Abstract
Importance
Hypertension is prevalent among patients with psoriasis. The effect of psoriasis and its severity on hypertension control is unknown.
Objective
To determine the association between uncontrolled blood pressure and psoriasis, both overall and according to objectively measured psoriasis severity, among patients with diagnosed hypertension.
Design, Setting, and Participants
Population-based cross-sectional study nested in a prospective cohort drawn from The Health Improvement Network (THIN), an electronic medical records database broadly representative of the general population in the United Kingdom. The study population included a random sample of patients with psoriasis (n = 1322) between the ages of 25 and 64 years in THIN who were included in the Incident Health Outcomes and Psoriasis Events prospective cohort and their age- and practice-matched controls without psoriasis (n = 11 977). All included patients had a diagnosis of hypertension; their psoriasis diagnosis was confirmed and disease severity was classified by their general practitioners.
Main outcomes and measures
Uncontrolled hypertension was defined as a systolic blood pressure of 140 mm Hg or higher or a diastolic blood pressure of 90 mm Hg or higher based on the blood pressure recorded closest in time to the assessment of psoriasis severity.
Results
There was a significant positive dose-response relationship between uncontrolled hypertension and psoriasis severity as objectively determined by the affected body surface area in both unadjusted and adjusted analyses that controlled for age, sex, body mass index, smoking and alcohol use status, presence of comorbid conditions, and current use of antihypertensive medications and nonsteroidal anti-inflammatory drugs (adjusted odds ratio [aOR], 0.97; 95% CI, 0.82-1.14 for mild psoriasis; aOR, 1.20; 95% CI, 0.99-1.45 for moderate psoriasis; and aOR, 1.48; 95% CI, 1.08-2.04 for severe psoriasis; P = .01 for trend). The likelihood of uncontrolled hypertension among psoriasis overall was also increased, although not statistically significantly so (aOR, 1.10; 95% CI, 0.98-1.24).
Conclusions and Relevance
Among patients with hypertension, psoriasis was associated with a greater likelihood of uncontrolled hypertension in a dose-dependent manner, with the greatest likelihood observed among those with moderate to severe psoriasis defined by 3% or more of the body surface area affected. Our data suggest a need for more effective blood pressure management, particularly among patients with more severe psoriasis.
Psoriasis is a chronic inflammatory disease of the skin that affects 2% to 4% of the general population.1 Cardiovascular risk factors,2 such as metabolic syndrome3 and its individual components including hypertension,4 obesity,5 dyslipidemia,6 and diabetes mellitus,7 are more prevalent among patients with psoriasis compared with those without psoriasis, and the prevalence of each risk factor generally increases with greater psoriasis severity. An expanding body of epidemiologic data8-15 suggests that psoriasis, especially when more severe, is associated with an increased risk of major adverse cardiovascular events (ie, myocardial infarction, stroke, and cardiovascular mortality) independent of traditional cardiovascular risk factors. Hypertension is a major risk factor for the development of cardiovascular disease and, thus, an important modifiable cause of premature morbidity and mortality.16
Previous studies17-21 have indicated that the presence of comorbid disease, specifically 2 or more comorbidities, is associated with poorer treatment of each disease. Among patients with hypertension, there are scant and inconsistent data22,23 to suggest that a variety of comorbid diseases affect blood pressure control. To our knowledge, no prior study has specifically assessed the effect of psoriasis on blood pressure control among hypertensive patients. Therefore, the purpose of the present study was to investigate the effect of psoriasis and psoriasis severity (as defined objectively by affected body surface area [BSA]) on blood pressure control among patients with diagnosed hypertension in a broadly representative population-based cohort. We hypothesized that, among patients with hypertension, those with psoriasis would be more likely to have uncontrolled blood pressure than would patients without psoriasis and that the relationship would be positively associated with greater psoriasis severity.
Methods
Study Design and Data Source
We conducted a population-based, cross-sectional study using The Health Improvement Network (THIN), a large (7.5 million patients from 415 practices) electronic medical records database maintained by general practitioners (GPs) and broadly representative of the United Kingdom. General practitioners, the gatekeepers of medicine in the United Kingdom, collect patients' demographic, diagnostic, treatment, and laboratory information using Vision software (In Practice Systems Ltd). THIN has been widely used and validated24 for the epidemiologic research of many diseases, including psoriasis. This study was conducted according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement and the Declaration of Helsinki and was granted exempt status by the University of Pennsylvania institutional review board. Study participants were registered in a practice with an Additional Information Services contract, which involves an agreement by the participants' GPs to complete questionnaires in exchange for financial compensation.
Study Population and Time of Observation
The Incident Health Outcomes and Psoriasis Events cohort was created by randomly sampling patients in THIN who were alive and aged 25 to 64 years at the time of sampling, had received at least 1 READ diagnostic code for psoriasis in the 2 years before sampling, and were registered in a practice with an Additional Information Services contract. Surveys with face and content validity designed by experts in epidemiology, dermatology, and primary care were sent to GPs of the sampled patients with psoriasis to verify the diagnosis and classify their disease extent via the National Psoriasis Foundation classification system as mild (limited disease with ≤2% BSA affected), moderate (scattered disease with 3%-10% BSA affected), or severe (extensive disease with >10% BSA affected), The exposed group consisted of patients with psoriasis READ diagnostic codes whose diagnosis and amount of skin involvement were verified by their GPs. The unexposed comparison group was constructed by randomly matching each psoriasis patient with up to 10 patients without psoriasis READ diagnostic codes who were from the same practice, in the same age category, and alive and actively registered with at least 1 GP visit within 2 years before sampling. To study the association between psoriasis severity and blood pressure control in patients with previously diagnosed hypertension, the study cohort was further restricted to patients with at least 1 READ diagnostic code for hypertension before or on the survey sampling date.
