Abstract
AIM: To identify a molecular marker for gastric cancer, and to investigate the relationship between the polymorphism of pepsinogen C (PGC) gene and the genetic predisposition to gastric cancer.
METHODS: A total of 289 cases were involved in this study. 115 cases came from Shenyang area, a low risk area of gastric cancer, including 42 unrelated controls and 73 patients with gastric cancer. 174 cases came from Zhuanghe area, a high-risk area of gastric cancer, including 113 unrelated controls, and 61 cases from gastric cancer kindred families. The polymorphism of PGC gene was detected by polymerase chain reaction (PCR) and the relation between the genetic polymorphism of PGC and gastric cancer was examined.
RESULTS: Four alleles, 310 bp (allele 1), 400 bp (allele 2), 450 bp (allele 3), and 480 bp (allele 4) were detected by PCR. The frequency of allele 1 was higher in patients with gastric cancer than that in controls. Genotypes containing homogenous allele 1 were significantly more frequent in patients with gastric cancer than that in controls (0.33, 0.14, χ2 = 3.86, P < 0.05). There was no significant difference between the control group of Zhuanghe and the group of gastric cancer kindred. But the frequency of allele 1 was higher in control group of Zhuanghe area than that in control group of Shenyang area and genotypes containing homogenous allele 1 were significantly more frequent in the control group of Zhuanghe area than those in control group of Shenyang area (0.33, 0.14, χ2 = 4.32, P < 0.05). In the group of gastric cancer kindred the frequency of allele 1 was significantly higher than that in control group of Shenyang area (0.5164, 0.3571, χ2 = 4.47, P < 0.05). Genotypes containing homogenous allele 1 were significantly more frequent in the group of gastric cancer kindred than those in control group of Shenyang area (0.36, 0.14, χ2 = 4.91, P < 0.05).
CONCLUSION: These results suggest that there is some relation between pepsinogen C gene polymorphism and gastric cancer, and the person with homogenous allele 1 predisposes to gastric cancer than those with other genotypes. Pepsinogen C gene polymorphism may be used as a genetic marker for a genetic predisposition to gastric cancer. The distribution of pepsinogen C gene polymorphism in Zhuanghe, a high-risk area of gastric cancer, is different from that in Shenyang, a low risk area of gastric cancer.
INTRODUCTION
Gastric cancer is the second most common cancer in the world. Especially in China and other eastern Asian countries, the mortality of gastric cancer is still in the leading status of all cancers. The 5-year survival rate of gastric cancer is low, and identification and a better control of risk factors seem to be the most effective means of prevention. It was showed that many factors were ascribing to the cause of gastric cancer, including the living habit, nutrition[1-3], microbe[4-6], and genetic predisposition[7-10]. Recently, following the primary completion of Human Genome Project, the association of genetic polymorphisms with diseases came to the study frontier[11-14]. Genetic polymorphisms are defined as variations in DNA that are observed in 1% or more of the population. The study of genetic polymorphisms promises to help define pathophysiologic mechanisms[15,16], to identify individuals at risk for disease[17-19] and to suggest novel targets for drug design and treatment[20-24].
Pepsinogen C (PGC), also known as progastricsin, is the precursor of pepsin C or gastricsin. PGC can be detected throughout the stomach and proximal duodenum from the period of late infant stages to adult. Therefore it is also considered to be a mature marker of stomach cells[25]. PGC consists of two electrophoretic isozymogens[26]. No genetic variation was reported at the protein level. At the DNA level, however, an about 100 bp insertion-deletion polymorphism was observed between exon 7 and exon 8 with several restriction enzymes. The polymorphism in PGC gene locus can be identified by both Southern blot and PCR methods.
In this study, we analyzed the PGC gene polymorphism of patients with gastric cancer and members with gastric cancer family history, and then examined the association between PGC gene polymorphism and gastric cancer.
