Abstract
AIM: To explore the status of extrahepatic hepatitis C virus (HCV) infection and replication in hepatitis C patients, and its potential implication in HCV infection and pathogenicity.
METHODS: By reverse-transcriptase poly-merase chain reaction (RT-PCR), in situ hybridization (ISH) and immunohistochemistry, HCV RNA, HCV replicative intermediate (minus-strand of HCV RNA), and HCV antigens were detected in 38 autopsy extrahepatic tissue specimens (including 9 kidneys, 9 hearts, 9 pancreas, 5 intestines, 2 adrenal glands, 2 spleens, 1 lymph node, and 1 gallbladder) from 9 hepatitis C patients, respectively; and the status of HCV replication in extrahepatic tissues was studied.
RESULTS: By RT-PCR, all 9 patients were positive for HCV RNA in kidney, heart, pancreas, and intestine, but only 6 (66.7%) patients were positive for HCV replicative intermediate. HCV RNA and HCV antigens were detected in kidney, heart, pancreas, intestine, adrenal gland, lymph node, and gallbladder in 5 (55.6%) and 6 (66.7%) patients by ISH and immunohistochemistry, respectively. HCV RNA and HCV antigens were not detected in these extrahepatic organs in 3 (33.3%) patients, although their livers were positive for HCV. HCV replicative intermediate detected by RT-PCR was consistent with HCV RNA and HCV antigens detected by ISH and immunohistochemistry (Kappa = 0.42-0.75). HCV RNA and HCV antigens were detected in myocardial cells, epithelial cells of intestinal gladular, interstitial cells of kidney, epithelial cells of tubules and glomerulus, pancreas acinar cells and epithelial cells of pancreatic duct, epithelial cells of mucous membrane sinus of gallbladder, cortex and medulla cells in adrenal gland, and mononuclear cells in lymph node. HCV RNA was also detected in bile duct epithelial cells, sinusoidal cells, and mononuclear cells in liver tissues by ISH.
CONCLUSION: HCV can infect extrahepatic tissues, and many various tissue cells may support HCV replication; extrahepatic HCV infection and replication may be of “concomitant state” in most of patients with hepati tis C. The infected extrahepatic tissues might act as a reservoir for HCV, and play a role in both HCV persistence and reactivation of infection. HCV as an etiologic agent replicating and expressing viral proteins in extrahepatic tissues itself contributes to extrahepatic syndrome associated-HCV infection in a few patients with chronic HCV infection.
Keywords: hepatitis C virus; hepatitis C antigens; in situ hybridization; immunohistochemistry; RNA; polymerase chain reaction; antibodies, monoclonal; digoxigenin
INTRODUCTION
Hepatitis C virus (HCV) was discovered in 1989[1], and is in a separate genus of the virus family Flavividae. Some aspects of cell tropism of Flavividae, such as the yellow fever virus and the dengue fever virus, have been clarified by in situ staining technique[2]. Different members of the Flavividae family infect a distinct, wide array of cells, resulting in multi-faceted disease expression. It should be noted that even a single amino acid change in the envelope molecule may modulate or even change the cell tropism and virulence of flaviviruses[3,4]. It may only be inferred that HCV may gain access to and replicate in extrahepatic tissues when compared with other flaviviruses. In 1985, the report of Hellings et al[5] showed that HCV, which was previously known as the non-A, non-B hepatitis virus, was successfully transmitted via the infusion of PBMC (peripheral blood mononuclear cells) purified from the patients; and then, Nouri Aria et al[6] reported that HCV RNA and HCV replicative intermediate (minus-strand of HCV RNA) were detected in the cytoplasm and nuclei of mononuclear and biliary epithelial cells in liver tissue by ISH. Other studies also indicated that HCV can transmit from mother to infant, possibly occurring in utero. These results suggested that HCV might replicate in PBMC and nonhepatic cells. In recent years, studies in extrahepatic HCV infection focused on readily obtainable tissue and body fluid samples such as PBMC[7-10], saliva, semen, urine, ascites, and biliary juices[11,12]. Subsequently, HCV replication in cells of the hematopoietic lineage has been demon-strated[8-10,13]. The results showed that it might be of pathological significance in HCV infection[8,10,14,15]. Recently, HCV RNA and HCV replicative intermediate in lymph node, pancreas, adrenal gland, thyroid, spleen, ovary, and uterus were detected by RT-PCR[16,17], but there was lack of in situ detection of HCV RNA in these extrahepatic tissues.