The start of the observation time was defined as the later of the acceptable mortality recording date and patient registration date in the practice. The end of the observation time was the date of GP survey sampling (November 2008 to September 2010). With the exception of blood pressure measurements, all covariates and laboratory values were assessed during the defined observation time window. Patients with any missing data or those who died or transferred out of the GP's practice before the end of the observation time were excluded from the analyses.
Blood Pressure Outcome and Covariate Definitions
Blood pressure is well recorded in THIN, and GPs are incentivized to record blood pressure measurements for new patient screening and chronic disease monitoring.25 Systolic and diastolic blood pressure records closest to (before or after) the survey sampling date were used to determine blood pressure control in patients with and without psoriasis. Based on the UK National Institute of Health and Care Excellence26 clinical guidelines, uncontrolled hypertension was defined as systolic blood pressure of 140 mm Hg or higher or diastolic blood pressure of 90 mm Hg or higher. In addition to primarily determining the proportions of patients with uncontrolled hypertension, differences in the proportions of patients currently receiving any antihypertensive treatment among those with and without psoriasis were assessed secondarily.
To investigate the effect of psoriasis severity on blood pressure control independent of other risk factors, multiple potential confounders and effect modifiers, including age, sex, body mass index (BMI), smoking and alcohol use status, medical comorbidities (ie, diabetes mellitus, hyperlipidemia, chronic kidney disease, and cardiovascular disease), treatment patterns (history of cyclosporine or systemic corticosteroid use, and current antihypertensive therapy or nonsteroidal anti-inflammatory drug [NSAID] use), duration of observation time, frequency of blood pressure recordings, and time between the most recent blood pressure record and survey sampling date, were assessed in multivariable models. Age and BMI were treated as continuous variables. Comorbid medical conditions were assessed as prevalent cases (eg, a patient was identified as having diabetes if he or she received a diabetes READ diagnostic code on or before the survey sampling date), and cardiovascular disease was an aggregated covariate that included coronary artery disease, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, and transient ischemic attack. The effect of NSAID use on blood pressure control was investigated because NSAIDs may increase blood pressure among hypertensive patients27 and they are used to treat psoriatic arthritis, a condition that affects approximately 10% of patients with psoriasis.28,29 Current NSAID use was defined by the presence of a prescription for an NSAID within 30 days before the date of the blood pressure recording used to define the study outcome.30 Similarly, current antihypertensive use was defined by the presence of a prescription within 90 days before the date of the blood pressure recording used to define the study outcome; prior antihypertensive therapies were not considered. The 6 classes of antihypertensive treatments studied were angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, diuretics (including thiazide, loop, and potassium-sparing diuretics), β-blockers, calcium channel blockers, and centrally acting agents. In addition, hypertension severity was classified according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) criteria31 from least to most severe as follows: 0, controlled with lifestyle modifications; 1, monotherapy (excluding centrally acting agents); 2, dual therapy (excluding centrally acting agents); 3, triple therapy (excluding centrally acting agents); and 4, quadruple therapy and/or centrally acting agents. For time-varying variables, such as BMI and smoking and alcohol use status, the most recent record before the survey sampling date was selected. The following potential confounders or effect modifiers were further explored in sensitivity analyses: duration of hypertension; frequency of blood pressure recordings; time between the most recent blood pressure record and the survey sampling date; hypertension severity; history of treatment with cyclosporine, of which hypertension is an adverse effect; history of systemic corticosteroid treatment, which may affect both psoriasis severity and blood pressure control; and comorbid joint disease.
Sample Size
The study was descriptive; thus, sample size was not determined a priori. All patients with psoriasis who met the inclusion criteria were included in the study, yielding 680, 469, and 173 patients with mild, moderate, and severe psoriasis, respectively. All effect measures are reported with 95% CIs.
Statistical Analysis
Descriptive statistics were used to summarize patient demographics. Groups were compared using the Wilcoxon rank sum test for continuous variables and the χ2 test for categorical variables. Odds ratios (ORs) and 95% CIs for the association of blood pressure control with psoriasis overall and according to psoriasis severity were calculated using logistic regression. In multivariable analyses, a purposeful selection approach was used in which predetermined covariates (ie, age, sex, BMI, current antihypertensive use, and current NSAID use) were included in the model regardless of their P values, and additional covariates were included if their univariate associations with the exposure and outcome were significant (P < .10). To achieve the most parsimonious model, a backward selection approach was used to eliminate covariates in the full model with P > .05 for which removal did not change the point estimates of the exposure variables by greater than 10%. Interactions between age and psoriasis and between sex and psoriasis were explored in the primary model and between hypertension severity and psoriasis in sensitivity analyses and were included in the models if significant (P < .10). Multiple sensitivity analyses were performed to assess the robustness of our results. All analyses were performed using Stata, version 12 (StataCorp).