MATERIALS AND METHODS
Patients
A total of 289 cases were involved in this study. 42 cases as health control came from the Blood Bank of the First Affiliated Hospital, China Medical University, whose health condition were checked up before blood was collected. 73 gastric cancer patients came from the Department of Oncology. 174 cases came from Zhuanghe, an area with high gastric cancer mortality, in the eastern Liaoning Province, China as described previously[27], including 61 members from seven gastric cancer kindred families and 113 health controls whose family do not have gastric cancer history. In every gastric cancer kindred, at least two persons of the family are gastric cancer patients.
Analysis of PGC gene polymorphism
The genomic DNA from peripheral blood was amplified by PCR. The primers used were: upstream, 5’-AGCCCTAAGCCTGTTTTTGG-3’; and the downstream, 5’-GGCCAGATCTGCGTGTTTTA-3’[28]. The reaction mixture including 32.15 pmol of each primer was subjected to 5 minutes at 95 °C; 35 cycles of one minute at 94 °C, one minute at 57 °C, one minute at 72 °C; with a final extension at 72 °C for 5 minutes. The amplification reaction proceeded in a thermocycler (PE-9600). 12 μl of reaction mixture (50 μl in total volume) underwent electrophoresis in 2% agarose gel, and the gel was stained with ethidium bromide.
Statistical analysis
The association between the polymorphism of PGC gene and gastric cancer was tested using χ2 test, with significance assigned to values below P < 0.05.
RESULTS
Detection of PGC gene polymorphism
After PCR, four alleles with different size were obtained: 310 bp (allele 1), 400 bp (allele 2), 450 bp (allele 3), and 480 bp (allele 4) (Figure 1). These results showed a little difference from the data showed by Southern blot, which demonstrated two different alleles (3.5 kb and 3.6 kb). According to the study of Ohtaki et al[28], the 400 bp, 450 bp and 480 bp of the PCR products correspond to the 3.6 kb, and the 310 bp correspond to 3.5 bp of EcoRI fragments in Southern blot.
Figure 1.
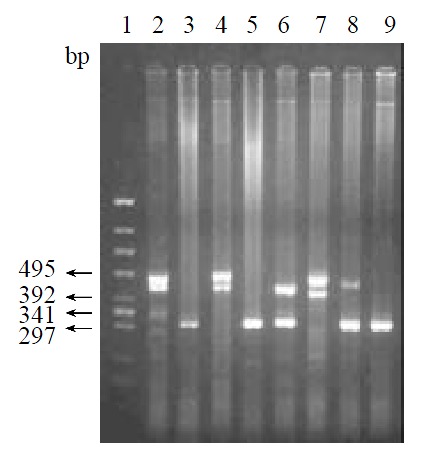
Analysis of PCR products. 1: The standard of molecule (174 HincII); 2-9: Genotype of samples--450 bp/480 bp; 310 bp/310 bp; 450 bp/480 bp; 310 bp/310 bp; 310 bp/400 bp; 400 bp/480 bp; 310 bp/480 bp; 310 bp/310 bp.
Distribution of PGC gene polymorphism
Ten different genotypes were obtained from the four alleles. Table 1 showed the distribution of these ten genotypes of PGC gene polymorphism in gastric cancer patients, members of gastric cancer kindred, health controls of Shenyang and Zhuanghe areas. Table 2 showed an estimated frequency of the four alleles in the four groups, and Table 3 showed the distribution of allele 1 homogenotype in above four groups. The frequency of allele 1 was higher in patients with gastric cancer than that in controls of Shenyang. Genotypes containing homogenous allele 1 were significantly more frequent in patients with gastric cancer than those in controls of Shenyang (P < 0.05). In the group of gastric cancer kindred the frequency of allele 1 was significantly higher than that in control group of Shenyang area (P < 0.05). Genotypes containing homogenous allele 1 were significantly more frequent in the group of gastric cancer kindred than those in control group of Shenyang area (P < 0.05). The frequency of allele 1 was higher in control group of Zhuanghe area than that in control group of Shenyang area and genotypes containing homogenous allele 1 were significantly more frequent in the control group of Zhuanghe area than those in control group of Shenyang area (P < 0.05). There was no significant difference between the control group of Zhuanghe area and the group of gastric cancer kindred.
Table 1.
