Abstract
Background and Purpose
The TRPC5 proteins assemble to create calcium‐permeable, non‐selective, cationic channels. We sought novel modulators of these channels through studies of natural products.
Experimental Approach
Intracellular calcium measurements and patch clamp recordings were made from cell lines. Compounds were generated by synthetic chemistry.
Key Results
Through a screen of natural products used in traditional Chinese medicines, the flavonol galangin was identified as an inhibitor of lanthanide‐evoked calcium entry in TRPC5 overexpressing HEK 293 cells (IC50 0.45 μM). Galangin also inhibited lanthanide‐evoked TRPC5‐mediated current in whole‐cell and outside‐out patch recordings. In differentiated 3T3‐L1 cells, it inhibited constitutive and lanthanide‐evoked calcium entry through endogenous TRPC5‐containing channels. The related natural flavonols, kaempferol and quercetin were less potent inhibitors of TRPC5. Myricetin and luteolin lacked effect, and apigenin was a stimulator. Based on structure–activity relationship studies with natural and synthetic flavonols, we designed 3,5,7‐trihydroxy‐2‐(2‐bromophenyl)‐4H‐chromen‐4‐one (AM12), which inhibited lanthanide‐evoked TRPC5 activity with an IC50 of 0.28 μM. AM12 also inhibited TRPC5 activity evoked by the agonist (−)‐Englerin A and was effective in excised outside‐out membrane patches, suggesting a relatively direct effect. It inhibited TRPC4 channels similarly, but its inhibitory effect on TRPC1–TRPC5 heteromeric channels was weaker.
Conclusions and Implications
The data suggest that galangin (a natural product from the ginger family) is a TRPC5 inhibitor and that other natural and synthetic flavonoids contain antagonist or agonist capabilities at TRPC5 and closely related channels depending on the substitution patterns of both the chromone core and the phenyl ring.
Abbreviations
- LPC
lysophosphatidylcholine
- S1P
sphingosine 1‐phosphate
Tables of Links
| LIGANDS |
|---|
| Apigenin |
| Englerin A |
| Galangin |
| LPC, lysophosphatidylcholine |
| Luteolin |
| Quercetin |
| S1P, sphingosine 1‐phosphate |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
In mammals, twenty‐eight genes encode transient receptor potential (TRP) proteins (Damann et al., 2008). These proteins assemble to form homotetrameric or heterotetrameric cationic channels, which are most commonly localized to the plasma membrane. Although there are similarities between different TRPs, they are diverse in sequence and the assembled channels are differentially activated or inhibited by physico‐chemical signals, including hot and cold temperatures and a plethora of chemicals, some of which are natural products such as capsaicin and menthol (Vriens et al., 2008). Several of the TRP channels have attracted attention as potential targets for drug discovery efforts, for example, TRPV1 or TRPA1 in the analgesia field.
The C subfamily of TRPs has seven members, TRPC1–7, although one of them, TRPC2, is not expressed in humans (Abramowitz and Birnbaumer, 2009; Beech, 2013; Birnbaumer, 2009; Bon and Beech, 2013). The TRPC subfamily is the one that is most closely related to the first‐identified TRP of photo‐transduction in Drosophila melanogaster: hence C, for canonical. The properties of the TRPCs have been reviewed (Abramowitz and Birnbaumer, 2009; Beech, 2013; Birnbaumer, 2009; Bon and Beech, 2013). Here, we focused on transient receptor potential canonical 5 (TRPC5), which, like most other TRPCs, assembles as homotetramers to form non‐selective Ca2+‐permeable cationic TRPC5 channels, but it also heteromerizes with other TRPCs such as transient receptor potential canonical 1 (TRPC1) (Beech, 2007; Zholos, 2014). TRPC5 is often noted for its expression in the CNS and is sometimes indicated as being exclusively neuronal. In this context, innate fear and pro‐epileptic roles have been suggested for TRPC5 as well as roles in growth cone formation and other neuronal functions (Greka et al., 2003; Phelan et al., 2013; Riccio et al., 2009). TRPC5 is, nevertheless, also expressed in peripheral tissues where non‐neuronal roles have been suggested, such as in podocyte barrier function, cancer cell multidrug resistance and adiponectin secretion from adipocytes (Ma et al., 2014; Schaldecker et al., 2013; Sukumar et al., 2012). TRPC5 channels can exhibit constitutive activity but are also modestly or strongly stimulated by various externally applied factors that are not specific to TRPC5 but include lanthanide ions (Gd3+ and La3+), sphingosine‐1‐phosphate (S1P) and lysophosphatidylcholine (LPC) (Flemming et al., 2006; Jung et al., 2003; Xu et al., 2006; Zeng et al., 2004). Lanthanides appear to act as direct activators or facilitators of channel opening, whereas S1P acts indirectly via G protein signalling (Jung et al., 2003; Xu et al., 2006). There is a view that TRPC5 forms a receptor‐activated channel and that this is its physiological purpose, but there is also the view that it is activated by stress factors without the need for receptor activation (Birnbaumer, 2009; Jiang et al., 2011). Both may be true and, indeed, TRPC5 channels, like several other TRP channels, show what is variously described as versatility, promiscuity or multiplicity of activation (Birnbaumer, 2009; Jiang et al., 2011; Vriens et al., 2008; Zeng et al., 2004).