During HCV infection, the viral burden and subsequent tissue damage were mainly confined to the liver[18-22]. However, several extrahepatic syndromes associated with HCV infection have been reported[23-26], which include mixed cryoglobulinaemia, glomerulonephritis, lymphoma, and other extrahepatic diseases such as porphyria cutanea tarda[27]. Up to now, little is known about the mechanisms and the role of HCV in the development of these extra hepatic syndromes; and the extrahepatic tissue cells that support viral replication have not been identified. Accordingly, we conducted reverse-transcriptase polymerase chain reaction (RT-PCR), in situ hybridization (ISH), and immunohistochemistry for HCV RNA, HCV replicative intermediate, and HCV antigens in several extrahepatic tissues from 9 hepatitis C patients in an attempt to explore the status of extrahepatic HCV infection and replication, and its potential implication in HCV infection and pathogenicity. We believe that the information so derived could lead to a better understanding of the mechanisms of both HCV persistence and reactivation of infection, and the role of HCV in the development of extrahepatic syndromes associated with HCV infection.
MATERIALS AND METHODS
Patients
Nine patients with severe viral hepatitis (1986-1994, diagnosed by autopsy, according to the Diagnostic Criteria of Viral Hepatitis Prevention and Cure Guideline, China’ 95), 8 male, 1 female, aged 25-48 years, Han nationality, were included in this study. All the patients were positive for HCV antigens and HCV RNA in livers, and their sera were positive for HBV markers. Thirty-eight paraffin-embedded autopsy extrahepatic tissue specimens (including 9 kidneys, 9 hearts, 9 pancreas, 5 intestines, 2 adrenal glands, 2 spleens, 1 lymph node, and 1 gallbladder) were provided by the Department of Pathology and Anatomy, Third Military Medical University, China.
Main reagents
Mouse monoclonal anti-HCV NS3, NS5 and CP10[28]. A digoxigenin-labeled cDNA antisense probe to the 5’-non-coding region of the HCV genome was 145 bp in length (provided by the Division of Clinical Immunology, Tongji Medical University). HCV genome primers were derived from the highly conserved 5’-non-coding region of the HCV genome, and were synthesized by Gibco BRL; outer primers: sense 5'-ACTCCACCATAGATCATCCC-3', antisense 5'-AACACTACTCGGCTAGCAGT-3'; inner primers: sense 5’-TTCACGCAGAAAGCGTCTAG-3’, antisense 5’-GTTGATCCAAGAAAGGACCC-3’.
RT-PCR
RNA was extracted from 2 to 3 pieces of 5 μm thick paraffin-embedded extrahepatic tissue sections (31 specimens, including 9 kidneys, 9 hearts, 9 pancreas, and 4 intestines) cut from the same pathological blocks as used for ISH and immunohistochemistry with TRIzol (Gibco BRL).
RT-PCR was performed as previously described[29,30]. The expected size of the amplified product was 145 bp. The PCR products were analyzed by agarose gel electrophoresis. The sera which were negative for HCV RNA and HCV replicative intermediate and the distilled water were used as negative control.
Immunohistochemistry and ISH
Immunohistochemistry and ISH were performed as previously described[28,31]. Liver specimens-positive for HCV RNA and HCV antigens were used as positive control. Ten autopsy extrahepatic tissue specimens from viral hepatitis patients, whose livers were negative for HCV antigens and HCV RNA, and 10 autopsy extrahepatic tissue specimens from patients with rabies were used as negative control. Furthermore, control slides were treated with ribonucleases (RNase) and deoxyribonuclease (DNase) before hybridization; and the digoxigenin-labeled probe was substituted with non-labeled HCV cDNA probe, which was used as control in ISH as well.
RESULTS
Detection rates of HCV RNA and HCV antigen in the tissues
By RT-PCR, all 9 (positive in 20 specimens, 64.5%) patients were positive for HCV RNA in kidney, heart, pancreas, and intestine, but only 6 (66.7%), (positive in 11 specimens, 35.5%) of 9 patients were positive for HCV replicative intermediate (Figure 1). By ISH and immunoh-istochemistry, positive staining for HCV RNA and HCV antigens in kidney, heart, pancreas, intestine, adrenal gland, lymph node, and gallbladder were found in 5 (55.6%), (positive in 11 specimens, 28.9%) of 9, and 6 (66.7%), (positive in 23 specimens, 60.5%) of 9 patients, respectively. Positive rates of HCV RNA and HCV antigens in single extrahepatic organ are shown in Table 1. Three (33.3%) of 9 patients were negative for HCV RNA and HCV antigens in these extrahepatic organs, although their livers were positive for HCV.
Figure 1.