The distribution of genotypes of PGC gene polymorphism in health control, gastric cancer patients, and gastric cancer kindred group
| Genotypes | Controls (Shenyang) | Gastric cancer patients | Controls (Zhuanghe) | Gastric cancer kindred |
| 1:1 | 6 (0.14) | 24 (0.33) | 37 (0.33) | 22 (0.36) |
| 1:2 | 10 (0.24) | 10 (0.14) | 9 (0.08) | 13 (0.21) |
| 1:3 | 7 (0.17) | 4 (0.05) | 14 (0.12) | 4 (0.07) |
| 1:4 | 1 (0.02) | 1 (0.01) | 9 (0.08) | 2 (0.03) |
| 2:2 | 4 (0.10) | 8 (0.11) | 10 (0.09) | 7 (0.11) |
| 2:3 | 5 (0.12) | 8 (0.11) | 9 (0.08) | 5 (0.08) |
| 2:4 | 1 (0.02) | 1 (0.01) | 13 (0.12) | 1 (0.16) |
| 3:3 | 2 (0.05) | 10 (0.14) | 2 (0.02) | 3 (0.05) |
| 3:4 | 3 (0.07) | 5 (0.07) | 8 (0.07) | 3 (0.05) |
| 4:4 | 3 (0.07) | 2 (0.03) | 2 (0.02) | 1 (0.16) |
| Total | 42 | 73 | 113 | 61 |
Table 2.
The frequency of four alleles of PGC gene polymorphism in health control, gastric cancer patients, and gastric cancer kindred group
| n |
Alleles of PGC gene polymorphism |
||||
| 1 (310 bp) | 2 (400 bp) | 3 (450 bp) | 4 (480 bp) | ||
| Controls (Shenyang) | 42 | 0.3571 | 0.2857 | 0.2262 | 0.1310 |
| Gastric cancer patients | 73 | 0.4315 | 0.2397 | 0.2534 | 0.0753 |
| Controls (Zhuanghe) | 113 | 0.4663 | 0.2389 | 0.1549 | 0.1504 |
| Gastric cancer kindred | 61 | 0.5164a | 0.2705 | 0.1475 | 0.0656 |
P < 0.05 vs compared with control group of Shenyang χ2 = 4.47.
Table 3.
The distribution of allele 1 homogenotype in health control, gastric cancer patients, and gastric cancer kindred
| n | 1:1 | others | |
| Controls (Shenyang) | 42 | 6 (0.14) | 36 |
| Gastric cancer patients | 73 | 24 (0.33)a | 49 |
| Controls (Zhuanghe) | 113 | 37 (0.33)c | 76 |
| Gastric cancer kindred | 61 | 22 (0.36)e | 39 |
aP < 0.05 vs compared with control group of Shenyang, χ2 = 3.86; cP < 0.05 vs compared with control group of Shenyang, χ2 = 4.32; eP < 0.05 vs compared with control group of Shenyang, χ2 = 4.91.
DISCUSSION
Family members of gastric cancer patients have been found to have a 1.5-fold to 3-fold increase in the risk of developing this cancer[29]. This familial aggregation may be due to genetic or environmental factors shared by family members[30-32]. To understand the genetic predisposition to gastric cancer, we selected pepsinogen C gene as a marker gene, and focused first on the distribution of the PGC gene polymorphism in gastric cancer patient group and health control. After PCR, four alleles of pepsinogen C gene with different size were obtained. The frequency of allele 1 was higher in patients with gastric cancer than that in controls. Genotypes containing homogenous allele 1 were significantly more frequent in patients with gastric cancer than those in controls. This result showed that there is relation between the pepsinogen C gene polymorphism and gastric cancer, and the person with homogenous allele 1 seems to predispose to gastric cancer than those with other genotypes.