As with other members of the TRPC subfamily, the pharmacology of low MW ligands for TRPC5 channels is relatively underdeveloped, often lacking potency and specificity and often not acting directly; various modulators of this type have been reviewed (Bon and Beech, 2013; Jiang et al., 2011). There is emerging evidence for synthetic low MW modulators, which include sigma‐1 receptor ligands, the antihistamine clemizole hydrochloride, riluzole, the 2‐aminoquinoline ML204 and the 2‐aminobenzimidazole derivative M084 (Amer et al., 2013; Miller et al., 2011; Richter et al., 2014a; Richter et al., 2014b; Zhu et al., 2015). There is also evidence for antagonist capability in dietary substances that include ω‐3 fatty acids and antioxidant chemicals, such as vitamin C, gallic acid and the polyphenol resveratrol (Naylor et al., 2011; Sukumar et al., 2012). Conversely, a remarkably potent and selective activator exists in (−)‐Englerin A, which derives from the plant Phyllanthus engleri (Akbulut et al., 2015). Such sensitivity to natural products aligns with the findings for other TRP subfamilies and a general concept for TRP channels as integrators of animal biology with physical factors of the external environment (Vriens et al., 2008). Here, we sought new information on TRPC5 channel modulators by testing a small set of natural products from traditional Chinese medicines.
Methods
Plasmids for TRPC1/TRPC5 and HEK‐TRPC3 experiments
Human TRPC3 was cloned into the pcDNA™4/TO expression vector (ThermoFisher Scientific, Waltham, MA, USA) between KpnI and XbaI restriction sites using hTRPC3/pcDNA3 (from M Zhu, Ohio State University) as a PCR template (forward primer: 5′ CAGTGGTACCGCCACCATGGAGGGAAGCCCATC 3′, and reverse primer: 5' ACACTCTAGATCATTCACATCTCAGCATGCTG 3′). To facilitate cloning of SYFP2–TRPC1 and mTurquoise2–TRPC5, a four amino acid linker (ASAS) flanked by AgeI and SacII restriction sites was introduced into pcDNA™4/TO between EcoRI and XhoI restriction sites using Gibson Assembly® (New England Biolabs, Ipswich, MA, USA) (forward oligonucleotide: 5′ CCACTAGTCCAGTGTGGTGGAATTCACCGGTGCCAGCGCATCCCGC 3′, and reverse oligonucleotide: 5′ GTTTAAACGGGCCCTCTAGACTCGAGCCGCGGGATGCGCTGGCACC 3′). Fluorophores were inserted upstream of the linker between KpnI and AgeI restriction sites using pSYFP2‐C1 (Addgene plasmid # 22878; Kremers et al., 2006) and pmTurquoise2‐C1 (Addgene plasmid # 60560; Goedhart et al., 2010), gifts from Dorus Gadella, as PCR templates (mTurquoise2/SYFP2 forward primer: 5′ TAATGGTACCGCCACCATGGTGAGC 3′, and mTurquoise2/SYFP2 reverse primer: 5′ TATTACCGGTCTTGTACAGCTCGTCCATGC 3′). Human TRPC1 (forward primer: 5′ ACTTCCGCGGCATGATGGCGGCCCTG 3′, and reverse primer: 5′ TTGCTCT AGAAAATGGTTAATTTCTTGGATAAAAC 3′) or human TRPC5 (forward primer: 5′ GATCCCGCGGAATGGCCCAACTGTACTACAAAAAG 3′, and reverse primer: 5′ GGGTCAAGGAAGGCACG 3′) was inserted downstream of the linker between SacII and XbaI restriction sites using hTRPC1/pIRES (Xu et al., 2006) and hTRPC5/pcDNA™4/TO (Zeng et al., 2004) as PCR templates. All constructs contained an N‐terminal Kozak sequence.