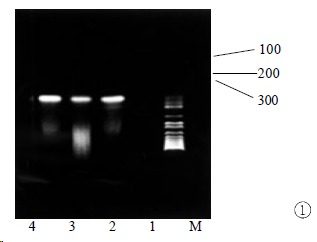
RT-PCR amplification result of minus-strand RNA in extrahepatic tissues. M: markers; 1: negative control; 2-4: amplification results of kidney, heart, and pancreas. The expected size of the amplification product is 145 bp
Table 1.
Results of HCV RNA and HCV antigens detection in extrahepatic organs and livers
| Tissue | Cases |
HCV RNA (+) |
HCV antigens (+) |
||||
| RT-PCR | ISH | NS3 | NS5 | CP10 | |||
| Kidney | 9 | 7 | 4* | 3 | 6 | 5 | 4 |
| Heart | 9 | 5 | 3* | 2 | 5 | 4 | 4 |
| Pancreas | 9 | 5 | 3* | 2 | 6 | 6 | 5 |
| Intestine | 5 | 3/4 | 1/4* | 1 | 3 | 2 | 0 |
| Adrenal gland | 2 | 1 | 1 | 1 | 0 | ||
| Spleen | 2 | 0 | 0 | 0 | 0 | ||
| Lymph node | 1 | 1 | 1 | 1 | 1 | ||
| Gallbladder | 1 | 1 | 1 | 0 | 1 | ||
| Liver | 9 | 9 | 7* | 9 | 8 | 7 | 7 |
Note:
Minus-strand RNA detected. The positivity of minus-strand RNA detected by RT-PCR was consistent with that of HCV RNA and HCV antigens detected by ISH and immunohistochemistry (Kappa = 0.42-0.75).
Expression of HCV RNA and HCV antigens in the tissues
HCV antigen staining was only seen within the cytoplasm with homogenous, inclusive or submembranous distribution. The hybridization signal was observed in both cytoplasm and nuclei, with a greater proportion of cytoplasm signal.
HCV existed in these extrahepatic organs except 2 spleens. There were HCV RNA and HCV antigen positive expressions in myocardial cells, epithelial cells of intestinal gladular, interstitial cells of kidney, epithelial cells of tubules and glomerulus, pancreas acinar cells and epithelial cells of pancreatic duct, epithelial cells of mucous membrane sinus of gallbladder, cortex and medulla cells in adrenal gland, and mononuclear cells in lymph node (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10). The amount of HCV positive cells in extrahepatic tissues was obviously less than that in liver tissues. In addition, there were some differences in HCV expression in various cells among different extrahepatic tissues, e.g. the amount of positive cells in heart tissue was less than that in kidney, pancreas, and intestine. In liver tissues, HCV antigens were only detected in hepatocytes, but hybridization signal of HCV RNA was seen in not only hepatocytes, but also bile duct epithelial cells, sinusoidal cells, and mononuclear cells (Figure 10, Figure 11, Figure 12). The amount of HCV positive cells in liver tissues was lower than 1%[28], but obviously higher than those in extrahepatic tissues.
Figure 2.
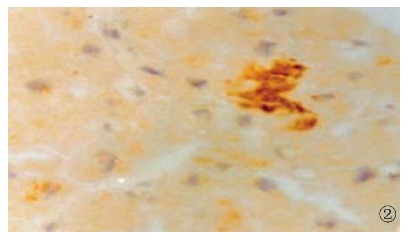
The expression of HCV NS3 in myocardial cells, show ing brown yellow. S-P (DAB) × 400
Figure 3.

The expression of HCV NS5 in epithelial cells of tubules, showing brown yellow. S-P (DAB) × 400
Figure 4.

The expression of HCV NS5 in the glomerulus, showing brown yellow. S-P (DAB) × 400
Figure 5.

The expression of HCV NS3 in mononuclear cells in lymph node, showing brown yellow. S-P (DAB) × 400
Figure 6.
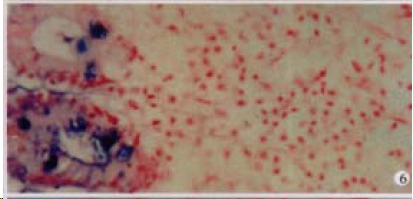
The expression of HCV RNA in epithelial cells of mucous membrane sinus of gallbladder, showing purple blue. ISH × 200
Figure 7.

The expression of HCV RNA in epithelial cells of intestinal gladular, showing purple blue. ISH × 400
Figure 8.

The expression of HCV RNA in the glomerulus, showing purple blue. ISH × 400
Figure 9.
