Abstract
Spindle sizes are different in diverse species and cell types. In frogs, the meiotic spindle size is positively correlated with the egg cell volume. Across species, relatively small mouse oocytes (70–80 μm) have a relatively large spindle while larger pig oocytes (about 120 μm) have a considerably smaller spindle. In this study we investigated whether species-specific oocyte spindle size was determined by cytoplasmic or nuclear factors. By exchanging the germinal vesicle between mouse and pig oocytes, we obtained two kinds of reconstructed oocytes: one with mouse ooplasm and pig GV (mCy-pGV oocyte), and the other with pig ooplasm and mouse GV (pCy-mGV oocyte). We show that the MII spindle size of the mCy-pGV oocyte is similar to that of the mouse meiotic spindle and significantly larger than that of the pig meiotic spindle. The timing of oocyte maturation also followed that of the species from which the oocyte cytoplasm arose, although some impact of the origin of the GV was observed. These data suggest that spindle size and the timing of meiotic progression are governed by cytoplasmic components rather than cytoplasmic volume and GV materials.
Oocyte maturation is a co-ordinated process involving nuclear (cell cycle) progression and cytoplasmic changes. In vitro oocyte developmental time courses are different in diverse species; for example, mouse oocytes take about 10–12 h, bovine oocytes require 20 h and pig oocytes require about 44 h to reach the MII stage1. The sizes of mouse and pig oocytes as well as the sizes and shapes of meiotic spindles of mouse oocytes and pig oocytes are significantly different. The smaller mouse oocyte contains an elongated spindle, while the larger pig oocyte contains a barrel-shaped small spindle. The mechanisms controlling spindle size and time course of meiotic maturation are unknown but can be addressed using oocyte reconstruction techniques. In mammals, oocytes are arrested naturally in the first prophase with a very large nucleus called the germinal vesicle (GV). GV material is important for oocyte development and subsequent cell reprogramming of the early embryo2,3,4. GV transfer technology has been used to rescue age-related aneuploidy in oocytes5,6. Previous work by others and us has shown that the reconstructed oocytes can be matured and fertilized by intracytoplasmic sperm injection (ICSI), which leads to live animals in some species such as the mouse7 and rabbit8.
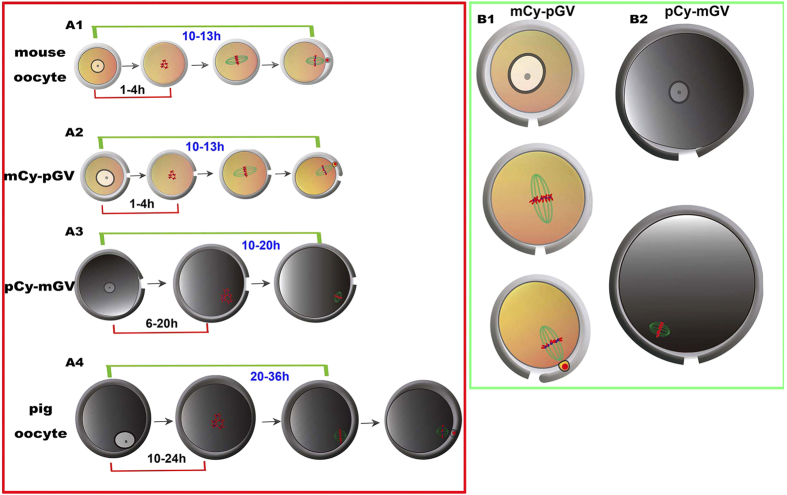
Thus, we have used the GV transfer technique, exchanged the GVs between mouse oocytes and pig oocytes to investigate the nuclear-cytoplasm interactions (Fig. 1), and to determine what controls meiotic time course and meiotic spindle size.
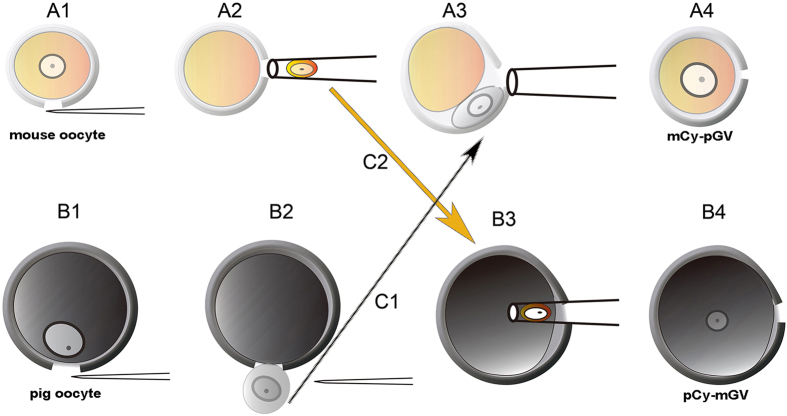
Figure 1. Procedure of GV transfer between mouse and pig oocytes.
(A1) Zona pellucida of mouse oocytes was cut with a sharp glass needle. (A2) Mouse GV was aspirated by a blunt-tip micropipette with an inner diameter of about 20 μm. (A3) Pig GV was transferred to the perivitelline space of enucleated mouse oocyte by a blunt-tip micropipette with an inner diameter of about 25 μm. (A4) Pig GV was fused to the mouse cytoplast by electric fusion. (B1) Zona pellucida of mouse oocyte was cut by using pressure with a sharp glass needle. (B2) Pig GV was squeezed out by pressing the zona pellucida. (B3) Mouse GV was directly injected into pig cytoplast by a blunt-tip micropipette with an inner diameter of about 20 μm with a piezo unit. (B4) A pCy-mGV oocyte was reconstructed with pig cytoplast and mouse GV. (C1) Pig GV was transferred to the micro-manipulation droplet with enucleated mouse oocyte. (C2) Mouse GV was transferred to the micro-manipulation droplet with enucleated pig oocyte.