To further study the relation of the PGC gene polymorphism to gastric cancer and the genetic background of Zhuanghe, the high-risk area of gastric cancer, we selected three groups as our next research objects: gastric cancer kindred group in Zhuanghe, the control group of Zhuanghe and the control group of Shenyang. There was no significant difference in the distribution of PGC gene polymorphism between the health control group of Zhuanghe and gastric cancer kindred group, though the frequency of allele 1 in gastric cancer kindred group was a little higher than that in the control group of Zhuanghe. Our understanding for this phenomenon was that the persons who lived in Zhuanghe did not move frequently because of the historical reason, and this consanguinity between the two groups was the main factor responsible for the above result. But the frequency of allele 1 was higher in control group of Zhuanghe area than that in control group of Shenyang area, and genotypes containing homogenous allele 1 were significantly more frequent in the control group of Zhuanghe area than those in control group of Shenyang area. The frequency of allele 1 in the group of gastric cancer kindred was significantly higher than that in control group of Shenyang area. Genotype containing homogenous allele 1 was significantly more frequent in the group of gastric cancer kindred than that in the control group of Shenyang area. The result showed the distribution of PGC gene polymorphism in Shenyang area was different from that in Zhuanghe area.
The mortality of gastric cancer in Zhuanghe area is more than 50 per five hundred thousand. In the low risk area of that cancer, such as Shenyang, however, the mortality is less than 10 per five hundred thousand. From gastric cancer kindred group, the control groups of Zhuanghe and Shenyang, the risk ratio of gastric cancer becomes lower in turn. The data in this study showed that the frequency of allele 1 and Genotype containing homogenous allele 1 in the gastric cancer kindred group, the control groups of Zhuanghe and Shenyang in turn decreases, which is consistent with the risk ratio of gastric cancer in the above three groups. Therefore, we could conclude that the polymorphism of PGC gene may be related to the predisposition of gastric cancer, and the allele 1 associated with the risk of gastric cancer.
The mechanism of the association between PGC gene polymorphism and gastric cancer is not clear, in this study however, several hypotheses can be proposed. One is that the PGC gene itself is one of the genes responsible for gastric cancer. PGC, distributed throughout the stomach and proximal duodenum, is an important enzyme in stomach. It was reported that PGC not only was a digestive enzyme, but also might be a growth factor during the healing of gastric lesions[33] and the change of serum PGC was associated with many gastric diseases[34-37]. The polymorphism of PGC gene is in the intron between exon 7 and exon 8. Whether this polymorphism could affect the expression of PGC gene or regulate the PGC gene expression when the stomach was attacked by some pathogenetic factors was not known. In the following study, we will concentrate on the association of the PGC gene polymorphism and the PGC gene expression. It is interesting to note that in this study the frequency of allele 1 of PGC in the group of gastric cancer kindred was also higher than that in the group of gastric cancer group. As we know, the members of the gastric cancer kindred all come from Zhuanghe, a place in which the incidence of gastric cancer is higher, and so is that of some other gastric diseases. In the study of Ohtaki’s group, their data showed the polymorphism of PGC gene was associated with gastric body ulcer[28]. Comparing with our data, we can conclude the polymorphism of PGC is related to gastric lesions.
Another possible explanation of the association between the PGC gene polymorphism and gastric cancer was that the PGC gene was not itself responsible for the predisposition but one of the responsible genes was closely linked to it. It is interesting to note that the PGC gene was localized to human chromosome 6p21.1-pter by analysis of mouse x human somatic cell hybrids. The recent linkage analysis demonstrated that the PGC gene is 22 cM proximal to HLA cluster which has been investigated to determine the associated with gastric disease[38-41], between D6S5 and D6S4, at a distance of 4.5 and 13.1 cM. Further molecular biological studies using polymorphism markers for this chromosome region will clarify whether PGC polymorphism is linked to disequilibria of the causative genetic variations for gastric cancer. Lastly, the presence of reduced penetrance, other modifier genes, or an interaction with the environment may explain the association.
