Abstract
AIM: To introduce an animal model of hepatocellular carcinoma (HCC) in ACI-rats, and to evaluate the therapeutic effects of Poly-lactide-co-glycolide(Plcg)-microspheres in the transarterial chemoembolization (TACE) in this model, as well the value of this model in the experiments of interventional therapy.
METHODS: Subcapsular implantation of a solid Morris Hepatoma 3924A (1 mm3) in the livers was carried out in 11 male ACI-rats. The tumor volume (V1) was measured by magnetic resonance imaging (MRI) (13 days after implantation). After laparotomy and retrograde placement of catheter into the gastroduodenal artery (14 days after implantation), the following protocols of interventional treatment were performed: (A) mitomycin C+Poly-lactide-co-glycolide(Plcg)-microspheres (n = 4); (B) 0.9% NaCl (control group, n = 7). 13 days after these therapies the change of the tumor volume (V2) was determined by MRI again.
RESULTS: The success rate of tumor implantation reached to 100%. The mean tumor volume before TACE (V1) were 0.082 cm3 in group A and 0.096 cm3 in group B respectively. The mean tumor volume after TACE (V2) were 0.230 cm3 in group A and 1.347 cm3 in group B respectively. The mean V2/V1 were 2.860 in group A and 27.120 in group B respectively. Compared to the control group (group B), groups A showed a significant reduction of tumor growth (P = 0.004) in the period of observation.
CONCLUSION: The growth of liver tumor could be obviously prevented by utilizing Plcg-mitomycin-microspheres in TACE in animal model. This rat model of HCC is suitable for the experimental studies of interventional therapy.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world, responsible for an estimated one million deaths annually[1,2]. In China, HCC has been ranked second of cancer mortality since 1990s[3]. It has a poor prognosis due to its rapid infiltrating growth and complicating liver cirrhosis[4]. Surgical resection is still the only potentially curative treatment for HCC, particularly for small HCC[3]. To date, the resection rate for HCC is unfortunately less than 30%[5,6]. Transarterial chemoembolization (TACE) is currently the first choice of treatment for most unresectable HCC and improve the survival rate in selected patients[7-13]. However, the technical variants of TACE have often been chosen on an empirical basis and it is not always safe and effective[14-16]. Although TACE advantageously combines arterial embolization of the vascular supply of HCC with controlled intra-arterial infusion of chemotherapeutic drugs, its application is limited by the lack of appropriate and reliable embolization materials[15,16]. Recently, poly-lactide-co-glycolide (Plcg)-microspheres has been proved to be a new promising embolic agent[17,18]. In order to study the interventional therapeutic strategies for HCC, it is necessary to establish a suitable and reproducible animal model. The aim of the present study was to introduce an animal model of HCC with the technique of tumor implantation in ACI-rats, and to evaluate the therapeutic effect of different methods of TACE, including the efficiency of Plcg-microspheres in this animal model. MRI was performed for measuring the tumor volume before and after the TACE in this study.
MATERIALS AND METHODS
Tumor
Morris hepatoma 3924A, a rapidly growing, poorly differentiated hepatocellular carcinoma, was induced by dietary administration of N-2-fluorenyldiacetamide in an ACI rat. The hepatoma specimens were obtained from the German Cancer Research Center in Heidelberg (DKFZ).
Animal
Inbred male ACI-rats weighing 200 to 220 g (n = 15) were obtained from the company of Harlan Winkelmann in Germany. The animals were kept under conventional conditions with a temperature of 22 ± 2 °C, a relative humidity of 55% ± 10%, a dark-light rhythm of 12 hr, and were fed standard laboratory chow and tap water ad libitum.
Anesthesia
All interventional and imaging procedures were carried out under intraperitoneally applied anesthesia with ketamine hydrochloride (Ketanest, Parke-Davis, Germany; 100 mg·kg-1), Xylazinhy-drochlorid (Rompun, Bayer, Germany; 15 mg·kg-1) and atropine sulfate (Atropinsulfat Braun, Braun, Germany; 0.1 mg·kg-1).