Cell culture
HEK 293 cells stably expressing tetracycline‐regulated human TRPC5 (Zeng et al., 2004) or TRPC4 (Akbulut et al., 2015) have been described. An equivalent cell line expressing TRPC3 was established. Cells were maintained in DMEM‐F12 + GlutaMAX‐1 (ThermoFisher Scientific) supplemented with 10% fetal calf serum (FCS) and penicillin/streptomycin at 37 °C in a 5% CO2 incubator. For selection, 400 μg·mL−1 zeocin and 5 μg·mL−1 blasticidin S were included in the cell medium. To induce expression, cells were incubated with 1 μg·mL−1 tetracycline (Sigma‐Aldrich, Gillingham, UK) for 24 h prior to experiments (Tet+). Non‐induced cells without addition of tetracycline (Tet−) were used as control. HEK 293 cells stably expressing tetracycline‐regulated human TRPM2 were prepared similarly and have also been described previously (McHugh et al., 2003). TRPV4 was studied in CHO K1 cells stably expressing human TRPV4 and maintained in Ham's F12 (ThermoFisher Scientific) in the presence of 1 mg·mL−1 G418 (Sigma‐Aldrich).
The 3T3‐L1 cell line was obtained from the American Type Culture Collection and cultured in DMEM‐F12 containing 10% fetal calf serum (FCS), 100 U·mL−1 penicillin and 100 μg·mL−1 streptomycin. To induce differentiation, cells were grown to confluence, and 2 days post‐confluence, the medium was changed to a medium containing 5 μg·mL−1 insulin, 0.25 μM dexamethasone and 0.5 mM IBMX with 10% FCS and antibiotics. After 48 h, medium was changed to a maintenance medium containing 5 μg·mL−1 insulin, 10% FCS and antibiotics. Cells were fed with fresh maintenance medium every 2 days until the day of experiments. For all experiments, cells were differentiated for 12–16 days.
Intracellular Ca2+ measurement
Induced (Tet+) and non‐induced (Tet−) cells were plated in poly‐d‐lysine‐coated black 96‐well plates (Corning, Corning, NY, USA) at a confluence of 90% 24 h before experimentation. Cells were incubated for 1 h in 4 μM fluo‐4‐AM, 2 μM fura‐2‐AM or 4 μM XRhod‐1‐AM in standard bath solution (SBS) at 37 °C in the presence of 0.01% pluronic acid (ThermoFisher Scientific) and, for fluo‐4‐AM and XRhod‐1‐AM, 2.5 mM probenecid. SBS contained (mM) the following: 130 NaCl, 5 KCl, 8 d‐glucose, 10 HEPES, 1.2 MgCl2 and 1.5 CaCl2; the pH was titrated to 7.4 with NaOH, and the osmolarity was ~290 mOsm. Cells were washed three times with SBS before measurements were made at room temperature (21 ± 2 °C) on a 96‐well fluorescence plate reader (FlexStation II384, Molecular Devices, Sunnyvale, CA, USA). Fura‐2 was excited at 340 and 380 nm, and emitted light was collected at 510 nm. Fluo‐4 was excited at 485 nm, and emitted light was collected at 525 nm. XRhod‐1 was excited at 580 nm, and emitted light was collected at 610 nm. Readings were made every 10 s. Fura‐2 measurements are shown as the fluorescence (F) ratio or change (Δ) in this ratio. Fluo‐4 and XRhod‐1 measurements are shown as absolute fluorescence in arbitrary units or changes in this fluorescence (ΔF). For experiments requiring no extracellular Ca2+, BaCl2 replaced the CaCl2 in SBS. When required, pretreatments with flavonoids were for 30 min at room temperature prior to recordings and maintained throughout. Control cells were treated with DMSO (vehicle) as appropriate.