Footnotes
Supported by The National Basic Research Program (973) of China, No.G1998051203 and National Natural Science Foundation of China, No.30171054
Edited by Zhang JZ
References
- 1.Botterweck AA, van den Brandt PA, Goldbohm RA. Vitamins, carotenoids, dietary fiber, and the risk of gastric carcinoma: results from a prospective study after 6.3 years of follow-up. Cancer. 2000;88:737–748. [PubMed] [Google Scholar]
- 2.Cai L, Yu SZ, Zhan ZF. Cytochrome P450 2E1 genetic polymorphism and gastric cancer in Changle, Fujian Province. World J Gastroenterol. 2001;7:792–795. doi: 10.3748/wjg.v7.i6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye WM, Yi YN, Luo RX, Zhou TS, Lin RT, Chen GD. Diet and gastric cancer: a casecontrol study in Fujian Province, China. World J Gastroenterol. 1998;4:516–518. doi: 10.3748/wjg.v4.i6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang ZF, Kurtz RC, Klimstra DS, Yu GP, Sun M, Harlap S, Marshall JR. Helicobacter pylori infection on the risk of stomach cancer and chronic atrophic gastritis. Cancer Detect Prev. 1999;23:357–367. doi: 10.1046/j.1525-1500.1999.99041.x. [DOI] [PubMed] [Google Scholar]
- 5.Melato M, Sidari L, Rizzardi C, Kovac D, Stimac D, Baxa P, Jonjic N. Gastric epithelium proliferation in early Hp+ and Hp- gastritis: a flow cytometry study. Anticancer Res. 2001;21:1347–1353. [PubMed] [Google Scholar]
- 6.Marinone C, Martinetti A, Mestriner M, Seregni E, Geuna M, Ferrari L, Strola G, Bonardi L, Fea E, Bombardieri E. p53 evaluation in gastric mucosa of patients with chronic Helicobacter pylori infection. Anticancer Res. 2001;21:1115–1118. [PubMed] [Google Scholar]
- 7.Oue N, Shigeishi H, Kuniyasu H, Yokozaki H, Kuraoka K, Ito R, Yasui W. Promoter hypermethylation of MGMT is associated with protein loss in gastric carcinoma. Int J Cancer. 2001;93:805–809. doi: 10.1002/ijc.1403. [DOI] [PubMed] [Google Scholar]
- 8.Deguchi R, Takagi A, Kawata H, Inoko H, Miwa T. Association between CagA+ Helicobacter pylori infection and p53, bax and transforming growth factor-beta-RII gene mutations in gastric cancer patients. Int J Cancer. 2001;91:481–485. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1088>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Yao XX, Yin L, Sun ZC. The expression of hTERT mRNA and cellular immunity in gastric cancer and precancerosis. World J Gastroenterol. 2002;8:586–590. doi: 10.3748/wjg.v8.i4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, Gao SS, Li YX, Fan ZM, Zhao X, Qi YJ, Wei JP, Zou JX, Liu G, Jiao LH, et al. Tumor suppressor gene p16 and Rb expression in gastric cardia precancerous lesions from subjects at a high incidence area in northern China. World J Gastroenterol. 2002;8:423–425. doi: 10.3748/wjg.v8.i3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 12.Yea SS, Yang YI, Jang WH, Lee YJ, Bae HS, Paik KH. Association between TNF-alpha promoter polymorphism and Helicobacter pylori cagA subtype infection. J Clin Pathol. 2001;54:703–706. doi: 10.1136/jcp.54.9.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen H, Xu Y, Zheng Y, Qian Y, Yu R, Qin Y, Wang X, Spitz MR, Wei Q. Polymorphisms of 5,10-methylenetetrahydrofolate reductase and risk of gastric cancer in a Chinese population: a case-control study. Int J Cancer. 2001;95:332–336. doi: 10.1002/1097-0215(20010920)95:5<332::aid-ijc1058>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Liu MR, Pan KF, Li ZF, Wang Y, Deng DJ, Zhang L, Lu YY. Rapid screening mitochondrial DNA mutation by using denaturing high-performance liquid chromatography. World J Gastroenterol. 2002;8:426–430. doi: 10.3748/wjg.v8.i3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfützer RH, Barmada MM, Brunskill AP, Finch R, Hart PS, Neoptolemos J, Furey WF, Whitcomb DC. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology. 2000;119:615–623. doi: 10.1053/gast.2000.18017. [DOI] [PubMed] [Google Scholar]
