–86. doi: 10.1136/gut.48.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosomi Y, Yokose T, Hirose Y, Nakajima R, Nagai K, Nishiwaki Y, Ochiai A. Increased cyclooxygenase 2 (COX-2) expression occurs frequently in precursor lesions of human adenocarcinoma of the lung. Lung Cancer. 2000;30:73–81. doi: 10.1016/s0169-5002(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 35.Tsubouchi Y, Mukai S, Kawahito Y, Yamada R, Kohno M, Inoue K, Sano H. Meloxicam inhibits the growth of non-small cell lung cancer. Anticancer Res. 2000;20:2867–2872. [PubMed] [Google Scholar]
- 36.Souza RF, Shewmake K, Beer DG, Cryer B, Spechler SJ. Selective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cells. Cancer Res. 2000;60:5767–5772. [PubMed] [Google Scholar]