Results
Patient Characteristics
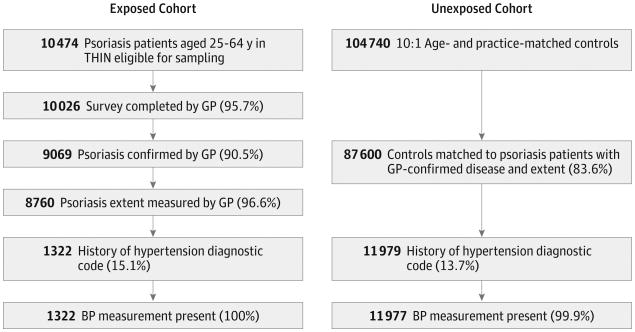
Of 10 474 eligible patients with psoriasis sampled, 10 026 had surveys returned by their GPs (95.7% response rate), of whom 9069 (90.5%) had their psoriasis diagnosis confirmed by their GP, and 8760 (96.6%) had their psoriasis extent determined (Figure). Of these patients, 1322 (15.1%) had a diagnosis of hypertension, all of whom had at least 1 blood pressure record during the time of observation. Among the patients with diagnosed hypertension, 680 (51.4%) had mild psoriasis (≤2% BSA), 469 (35.5%) had moderate psoriasis (3%-10% BSA), and 173 (13.1%) had severe psoriasis (BSA >10%). Psoriatic arthritis was diagnosed in 122 (9.2%) patients with hypertension, and 610 (46.1%) patients had a psoriasis diagnosis for at least 10 years. A similar selection algorithm for the comparison group consisting of patients without psoriasis yielded 11 977 patients with diagnosed hypertension and at least 1 blood pressure recorded.
Figure. Study Population Selection.
Flow diagram of exposed (psoriasis) and unexposed (no psoriasis) patients included in the study. BP indicates blood pressure; GP, general practitioner; and THIN, The Health Improvement Network.
Compared with patients without psoriasis, those with psoriasis had a higher median BMI (P < .001), more blood pressure records (P = .004), and a shorter time between the blood pressure record and survey sampling date (P = .01). In addition, patients with psoriasis were more likely to be former or active smokers (P < .001), have a history of cyclosporine (P < .001) or systemic corticosteroid use (P = .002), be current users of NSAIDs (P < .001), and be receiving quadruple therapy and/or a centrally acting agent for hypertension (P = .01) (Table 1). Higher prevalences of diabetes (P < .001) and cardiovascular disease (P = .04) were observed in patients with psoriasis than in patients without psoriasis.
Table 1. Characteristics of the Study Population.
| Characteristic | No.(%) | P Valuea | |
|---|---|---|---|
| Control (n = 11 977) | Psoriasis (n = 1322) | ||
| Age, median (IQR), y | 57.0 (50.0-62.0) | 57.0 (50.0-61.0) | .56b |
| Male sex | 6109 (51.0) | 711 (53.8) | .06 |
| Duration of observation, median (IQR), y | 12.5 (8.9-18.2) | 12.5 (9.2-18.2) | .71b |
| Time of BP recording from survey sampling date, median (IQR), d | 52 (18-109) | 46 (17-100) | .01b |
| Total No. of BP recordings, median (IQR) | 26 (17-38) | 27 (17-39) | .004b |
| BMI, median (IQR)c | 28.6 (25.3-32.7) | 29.8 (26.3-34.1) | <.001b |
| Smoking statusc | |||
| Never | 5555 (46.4) | 462 (34.9) | <.001 |
| Former | 4228 (35.3) | 568 (43.0) | |
| Active | 2176 (18.2) | 291 (22.0) | |
| Alcohol usec | |||
| Never | 1067 (8.9) | 94 (7.1) | .08 |
| Former | 1094 (9.1) | 127 (9.6) | |
| Active | 9341 (78.0) | 1054 (79.7) | |
| Psoriasis severity | |||
| Mild(BSA≤2%) | NA | 680 (51.4) | NA |
| Moderate (BSA 3%-10%) | 469 (35.5) | ||
| Severe (BSA >10%) | 173 (13.1) | ||
| Psoriasis duration, yd | |||
| <2-9 | NA | 704 (53.3) | NA |
| 10-29 | 468 (35.4) | ||
| ≥30 | 142 (10.7) | ||
| Psoriatic arthritis | NA | 122 (9.2) | NA |
| History of cyclosporine use | 26 (0.22) | 27 (2.0) | <.001 |
| History of systemic corticosteroid use | 2125 (17.7) | 282 (21.3) | .002 |
| Current NSAID usee | 1762 (14.7) | 243 (18.4) | <.001 |
| Current antihypertensive usef,g | 9426 (78.7) | 1057 (79.9) | .29 |
| No. of antihypertensive therapies as a measure of hypertension severityh | |||
| None | 2551 (21.3) | 265 (20.1) | .01 |
| Monotherapy | 4108 (34.3) | 459 (34.7) | |
| Dual therapy | 3134 (26.2) | 322 (24.4) | |
| Triple therapy | 1171 (9.8) | 127 (9.6) | |
| Quadruple therapy and/or centrally acting agent | 1013 (8.5) | 149 (11.3) | |
| Hypertension duration, median (IQR), y | 4.9 (2.3-8.1) | 4.8 (2.0-8.3) | .69b |
| Comorbidities | |||
| Diabetes mellitus | 1943 (16.2) | 265 (20.1) | <.001 |
| Hyperlipidemia | 3015 (25.2) | 344 (26.0) | .50 |
| Chronic kidney disease | 1041 (8.7) | 135 (10.2) | .07 |
| Cardiovascular diseasei | 1603 (13.4) | 204 (15.4) | .04 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; BSA, body surface area; IQR, interquartile range; NA, not applicable; NSAID, nonsteroidal anti-inflammatory drug.