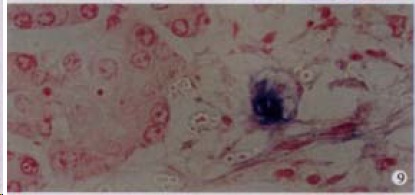
The expression of HCV RNA in the pancreas acinar cells, showing purple blue. ISH × 400
Figure 10.

The expression of HCV RNA in cortex cells in adrenal gland, showing purple blue. ISH × 400
Figure 11.
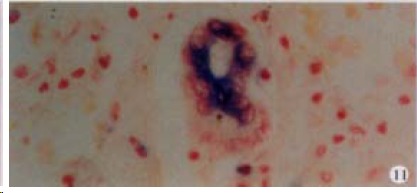
The expression of HCV RNA in bile duct epithelial cells in liver, showing purple blue. ISH × 400
Figure 12.

The expression of HCV RNA in bile duct cells (BDC), sinusoidal cells (SC) in liver, showing purple blue. ISH × 400
Negative control
The samples (such as extrahepatic tissues, sera, and the distilled water) from the subjects of control groups were negative for HCV RNA and HCV antigens detected by RT-PCR, ISH, and immunohistochemistry, respectively. Using RNase treatment of the extrahepatic tissues before hybridization, the HCV RNA staining in these tissue cells faded. DNase treatment did not reduce HCV RNA signal in these tissue cells. Using non-labeled cDNA probe, HCV RNA hybridization signal was not observed in these tissues.
DISCUSSION
Extrahepatic HCV infection was found in hepatitis C patients, recently. But as RT-PCR is fraught with problems[32,33], contradictory data rela ted to extrahepatic HCV infection have been reported[16,17,34-36], and there have been few in situ detection of HCV in extrahepatic tissues, the identification of extrahepatic HCV infection and replication has been controversial.
In this study, HCV RNA and HCV replicative intermediate in kidney, heart, pancreas, and intestine were detected by RT-PCR, but more importantly, the localization of HCV RNA and HCV antigens (NS3, NS5 and CP10) in kidney, heart, pancreas, intestine, adrenal gland, lymph node, and gallbladder was demonstrated convincingly by ISH and immunohistochemistry, and various tissue cell types harboring HCV such as pancreas acinar cells were also identified. Moreover, the positivity of HCV replicative intermediate detected by RT-PCR was consistent with that of HCV RNA and HCV antigens in these tissue cells detected by ISH and immunohistochemistry (Kappa = 0.42-0.75). More recently, we used a digoxigenin-labeled HCV oligonucleotide sense probe and detected HCV replicative intermediate in the extrahepatic tissues-positive for HCV RNA and HCV antigens by ISH (data being summarized). These results showed that HCV could infect extrahepatic tissues, and various tissue cells might support viral replication. Recently, Loriot et al[37] reported that gallbladder epithelial cells from HCV-negative subjects were successfully infected by HCV in vitro, and ISH and immunohistochemical studies identified pancreas and gastric mucosa as the sites of HCV infection[38,39]. These reports support the results of our studies.
So far, there have been few reports on the status of extrahepatic HCV infection and replication in hepatitis C patients. In order to explore this issue, we compared with the status of HCV expression between extrahepatic tissue and liver, and also among different extrahepatic tissues, the results indicated that the detection rates of HCV in extrahepatic tissues were low[40], and the amount of HCV positive staining cells in extrahepatic tissues was also obviously lower than that in livers; and there were differences in HCV expression in various cells among different extrahepatic tissues. These results suggested that the levels of extrahepatic HCV infection and replication were relatively low as compared with those of livers; and there were differences in the status of HCV replication among different extrahepatic tissues. Whether HCV can bring about cell injury of infected extrahepatic tissues is not well understood because of the lack of extrahepatic histopathological observation. Recently, some reports showed that HCV is an causal agent in the pathogenesis of hypertrophic cardiomyopathy and chronic myocarditis[41,42], and that the patients’ gastroduodenal mucosal lesions might be associated with HCV infection[43]. However, most of the patients with hepatitis C did not have extrahepatic clinical manifestations[44]. In our previousl studies, we found no relationship between the HCV expression in extrahepatic tissue cells and tissue lesions[45]. We speculate that extrahepatic HCV infection and replication may be of “concomitant state” in most of patients with hepatitis C, while liver was a key organ in HCV infection and pathogenicity[18,21,31,45], i.e. HCV may replicate in various tissue cells, but viral replication may not cause obvious cell injury of these infected tissues.