Tumor implantation (At day 1)
The technique for tumor implantation was basically similar to that described by Yang et al[19,20] with minor modifications[21]. The Morris Hepatoma 3924A tumor tissue, recovered from the passaged animals 2 weeks after subcutaneous implantation (corresponding to 5 × 106 tumor cells), was cut into small cubes about 1 mm3.
The recipient ACI-rats were intraperitoneally anesthetized, and the upper abdomen was shaved. A small subcapsular incision on the left lateral lobe of the liver was made. The tumor fragment was gently placed into the pocket with a small cotton swab on the liver surface as hemostasis and the abdominal wall was then closed.
Catheterization and TACE (At day 14)
A PE-10 polyethylene catheter (inner diameter 0.28 mm, outer diameter 0.61 mm; Wenzel/Heidelberg, Germany) was used for experiments of TACE under a second laparotomy. By using a binocular operative microscope (M651, Leica/Wetzler, Germany), the catheter was retrograde inserted into the gastroduodenal artery (Figure 1). After slightly drawing the thin rope around the common hepatic artery, the following different agents were injected through the catheter: Group A (n = 4): TACE with mitomycin C (0.25 mg·kg-1) and Plcg-microspheres (200 mg·kg-1, diameter: 40 µm; Institute of Pharmacological Technology, Philipps University, Marburg/Germany).
Figure 1.
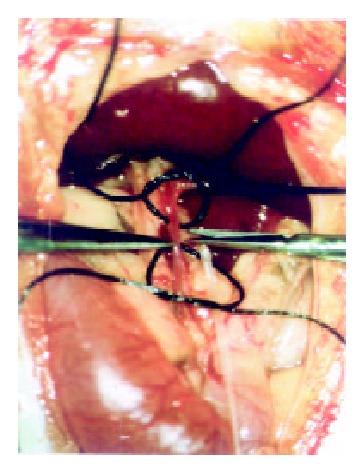
Catheterization of the gastroduodenal artery in vivo
Group B (control group, n = 7): injection of 0.9% NaCl alone.
MRI
MRI was performed in a 1.0 Tesla Magnetom superconducting system (Siemens, Erlangen, Germany) supplemented by a commercial coil (Small Field of View) before and after the catheterization (At day 13 and 27). T1-weighted (TR/TE, 400/14 ms) and T2-weighted (TR/TE, 3000/96 ms) axial SE images with spatial presaturation for controlling the flow artifact were acquired. Slice thickness was 2.0 mm, Matrix was 192 × 256. There was no gap between sections. The findings on T1- and T2-weighted SE images were examined in all 11 rats. Tumor volume was determined and evaluated according to the formula: Tumor volume (mm3) = Length (mm) × Width2 (mm)2/2[22].
RESULTS
In all the rats receiving tumor implantation with Morris Hepatoma 3924A, the rate of tumor implantation reached 100%. None of the animals died during implantation or in the postoperative period.
The sensitivity of MRI for detecting HCC reached 100%. HCC showed a hypointense pattern on T1-weighted images and a hyperintense pattern on T2-weighted images in the left lateral lobe of the liver (Figure 2, Figure 3). Necrosis, hemorrhage and metastase of the tumor were not observed. The liver tumor was well discernible from the surrounding liver tissue on each image.
Figure 2.
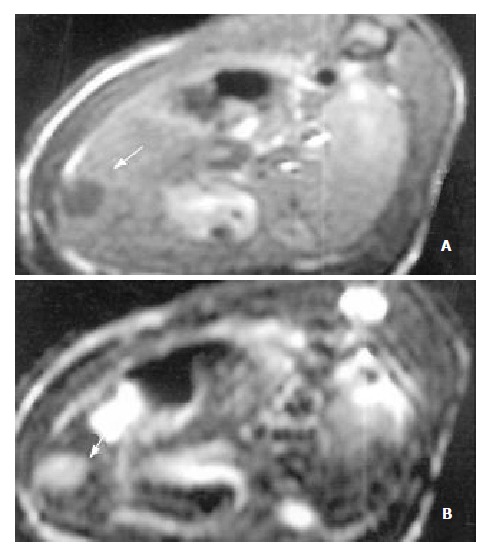
MR images of HCC before the application of Plcg-Mitomycin-microspheres: (a) T1-weighted image (400/14 ms) showed a hypointense pattern in the left lateral lobe of the liver, (b) T2-weighted image (3000/96 ms) showed a hyperintense pattern in the liver. Necrosis, hemorrhage and metastase of the tumor were not observed.