Results
In vitro developmental time course and spindle size of mouse and pig oocyte
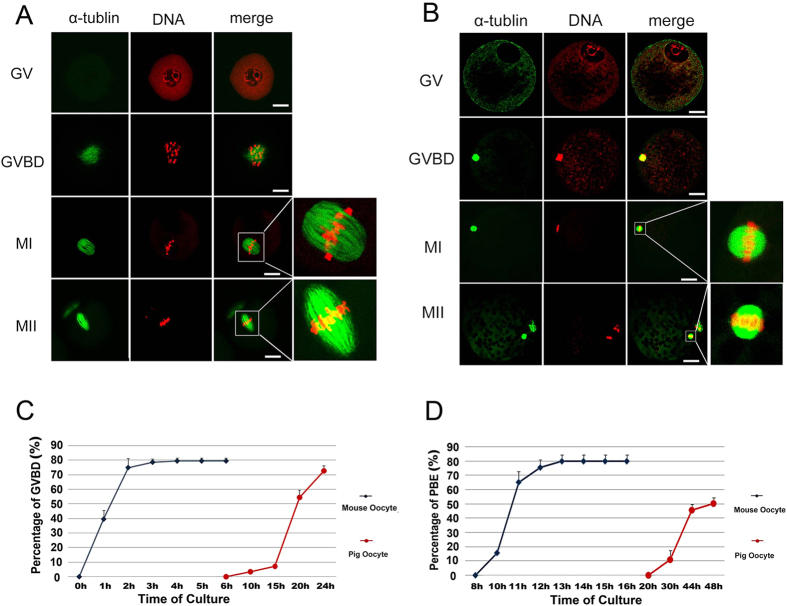
Mouse and pig oocytes were stained by PI and anti-α-tubulin antibody to detect chromosome condensation and spindle assembly. MI and MII spindles of mouse oocytes were larger than the spindles of pig oocytes (Fig. 2A,B). Mouse oocyte GVBD took place in less than 4 h of in vitro culture and first polar body extrusion took place at 10–13 h of in vitro culture. However, the pig oocytes took 10–24 h to undergo GVBD and 30–48 h for first polar body extrusion (Fig. 2C,D).
Figure 2. Overview of the difference in spindle size and in vitro maturational time course in pig and mouse oocytes.
(A) Mouse oocytes at the GV, GVBD, MI and MII stages were stained with PI and anti-α-tublin antibody to detect mouse meiotic spindle shape and size. (B) Pig oocytes at the GV, GVBD, MI and MII stages were stained with PI and anti-α-tubulin antibody to detect pig meiotic spindle shape and size. (C) Percentages of mouse oocyte GVBDs (0 h: 0%; 1 h: 39.53 ± 6.10%; 2 h: 74.49 ± 6.26%; 3 h: 78.57 ± 2.18%; 4 h: 79.41 ± 2.03%; 5 h: 79.41 ± 2.03%; 6 h: 79.41 ± 2.03%) were observed each hour from 0–6 h of in vitro culture, and percentages of pig oocyte GVBDs (6 h: 0%; 10 h: 3.53 ± 1.22; 15 h: 7.11 ± 1.24; 20 h: 54.44 ± 5.09; 24 h: 72.67 ± 3.48) were observed at 6 h, 10 h, 15 h, 20 hand 24 h of in vitro culture. (D) Percentages of mouse oocytes with first polar body extrusion (8 h: 0%; 10 h: 15.70 ± 0.93%; 11 h: 65.22 ± 7.25%; 12 h: 75.56 ± 5.18%; 13 h: 79.94 ± 4.38%; 14 h: 79.94 ± 4.38%; 15 h: 79.94 ± 4.38%; 16 h: 79.94 ± 4.38%) were observed one or two hours from 8 h to 16 h of in vitro culture, and percentages of pig oocytes with first polar body extrusion (20 h: 0%; 30 h: 11.01 ± 6.27%; 44 h: 45.55 ± 3.85%; 48 h: 50.14 ± 4.07%) were observed at 20 h, 30 h, 44 hand 48 h of in vitro culture (bar = 30 μm).
Maturation of the mCy-pGV oocyte reconstructed by fusing mouse cytoplast with pig GV
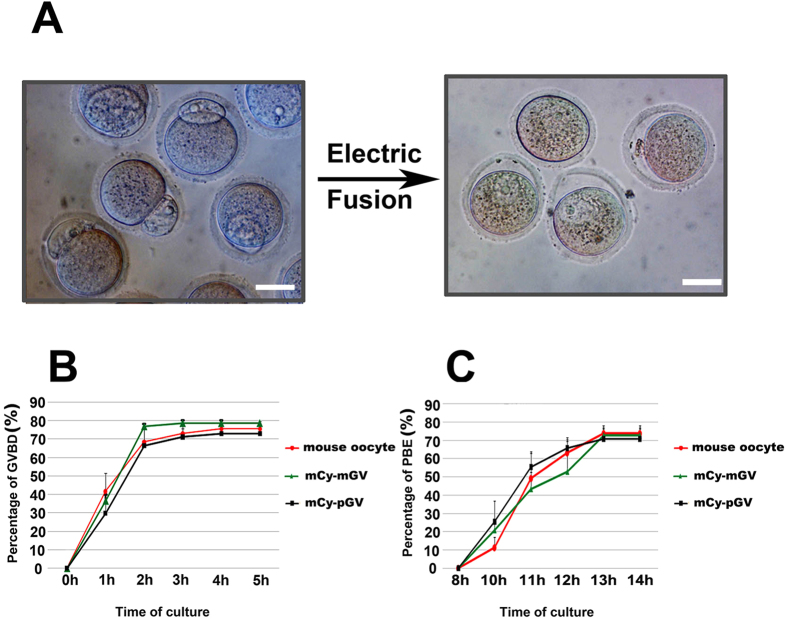
Mouse and pig oocytes were enucleated by micro-manipulation, and then the pig GV was transferred into the perivitelline space of the enucleated mouse oocyte. The pig GV and mouse cytoplast were fused by electric fusion (Fig. 3A). Mouse GV and mouse cytoplast were also fused as a mock control (mCy-mGV oocytes). mCy-pGV oocytes were cultured in vitro and observed every 1 or 2 hours from 0 h to 14 h. The developmental time course of the mCy-pGV oocytes was similar to that of the mouse oocytes and mCy-mGV oocytes (Fig. 3B,C). The mCy-pGV oocytes underwent GVBD within 4 h, and the first polar body extrusion took place at 10–13 h after release from milrinone inhibition. Percentages of the oocyte GVBD and the first polar body extrusion were not significantly different between the mCy-pGV oocytes, control mouse oocytes and mCy-mGV oocytes (Fig. 3B,C).