The χ2 test was used for analysis.
The Wilcoxon rank sum test was used for analysis.
Data for BMI, smoking, and alcohol use were available for 97.1%, 99.9%, and 96.1% of the patients, respectively, with no significant differences between patients with and without psoriasis.
Data for psoriasis duration were available for 99.4% of the patients.
Patients were considered to be currently receiving an NSAID if they had received a prescription within 30 days before the date of BP measurement.
Patients were considered to be currently receiving antihypertensive medication if they had received a prescription within 90 days before the date of BP measurement.
Classes of antihypertensive medications included angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, calcium channel blockers, diuretics, and centrally acting agents.
Using the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure criteria,31 hypertension severity was defined as follows from, least to most severe: 0, controlled with lifestyle modifications; 1, monotherapy (excluding centrally acting agents); 2, dual therapy (excluding centrally acting agents); 3, triple therapy (excluding centrally acting agents); and 4, quadruple therapy and/or centrally acting agent.
Included a history of coronary artery disease, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, or transient ischemic attack.
Blood Pressure Control in Psoriasis Patients With Hypertension
In unadjusted analyses, among psoriasis patients with diagnosed hypertension, there was a significant dose-response relationship between uncontrolled hypertension and objective measures of psoriasis severity as determined by the affected BSA: 344 (50.6%), 265 (56.5%), and 103 (59.5%) patients with mild (BSA ≤2%), moderate (BSA 3%-10%), and severe (BSA >10%) psoriasis, respectively, had uncontrolled hypertension (P = .02 for trend).
After adjusting for age, sex, BMI, smoking and alcohol use status, the presence of comorbid conditions (diabetes mellitus, chronic kidney disease, hyperlipidemia, and cardiovascular disease), and current use of antihypertensive medications and NSAIDs, the dose-response relationship between uncontrolled hypertension and objectively measured psoriasis severity remained significant, with ORs of 0.97 (95% CI, 0.82-1.14), 1.20 (95% CI, 0.99-1.45), and 1.48 (95% CI, 1.08-2.04) in patients with mild, moderate, and severe psoriasis, respectively (P = .01 for trend) (Table 2). The likelihood of having uncontrolled hypertension among all patients with psoriasis compared with those without psoriasis was similar in unadjusted and adjusted analyses and was found to be increased, although not statistically significantly so (adjusted OR, 1.10; 95% CI 0.98-1.24). The likelihood of uncontrolled hypertension was the greatest among patients with moderate and severe psoriasis. No significant interactions between age and psoriasis and between sex and psoriasis were identified in the adjusted model. Furthermore, in similarly adjusted analyses, patients with psoriasis were equally as likely to be currently receiving antihypertensive treatment as were patients without psoriasis (OR, 1.01; 95% CI, 0.86-1.18), and the likelihood of receiving antihypertensive therapy did not differ significantly by psoriasis severity (P = .38).
Table 2. Association Between Psoriasis and Uncontrolled Hypertensiona.
| Analysis | Psoriasis, Odds Ratio (95% CI) | P Value for Trendb | |||
|---|---|---|---|---|---|
| All | Mild | Moderate | Severe | ||
| Unadjusted | 1.09 (0.97-1.22) | 0.96 (0.82-1.12) | 1.20 (0.99-1.45) | 1.39 (1.02-1.91) | .02 |
| Adjustedc | 1.10 (0.98-1.24) | 0.97 (0.82-1.14) | 1.20 (0.99-1.45) | 1.48 (1.08-2.04) | .01 |
Logistic regression.
Trend analysis was conducted by coding psoriasis severity as a linear variable (0, no psoriasis; 1, mild; 2, moderate; and 3, severe).
Adjusted for age, sex, body mass index, smoking and alcohol use status, presence of diabetes mellitus, chronic kidney disease, hyperlipidemia, cardiovascular disease, current antihypertensive treatment, and current nonsteroidal anti-inflammatory drug use.
Sensitivity Analyses
We tested the robustness of our observations with multiple sensitivity analyses (Table 3). Our results were not altered by redefining the end of observation by the date of blood pressure records used in the primary analysis, which ensured that all covariates were present before the outcome assessment. To further minimize misclassification of psoriasis, we evaluated the main effects among patients whose psoriasis diagnoses were made by dermatologists and observed a strengthened overall association (OR, 1.26; 95% CI, 1.06-1.50). Similarly, using a stricter definition of hypertension that required at least 2 hypertension READ diagnostic codes did not affect our findings, nor did adjusting for timing or frequency of blood pressure recordings. The results were also robust to exclusion of comorbid arthritic conditions (ie, psoriatic, rheumatoid, and osteoarthritis), indicating that the results were due to skin disease and not joint disease. Exclusion of patients with a history of cyclosporine treatment, of which hypertension is a potential adverse effect, did not attenuate our findings, nor did exclusion of patients with a history of systemic corticosteroid treatment, which may alter psoriasis severity and contribute to increased blood pressure. Alternative adjustment for antihypertensive therapy according to the JNC 7 criteria,31 which defines the severity of hypertension by number and classes of antihypertensive agents, also did not change the results. Finally, defining uncontrolled hypertension based on the mean of all blood pressure recordings within 12 months before the date of blood pressure measurement used in the primary analysis resulted in a stronger overall association (OR, 1.15; 95% CI, 1.02-1.30), suggesting that use of a single blood pressure measurement is unlikely to result in overestimation of the association between psoriasis and uncontrolled hypertension.