Figure 3.
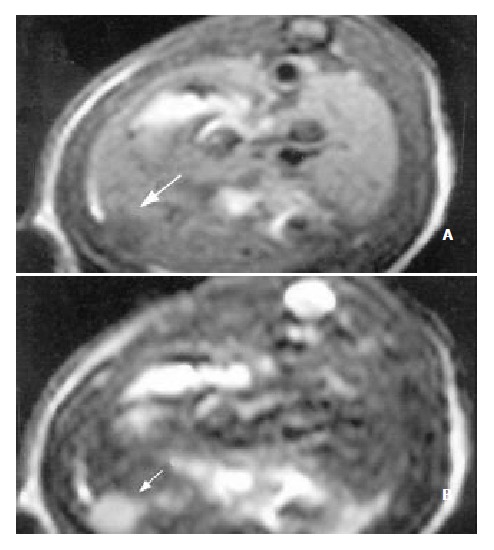
MR images of HCC after the application of Plcg-Mitomycin-microspheres: (a) MRI T1WI (400/14 ms); (b) MRI T2WI (3000/96 ms). The tumor volume after the application of Plcg-Mitomycin-microspheres was not changed compared to that before the application.
The efficiencies of Plcg-Mitomycin-microspheres in the interventional therapy of HCC in ACI-rats were shown in Table 1.
Table 1.
The efficiencies of Plcg-Mitomycin-microspheres in the interventional therapy of HCC in ACI-rats
| Group A (Plcg) | Group B (control group) | |
| Mean volume (V1) before TACE (cm3) | 0.082 | 0.096 |
| Mean volume (V2) after TACE (cm3) | 0.230 | 1.347 |
| Mean V2/V1 | 2.86 | 27.12 |
Compared to the control group (group B), groups A showed a significant reduction of tumor growth (P = 0.004) in the period of observation by t-test.
DISCUSSION
In order to investigate the efficiency of TACE for HCC, it is necessary to have a suitable and reproducible animal model. Various animal models of liver tumor have been established. The diethylnitrosamine model for hepatic tumor induction was simple, and provided a more representative range of tumors for experimental evaluation. However, the high mortality of the animals and various localization/number/size of the tumor in the liver were the major shortcomings. Therefore, the application of this model was extremely limited. The technique with a needle injection of tumor cells into hepatic parenchyma often caused tumor spill from the puncture channel and might result direct injection of the tumor cells into the circulation[19]. Although a relative high tumor take rate could be obtained by using the Walker-256/VX2 model in rats or rabbits with the technique of tumor implantation, these animal models belonged to carcinosarcoma or adenocarcinoma and were usually utilized for the study of liver metastases[23-27]. It is well known that the metastasis way and the therapeutic strategies as well the related effects of HCC and sarcoma are quite different from the aspect of histopathology, and a characteristic of HCC which distinguishes it from most metastases to the liver is that it is a highly vascular tumor[28], so that these animal models are unsatisfactory and not suitable for studying the interventional therapy.