Figure 3. In vitro maturational time course is similar between mCy-pGV oocytes and mouse oocytes.
(A) Pig GV was fused to the mouse enucleated oocyte by electric fusion. (B) Percentages of GVBD in mouse oocytes (0 h: 0%; 1 h: 41.61 ± 11.35% 2 h: 68.51 ± 9.84%; 3 h: 73.11 ± 6.68%; 4 h: 75.53 ± 2.57%;5 h: 75.53 ± 2.57%), mCy-mGV oocytes (0 h: 0%; 1 h: 36.62 ± 0.24%; 2 h: 76.99 ± 3.17%; 3 h: 78.74 ± 1.38%; 4 h: 78.74 ± 1.38%; 5 h: 78.74 ± 1.38%) and mCy-pGV oocytes (0 h: 0%; 1 h: 29.81 ± 6.05%; 2 h: 66.37 ± 12.46%; 3 h: 71.64 ± 8.91%; 4 h: 72.92 ± 6.82%; 5 h72.91 ± 6.82%) were observed each hour from 0 to 5 h of in vitro culture. (C) Percentages of mouse oocytes (8 h: 0%; 10 h: 10.57 ± 4.41%; 11 h: 49.17 ± 13.72%; 12 h: 63.10 ± 8.22%; 13 h: 73.69 ± 3.04%; 14 h: 73.69 ± 3.04%), mCy-mGV oocyte(8 h: 0%; 10 h: 20.88 ± 3.76%; 11 h: 43.46 ± 8.90%; 12 h: 52.65 ± 5.84%; 13 h: 72.71 ± 5.28%; 14 h: 72.71 ± 5.29%) and mCy-pGV oocytes (8 h: 0%; 10 h: 25.56 ± 11.16%; 11 h: 55.27 ± 8.33%; 12 h: 65.65 ± 3.71%; 13 h: 70.78 ± 5.42%; 14 h: 70.78 ± 5.42%) with first polar body extrusion were detected 8 h, 10 h, 12 h, 13 h, and 14 h of in vitro culture (bar = 30 μm).
Spindle size of the mCy-pGV oocyte reconstructed by fusion of mouse cytoplast with pig GV
In mCy-pGV oocytes, normal chromosome condensation could be detected and spindles were well assembled in both MI and MII stages. The size of spindles of mCy-pGV oocytes was similar to that of the mouse oocytes but significantly larger than that of the pig oocytes (Fig. 4A). The sizes of the MI and MII spindles in mCy-pGV oocytes were 34.25 ± 4.17 μm and 31.57 μm ± 4.90 μm, respectively, which were similar to those of the mouse MI oocytes (36.23 ± 4.99 μm) and MII oocytes (30.50 ± 4.91 μm), but significantly larger than those of pig MI oocytes (11.94 ± 1.80 μm) and MII oocytes (13.32 ± 1.10 μm) (p < 0.05) (Fig. 4B).
Figure 4. Spindle size of mCy-pGV oocytes.
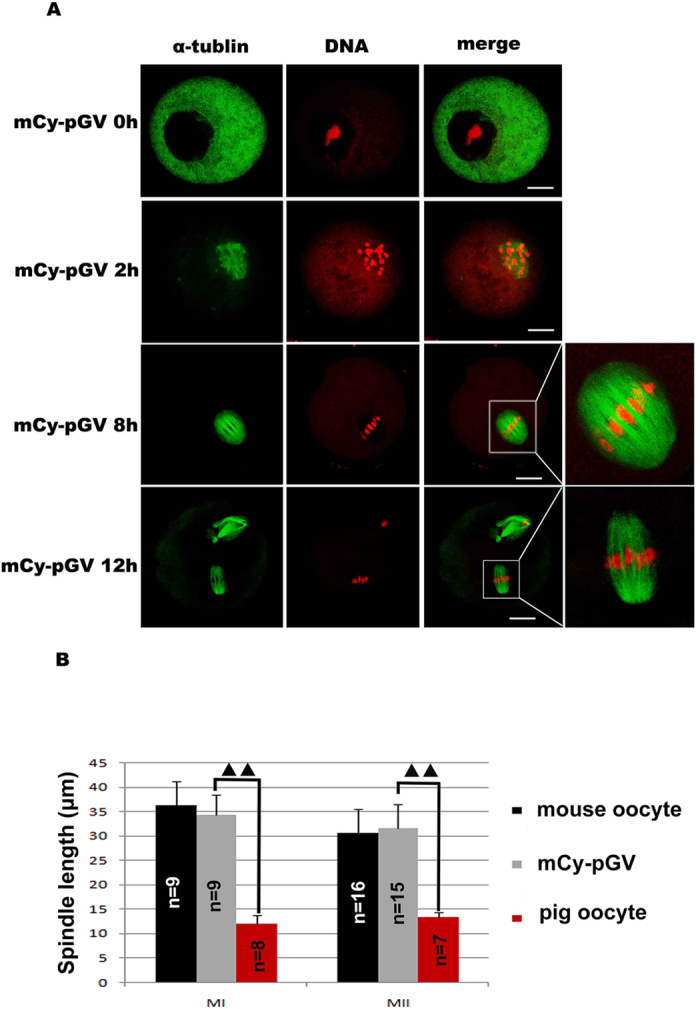
(A) mCy-pGV oocytes were stained with PI and anti-α-tubulin antibody at 0 h(GV stage), 2 h(GVBD stage), 8 h(MI stage) and12 h(MII stage) of culture. (B) Meiotic spindle size was measured; the sizes of MI (34.25 ± 4.17 μm) and MII (31.57 μm ± 4.90 μm) spindles of the mCy-pGV oocytes were similar to those of MI (36.23 ± 4.99 μm) and MII (30.50 ± 4.91 μm) spindles of mouse oocytes, but significantly larger than those of the pig oocytes (MI: 11.94 ± 1.80 μm; MII: 13.32 ± 1.10 μm) (bar = 30 μm).