Zignego et al[46] reported that the infected PBMC might act as a reservoir for HCV. In this study, various tissue cells may support viral replication. These extrahepatic tissue and PBMC reservoirs are considered to provide the source of HCV for the patient’s liver reinfection and recurrence of infection after liver transplantation for hepatitis C. Once the immunologically privileged sites are infected, HCV is more difficultly eliminated by the host and/or antivirus therapy, which might play a role in both HCV persistence and reactivation of infection after interferon therapy[8,47].
At present, little is known of the mechanisms and the HCV role in the development of HCV-related extrahepatic syndromes. Some reports showed that the vasculitic and kidney damage in mixed cryoglobulinemia and glomerulonephritis associated with HCV infection might be mediated by immune mechanisms[48,49]. However, in this study, HCV may replicate and express viral proteins in many tissue cells such as interstitial cells of kidney, epithelial cells of tubules, and glomerulus. It is noticeable that some of the cell types found to harbor HCV are also associated with HCV-related extrahepatic syndromes such as glomerulonephritis. In addition, HCV antigens were also found in mesangial and paramesangial cells and epithelial cells of tubules in glomerulonephritis kidney tissues[24,48], and in lymphoid cells in hyperplastic reactive lymphadenopathy[50]; HCV RNA and/or HCV replicative intermediate were detected in residual parotid epithelial cells in the parotid non-Hodgkin’s lymphoma lesion by ISH[51], and in epineurial cells in mixed cryoglobulinemia-associated neuropathy by in situ RT-PCR[52]. HCV as an etiologic agent replicating and expressing viral proteins in extrahepatic tissues itself contributes to extrahepatic syndrome associated-HCV infection in a few patients with chronic HCV infection. For example, a pathogenetic role of HCV as an exogenous trigger might be hypothesized in the parotid non-Hodgkin’s lymphoma[51], or in hypertrophic cardiomypathy[41]. In hepatitis B patients, several extrahepatic syndromes associated-HBV infection have been considered to be mediated by immune complexes or other immunological mechanisms, but various vasculitic and other HBV-related skin lesions are associated with local HBV replication. So Mason et al[53] suggested that once the HBV-related immune complexes are captured by the vascular endothelium, the vasculitic damage might also be mediated by viral replication and immune mechanisms. We speculate that HCV might use a similar mechanism to bring about the vasculitic and kidney damage in the development of mixed cryoglobulinemia and glomerulonephritis associated-HCV infection.
Footnotes
Edited by Ma JY
Supported by the Medical and Health Sciences Foundation of Chinese PLA, No.98D066
This work was accomplished in Tongji Hospital of Tongji Medical Universi ty and Southwest Hospital of Third Military Medical University
References
- 1.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood borne non A, non B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 2.Hall WC, Crowell TP, Watts DM, Barros VL, Kruger H, Pinheiro F, Peters CJ. Demonstration of yellow fever and dengue antigens in formalin-fixed paraffin-embedded human liver by immunohistochemical analysis. Am J Trop Med Hyg. 1991;45:408–417. doi: 10.4269/ajtmh.1991.45.408. [DOI] [PubMed] [Google Scholar]
- 3.Sánchez IJ, Ruiz BH. A single nucleotide change in the E protein gene of dengue virus 2 Mexican strain affects neurovirulence in mice. J Gen Virol. 1996;77(Pt 10):2541–2545. doi: 10.1099/0022-1317-77-10-2541. [DOI] [PubMed] [Google Scholar]
- 4.Chen LK, Lin YL, Liao CL, Lin CG, Huang YL, Yeh CT, Lai SC, Jan JT, Chin C. Generation and characterization of organ-tropism mutants of Japanese encephalitis virus in vivo and in vitro. Virology. 1996;223:79–88. doi: 10.1006/viro.1996.0457. [DOI] [PubMed] [Google Scholar]