In our present study, an animal model of HCC with the technique of tumor implantation previously described by Yang et al[19,20] was established. The rate of tumor implantation reached 100%[17]. Necrosis, hemorrhage and metastases of the tumor were not observed. Histopathologically, Morris hepatoma 3924A was a poorly differentiated hepatocellular carcinoma, mimicking the Edmondson grade-III hepatoma in humans[20] (Edmonson’s classification is based on the degree of differentiation of HCC). The appropriate growth speed of the liver tumor made it easy for MRI examination[29]. The blood supply of the tumor mainly came from hepatic arteries which was similar to that in human liver cancers[19,29]. As is known to all, HCC was usually hypervascular except for differentiated HCC and TACE was usually effective only for hypervascular HCC[30-33]. Compared to other animal models, our rat model was more suitable for the study of interventional therapy of HCC, and the related conclusion of the study was more convincing.
MRI examination for measuring the tumor volume without utilizing contrast media was carried out in our experiments. There was no single parameter better than tumor growth rate that could give information on the effects of different therapeutic maneuvers on tumor growth[22]. In another study of ours, we have demonstrated that MRI as an invasive imaging modality was superior to CT, DSA in the diagnosis of HCC in experiments[29]. It was supported by histological examination that MRI was also superior to ultrasonography for judging the tumor dimensions[29]. Another advantage of MRI was its excellent soft-tissue contrast resolution[34]. T1-weighted imaging was superior to T2-weighted imaging in depicting early HCC, but the latter could be useful in evaluating the progression of HCC in the histopathologically early stages[35]. The signal intensity on T2-weighted images correlated with the histological grade and histopathological change of HCC[35-37]. The detectability of MRI was 100% in the present study. HCC showed a hypointense pattern on T1-weighted images and a hyperintense pattern on T2-weighted images in the left lateral lobe of the liver. Necrosis, hemorrhage and metastase of the tumor were not observed. The contrast between the tumor and surrounding normal liver parenchyma was clear to observe. Based on these results, we concluded that MRI was useful in the assessment of the therapeutic effects of TACE in HCC.
TACE is one choice of the palliative treatment for unresectable HCC, particularly for patients with multifocal HCC and with acceptable liver functions. TACE caused tumor necrosis by occlusion of the feeding artery of HCC, and its clinical efficiencies have been generally recognized[38-41]. By using TACE with a combination of cytostatic drugs (mitomycin, doxorubicin, epirubicin, cisplatin, 5-Fu), a reduction of vital tumor tissue could be achieved[42-44], although the prolongation of survival remained questionable[45,46]. As stated before, although TACE advantageously combined arterial embolization of the vascular supply of a neoplasm with controlled intra-arterial infusion of chemotherapeutic drugs, its application was limited by the lack of appropriate and reliable embolic agents. The major problem with embolic agents are twofold[47]: first, they could often completely obstruct the hepatic artery, leading to difficulties in administration of subsequent courses of hepatic artery chemotherapy. With a relative short half-life of embolic agents, the effectiveness of TACE was not significantly improved; and second, it was easy to aggravate the liver cirrhosis and lead to hepatic failure after repeated TACE. The optimal treatment modality of TACE was unknown[14,48].
Recently, Plcg-microspheres have been proved to be a new promising embolic agent in TACE in experiments[17,18]. Verrijk and co-workers[49,50] reported that TACE with Plcg-microspheres and CDDP significantly improved the delivery of cytotoxic drugs to liver tumor and simultaneously reduced systemic toxicity in animals. Plcg-microspheres are biodegradable polymers with high molecular size and possess a good tissue compatibility. The degradation rate of polylactides is known to depend on the polymer microstructure that may be of the L, D or D, L type. The polyester lifetime could be controlled in a range of a few days or months[49-51]. It has been suggested that this drug could slowly release into the tumor tissues principally by a diffusion mechanism. It has also been indicated that the microspheres size of 40 µm allows a distal and homogeneous migration of the embolic agent within the targeted organ, without passing through the capillary filter[18], it is this reason that Plcg-microspheres with the size of 40 µm were chosen in our experiments. Verrijk et al[49,50] also demonstrated, that the absence of a burst effect and an adequate CDDP release from Plcg-CDDP-microspheres then significantly prolonged the first-pass effect, with the result that low systemic platinum levels were maintained while a liver platinum concentration was achieved which was high enough for an antitumor effect. The results of our present studies indicated that Plcg-microspheres could effectively retard the growth of HCC. Compared to the control group (group B), groups A (Plcg) showed a significant reduction of tumor growth (P = 0.004) in the period of observation by t-test. The therapeutic efficiencies of Plcg-mitomycin-microspheres was similar to Gelform+Mitomycin+Lipiodol in our another study (Mean V2/V1 = 2.86, P = 0.748, unpublished results).