Maturation of the pCy-mGV oocyte reconstructed by microinjection of mouse GV into pig cytoplast
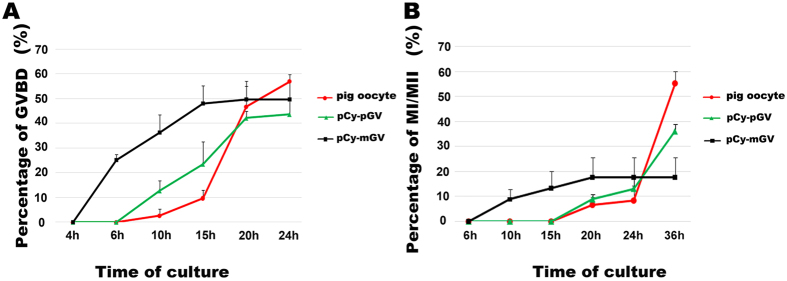
Mouse GV was directly injected to the pig enucleated oocyte using a piezo unit. GVBD of the pCy-mGV oocytes took place at 6–20 h of culture in vitro, which was significantly extended compared to the time course of mouse oocyte GVBD, although it was 4 hours earlier than that of the pig oocyte GVBD (10–24 h) (Fig. 5A). The reconstructed oocytes developed to the MI stage at 10–20 h of in vitro culture, which was significantly longer than in mouse oocytes, but 10 hearlier than the pig oocytes reaching the MI stage (20–36 h) (Fig. 5B). However, probably due to the removal of cumulus cells, the reconstructed oocytes failed to develop to the MII stage in our culture system. It has been previously reported that the pig oocytes without surrounding cumulus cells have a limited developmental capability9. Pig GV and pig cytoplast were fused as a mock control (pCy-pGV oocytes), which also had a compromised maturational capability, and failed to reach the MII stage (Fig. 5B).
Figure 5. In vitro maturational time course of pig oocyte and pCy-mGV oocytes.
(A) Percentages of GVBD in pig oocytes (4 h: 0%; 6 h: 0%; 10 h: 3.54 ± 1.62%; 15 h: 9.71 ± 3.31%; 20 h: 46.73 ± 8.44%; 24: 56.94 ± 2.98%), pCy-pGV oocytes (0 h: 0%; 6 h: 0%; 10 h: 12.58 ± 3.09%; 15 h: 23.48 ± 9.19%; 20 h: 42.12 ± 2.92%; 24 h: 43.64 ± 5.53%) and pCy-mGV oocytes (4 h: 0%; 6 h: 25.24 ± 2.20%; 10 h: 36.27 ± 7.17%; 15 h: 48.19 ± 7.11%; 20 h: 49.77 ± 7.44%; 24: 49.77 ± 7.44%) were observed at 4 h, 6 h, 10 h, 15 h, 20 hand 24 h of in vitro culture. pCy-mGV oocytes reached the GVBD stage at 6–20 h of culture which was 4 hearlier than the pig oocytes (20–24 h). B: Percentages of pig oocytes (6 h: 0%; 10 h: 0%; 15 h: 0%; 20 h: 6.67 ± 2.89%; 24 h: 8.33 ± 5.77%; 36 h: 55.00 ± 5.00%), pCy-pGV oocytes (6 h: 0%; 10 h: 0%; 15 h: 0%; 20 h: 8.86 ± 2.04%; 24 h: 13.10 ± 4.29%; 36 h: 35.98 ± 2.79%) and pCy-mGV oocytes (6 h: 0%; 10 h: 8.75 ± 3.98%; 15 h: 13.19 ± 6.88%; 20 h: 17.50 ± 7.95%; 24 h: 17.50 ± 7.95%; 36 h: 17.50 ± 7.95%) reaching the MI stage were detected at 6 h, 10 h, 15 h, 20 h, 24 hand 36 h of in vitro culture. pCy-mGV oocytes reached the MI stage at 10–20 h of in vitro culture which was 10 hearlier than the pig oocyte (20–36 h).
Spindle size of pCy-mGV oocyte reconstructed by microinjection of mouse GV into pig GV cytoplast
Chromosomes condensed normally when pCy-mGV oocytes reached the GVBD stage at 6–10 h of in vitro culture (Fig. 6A). A small spindle was assembled in the reconstructed pCy-mGV oocyte at the MI stage (Fig. 6A). Spindle size of the pCy-mGV oocyte was 12.80 ± 1.27 μm, which was similar to that of the pig MI spindle (13.17 ± 0.74 μm) and significantly shorter than that of the mouse MI spindle (33.33 ± 4.80 μm) (Fig. 6B).
Figure 6. Spindle size of pCy-mGV oocytes.