Electrophysiology
Current recordings were made under voltage clamp using the whole‐cell or outside‐out configuration of the patch clamp technique at room temperature. Cells were seeded on glass coverslips at 20–30% density. Signals were amplified and sampled using an Axopatch 200B amplifier and pCLAMP 8 or 10 software (Molecular Devices). Data were filtered at 2 kHz and digitally sampled at 4 kHz. The voltage protocol comprised voltage ramps applied from −100 to +100 mV or every 10 s from a holding potential of 0 mV. The extracellular solution was SBS, and the patch pipette solution contained (mM) the following: 135 CsCl, 2 MgCl2, 1 EGTA, 10 HEPES, 5 Na2ATP and 0.1 Na2GTP, titrated to pH 7.2 with NaOH. All solutions were filtered using a 0.2 μm filter (Sartorius, Göttingen, Germany). TRPC4 or TRPC5 cells were induced by tetracycline 24 h before experiments. For TRPC1–TRPC5 whole‐cell recordings, HEK 293‐MSR cells were transiently transfected with SYFP2–TRPC1 and mTurquoise2–TRPC5 according to the manufacturer's instructions, with the following modifications: 4.5 μg of each construct and 5.5 μL Lipofectamine®2000 (ThermoFisher Scientific) were used. Cells were transfected at 90–95% confluence in a 35 mm culture dish, and transfection was performed for 4 h. Patch clamp experiments were performed on the cells 24–48 h post‐transfection.
Chemical syntheses
A library of 41 mono‐substituted flavonols 4 was prepared using a two‐step synthetic procedure (Scheme S1). The galangin analogue, 3,5,7‐trihydroxy‐2‐(2‐bromophenyl)‐4H‐chromen‐4‐one (AM12) was synthesized in four steps (Scheme S2). All synthesized chemicals were >97% pure according to 1H NMR and 13C NMR analyses. Synthetic and analytical details are reported in the Supporting Information.
Chemicals and stock solutions
Commercially available chemicals were purchased from Sigma‐Aldrich, unless stated otherwise. Stocks of chemicals were reconstituted in an appropriate vehicle: fluo‐4‐AM, fura‐2‐AM and X‐Rhod‐AM (ThermoFisher Scientific) were dissolved at 1 mM in DMSO; pluronic acid F‐127 (ThermoFisher Scientific) was stored at 10% w/v in DMSO at room temperature; probenecid was freshly prepared at 0.5 M in 1 M NaOH and diluted to 1:200 to give a working concentration of 2.5 mM; galangin, apigenin, kaempferol, quercetin, myricetin and luteolin were used as 10 mM stock solutions in ethanol. All other flavonols were synthesized and purified (for details, see the Supporting Information) and used as 10 mM stock solutions in DMSO. Stock solutions were diluted to 1:1000 into the recording solution, giving a final working concentration of 0.01% solvent. Gd3+ and La3+ were used as aqueous solutions of GdCl3 and LaCl3 respectively. 1‐Oleoyl‐2‐acetyl‐sn‐glycerol, thapsigargin and 4α‐phorbol 12,13‐didecanoate were all dissolved in DMSO and stored as 50, 5 and 10 mM stocks respectively. l‐α‐LPC from egg yolk and S1P were dissolved in methanol and stored as stock concentrations of 5 and 10 mM respectively. ATP and H2O2 were stored as aqueous stock solutions. Englerin A was prepared as a 10 mM stock solution in DMSO, stored in aliquots at −80 °C and diluted to working concentrations in experimental buffer (e.g. SBS) containing 0.1% DMSO and 0.01% pluronic acid. Pluronic acid was used as a dispersing agent to minimize aggregation of Englerin A.
Data analysis
Data are presented as mean ± SEM, where n represents the number of independent experiments and the N represents the total number of wells of a 96‐well plate used for n experiments. For patch clamp experiments, n was the number of recordings from individual cells. For patch clamp experiments, currents were normalized to the maximum current. Data subjected to statistical analysis are based on at least five individual experiments (n). Data points in individual calcium imaging experiments were based on at least four replicates each. Student's t‐tests were used for comparisons between two sets of data and statistically significant differences are indicated when P < 0.05; no significant difference by NS. For IC50 determinations, data were normalized to the vehicle controls (DMSO or ethanol), and the Hill equation was fitted using Origin software (OriginLab, Northampton, MA, USA).
Results
Galangin inhibits TRPC5 channels overexpressed in HEK 293
We screened natural products from traditional Chinese medicines for effects on Ca2+ entry in HEK 293 cells overexpressing TRPC5 (Figure S1). Each natural product was pre‐incubated with cells for 30 min and maintained throughout each recording at 10 μM. During the recordings, the lanthanide gadolinium (50 μM Gd3+) was applied to stimulate the TRPC5‐mediated Ca2+ entry in the presence of each natural product. Through this screen, galangin was found to be inhibitory against the Gd3+‐evoked signal (Figure 1A). Galangin is from Alpinia officinarum and other members of the ginger family.
Figure 1.