To conclude, the growth of liver tumor was markedly prevented by the use of Plcg-mitomycin-microspheres in the TACE in an animal model. This effective and reliable embolic agent might be useful in clinic in future. However, the mechanism of effect of Plcg-microspheres is not clear so far. Data on a large number of animals and the test of application of various cytotoxic agents in the TACE, as well the repeated TACE will be required.
Footnotes
Supported by Bundesministerium fuer Bildung, Wissenschaft, Forschung und Technologie (Germany), No. 01KS9602
Edited by Pang LH
References
- 1.Cha C, DeMatteo RP, Blumgart LH. Surgery and ablative therapy for hepatocellular carcinoma. J Clin Gastroenterol. 2002;35:S130–S137. doi: 10.1097/00004836-200211002-00009. [DOI] [PubMed] [Google Scholar]
- 2.Teo EK, Fock KM. Hepatocellular carcinoma: an Asian perspective. Dig Dis. 2001;19:263–268. doi: 10.1159/000050692. [DOI] [PubMed] [Google Scholar]
- 3.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31:330–335. doi: 10.1002/hep.510310211. [DOI] [PubMed] [Google Scholar]
- 5.Sturm JW, Keese MA, Bönninghoff RG, Wüstner M, Post S. [Locally ablative therapies of hepatocellular carcinoma] Onkologie. 2001;24 Suppl 5:35–45. doi: 10.1159/000055185. [DOI] [PubMed] [Google Scholar]
- 6.Chung JW. Transcatheter arterial chemoembolization of hepatocellular carcinoma. Hepatogastroenterology. 1998;45 Suppl 3:1236–1241. [PubMed] [Google Scholar]
- 7.Acunaş B, Rozanes I. Hepatocellular carcinoma: treatment with transcatheter arterial chemoembolization. Eur J Radiol. 1999;32:86–89. doi: 10.1016/s0720-048x(99)00117-5. [DOI] [PubMed] [Google Scholar]
- 8.Rose DM, Chapman WC, Brockenbrough AT, Wright JK, Rose AT, Meranze S, Mazer M, Blair T, Blanke CD, Debelak JP, et al. Transcatheter arterial chemoembolization as primary treatment for hepatocellular carcinoma. Am J Surg. 1999;177:405–410. doi: 10.1016/s0002-9610(99)00069-0. [DOI] [PubMed] [Google Scholar]
- 9.Lladó L, Virgili J, Figueras J, Valls C, Dominguez J, Rafecas A, Torras J, Fabregat J, Guardiola J, Jaurrieta E. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50–57. doi: 10.1002/(sici)1097-0142(20000101)88:1<50::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Zangos S, Gille T, Eichler K, Engelmann K, Woitaschek D, Balzer JO, Mack MG, Thalhammer A, Vogl TJ. [Transarterial chemoembolization in hepatocellular carcinomas: technique, indications, results] Radiologe. 2001;41:906–914. doi: 10.1007/s001170170062. [DOI] [PubMed] [Google Scholar]
- 11.Tang ZY. Hepatocellular carcinoma. J Gastroenterol Hepatol. 2000;15 Suppl:G1–G7. doi: 10.1046/j.1440-1746.2000.02257.x. [DOI] [PubMed] [Google Scholar]
- 12.Livraghi T. Treatment of hepatocellular carcinoma by interventional methods. Eur Radiol. 2001;11:2207–2219. doi: 10.1007/s003300100889. [DOI] [PubMed] [Google Scholar]
- 13.Loewe C, Cejna M, Schoder M, Thurnher MM, Lammer J, Thurnher SA. Arterial embolization of unresectable hepatocellular carcinoma with use of cyanoacrylate and lipiodol. J Vasc Interv Radiol. 2002;13:61–69. doi: 10.1016/s1051-0443(07)60010-4. [DOI] [PubMed] [Google Scholar]