Table 3. Sensitivity Analyses.
| Analysis | No. of Patients | Psoriasis, Odds Ratio (95% CI) | P Value for Trenda | ||||
|---|---|---|---|---|---|---|---|
| Control | Psoriasis | All | Mild | Moderate | Severe | ||
| Primary model | 11 977 | 1322 | 1.10 (0.98-1.24) | 0.97 (0.82-1.14) | 1.20 (0.99-1.45) | 1.48 (1.08-2.04) | .01 |
| Redefined end of observation by date of BP record used in primary analysis | 11 977 | 1322 | 1.10 (0.98-1.24) | 0.97 (0.82-1.14) | 1.19 (0.98-1.45) | 1.47 (1.07-2.03) | .01 |
| Adjusted for time between BP record and survey sampling date | 11 977 | 1322 | 1.10 (0.98-1.24) | 0.96 (0.82-1.13) | 1.20 (0.99-1.45) | 1.47 (1.07-2.02) | .01 |
| Restricted to patients with BP record | |||||||
| Within 12 mo of survey sampling date | 11 455 | 1282 | 1.08 (0.96-1.22) | 0.95 (0.81-1.12) | 1.16 (0.95-1.42) | 1.49 (1.08-2.06) | .02 |
| Within 1 mo of survey sampling date | 4250 | 501 | 1.15 (0.94-1.40) | 0.98 (0.75-1.28) | 1.25 (0.90-1.73) | 1.71 (1.02-2.86) | .04 |
| Adjusted for No. of BP records | 11 977 | 1322 | 1.10 (0.97-1.24) | 0.96 (0.82-1.13) | 1.20 (0.98-1.45) | 1.46 (1.06-2.01) | .01 |
| Adjusted for duration of hypertension | 11 977 | 1322 | 1.10 (0.98-1.24) | 0.97 (0.82-1.13) | 1.20 (0.99-1.45) | 1.48 (1.08-2.04) | .01 |
| Excluded patients | |||||||
| With psoriatic arthritis | 11 977 | 1200 | 1.10 (0.97-1.25) | 0.94 (0.80-1.11) | 1.25 (1.01-1.53) | 1.62 (1.12-2.34) | .01 |
| With rheumatoid arthritis | 11 843 | 1293 | 1.10 (0.97-1.24) | 0.96 (0.82-1.13) | 1.19 (0.98-1.45) | 1.46 (1.06-2.01) | .02 |
| With osteoarthritis | 10 132 | 1074 | 1.19 (1.04-1.36) | 1.07 (0.90-1.29) | 1.24 (1.00-1.54) | 1.59 (1.12-2.25) | .001 |
| Receiving cyclosporine | 11 951 | 1295 | 1.10 (0.98-1.24) | 0.97 (0.82-1.14) | 1.19 (0.98-1.45) | 1.54 (1.10-2.16) | .01 |
| With any history of systemic corticosteroid treatment | 9852 | 1,040 | 1.09 (0.95-1.24) | 0.95 (0.79-1.14) | 1.17 (0.95-1.46) | 1.55 (1.07-2.26) | .03 |
| Redefined hypertension diagnosis by presence of ≥2 READ diagnostic codes | 10 354 | 1151 | 1.09 (0.95-1.23) | 0.93 (0.78-1.11) | 1.20 (0.97-1.48) | 1.50 (1.07-2.11) | .02 |
| Restricted to dermatologist-confirmed psoriasis | 11 977 | 594 | 1.26 (1.06-1.50) | 1.27 (0.90-1.80) | 1.18 (0.92-1.50) | 1.43 (1.03-1.98) | .01 |
| Adjusted for JNC 7 criteriab as a replacement for current antihypertensive therapy | 11 977 | 1322 | 1.10 (0.97-1.24) | 0.96 (0.82-1.13) | 1.19 (0.98-1.44) | 1.48 (1.07-2.03) | .01 |
| Defined uncontrolled hypertension by mean of all BP measurements within 12 mo before date of BP used in primary analysis | 11 977 | 1322 | 1.15 (1.02-1.30) | 1.08 (0.92-1.27) | 1.19 (0.98-1.44) | 1.39 (1.01-1.92) | .01 |
Abbreviations: BP, blood pressure; JNC 7, Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.
Trend analysis was conducted by coding psoriasis severity as a linear variable (0, no psoriasis; 1, mild; 2, moderate; 3, severe).
Using JNC 7 criteria,31 hypertension severity was defined as follows from least to most severe: 0, controlled with lifestyle modifications; 1, monotherapy (excluding centrally acting agents); 2, dual therapy (excluding centrally acting agents); 3, triple therapy (excluding centrally acting agents); and 4, quadruple therapy and/or centrally acting agent.