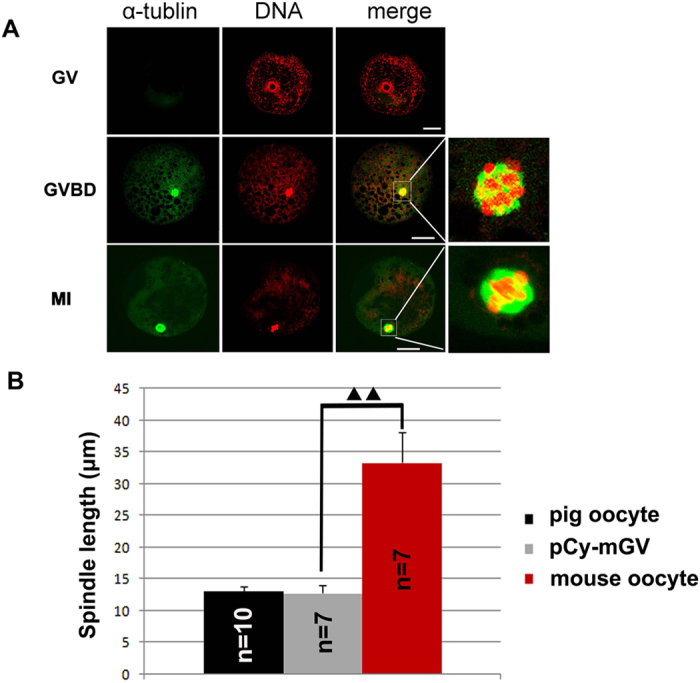
(A) pCy-mGV oocytes at GV, GVBD and MI stages were stained with PI and anti-α-tubulin antibody. Normal chromosome condensation could be detected in GVBD stage oocytes. A small spindle was assembled in the pCy-mGV oocytes. (B) Spindle size of the MI stage pCy-mGV oocytes were12.80 ± 1.27 μm, significantly small than that of mouse oocytes (33.33 ± 4.80 μm) (p < 0.05), but similar to that of pig oocytes (13.17 ± 0.74 μm) (bar = 30 μm).
Discussion
The spindle is an important cellular structure that functions to segregate chromosomes in both mitotic and meiotic cells. Spindle size is determined by a series of microtubule-associated proteins as well as the cell size. Molecular mechanisms of spindle length control in mitotic cells have been widely studied. The balance between microtubule (MT) polymeriztion and depolymerization is critical for the spindle length control10,11. Spindle size decreases or stops growing after inhibition of MT polymerization factors such as TOG12, EB113,CLASP14. However, knockdown of the MT depolymerization-related proteins such as, kinesin-815 and kinesin-1316 results in elongated and monopolar spindles. The microtubule-severing ATPase katanin is also important in mitotic and meiotic spindle length control. Inhibition of katanin severing activity reduces nocodazole-induced spindle microtubule depolymerization17,18. The spindle size also varies greatly to accommodate rapid changes in cell size. In Xenopus, the diameter of the X. laevis eggs is 1.2 mm which is five-fold large than the X. tropicalis eggs (diam ~ 0.6 mm), and the spindle of the X. laevis is larger, too19. Two recent studies showed that cell volume is related to spindle size in mitotic Xenopus embryo cells, and changes in cytoplasmic volume are sufficient to drive spindle scaling.20,21. Thus, availability of cytoplasmic components, together with cell size, determines spindle size in mitotic embryonic cells.
On the other hand, nuclear materials are quite important for cellular activities. If the GV was replaced by primary spermatogonium, fibroblast or cumulus cells the reconstructed oocyte could not adequately complete the meiotic cell cycle22,23. The cytoplasm of GV stage oocytes3 and pronuclear stage zygotes24,25 were not suitable for nuclear transfer experiments. Enucleated MII oocytes, the cytoplasm of metaphase zygotes and metaphase blastomeres of 2-cell embryos can successfully reprogram the somatic cell, suggesting that the material enclosed in the pronucleus may be involved in the successful reprogramming, However, a recent investigation using the interphase cytoplasm of two-cell mouse embryos as the nuclear transfer recipients resulted in live offspring26. This suggests that the materials in the germinal vesicle and the pronuclei may be more important than in the somatic cell (blastomere of 2-cell embryo) nucleus. In addition, the nucleolus of the GV stage oocyte which is localized in the germinal vesicle is essential for nucleolus formation of the early embryo4, which explains the importance of the GV materials for the oocyte and subsequent embryo.
Interestingly, the pig oocyte has a relatively large cell size compared to the mouse oocyte. However, the spindle size of the pig oocyte is much smaller than that of the mouse oocyte. In addition, fully grown pig oocytes need much longer time to resume meiosis and complete meiotic maturation compared to mouse oocytes. We wondered if the germinal vesicle material or the cytoplasm plays critical functions in determining the spindle size and meiotic maturation time course in meiotic mammalian oocytes.
Sendai-virus-mediated fusion of GV-stage oocyte was used to investigate the interaction between the germinal vesicle and cytoplasm27,28,29. After cell fusion, it was found that the MPF (maturation-promoting factor) function was not species-specific. However, this cell fusion approach could not distinguish between the cytoplasmic and nuclear influence. We thus used GV transfer technology to reconstruct the oocyte with GV and cytoplast derived from mouse and pig oocytes. In the mCy-pGV and pCy-mGV oocytes, the spindle can be assembled normally. mCy-pGV oocytes can develop to the MII stage like mouse oocytes. The pCy-mGV oocyte has a compromised maturational capability compared to the pig oocyte, which may be a result of the removal of the granulosa cells surrounding the oocyte before micro-manipulation. Centrifugation of the pig oocytes before enucleation of the oocyte may be another reason for the weak maturational capability. Although the pCy-mGV oocyte cannot develop to the MII stage, the MI spindle assembled normally in the pCy-mGV oocyte. Interestingly, when the mouse oocyte GV was transferred into the enucleated pig oocyte, a small barrel-shaped spindle was formed, while when the pig oocyte GV was transferred into the enucleated mouse oocyte, a large spindle was formed (Fig. 7). Our results suggest that it is the cytoplasmic components rather than the GV materials that determine the meiotic spindle size and shape in mammalian oocytes.