- 14.Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 15.Poon RT, Ngan H, Lo CM, Liu CL, Fan ST, Wong J. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol. 2000;73:109–114. doi: 10.1002/(sici)1096-9098(200002)73:2<109::aid-jso10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Trinchet JC, Ganne-Carrie N, Beaugrand M. Intra-arterial chemoembolization in patients with hepatocellular carcinoma. Hepatogastroenterology. 1998;45 Suppl 3:1242–1247. [PubMed] [Google Scholar]
- 17.Qian J, Feng GS, Truebenbach J, Pereira PL, Huppert PE, Graepler F, Eul T, Claussen CD. Experimentelle Untersuchung zur Effektivitat der Transarteriellen Chemoembolisation (TACE) im Tiermodell des hepatozellularen Karzinom. Acta Universitatis Medicinae Tongji. 2001;21:115–118. doi: 10.1007/BF02888072. [DOI] [PubMed] [Google Scholar]
- 18.Bastian P, Bartkowski R, Köhler H, Kissel T. Chemo-embolization of experimental liver metastases. Part I: distribution of biodegradable microspheres of different sizes in an animal model for the locoregional therapy. Eur J Pharm Biopharm. 1998;46:243–254. doi: 10.1016/s0939-6411(98)00047-2. [DOI] [PubMed] [Google Scholar]
- 19.Yang R, Rescorla FJ, Reilly CR, Faught PR, Sanghvi NT, Lumeng L, Franklin TD, Grosfeld JL. A reproducible rat liver cancer model for experimental therapy: introducing a technique of intrahepatic tumor implantation. J Surg Res. 1992;52:193–198. doi: 10.1016/0022-4804(92)90072-8. [DOI] [PubMed] [Google Scholar]
- 20.Yang R, Liu Q, Rescorla FJ, Grosfeld JL. Experimental liver cancer: improved response after hepatic artery ligation and infusion of tumor necrosis factor-alpha and interferon-gamma. Surgery. 1995;118:768–772; discussion 772-774. doi: 10.1016/s0039-6060(05)80048-0. [DOI] [PubMed] [Google Scholar]
- 21.Trübenbach J, Pereira PL, Graepler F, Huppert PE, Eul T, König CW, Duda SH, Claussen CD. [Animal experiment studies on the effectiveness of permanent occlusion of the hepatic artery in transarterial chemoembolization] Rofo. 2000;172:274–277. doi: 10.1055/s-2000-112. [DOI] [PubMed] [Google Scholar]
- 22.Carlsson G, Gullberg B, Hafström L. Estimation of liver tumor volume using different formulas - an experimental study in rats. J Cancer Res Clin Oncol. 1983;105:20–23. doi: 10.1007/BF00391826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarmenitis SD, Kalogeropoulou CP, Hatjikondi O, Ravazoula P, Petsas T, Siamblis D, Kalfarentzos F. An experimental approach of the Doppler perfusion index of the liver in detecting occult hepatic metastases: histological findings related to the hemodynamic measurements in Wistar rats. Eur Radiol. 2000;10:417–424. doi: 10.1007/s003300050068. [DOI] [PubMed] [Google Scholar]
- 24.Ishida H, Murata N, Yamada H, Nomura T, Shimomura K, Fujioka M, Idezuki Y. Effect of CO(2) pneumoperitoneum on growth of liver micrometastases in a rabbit model. World J Surg. 2000;24:1004–1008. doi: 10.1007/s002680010169. [DOI] [PubMed] [Google Scholar]
- 25.Kuszyk BS, Boitnott JK, Choti MA, Bluemke DA, Sheth S, Magee CA, Horton KM, Eng J, Fishman EK. Local tumor recurrence following hepatic cryoablation: radiologic-histopathologic correlation in a rabbit model. Radiology. 2000;217:477–486. doi: 10.1148/radiology.217.2.r00nv41477. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg SN, Walovitch RC, Straub JA, Shore MT, Gazelle GS. Radio-frequency-induced coagulation necrosis in rabbits: immediate detection at US with a synthetic microsphere contrast agent. Radiology. 1999;213:438–444. doi: 10.1148/radiology.213.2.r99nv17438. [DOI] [PubMed] [Google Scholar]