- 16.Prkacin I, Novak B, Sertić J, Mrzljak A. Angiotensin-converting enzyme gene polymorphism in patients with systemic lupus. Acta Med Croatica. 2001;55:73–76. [PubMed] [Google Scholar]
- 17.Wu MS, Huang SP, Chang YT, Lin MT, Shun CT, Chang MC, Wang HP, Chen CJ, Lin JT. Association of the -160 C --& gt; a promoter polymorphism of E-cadherin gene with gastric carcinoma risk. Cancer. 2002;94:1443–1448. doi: 10.1002/cncr.10371. [DOI] [PubMed] [Google Scholar]
- 18.Takezaki T, Gao CM, Wu JZ, Li ZY, Wang JD, Ding JH, Liu YT, Hu X, Xu TL, Tajima K, et al. hOGG1 Ser(326)Cys polymorphism and modification by environmental factors of stomach cancer risk in Chinese. Int J Cancer. 2002;99:624–627. doi: 10.1002/ijc.10400. [DOI] [PubMed] [Google Scholar]
- 19.Hiyama T, Tanaka S, Kitadai Y, Ito M, Sumii M, Yoshihara M, Shimamoto F, Haruma K, Chayama K. p53 Codon 72 polymorphism in gastric cancer susceptibility in patients with Helicobacter pylori-associated chronic gastritis. Int J Cancer. 2002;100:304–308. doi: 10.1002/ijc.10483. [DOI] [PubMed] [Google Scholar]
- 20.Tsukino H, Kuroda Y, Qiu D, Nakao H, Imai H, Katoh T. Effects of cytochrome P450 (CYP) 2A6 gene deletion and CYP2E1 genotypes on gastric adenocarcinoma. Int J Cancer. 2002;100:425–428. doi: 10.1002/ijc.10492. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Zhang X, Hou G, Sha W, Reynolds GP. The increased activity of plasma manganese superoxide dismutase in tardive dyskinesia is unrelated to the Ala-9Val polymorphism. J Psychiatr Res. 2002;36:317–324. doi: 10.1016/s0022-3956(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 22.Wootton JC, Feng X, Ferdig MT, Cooper RA, Mu J, Baruch DI, Magill AJ, Su XZ. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- 23.Clark AG. Population genetics: malaria variorum. Nature. 2002;418:283–285. doi: 10.1038/418283a. [DOI] [PubMed] [Google Scholar]
- 24.Feng Z, Curtis J, Minchella DJ. The influence of drug treatment on the maintenance of schistosome genetic diversity. J Math Biol. 2001;43:52–68. doi: 10.1007/s002850100092. [DOI] [PubMed] [Google Scholar]
- 25.Kageyama T, Ichinose M, Tsukada-Kato S, Omata M, Narita Y, Moriyama A, Yonezawa S. Molecular cloning of neonate/infant-specific pepsinogens from rat stomach mucosa and their expressional change during development. Biochem Biophys Res Commun. 2000;267:806–812. doi: 10.1006/bbrc.1999.2047. [DOI] [PubMed] [Google Scholar]
- 26.Kageyama T. Pepsinogens, progastricsins, and prochymosins: structure, function, evolution, and development. Cell Mol Life Sci. 2002;59:288–306. doi: 10.1007/s00018-002-8423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo XL, Wang LE, Wang L, Dong M, Yuan Y. The significant of measuring serum Hp CagA on the high risk population in the high-risk area of gastric cancer. Shijie Huaren Xiaohua Zazhi. 2001;9:595–596. [Google Scholar]
- 28.Ohtaka Y, Azuma T, Konishi J, Ito S, Kuriyama M. Association between genetic polymorphism of the pepsinogen C gene and gastric body ulcer: the genetic predisposition is not associated with Helicobacter pylori infection. Gut. 1997;41:469–474. doi: 10.1136/gut.41.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner H, Arndt V, Stürmer T, Stegmaier C, Ziegler H, Dhom G. Individual and joint contribution of family history and Helicobacter pylori infection to the risk of gastric carcinoma. Cancer. 2000;88:274–279. doi: 10.1002/(sici)1097-0142(20000115)88:2<274::aid-cncr5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Kim JC, Kim HC, Roh SA, Koo KH, Lee DH, Yu CS, Lee JH, Kim TW, Lee HL, Beck NE, et al. hMLH1 and hMSH2 mutations in families with familial clustering of gastric cancer and hereditary non-polyposis colorectal cancer. Cancer Detect Prev. 2001;25:503–510. [PubMed] [Google Scholar]