- 5.Hao F, Li MD, Chen GZ. Intrauterine HCV infection identified by sequencing a segment of envelope glycoprotein. Xin Xiaohuabingxue Zazhi. 1997;5:346–347. [Google Scholar]
- 6.Sun DG, Liu CY, Meng ZD, Sun YD, Wang SC, Yang YQ, Liang ZL, Zhuang H. A prospective study of vertical transmissionof hepatitis C virus. China Natl J New Gastroenterol. 1997;3:111–113. doi: 10.3748/wjg.v3.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou P, Cai Q, Chen YC, Zhang MS, Guan J, Li XJ. Hepatitis C virus RNA detection in serum and peripheral blood mononuclear cells of patients with hepatitis C. China Natl J New Gastroenterol. 1997;3:108–110. doi: 10.3748/wjg.v3.i2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu HB, Li ZW, Li Y. Clinical significance of detection of positive and negative strands of HCV RNA in peripheral bloodmononuclear cells. Shijie Huaren Xiaohua Zazhi. 1999;7:220–221. [Google Scholar]
- 9.Cheng JL, Chen LB, Tong WB, Chen PL, Liu BL, Gao JE, Du SC, Feng BF. Persistence of hepatitis C virus type II in patient's peripheral blood B lymphocytes transformed by Epstein-Barr virus. Zhonghua Yixue Zazhi. 2000;80:349–353. [PubMed] [Google Scholar]
- 10.Bronowicki JP, Loriot MA, Thiers V, Grignon Y, Zignego AL, Bréchot C. Hepatitis C virus persistence in human hematopoietic cells injected into SCID mice. Hepatology. 1998;28:211–218. doi: 10.1002/hep.510280127. [DOI] [PubMed] [Google Scholar]
- 11.Liou TC, Chang TT, Young KC, Lin XZ, Lin CY, Wu HL. Detection of HCV RNA in saliva, urine, seminal fluid, and ascites. J Med Virol. 1992;37:197–202. doi: 10.1002/jmv.1890370309. [DOI] [PubMed] [Google Scholar]
- 12.Kuan SF, Garcia-Tsao G, Cartun RW, Emanuel JR, West AB. Viral RNA in duodenal bile of cirrhotic patients with chronic hepatitis C. Arch Pathol Lab Med. 1997;121:847–852. [PubMed] [Google Scholar]
- 13.Chen L, Chen P, Tian H. [Identification and visualization of virus-like particles in peripheral blood mononuclear cells] Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2000;14:14–8, 101. [PubMed] [Google Scholar]
- 14.Fan XG, Tang FQ, Ou ZM, Zhang JX, Liu GC, Hu GL. Lymphoproliferative response to hepatitis C virus (HCV) antigens in patients with chronic HCV infection. Shijie Huaren Xiaohua Zazhi. 1999;7:1038–1040. [Google Scholar]
- 15.Nie QH, Li MD, Hu DR, Chen GZ. Study on the cause of human protective immunodeficiency after HCV infection. Shijie Huaren Xiaohua Zazhi. 2000;8:28–30. [Google Scholar]
- 16.Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. Search for hepatitis C virus extrahepatic replication sites in patients with acquired immunodeficiency syndrome: specific detection of negative-strand viral RNA in various tissues. Hepatology. 1998;28:1398–1401. doi: 10.1002/hep.510280531. [DOI] [PubMed] [Google Scholar]
- 17.Sugiyama K, Kato N, Ikeda M, Mizutani T, Shimotohno K, Kato T, Sugiyama Y, Hasumi K. Hepatitis C virus in pelvic lymph nodes and female reproductive organs. Jpn J Cancer Res. 1997;88:925–927. doi: 10.1111/j.1349-7006.1997.tb00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu JG, Zhang GS, Yang DL, Zhou XM, Chen ZX. Survey on the liver pathology of chronic active hepatitis C. Xin Xiaohuabingxue Zazhi. 1996;4(Suppl 5):41–42. [Google Scholar]
- 19.Zhang SL, Liang XS, Lin SM, Qiu PC. Relation between viremia level and liver disease in patients with chronic HCV infection. China Natl J New Gastroenterol. 1996;2:115–117. [Google Scholar]
- 20.Assy N, Minuk G. A comparison between previous and present histologic assessments of chronic hepatitis C viral infections in humans. World J Gastroenterol. 1999;5:107–110. doi: 10.3748/wjg.v5.i2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan FM, Hao F, Gu CH, Zhang XQ, Zhao LB. Characteristics and significance of expression of hepatitis C virus antigens inextrahepatic tissue and liver. Disan Junyi Daxue Xuebao. 1999;21:443–445. [Google Scholar]