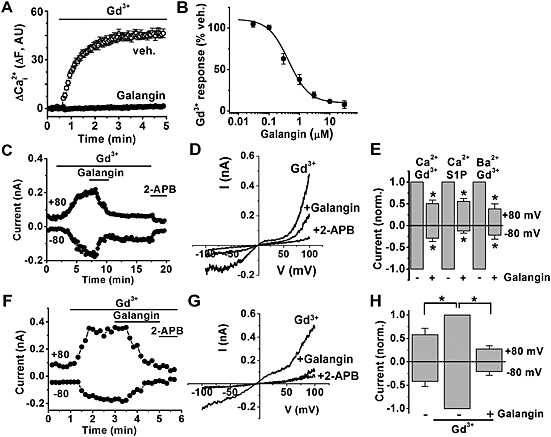
Galangin inhibits TRPC5. Recordings were from TRPC5‐expressing (Tet+) HEK 293, and extracellular Ca2+ was present at 1.5 mM unless indicated otherwise. (A) Free intracellular calcium ion (Ca2+i) concentration shown by fluo‐4 fluorescence intensity (F) in arbitrary units (AUs). Cells were incubated with 10 μM galangin or ethanol vehicle control (veh.) for 30 min before 50 μM Gd3+ was applied. (B) As for (A) but concentration–response data, showing an IC50 of the fitted Hill equation of 0.45 μM (n/N = 5/22). (C) Whole‐cell currents obtained during ramp changes in voltage from −100 to +100 mV every 10 s and during application of 30 μM Gd3+ and 10 μM galangin and then 75 μM 2‐aminoethoxydiphenylborate (2‐APB) alone. Ba2+ was present in the bath solution rather than Ca2+. (D) Example I‐Vs for the experiment in (C). (E) Normalized (norm.) data for whole‐cell currents evoked by 30 μM Gd3+ or 5 μM S1P in the absence (−) and presence (+) of 10 μM galangin. Ba2+ indicates when Ca2+ was substituted by barium ions in the bath solution. Currents were normalized to the amplitude prior to galangin application (n = 5, n = 5, n = 5). (F) Example outside‐out patch currents during application of 100 μM Gd3+, 10 μM galangin and 75 μM 2‐APB. (G) Example I‐Vs for the experiment in (F). (H) Normalized (norm.) data for outside‐out patch currents before and after application of 100 μM Gd3+ and after the addition of 10 μM galangin (n = 6). *P < 0.05.
Galangin had a concentration‐dependent inhibitory effect against the Gd3+‐evoked Ca2+ signal, acting with an IC50 of 0.45 μM (Figure 1B). It was also effective against Gd3+‐evoked TRPC5‐mediated current in whole‐cell voltage clamp recordings (Figure 1C). Its effect occurred within 2 min and was not readily reversed on washout (Figure 1C). The TRPC5 current–voltage relationship (I‐V) characteristically showed inward rectification at negative voltages and outward rectification at positive voltages with a plateau between 0 and +40 mV, which gave an approximate inverted S‐shape and seat‐like effect at positive voltages (Figure 1D). This signature I‐V was suppressed by galangin, consistent with it acting as a TRPC5 channel inhibitor (Figure 1D). Subsequent application of the TRPC5 inhibitor 2‐aminoethoxydiphenylborate (75 μM) (Xu et al., 2005) further inhibited the current (Figure 1C and D). Galangin was effective whether Gd3+ activated the channel in the presence or absence of Ca2+ (Ca2+ was substituted by Ba2+), suggesting that its action was Ca2+ independent (Figure 1C–E). Galangin was also effective against TRPC5 current stimulated by S1P, suggesting that its effect was not restricted to inhibition of the Gd3+ effect (Figure 1e). Galangin inhibited the TRPC5‐mediated current evoked in excised outside‐out membrane patches, suggesting a relatively direct effect (Figure 1F–H). The data suggest that galangin is an inhibitor of TRPC5 channels.
Galangin inhibits endogenous TRPC5‐containing channels
To determine if galangin also inhibits endogenous channels, we investigated differentiated 3T3‐L1 cells, which are a model of mature adipocytes and contain Ca2+ signals mediated by TRPC5‐containing channels (Sukumar et al., 2012). These endogenous channels exhibit constitutive activity leading to elevated basal intracellular Ca2+ concentration and further elevation in response to lanthanum (La3+), another lanthanide ion that was previously used in place of Gd3+ to activate TRPC5‐containing channels in 3T3‐L1 cells (Sukumar et al., 2012; Xu et al., 2008a). Galangin suppressed the basal Ca2+ signal and the La3+ response with estimated IC50s of 1.85 and 6.05 μM respectively (Figure 2A–C). The data suggest that galangin is an inhibitor of endogenous channels that contain TRPC5.