- 31.Bakir T, Can G, Erkul S, Siviloglu C. Stomach cancer history in the siblings of patients with gastric carcinoma. Eur J Cancer Prev. 2000;9:401–408. doi: 10.1097/00008469-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Dhillon PK, Farrow DC, Vaughan TL, Chow WH, Risch HA, Gammon MD, Mayne ST, Stanford JL, Schoenberg JB, Ahsan H, et al. Family history of cancer and risk of esophageal and gastric cancers in the United States. Int J Cancer. 2001;93:148–152. doi: 10.1002/ijc.1294. [DOI] [PubMed] [Google Scholar]
- 33.Kishi K, Kinoshita Y, Matsushima Y, Okada A, Maekawa T, Kawanami C, Watanabe N, Chiba T. Pepsinogen C gene product is a possible growth factor during gastric mucosal healing. Biochem Biophys Res Commun. 1997;238:17–20. doi: 10.1006/bbrc.1997.7231. [DOI] [PubMed] [Google Scholar]
- 34.Fernández R, Vizoso F, Rodríguez JC, Merino AM, González LO, Quintela I, Andicoechea A, Truan N, Díez MC. Expression and prognostic significance of pepsinogen C in gastric carcinoma. Ann Surg Oncol. 2000;7:508–514. doi: 10.1007/s10434-000-0508-9. [DOI] [PubMed] [Google Scholar]
- 35.Kikuchi S, Kurosawa M, Sakiyama T, Tenjin H, Miki K, Wada O, Inaba Y. Long-term effect of Helicobacter pylori infection on serum pepsinogens. Jpn J Cancer Res. 2000;91:471–476. doi: 10.1111/j.1349-7006.2000.tb00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujisawa T, Kumagai T, Goto A, Fujimori K, Akamatsu T, Kiyosawa K. [Investigation about usefulness of serum antibody of Helicobacter pylori and serum pepsinogen I/II ratio as a marker of the judgment after eradication therapy] Nihon Rinsho. 1999;57:101–106. [PubMed] [Google Scholar]
- 37.Araki H, Miyazaki R, Matsuda T, Gejyo F, Koni I. Significance of serum pepsinogens and their relationship to Helicobacter pylori infection and histological gastritis in dialysis patients. Nephrol Dial Transplant. 1999;14:2669–2675. doi: 10.1093/ndt/14.11.2669. [DOI] [PubMed] [Google Scholar]
- 38.Magnusson PKE H, Eriksson I, Held M, Nyrén O, Engstrand L, Hansson LE, Gyllensten UB. Gastric cancer and human leukocyte antigen: distinct DQ and DR alleles are associated with development of gastric cancer and infection by Helicobacter pylori. Cancer Res. 2001;61:2684–2689. [PubMed] [Google Scholar]
- 39.Archimandritis A, Sougioultzis S, Foukas PG, Tzivras M, Davaris P, Moutsopoulos HM. Expression of HLA-DR, costimulatory molecules B7-1, B7-2, intercellular adhesion molecule-1 (ICAM-1) and Fas ligand (FasL) on gastric epithelial cells in Helicobacter pylori gastritis; influence of H. pylori eradication. Clin Exp Immunol. 2000;119:464–471. doi: 10.1046/j.1365-2249.2000.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakai T, Aoyama N, Satonaka K, Shigeta S, Yoshida H, Shinoda Y, Shirasaka D, Miyamoto M, Nose Y, Kasuga M. HLA-DQB1 locus and the development of atrophic gastritis with Helicobacter pylori infection. J Gastroenterol. 1999;34 Suppl 11:24–27. [PubMed] [Google Scholar]
- 41.Yoshitake S, Okada M, Kimura A, Sasazuki T. Contribution of major histocompatibility complex genes to susceptibility and resistance in Helicobacter pylori related diseases. Eur J Gastroenterol Hepatol. 1999;11:875–880. doi: 10.1097/00042737-199908000-00011. [DOI] [PubMed] [Google Scholar]