- 27.Guo WJ, Li J, Ling WL, Bai YR, Zhang WZ, Cheng YF, Gu WH, Zhuang JY. Influence of hepatic arterial blockage on blood perfusion and VEGF, MMP-1 expression of implanted liver cancer in rats. World J Gastroenterol. 2002;8:476–479. doi: 10.3748/wjg.v8.i3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carr BI. Hepatic artery chemoembolization for advanced stage HCC: experience of 650 patients. Hepatogastroenterology. 2002;49:79–86. [PubMed] [Google Scholar]
- 29.Trübenbach J, Graepler F, Pereira PL, Ruck P, Lauer U, Gregor M, Claussen CD, Huppert PE. Growth characteristics and imaging properties of the morris hepatoma 3924A in ACI rats: a suitable model for transarterial chemoembolization. Cardiovasc Intervent Radiol. 2000;23:211–217. doi: 10.1007/s002700010045. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima Y, Nakashima O, Hsia CC, Kojiro M, Tabor E. Vascularization of small hepatocellular carcinomas: correlation with differentiation. Liver. 1999;19:12–18. doi: 10.1111/j.1478-3231.1999.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 31.Tang ZY. Treatment of hepatocellular carcinoma. Digestion. 1998;59:556–562. doi: 10.1159/000007531. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi M, Matsui O, Ueda K, Kawamori Y, Gabata T, Kadoya M. Progression to hypervascular hepatocellular carcinoma: correlation with intranodular blood supply evaluated with CT during intraarterial injection of contrast material. Radiology. 2002;225:143–149. doi: 10.1148/radiol.2251011298. [DOI] [PubMed] [Google Scholar]
- 33.Kamura T, Kimura M, Sakai K, Ichida T, Seki H, Yamamoto S, Ozaki T. Small hypervascular hepatocellular carcinoma versus hypervascular pseudolesions: differential diagnosis on MRI. Abdom Imaging. 2002;273:15–24. doi: 10.1007/s00261-001-0074-z. [DOI] [PubMed] [Google Scholar]
- 34.Mueller-Lisse UG, Heuck AF. [Control and monitoring of focal thermotherapy with magnetic resonance tomography. An overview] Radiologe. 1998;38:200–209. doi: 10.1007/s001170050343. [DOI] [PubMed] [Google Scholar]
- 35.Honda H, Kaneko K, Maeda T, Kuroiwa T, Fukuya T, Yoshimitsu K, Irie H, Aibe H, Takenaka K, Masuda K. Small hepatocellular carcinoma on magnetic resonance imaging. Relation of signal intensity to angiographic and clinicopathologic findings. Invest Radiol. 1997;32:161–168. doi: 10.1097/00004424-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Inoue E, Kuroda C, Fujita M, Narumi Y, Kadota T, Kuriyama K, Ishiguro S, Kasugai H, Sasaki Y, Imaoka S. MR features of various histological grades of small hepatocellular carcinoma. J Comput Assist Tomogr. 1993;17:75–79. doi: 10.1097/00004728-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Yan FH, Zhou KR, Cheng JM, Wang JH, Yan ZP, Da RR, Fan J, Ji Y. Role and limitation of FMPSPGR dynamic contrast scanning in the follow-up of patients with hepatocellular carcinoma treated by TACE. World J Gastroenterol. 2002;8:658–662. doi: 10.3748/wjg.v8.i4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogl TJ, Schroeder H, Trapp M, Straub R, Schuster A, Schuster M, Mack M, Souchon F, Neuhaus P. [Multi-sequential arterial chemoembolization of advanced hepatocellular carcinomas: computerized tomography follow-up parameters for evaluating effectiveness of therapy] Rofo. 2000;172:43–50. doi: 10.1055/s-2000-279. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Wu PH, Li JQ, Zhang WZ, Lin HG, Zhang YQ. Segmental transcatheter arterial embolization for primary hepatocellular carcinoma. World J Gastroenterol. 1998;4:511–512. doi: 10.3748/wjg.v4.i6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen MS, Li JQ, Zhang YQ, Lu LX, Zhang WZ, Yuan YF, Guo YP, Lin XJ, Li GH. High-dose iodized oil transcatheter arterial chemoembolization for patients with large hepatocellular carcinoma. World J Gastroenterol. 2002;8:74–78. doi: 10.3748/wjg.v8.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan J, Ten GJ, He SC, Guo JH, Yang DP, Wang GY. Arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 1998;4:33–37. doi: 10.3748/wjg.v4.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamada K, Nakanishi T, Kitamoto M, Aikata H, Kawakami Y, Ito K, Asahara T, Kajiyama G. Long-term prognosis of patients undergoing transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: comparison of cisplatin lipiodol suspension and doxorubicin hydrochloride emulsion. J Vasc Interv Radiol. 2001;12:847–854. doi: 10.1016/s1051-0443(07)61510-3. [DOI] [PubMed] [Google Scholar]
- 43.Gattoni F, Dova S, Uslenghi CM. Three-year follow-up of 62 cirrhotic patients with hepatocellular carcinoma treated with chemoembolization. Minerva Chir. 2000;55:31–37. [PubMed] [Google Scholar]
- 44.Poyanli A, Rozaneş I, Acunaş B, Sencer S. Palliative treatment of hepatocellular carcinoma by chemoembolization. Acta Radiol. 2001;42:602–607. doi: 10.1080/028418501127347278. [DOI] [PubMed] [Google Scholar]
- 45.Roche A. Therapy of HCC--TACE for liver tumor. Hepatogastroenterology. 2001;48:3–7. [PubMed] [Google Scholar]
- 46.Yip D, Findlay M, Boyer M, Tattersall MH. Hepatocellular carcinoma in central Sydney: a 10-year review of patients seen in a medical oncology department. World J Gastroenterol. 1999;5:483–487. doi: 10.3748/wjg.v5.i6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwai K, Maeda H, Konno T. Use of oily contrast medium for selective drug targeting to tumor: enhanced therapeutic effect and X-ray image. Cancer Res. 1984;44:2115–2121. [PubMed] [Google Scholar]
- 48.Lorenz M, Liermann D, Staib-Sebler E, Gog C, Encke A, Kollath J. [Noradrenaline-assisted selective chemoembolization of hepatocellular carcinoma] Zentralbl Chir. 1994;119:777–786. [PubMed] [Google Scholar]
- 49.Verrijk R, Smolders IJ, Bosnie N, Begg AC. Reduction of systemic exposure and toxicity of cisplatin by encapsulation in poly-lactide-co-glycolide. Cancer Res. 1992;52:6653–6656. [PubMed] [Google Scholar]
- 50.Verrijk R, Smolders IJ, McVie JG, Begg AC. Polymer-coated albumin microspheres as carriers for intravascular tumour targeting of cisplatin. Cancer Chemother Pharmacol. 1991;29:117–121. doi: 10.1007/BF00687320. [DOI] [PubMed] [Google Scholar]
- 51.Hagiwara A, Sakakura C, Tsujimoto H, Imanishi T, Ohgaki M, Yamasaki J, Sawai K, Takahashi T, Fujita T, Yamamoto A, et al. Selective delivery of 5-fluorouracil (5-FU) to i.p. tissues using 5-FU microspheres in rats. Anticancer Drugs. 1997;8:182–188. doi: 10.1097/00001813-199702000-00009. [DOI] [PubMed] [Google Scholar]