Figure 2.

Galangin inhibits Ca2+ entry through endogenous channels in differentiated 3T3‐L1 cells. Intracellular Ca2+ was measured using fluo‐4. (A) Example data from a single 96‐well plate showing basal Ca2+ and then 20 μM La3+‐evoked Ca2+ entry in the presence of vehicle (veh.) and 0.1, 1, 3 and 10 μM galangin. (B, C) Summarized concentration–response data for experiments of the type shown in (A) for (B) basal Ca2+ and (C) La3+‐evoked Ca2+ entry (n/N = 10/59). The IC50s for inhibition of basal and La3+‐evoked Ca2+ entry were 1.85 and 6.05 μM respectively.
Diverse effects of different but closely related natural flavonoids
Galangin is one in a series of compounds found in plants and commonly in diets and traditional remedies. A screen of natural flavonoids revealed that kaempferol and quercetin were inhibitors of Gd3+‐evoked Ca2+ entry in HEK 293 cells overexpressing TRPC5 but myricetin, apigenin and luteolin were not (Figure S2). Although kaempferol and quercetin were inhibitors, they were less potent than galangin (Figure 3A and B cf. Figure 1B). Apigenin had a stimulatory effect in Ca2+ measurement experiments (Figure S2) and was investigated further by whole‐cell voltage clamp recording. Apigenin was able to stimulate TRPC5‐mediated current, which could then be further enhanced by Gd3+ and blocked by 2‐aminoethoxydiphenylborate (Figure 3C–E). The apigenin‐activated current exhibited the characteristic I‐V shape of TRPC5, suggesting that it is indeed an activator of TRPC5 channels (Figure 3D). The data suggest that flavonoids inhibit, stimulate or have no effect on TRPC5 activity depending on small differences in substituent pattern.
Figure 3.

Negative or positive modulation of TRPC5 by natural flavonols. Recordings were from TRPC5‐expressing (Tet+) HEK 293 cells (A, B). Intracellular Ca2+ was measured using XRhod‐1. (A) Concentration–response data for kaempferol (IC50 3.9 μM, n/N = 5/22). (B) Concentration–response data for quercetin (IC50 6.5 μM, n/N = 5/25). (C–E) Whole‐cell data obtained from ramp changes in voltage from −100 to +100 mV every 10 s. (C) Example single‐cell recording showing responses to 10 μM apigenin and 30 μM Gd3+ and then 75 μM 2‐aminoethoxydiphenylborate (2‐APB) alone. (D) Example I‐Vs for the experiment in (C) [vehicle (veh.) indicates current before the application of apigenin]. (E) Mean current amplitudes for experiments of the type shown in (C) (n = 5 each). *P < 0.05.
Identification of AM12 as a synthetic flavonol that inhibits Ca2+ entry evoked by Gd3+
To further investigate structure–activity relationships of flavonols, a library of 41 mono‐substituted flavonols was synthesized using a two‐step procedure (Scheme S1) and screened at a concentration of 10 μM against Gd3+‐evoked Ca2+ entry in TRPC5 overexpressing HEK 293 cells (Figure S1). Guided by the results with natural and synthetic flavonols (Figure 4A and B), we designed AM12 (Figure 4c), which was synthesized in four steps (Scheme S2). AM12 inhibited the Gd3+‐evoked Ca2+ signal with an IC50 of 0.28 μM (Figure 4D and E). The data suggest that AM12 is slightly more potent than the natural product galangin as an inhibitor of the Gd3+‐evoked signal.
Figure 4.

Design of AM12 and its inhibition of Gd3+‐evoked TRPC5 activity. (A–C) Structure–activity relationships (SARs) of natural flavonoids (A) and synthetic, mono‐substituted flavonols (B). Comparison of natural flavonoids with compound 4‐A0 reveals the influence of hydroxyl substituents on inhibitor potency. Comparison of compound series 4 (Figure S3) reveals that most TRPC5 inhibitors in this series (inhibition >50% at 10 μM) had an ortho‐substituted (R2) phenyl ring, while compounds with R1, R3 or R4 substituents had weak or variable stimulatory effects. (C) Flavonol AM12 combines the hydroxylation pattern of galangin, the strongest natural TRPC5 inhibitor found to date, with an ortho‐substituted phenyl ring. The structure of AM12 is drawn as a twisted conformer to highlight the proposed effect of an ortho‐Br substituent. (D, E) Intracellular Ca2+ was measured using fura‐2 in TRPC5‐expressing (Tet+) HEK 293. (D) Example data from a single 96‐well plate showing basal Ca2+ and then Gd3+‐evoked Ca2+ entry in the presence of vehicle (veh.) and 0.1, 1, 3 and 10 μM AM12. (E) Summarized concentration–response data for experiments of the type shown in (D) for Gd3+‐evoked Ca2+ entry (IC50 0.28 μM, n/N = 5/30).