Figure 7. Summary of in vitro maturational time course and spindle size determination in mCy-pGV and pCy-mGV oocytes.
(A1) Normal mouse oocytes take 1–4 h for GVBD and 10–13 h for PBE. (A2) mCy-pGV oocytes take 1–4 h for GVBD and 10–13 h for PBE, which is similar to the mouse oocyte in vitro maturational time course. (A3) pCy-mGV oocytes take 6–20 h to reach GVBD, and 10–20 h to reach the MI stage, which is significantly longer than that of mouse oocytes; (A4) Pig oocytes take 10–24 h for GVBD and 20–36 h for PBE, which is longer than that of in vitro maturation of pCy-mGV oocytes. (B1) mCy-pGV oocytes contain a large spindle similar to mouse oocyte spindles. (B2) pCy-mGV oocytes contain small spindles similar to the pig oocyte spindles.
The maturational time course of oocytes was also largely determined by the ooplasm. The mCy-pGV oocyte has a considerable developmental capability like the mouse oocyte. The percentage of GVBD and the maturational time course were similar to the mouse oocyte. Most mCy-pGV oocytes underwent GVBD at the same time as mouse oocytes and could develop to the MII stage at 10–13 h of in vitro culture. When the GV of a mouse oocyte was transferred into an enucleated pig oocyte, the in vitro developmental time to GVBD and the MI stage were also significantly extended. GVBD of the pCy-mGV oocyte took place at 6–20 h of in vitro culture, which is much slower compared to mouse oocyte GVBD, but 4 h faster than pig oocyte GVBD (10–24 h) (Fig. 7). Therefore, the oocyte meiotic maturational time course is mainly determined by the cytoplasm, while the GV materials may also regulate this process to a certain extent.
Conclusions
The spindle sizes of mCy-pGV oocytes and pCy-mGV oocytes are both similar to those of oocytes which contribute the cytoplasm. Unlike in Xenopus mitotic embryonic cells, it is the ooplasmic components but not the cytoplasmic volume that determine the spindle size in mammalian oocytes. Although GV materials are important for somatic cell reprogramming and subsequent development of the embryo, it is not related to spindle size control.
Methods
Reagents and animals
All reagents were purchased from Sigma unless otherwise noted. All mice were housed under specific pathogen-free controlled conditions with a 14L:10D light/dark cycle. The animal maintenance, handling and all the relevant details in the methods section were carried out in accordance with the Institutional Guidelines for Animal Use and Care at the Institute of Zoology, Chinese Academy of Sciences. All experimental protocols were approved by the ethical committee of the Institute of Zoology, Chinese Academy of Sciences (approval number IOZ12017).
Mouse oocyte collection and in vitro maturation
Immature oocytes at the germinal vesicle (GV) stage were collected from ovaries of 6–8-wk-old ICR female mice in M2 medium. Oocytes used for micro-manipulation were cultured in M2 medium supplemented with 2.5 μM milrinone to maintain the oocytes at the GV stage during micro-manipulation. After micro-manipulation, oocytes were washed thoroughly and further cultured in M16 medium covered with liquid paraffin oil at 37 °C in an atmosphere of 5% CO2 in air until proceeding to the GV, GVBD, MI and MII stages.
Pig oocyte collection and in vitro maturation
Collection and in vitro maturation of pig oocytes were conducted as previously reported30. In brief, pig ovaries were obtained from our local abattoir and transported to the laboratory at 35 °C. Cumulus-oocytes complexes (COCs) were aspirated from antral follicles (3–6 mm in diameter) of ovaries with an 18-gauge needle fixed to a 20 ml disposable syringe. After three rinses in washing medium (TCM-199 medium (Invitrogen) supplemented with 2.2% NaHCO3), COCs with uniform cytoplasm and at least four layers of compact cumulus cells were selected for culture. To prepare cumulus cell-denuded oocytes (DOs), cumulus cells were freed from the collected COCs after treatment for 3 min with 300 IU/ml hyaluronidase, followed by repeated pipetting using a narrow-bore micropipette. COCs and DOs were cultured in the basic medium with hormone. Basic medium was TCM-199, supplemented with 0.1% PVA (w/v), 0.91 mM sodium pyruvate, 75 μg/ml potassium penicillin G and 50 μg/ml streptomycin sulphate. Pig oocytes were cultured in basic medium with 0.57 mM cysteine, 0.5 μg/ml FSH, 0.5 μg/ml LH, and 10 ng/ml EGF. 4 mM HX was added to the culture medium to prevent the oocytes from GVBD for micro-manipulation. Oocytes of the control group or after micro-manipulation were cultured in 500 μl maturation medium in 4-well dishes, covered by liquid paraffin oil for up to 44 h at 38.5 °C in an atmosphere of 5% CO2 in air and saturated humidity.
Preparation of mouse GVs and cytoplasts
Germinal vesicles and cytoplasts for GV transfer were obtained as described in previous reports6,31. GV stage oocytes were kept in M2 medium supplemented with 2.5 μM milrinone to maintain the oocytes at the GV stage. Then the oocytes were cultured in M16 medium supplemented with 2.5 M milrinone for 2 h. After 2 h of culture in milrinone-supplemented M16 medium, GV oocytes have developed a perivitelline space, facilitating manipulation and enucleation. Enucleation and GV transplantation were performed in manipulation drops of M2 medium supplemented with 2.5 μM milrinone, 5 μg/ml cytochalasin B (CB) and 0.2 mg/ml demecolcine (M-Enu-M2). Immature oocytes were placed in manipulation drops for 30 min before GV removal. A slit was made in the zona pellucida by pressing a sharp glass needle through the perivitelline space against the holding pipette (See Supplementary file 1). A blunt-tip micropipette with an inner diameter of about 20 μm was inserted to remove the GV (See Supplementary file 2). The micropipette was rinsed in 10% PVP before GV removal to reduce sticking of the membranes within the pipette, then two or three droplets of mineral oil were aspirated to help control the fluid flow during the manipulation. Mouse GVs and enucleated mouse oocytes (cytoplasts) were placed in the milrinone-supplemented M16 medium for further manipulation.