- 22.Lin XT, Luo KX, Ren XF, He HT, Zhu YF, Zhang L. Fas antigen and Fas ligand expression in liver tissues of patients withchronic hepatitis C. Huaren Xiaohua Zazhi. 1998;6:298–299. [Google Scholar]
- 23.Nguyen QT, Leruez-Ville M, Ferrière F, Cohen P, Roulot-Marullo D, Coste T, Dény P, Guillevin L. Hepatitis C virus genotypes implicated in mixed cryoglobulinemia. J Med Virol. 1998;54:20–25. doi: 10.1002/(sici)1096-9071(199801)54:1<20::aid-jmv4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 24.Lang ZW, Guo XH, Meng X, Zhang SJ, Li BG, Wei M. Hepatitis C virus-induced glomerulonephritis. Zhonghua Neike Zazhi. 1998;37:320–322. [Google Scholar]
- 25.Rasul I, Shepherd FA, Kamel-Reid S, Krajden M, Pantalony D, Heathcote EJ. Detection of occult low-grade b-cell non-Hodgkin's lymphoma in patients with chronic hepatitis C infection and mixed cryoglobulinemia. Hepatology. 1999;29:543–547. doi: 10.1002/hep.510290224. [DOI] [PubMed] [Google Scholar]
- 26.Ji XL. Hepatitis C closely relevant to gastrointestinal lymphoma. Xin Xiaohuabingxue Zazhi. 1997;5:279. [Google Scholar]
- 27.Chuang TY, Brashear R, Lewis C. Porphyria cutanea tarda and hepatitis C virus: a case-control study and meta-analysis of the literature. J Am Acad Dermatol. 1999;41:31–36. doi: 10.1016/s0190-9622(99)70402-0. [DOI] [PubMed] [Google Scholar]
- 28.Yan FM, Hao F, Gu CH. Study of expression of hepatitis C virus antigens in liver from patients with severe viral hepatitis. Disan Junyi Daxue Xuebao. 1998;20:412–414. [Google Scholar]
- 29.Tang ZY, Yang DL, Wang YK, Yu ZQ, Hao LJ. Establishment of reverse transcription polymerase chain reaction for detection of hepatitis C virus RNA and analysis of experimental factors. Tongji Yike Daxue Xuebao. 1995;24:327–329. [Google Scholar]
- 30.Tang W, Du SC, Tao QM, Zhu L. A study on anti-contamination of RT-PCR in detection of HCV-RNA. Xin Xiaohuabingxue Zazhi. 1997;5:638–639. [Google Scholar]
- 31.Zhao XP, Shen HX, Tian DY, Zhang DS, Peng ZH, Yang DL, Hao LJ. Expression and significance of HCV RNA and HCV NS5 antigen in liver tissues of patients with hepatitis C. Shijie Huaren Xiaohua Zazhi. 1999;7:516–518. [Google Scholar]
- 32.Komminoth P, Adams V, Long AA, Roth J, Saremaslani P, Flury R, Schmid M, Heitz PU. Evaluation of methods for hepatitis C virus detection in archival liver biopsies. Comparison of histology, immunohistochemistry, in situ hybridization, reverse transcriptase polymerase chain reaction (RT-PCR) and in situ RT-PCR. Path Res Pract. 1994;190:1017–1025. doi: 10.1016/s0344-0338(11)80896-4. [DOI] [PubMed] [Google Scholar]
- 33.Sangar DV, Carroll AR. A tale of two strands: reverse-transcriptase polymerase chain reaction detection of hepatitis C virus replication. Hepatology. 1998;28:1173–1176. doi: 10.1002/hep.510280501. [DOI] [PubMed] [Google Scholar]
- 34.Lanford RE, Chavez D, Chisari FV, Sureau C. Lack of detection of negative-strand hepatitis C virus RNA in peripheral blood mononuclear cells and other extrahepatic tissues by the highly strand-specific rTth reverse transcriptase PCR. J Virol. 1995;69:8079–8083. doi: 10.1128/jvi.69.12.8079-8083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takamatsu K, Okayasu I, Koyanagi Y, Yamamoto N. Hepatitis C virus propagates in salivary glands. J Infect Dis. 1992;165:973–974. doi: 10.1093/infdis/165.5.973. [DOI] [PubMed] [Google Scholar]
- 36.Taliani G, Celestino D, Badolato MC, Pennica A, Bozza A, Poliandri G, Riccieri V, Benfari G, Sebastiani A, De Bac C, et al. Hepatitis C virus infection of salivary gland epithelial cells. Lack of evidence. J Hepatol. 1997;26:1200–1206. doi: 10.1016/s0168-8278(97)80452-7. [DOI] [PubMed] [Google Scholar]
- 37.Loriot MA, Bronowicki JP, Lagorce D, Lakehal F, Persico T, Barba G, Mergey M, Vons C, Franco D, Belghiti J, et al. Permissiveness of human biliary epithelial cells to infection by hepatitis C virus. Hepatology. 1999;29:1587–1595. doi: 10.1002/hep.510290527. [DOI] [PubMed] [Google Scholar]