AM12 inhibits TRPC1/4/5 channels relatively directly
To investigate if AM12 might directly inhibit TRPC5, we used outside‐out patch recordings and bath‐applied AM12 to the extracellular face of the membrane. Moreover, to address the possibility that the effect of AM12 might be specific to Gd3+‐activated channel activity, we used an alternative, newly described, TRPC5 activator, (−)‐Englerin A (Akbulut et al., 2015). (−)‐Englerin A is considerably more potent and efficacious than Gd3+ (Figure 5A cf. Figure 1F). AM12 caused prompt inhibition of (−)‐Englerin A‐activated TRPC5 activity, and there was fast recovery on washout (Figure 5A–C). The average inhibition was ~65% at 5 μM AM12. AM12 also inhibited current through TRPC1–TRPC5 heteromeric channels, which were studied in whole‐cell recordings because of difficulty in obtaining outside‐out patches from TRPC1‐expressing cells (Figure 5D‐F). HEK 293 cells were transiently transfected with SYFP2–TRPC1 and mTurquoise2–TRPC5, and the expression of both proteins was detected by fluorescence microscopy. In whole‐cell patches of cells overexpressing both proteins and stimulated with (−)‐Englerin A, the characteristic seat‐like inflection of the TRPC5 I‐V was missing (Figure 5E cf. Figure 5B), which was consistent with the presence of heteromeric TRPC1–TRPC5 channels (Akbulut et al., 2015). AM12 was notably less effective against these heteromeric channels, giving only ~20% inhibition at 5 μM (Figure 5D‐F). Outside‐out patch recordings were also made from cells overexpressing TRPC4 homomeric channels, which are the most closely related to TRPC5 channels (Figure 5G–I). As with TRPC5 homomers, AM12 promptly inhibited TRPC4, and the average inhibition by 5 μM AM12 was ~80% (Figure 5G–I). The data suggest that AM12 inhibits TRPC5 and TRPC4 channels via a site accessible from the extracellular face of the membrane, acting directly on either the channel or a site closely associated with it. AM12 has an effect on heteromeric TRPC1–TRPC5 channels, but it is a relatively weak effect.
Figure 5.
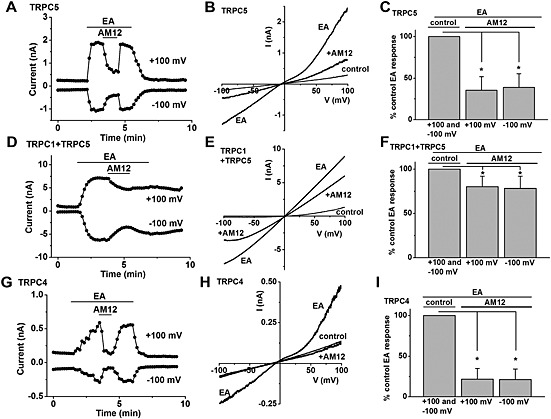
Inhibitory effect of AM12 on TRPC1/4/5 channels stimulated by (−)‐Englerin A (EA). Recordings were made from outside‐out membrane patches from TRPC5 Tet+ cells (A–C) or TRPC4 Tet+ cells (G–I) or from whole‐cell expressing SYFP2–TRPC1 and mTurquoise2–TRPC5 (D–F). (A) Example TRPC5 outside‐out patch currents during ramp changes in voltage from −100 to +100 mV every 10 s and application of 100 nM (−)‐EA and 5 μM AM12. (B) Example I‐Vs for the experiment in (A). (C) Mean normalized data for TRPC5 outside‐out patch currents evoked by 100 nM (−)‐EA and 5 μM AM12 (n = 5). (D) Example TRPC1–TRPC5 whole‐cell currents obtained during ramp changes in voltage from −100 to +100 mV every 10 s and during application of 100 nM (−)‐EA and 5 μM AM12. (E) Example I‐Vs for the experiment in (D). (F) Mean normalized data for TRPC1–TRPC5 whole‐cell currents evoked by 100 nM (−)‐EA and 5 μM AM12 (n = 5). (G) Example TRPC4 outside‐out patch currents during ramp changes in voltage from −100 to +100 mV every 10 s and application of 100 nM (−)‐EA and 5 μM AM12. (H) Example I‐Vs for the experiment in (G). (I) Mean normalized data for TRPC4 outside‐out patch currents evoked by 100 nM (−)‐EA and 5 μM AM12 (n = 5). *P < 0.05.