Preparation of pig GVs and cytoplasts
The protocol of pig GV removal was based on previous reports of pig32 and bovine33 oocyte GV transfer procedure with some modifications. Pig DOs were exposed to basic medium supplemented with 15 μg/ml CB and 0.1 μg/ml demecolcine for 30 min at 38.5 °C, with 5% CO2 in air. Micro-manipulation of pig oocyte GV transfer was carried out in P-Enu-M2 medium which was M2 medium supplemented with 15 μg/ml cytochalasin B and 0.1 μg/ml demecolcine. Pig oocytes were washed several times, transferred into P-Enu-M2, and centrifuged for 10 min at 4000 g for visualization of the GV. Then a slit was made in the zona pellucida by pressing a sharp glass needle through the perivitelline space against the holding pipette near the position of the germinal vesicle. The germinal vesicle was then squeezed out by pressing on the zona pellucida (See Supplementary file 3). Oocytes were then carefully pipetted to separate the removed GV and the oocyte.
GV transfer by Piezo manipulation and electric fusion
A pig or mouse GV was transferred into the perivitelline space of a pig or mouse cytoplast by a blunt-tip micropipette with an inner diameter of about 25 μm (for mouse GV transfer) or 30 μm (for pig GV transfer). The pig GV-mouse cytoplast complexes (mouse GV-mouse cytoplast, pig GV-pig cytoplast) were transferred to a drop of fusion medium (0.3 M mannitol, 0.1 mM CaCl2 and 0.05 mM MgSO4 in sterile water). Electro-fusion was stimulated with two electrical pulses (160 V/mm DC for 60 ms) delivered by a Kefa Electro Cell manipulator (Academia Sinica). The fusion rate was examined 30 min later. The mouse GV was directly injected into pig enucleated oocyte cytoplasm (cytoplast) by a piezo-actuated micromanipulator. First, a single weak piezo pulse was applied to break the cytoplasmic membrane around the GV in a 20 μm pipette. The pipette was inserted from the incision of the zona pellucida, and advanced until it almost reached the opposite side of the oocyte. One weak piezo pulse was applied to puncture the oolemma at the pipette tip, and the mouse GV was immediately expelled into the enucleated pig oocyte (cytoplast) (See Supplementary file 4).
Antibodies and immunofluorescence
Oocytes were first fixed in 4% paraformaldehyde for 30 min. The permeabilization procedures were different in diverse oocytes: mouse or mCy-pGV oocytes were treated with 0.5% Triton X-100 for 20 min at room temperature; pig or pCy-mGV oocytes were treated with 0.5% Triton X-100 over night at 37 °C covered with mineral oil. After blocking in 1% BSA for 1 h, the oocytes were further washed four times in PBS with 0.05% Tween 20 and stained with anti-α-tubulin antibody (1:100 in PBS with 0.05% Tween 20, Sigma) over night at 4 °C. After four washes in PBS with 0.05% Tween 20, the oocytes were incubated with PI (propidium iodide) in PBS containing 0.05% Tween 20 for 10 min. Finally, the oocytes were mounted on glass slides and examined with a laser scanning confocal microscope (Zeiss LSM 510 and 710 META). Spindle sizes were measured by the software ZEN 2009 Light Edition (Carl Zeiss MicroImaging GmbH).
Statistical analysis
All experiments used at least three independent samples and were repeated at least three times; results are given as means ± SD. Group comparisons were made by two-tailed unpaired Student’s t-tests. P-values of <0.05 were considered significant.
Additional Information
How to cite this article: Wang, Z.-W. et al. Cytoplasmic Determination of Meiotic Spindle Size Revealed by a Unique Inter-Species Germinal Vesicle Transfer Model. Sci. Rep. 6, 19827; doi: 10.1038/srep19827 (2016).
Supplementary Material
Acknowledgments
This study was supported by Major Basic Research Program (2012CB944404) and National Natural Science Foundation of China (30930065, 31530049) to Q.Y.S.
Footnotes
Author Contributions Z.-W.W., and Q.-Y.S. designed research; Z.-W.W., G.-L.Z. performed research; Z.-W.W., and Q.-Y.S. analyzed data; and Z.-W.W., H.S., J.C. and Q.-Y.S. wrote the paper. All authors read and approved the final manuscript.