Discussion
In our large population-based study of patients with psoriasis and diagnosed hypertension in the United Kingdom, we found a significant positive dose-response relationship between objective measures of psoriasis severity and uncontrolled hypertension as defined by a systolic blood pressure of 140 mm Hg or higher or a diastolic blood pressure of 90 mm Hg or higher measured in routine clinical practice, independent of potential risk factors for poor blood pressure control. The likelihood of uncontrolled hypertension in patients with vs without psoriasis was greatest among those with moderate and severe skin disease, representing nearly half of the psoriasis patients seen by GPs in the United Kingdom. Our results were robust to multiple sensitivity analyses performed to minimize misclassification of psoriasis and hypertension diagnoses, account for confounding owing to antihypertensive therapy, minimize detection bias by adjusting for the timing and number of blood pressure measurements, minimize any contribution from comorbid joint disease, and minimize any effect from cyclosporine or systemic corticosteroid treatment. Thus, collectively, our results suggest that, among patients with a diagnosis of hypertension, psoriasis (particularly moderate to severe disease), is independently associated with poorly controlled blood pressure.
Prior studies of hypertension in patients with psoriasis have primarily focused on measurements of the prevalence and incidence of hypertension. A meta-analysis4 of observational studies that assessed the association between psoriasis and hypertension confirmed an increased prevalence of hypertension among patients with psoriasis (OR, 1.58; 95% CI, 1.42-1.76). In the same meta-analysis, the prevalence of hypertension was reported to increase with greater psoriasis severity (mild psoriasis: OR, 1.30; 95% CI, 1.15-1.47; severe psoriasis: OR, 1.49; 95% CI, 1.20-1.86). It is important to note that in these studies included in the meta-analysis, psoriasis severity was determined indirectly by psoriasis treatment patterns, and potential confounding variables were rarely adjusted for in multivariable analyses. Studies32,33 of incident hypertension have also suggested an increased risk of developing hypertension among patients with an established diagnosis of psoriasis. A single study34 assessed the effect of psoriasis on hypertension severity (as defined by the JNC 7 criteria). In that clinic-based, case-control study, patients with psoriasis were more likely to require complex antihypertensive management than were those without psoriasis; the effect of psoriasis severity was not assessed. Our findings advance the current literature by showing a novel dose-dependent association between direct measures of psoriasis severity and uncontrolled hypertension as determined by routine clinic-based blood pressure measurements. Ours is also one of the few studies to examine the effect of a comorbid chronic inflammatory disease, such as psoriasis, on blood pressure control among patients with hypertension.
The pathophysiologic mechanisms of hypertension in patients with psoriasis remain unknown, although several biological pathways have been implicated, including overexpression of endothelin 1,35 a potent vasoconstrictor expressed in both vascular endothelium and keratinocytes; increased oxidative stress; and common inflammatory pathways, including key cytokines such as tumor necrosis factor and interleukin 17.36-39 Upregulation of the renin-angiotensin signaling pathway may also promote the development of more difficult-to-control hypertension in patients with psoriasis. This hypothesis is supported by the observations of increased expression of the renin gene in lesional skin of patients with moderate to severe psoriasis compared with matched nonlesional skin40 and greater plasma renin activity and increased urinary aldosterone excretion in patients with psoriasis.41
The results of our study must be interpreted in light of its strengths and limitations. The strengths include the use of a large, population-based cohort broadly representative of the United Kingdom's general population and a high survey response rate for assessing psoriasis, which minimizes selection bias and enhances the external validity (ie, generalizability) of our findings. General practitioners were unaware of the hypotheses being tested, and ascertainment of blood pressure was nearly 100%, thus minimizing the potential for information bias. Misclassification of psoriasis severity was unlikely based on a previous finding42 that even untrained patients can reliably classify the severity of their psoriasis using methods similar to those used by the GPs in the present study. Limitations include the cross-sectional design, which prevents us from determining the directionality of the relationship between psoriasis severity and blood pressure control. Although we hypothesized that more severe psoriasis contributes to poorer blood pressure control, we cannot rule out the possibility that uncontrolled hypertension promotes more severe psoriasis. Misclassification of the outcome is another potential limitation because the determination of uncontrolled hypertension was based on a single blood pressure measurement recorded in the clinic rather than an aggregate of multiple blood pressure measurements and thus may overestimate poor blood pressure control. However, previous studies43,44 assessing the prevalence of uncontrolled hypertension found that using single vs multiple blood pressure readings to define uncontrolled hypertension resulted in similar prevalences, indicating that a single blood pressure measurement is representative of overall blood pressure control. Furthermore, any misclassification of uncontrolled hypertension would likely be nondifferential (ie, equally as likely in patients with and without psoriasis) and would have biased our results toward the null, resulting in conservative estimates of the true association. Indeed, our sensitivity analysis substituting the 12-month mean blood pressure reading for the single reading used in the primary analysis supports this conjecture. Our findings may also be partially attributable to the choice of psoriasis therapy, most concerning of which would be cyclosporine, considering its association with hypertension. However, our results were robust to the exclusion of patients who had received cyclosporine, suggesting a minimal contribution of cyclosporine treatment to the observed association between psoriasis and uncontrolled hypertension. Based on prior data45,46 that suggest undertreatment of high blood pressure in psoriasis patients with diagnosed hypertension, our results may be interpreted to be attributable to differential management of hypertension in patients with and without psoriasis. However, in our adjusted multivariable analyses, we found patients with psoriasis to be equally as likely to receive antihypertensive treatment as those without psoriasis. Finally, although we controlled for several potential confounding factors, there may be additional unmeasured confounders, such as diet, physical activity, stress, and antihypertensive medication adherence, that were not included in our analyses and may have important effects on the association between psoriasis and uncontrolled hypertension.45
Conclusions
To our knowledge, this is the first study to investigate the effect of objectively measured psoriasis severity on blood pressure control and demonstrate a significant and increasing likelihood of uncontrolled hypertension among patients with more severe psoriasis, independent of other risk factors for poor blood pressure control. Adding to the currently limited understanding of the effects of comorbid disease on hypertension, our findings have important clinical implications, suggesting a need for more effective management of blood pressure in patients with psoriasis, especially those with more extensive skin involvement (ie, ≥3% BSA affected). Additional studies are needed to further characterize the effect of chronic inflammatory diseases, such as psoriasis, and its therapies on coexisting hypertension and its treatment in a longitudinal manner;to better understandthemechanisms underlying poor blood pressure control among patients with psoriasis; and to determine whether an improvement in hypertension management affects psoriasis severity.