- 38.Lang ZW, Huang DZ, Guo XH, Yan HP, Meng X, Zhang SJ. Detection of HCV in pancreas tissues from patients with HCV infection. Linchuang Gandanbing Zazhi. 1997;13:137–139. [Google Scholar]
- 39.Yuan GH, Luo ZX, Huang QT, Lang ZW, An DR, Tu DM. Detection of HCV antigens in the gastric mucosa of patients withchronic hepatitis C. Zhonghua Chuanranbing Zazhi. 1998;16:148–150. [Google Scholar]
- 40.Yan F, Hao F, Zhao L. [Study of expression of hepatitis C virus antigens and viral replication in extrahepatic tissues] Zhonghua Gan Zang Bing Za Zhi. 2000;8:40–42. [PubMed] [Google Scholar]
- 41.Matsumori A, Ohashi N, Nishio R, Kakio T, Hara M, Furukawa Y, Ono K, Shioi T, Hasegawa K, Sasayama S. Apical hypertrophic cardiomyopathy and hepatitis C virus infection. Jpn Circ J. 1999;63:433–438. doi: 10.1253/jcj.63.433. [DOI] [PubMed] [Google Scholar]
- 42.Okabe M, Fukuda K, Arakawa K, Kikuchi M. Chronic variant of myocarditis associated with hepatitis C virus infection. Circulation. 1997;96:22–24. doi: 10.1161/01.cir.96.1.22. [DOI] [PubMed] [Google Scholar]
- 43.You J, Zhuang L, Tang BZ, Tang WH, Liu BY. Relationship between chronic viral hepatitis Cand gastroduodenal mucosal lesions. Huaren Xiaohua Zazhi. 1998;6:963–965. [Google Scholar]
- 44.Cacoub P, Renou C, Rosenthal E, Cohen P, Loury I, Loustaud-Ratti V, Yamamoto AM, Camproux AC, Hausfater P, Musset L, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d'Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l'Hepatite C. Medicine ( Baltimore) 2000;79:47–56. doi: 10.1097/00005792-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Yan FM, Hao F, Gu CH, Zhao LB, Chen AS, Zhao XP, Hao LJ. Immunohistochemical study on the state of extrahepatic hepatitis C virus infection in severe hepatitis C. Zhonghua Chuanranbing Zazhi. 1999;17:231–233. [Google Scholar]
- 46.Zignego AL, Macchia D, Monti M, Thiers V, Mazzetti M, Foschi M, Maggi E, Romagnani S, Gentilini P, Bréchot C. Infection of peripheral mononuclear blood cells by hepatitis C virus. J Hepatol. 1992;15:382–386. doi: 10.1016/0168-8278(92)90073-x. [DOI] [PubMed] [Google Scholar]
- 47.Yan FM, Hao F, Zhao LB, Gu CH, Chen AS, Zhao XP, Hao LJ. Study on the expression of HCV RNA and antigens in multiextrahepatic tissues. Zhonghua Neike Zazhi. 1999;38:669. [Google Scholar]
- 48.Sansonno D, Gesualdo L, Manno C, Schena FP, Dammacco F. Hepatitis C virus-related proteins in kidney tissue from hepatitis C virus-infected patients with cryoglobulinemic membranoproliferative glomerulonephritis. Hepatology. 1997;25:1237–1244. doi: 10.1002/hep.510250529. [DOI] [PubMed] [Google Scholar]
- 49.Sansonno D, Cornacchiulo V, Iacobelli AR, Di Stefano R, Lospalluti M, Dammacco F. Localization of hepatitis C virus antigens in liver and skin tissues of chronic hepatitis C virus-infected patients with mixed cryoglobulinemia. Hepatology. 1995;21:305–312. [PubMed] [Google Scholar]
- 50.Sansonno D, De Vita S, Cornacchiulo V, Carbone A, Boiocchi M, Dammacco F. Detection and distribution of hepatitis C virus-related proteins in lymph nodes of patients with type II mixed cryoglobulinemia and neoplastic or non-neoplastic lymphoproliferation. Blood. 1996;88:4638–4645. [PubMed] [Google Scholar]
- 51.De Vita S, Sansonno D, Dolcetti R, Ferraccioli G, Carbone A, Cornacchiulo V, Santini G, Crovatto M, Gloghini A, Dammacco F, et al. Hepatitis C virus within a malignant lymphoma lesion in the course of type II mixed cryoglobulinemia. Blood. 1995;86:1887–1892. [PubMed] [Google Scholar]
- 52.Bonetti B, Scardoni M, Monaco S, Rizzuto N, Scarpa A. Hepatitis C virus infection of peripheral nerves in type II cryoglobulinaemia. Virchows Arch. 1999;434:533–535. doi: 10.1007/s004280050380. [DOI] [PubMed] [Google Scholar]
- 53.Mason A, Wick M, White H, Perrillo R. Hepatitis B virus replication in diverse cell types during chronic hepatitis B virus infection. Hepatology. 1993;18:781–789. doi: 10.1002/hep.1840180406. [DOI] [PubMed] [Google Scholar]