References
- Fulka J. Jr., First N. L. & Moor R. M. Nuclear and cytoplasmic determinants involved in the regulation of mammalian oocyte maturation. Mol Hum Reprod 4, 41–49 (1998). [DOI] [PubMed] [Google Scholar]
- Miyamoto K. et al. Nuclear Wave1 is required for reprogramming transcription in oocytes and for normal development. Science 341, 1002–1005 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S. et al. Germinal vesicle material is essential for nucleus remodeling after nuclear transfer. Biol Reprod 67, 928–934 (2002). [DOI] [PubMed] [Google Scholar]
- Ogushi S. et al. The maternal nucleolus is essential for early embryonic development in mammals. Science 319, 613–616 (2008). [DOI] [PubMed] [Google Scholar]
- Liu H., Wang C. W., Grifo J. A., Krey L. C. & Zhang J. Reconstruction of mouse oocytes by germinal vesicle transfer: maturity of host oocyte cytoplasm determines meiosis. Hum Repord 14, 2357–2361 (1999). [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Ergun B., Huang T. H., Rosenwaks Z. & Palermo G. D. A reliable technique of nuclear transplantation for immature mammalian oocytes. Hum Repord 14, 1312–1317 (1999). [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Rosenwaks Z. & Palermo G. D. A successful model to assess embryo development after transplantation of prophase nuclei. Hum Repord 19, 975–981 (2004). [DOI] [PubMed] [Google Scholar]
- Li G. P. et al. Viable rabbits derived from reconstructed oocytes by germinal vesicle transfer after intracytoplasmic sperm injection (ICSI). Mol Reprod Dev 58, 180–185 (2001). [DOI] [PubMed] [Google Scholar]
- Sun Q. Y., Lai L., Bonk A., Prather R. S. & Schatten H. Cytoplasmic changes in relation to nuclear maturation and early embryo developmental potential of porcine oocytes: effects of gonadotropins, cumulus cells, follicular size, and protein synthesis inhibition. Mol Reprod Dev 59, 192–198 (2001). [DOI] [PubMed] [Google Scholar]
- Goshima G. & Scholey J. M. Control of mitotic spindle length. Annu Rev Cell Dev 26, 21–57 (2010). [DOI] [PubMed] [Google Scholar]
- Alfaro-Aco R. & Petry S. Building the Microtubule Cytoskeleton Piece by Piece. J Biol Chem 290, 17154–17162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman L. P., Harry E. F., McAinsh A. D., Prior I. A. & Royle S. J. Specific removal of TACC3-ch-TOG-clathrin at metaphase deregulates kinetochore fiber tension. J Cell Sci 126, 2102–2113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova Y. et al. Mammalian end binding proteins control persistent microtubule growth. J Cell Biol 184, 691–706 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espiritu E. B., Krueger L. E., Ye A. & Rose L. S. CLASPs function redundantly to regulate astral microtubules in the C. elegans embryo. Dev Biol 368, 242–254 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Brust-Mascher I., Cheerambathur D. & Scholey J. M. Coupling between microtubule sliding, plus-end growth and spindle length revealed by kinesin-8 depletion. Cytoskeleton 67, 715–728 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do K. K., Hoang K. L. & Endow S. A. The kinesin-13 KLP10A motor regulates oocyte spindle length and affects EB1 binding without altering microtubule growth rates. Bio Open 3, 561–570 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally K., Audhya A., Oegema K. & McNally F. J. Katanin controls mitotic and meiotic spindle length. J Cell Biol 175, 881–891 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buster D., McNally K. & McNally F. J. Katanin inhibition prevents the redistribution of gamma-tubulin at mitosis. J Cell Sci 115, 1083–1092 (2002). [DOI] [PubMed] [Google Scholar]
- Brown K. S. et al. Xenopus tropicalis egg extracts provide insight into scaling of the mitotic spindle. J Cell Biol 176, 765–770 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. C., Vahey M. D., Skandarajah A., Fletcher D. A. & Heald R. Cytoplasmic volume modulates spindle size during embryogenesis. Science 342, 856–860 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel J. et al. Changes in cytoplasmic volume are sufficient to drive spindle scaling. Science 342, 853–856 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulka J. Jr., Martinez F., Tepla O., Mrazek M. & Tesarik J. Somatic and embryonic cell nucleus transfer into intact and enucleated immature mouse oocytes. Hum Reprod 17, 2160–2164 (2002). [DOI] [PubMed] [Google Scholar]
- Kubelka M. & Moor R. M. The behaviour of mitotic nuclei after transplantation to early meiotic ooplasts or mitotic cytoplasts. Zygote 5, 219–227 (1997). [DOI] [PubMed] [Google Scholar]
- McGrath J. & Solter D. Inability of mouse blastomere nuclei transferred to enucleated zygotes to support development in vitro. Science 226, 1317–1319 (1984). [DOI] [PubMed] [Google Scholar]
- Wakayama T., Tateno H., Mombaerts P. & Yanagimachi R. Nuclear transfer into mouse zygotes. Nat Genet 24, 108–109 (2000). [DOI] [PubMed] [Google Scholar]
- Kang E. et al. Nuclear reprogramming by interphase cytoplasm of two-cell mouse embryos. Nature 509, 101–104 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulka J. Jr. Nuclear maturation in pig and rabbit oocytes after interspecific fusion. Exp Cell Res 146, 212–218 (1983). [DOI] [PubMed] [Google Scholar]
- Fulka J. Jr., Motlik J., Fulka J. & Crozet N. Inhibition of nuclear maturation in fully grown porcine and mouse oocytes after their fusion with growing porcine oocytes. J Exp Zool 235, 255–259 (1985). [DOI] [PubMed] [Google Scholar]
- Fulka J. Jr., Leibfried-Rutledge M. L. & First N. L. Control of germinal vesicle breakdown in bovine x murine hybrid oocytes. Reprod Nutri Dev 33, 411–417 (1993). [DOI] [PubMed] [Google Scholar]
- Yang C. R. et al. The G protein coupled receptor 3 is involved in cAMP and cGMP signaling and maintenance of meiotic arrest in porcine oocytes. PLoS One 7, e38807 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z., Cheng Y., Liang C. G. & Latham K. E. Nuclear transfer in mouse oocytes and embryos. Methods Enzymol 476, 171–184 (2010). [DOI] [PubMed] [Google Scholar]
- Nakagawa S. et al. Vitrification of fully grown and growing porcine oocytes using germinal vesicle transfer. J Reprod Dev 57, 335–341 (2011). [DOI] [PubMed] [Google Scholar]
- Franciosi F., Perazzoli F., Lodde V., Modina S. C. & Luciano A. M. Developmental competence of gametes reconstructed by germinal vesicle transplantation from fresh and cryopreserved bovine oocytes. Fertil Steril 93, 229–238 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.