Acknowledgments
Funding/Support: This study was supported by grant R01-HL089744 from the National Heart, Lung, and Blood Institute (Dr Gelfand), grant K24-AR064310 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Dr Gelfand), a Dermatology Foundation Career Development Award (Dr Takeshita), and a National Psoriasis Foundation Fellowship Award (Dr Takeshita).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Takeshita and Mr Shin had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Takeshita, Wang, Margolis, Gelfand.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Takeshita, Wang, Shin.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Takeshita, Wang, Shin, Kimmel, Troxel, Gelfand.
Administrative, technical, or material support: Shin, Mehta.
Study supervision: Shin, Mehta, Margolis, Gelfand.
Conflict of Interest Disclosures: Dr Takeshita received payment for continuing medical education work related to psoriasis. Dr Gelfand received honoraria serving as a consultant for Abbvie, Amgen Inc, Celgene Corp, Eli Lilly, Janssen Biologics (formerly Centocor), Merck, Novartis Corp, and Pfizer Inc; obtained grants or has pending grants from Abbvie, Amgen Inc, Eli Lilly, Genentech Inc, Janssen, Novartis Corp, and Pfizer Inc; and received payment for continuing medical education work related to psoriasis. No other disclosures were reported.
Previous Presentations: An abstract of the data contained in this manuscript was presented at the American Academy of Dermatology Annual Meeting; March 22, 2014, Denver, Colorado; and the American Society for Clinical Investigation/ American Association of American Physicians Joint Meeting; April 26, 2014; Chicago, Illinois.
References
- 1.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55(5):829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 3.Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3, pt 1):556–562. doi: 10.1038/jid.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31(3):433–443. doi: 10.1097/HJH.0b013e32835bcce1. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:e54. doi: 10.1038/nutd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma C, Harskamp CT, Armstrong EJ, Armstrong AW. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol. 2013;168(3):486–495. doi: 10.1111/bjd.12101. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(1):84–91. doi: 10.1001/2013.jamadermatol.406. [DOI] [PubMed] [Google Scholar]
- 8.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 9.Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129(10):2411–2418. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the UK. Br J Dermatol. 2010;163(3):586–592. doi: 10.1111/j.1365-2133.2010.09941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2013;2(2):e000062. doi: 10.1161/JAHA.113.000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Invest Dermatol. 2013;133(10):2340–2346. doi: 10.1038/jid.2013.149. [DOI] [PubMed] [Google Scholar]
- 14.Gu WJ, Weng CL, Zhao YT, Liu QH, Yin RX. Psoriasis and risk of cardiovascular disease: a meta-analysis of cohort studies. Int J Cardiol. 2013;168(5):4992–4996. doi: 10.1016/j.ijcard.2013.07.127. [DOI] [PubMed] [Google Scholar]
- 15.Horreau C, Pouplard C, Brenaut E, et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):12–29. doi: 10.1111/jdv.12163. [DOI] [PubMed] [Google Scholar]
- 16.Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 17.Lund L, Jacobsen J, Nørgaard M, et al. The prognostic impact of comorbidities on renal cancer, 1995 to 2006: a Danish population based study. J Urol. 2009;182(1):35–40. doi: 10.1016/j.juro.2009.02.136. [DOI] [PubMed] [Google Scholar]
- 18.Bautista LE, Vera-Cala LM, Colombo C, Smith P. Symptoms of depression and anxiety and adherence to antihypertensive medication. Am J Hypertens. 2012;25(4):505–511. doi: 10.1038/ajh.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128(4):2099–2107. doi: 10.1378/chest.128.4.2099. [DOI] [PubMed] [Google Scholar]
- 20.Piccirillo JF, Vlahiotis A. Comorbidity in patients with cancer of the head and neck: prevalence and impact on treatment and prognosis. Curr Oncol Rep. 2006;8(2):123–129. doi: 10.1007/s11912-006-0047-z. [DOI] [PubMed] [Google Scholar]
- 21.Corsonello A, Antonelli Incalzi R, Pistelli R, Pedone C, Bustacchini S, Lattanzio F. Comorbidities of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2011;17(suppl 1):S21–S28. doi: 10.1097/01.mcp.0000410744.75216.d0. [DOI] [PubMed] [Google Scholar]
- 22.Wong ND, Lopez VA, L'Italien G, Chen R, Kline SE, Franklin SS. Inadequate control of hypertension in US adults with cardiovascular disease comorbidities in 2003-2004. Arch Intern Med. 2007;167(22):2431–2436. doi: 10.1001/archinte.167.22.2431. [DOI] [PubMed] [Google Scholar]
- 23.Paulsen MS, Andersen M, Thomsen JL, et al. Multimorbidity and blood pressure control in 37 651 hypertensive patients from Danish general practice. J Am Heart Assoc. 2013;2(1):e004531. doi: 10.1161/JAHA.112.004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seminara NM, Abuabara K, Shin DB, et al. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol. 2011;164(3):602–609. doi: 10.1111/j.1365-2133.2010.10134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serumaga B, Ross-Degnan D, Avery AJ, et al. Effect of pay for performance on the management and outcomes of hypertension in the United Kingdom: interrupted time series study. BMJ. 2011;342:d108. doi: 10.1136/bmj.d108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hypertension: the clinical management of primary hypertension in adults. [Accessed June 20, 2013];NICE Clinical Guideline. 2011 Aug;127 http://www.nice.org.uk/nicemedia/live/13561/56008/56008.pdf. [Google Scholar]
- 27.Armstrong EP, Malone DC. The impact of nonsteroidal anti-inflammatory drugs on blood pressure, with an emphasis on newer agents. Clin Ther. 2003;25(1):1–18. doi: 10.1016/s0149-2918(03)90003-8. [DOI] [PubMed] [Google Scholar]
- 28.Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53(4):573. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 29.Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford) 2013;52(3):568–575. doi: 10.1093/rheumatology/kes324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White WB, West CR, Borer JS, et al. Risk of cardiovascular events in patients receiving celecoxib: a meta-analysis of randomized clinical trials. Am J Cardiol. 2007;99(1):91–98. doi: 10.1016/j.amjcard.2006.07.069. [DOI] [PubMed] [Google Scholar]
- 31.The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) [Accessed June 20, 2013];2004 http://www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. [PubMed]
- 32.Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159(4):895–902. doi: 10.1111/j.1365-2133.2008.08707.x. [DOI] [PubMed] [Google Scholar]
- 33.Qureshi AA, Choi HK, Setty AR, Curhan GC. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145(4):379–382. doi: 10.1001/archdermatol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong AW, Lin SW, Chambers CJ, Sockolov ME, Chin DL. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6(3):e18227. doi: 10.1371/journal.pone.0018227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonifati C, Mussi A, Carducci M, et al. Endothelin-1 levels are increased in sera and lesional skin extracts of psoriatic patients and correlate with disease severity. Acta Derm Venereol. 1998;78(1):22–26. doi: 10.1080/00015559850135779. [DOI] [PubMed] [Google Scholar]
- 36.Sowers JR. Hypertension, angiotensin II, and oxidative stress. N Engl J Med. 2002;346(25):1999–2001. doi: 10.1056/NEJMe020054. [DOI] [PubMed] [Google Scholar]
- 37.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II–mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51(5):1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madhur MS, Lob HE, McCann LA, et al. Interleukin 17 promotes angiotensin II–induced hypertension and vascular dysfunction. Hypertension. 2010;55(2):500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suárez-Fariñas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol. 2012;132(11):2552–2564. doi: 10.1038/jid.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ena P, Madeddu P, Glorioso N, Cerimele D, Rappelli A. High prevalence of cardiovascular diseases and enhanced activity of the renin-angiotensin system in psoriatic patients. Acta Cardiol. 1985;40(2):199–205. [PubMed] [Google Scholar]
- 42.Dommasch ED, Shin DB, Troxel AB, Margolis DJ, Gelfand JM. Reliability, validity and responsiveness to change of the Patient Report of Extent of Psoriasis Involvement (PREPI) for measuring body surface area affected by psoriasis. Br J Dermatol. 2010;162(4):835–842. doi: 10.1111/j.1365-2133.2009.09589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meissner I, Whisnant JP, Sheps SG, et al. Detection and control of high blood pressure in the community: do we need a wake-up call? Hypertension. 1999;34(3):466–471. doi: 10.1161/01.hyp.34.3.466. [DOI] [PubMed] [Google Scholar]
- 44.Alexander M, Tekawa I, Hunkeler E, et al. Evaluating hypertension control in a managed care setting. Arch Intern Med. 1999;159(22):2673–2677. doi: 10.1001/archinte.159.22.2673. [DOI] [PubMed] [Google Scholar]
- 45.Kimball AB, Szapary P, Mrowietz U, et al. Underdiagnosis and undertreatment of cardiovascular risk factors in patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;67(1):76–85. doi: 10.1016/j.jaad.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 46.Ahlehoff O, Skov L, Gislason G, et al. Pharmacological undertreatment of coronary risk factors in patients with psoriasis: observational study of the Danish nationwide registries. PLoS One. 2012;7(4):e36342. doi: 10.1371/journal.pone.0036342. [DOI] [PMC free article] [PubMed] [Google Scholar]