Selectivity of AM12
At 10 μM, AM12 had a modest inhibitory effect on Ca2+ entry through TRPC3 channels in some recordings, but overall, the effect did not reach statistical significance (Figure 6A). There was a significant stimulatory effect on Ca2+ entry through TRPV4 channels, but no effect on TRPM2 channels (Figure 6A–C). AM12 had no effect on the endogenous Ca2+ release signal evoked by thapsigargin or ATP (Figure 6D and E). Thapsigargin causes Ca2+ release by inhibiting the smooth endoplasmic reticular Ca2+ ATPase, whereas A T P causes release via a GPCR and inositol 1,4,5‐trisphosphate production, via PLC activity. The data suggest AM12 has a degree of selectivity for TRPC5 and TRPC4 channels but is not completely specific.
Figure 6.

AM12 effects on TRPC3 channels, TRPV4 channels, TRPM2 channels and Ca2+ release. Intracellular Ca2+ was measured using fluo‐4 (B) or fura‐2 (A, C, D, E). (a) Cells were stably overexpressing TRPC3 and the TRPC3 agonist 1‐oleoyl‐2‐acetyl glycerol (OAG, 50 μM) was used to activate the channels in the presence of the vehicle control (veh.) or 10 μM AM12. On the left are example data from a single 96‐well plate and on the right are mean data at two different time points for multiple plates of this type (n/N = 5/26). (B) Cells were stably overexpressing TRPV4 and the TRPV4 agonist 4α‐phorbol 12,13‐didecanoate (4αPDD, 1 μM) was used to activate the channels in the presence of the veh. or 10 μM AM12. On the left are example data from a single 96‐well plate, and on the right are mean data for multiple plates of this type (n/N = 5/35). (C) Cells were overexpressing TRPM2, and the TRPM2 activator H2O2 (1 mM) was used to activate the channels in the presence of the veh. or 10 μM AM12. On the left are example data from a single 96‐well plate, and on the right are mean data for multiple plates of this type (n/N = 5/24). (D) Cells were non‐induced (Tet−) TRPC5 HEK cells in the absence of extracellular Ca2+. Thapsigargin (TG, 3 μM) was applied to release intracellular Ca2+ in the presence of the veh. or 10 μM AM12. On the left are example data from a single 96‐well plate, and on the right are mean data for multiple plates of this type (n/N = 5/32). (E) Cells were non‐induced (Tet−) TRPC5 HEK cells in the absence of extracellular Ca2+. A TP (100 μM) was applied to release intracellular Ca2+ in the presence of the veh. or 10 μM AM12. On the left are example data from a single 96‐well plate, and on the right are mean data for multiple plates of this type (n/N = 5/25). *P < 0.05; NS, not significant; AU, arbitrary unit.
Stimulatory effect of AM12
Gd3+ and (−)‐Englerin A are not endogenous stimulators of TRPC5. Therefore, we next investigated the effect of AM12 against TRPC5 activity evoked by the physiological substance S1P. Unexpectedly, 10 μM AM12 stimulated rather than inhibited S1P‐evoked Ca2+ entry in HEK 293 cells overexpressing TRPC5 (Figure 7A). Similarly, TRPC5‐mediated Ca2+ entry evoked by the endogenous substance LPC was enhanced by AM12 (10 μM) (Figure 7B). In control cells without induction of TRPC5 expression (Tet− cells), there were no effects of AM12 (Figure 7C), but to further investigate if there were effects of AM12 in Tet‐ cells, we investigated the effect of ATP in the presence of extracellular Ca2+ and in the presence or absence of AM12 (Figure 7D). AM12 had no effect on A TP‐ evoked Ca2+ release (Figure 6E) but enhanced the A T P response in the presence of Ca2+ (Figure 7D). The data suggest that AM12 had a stimulatory effect on an endogenous Ca2+ entry mechanism. Stimulation of endogenous Ca2+ entry could potentially explain the stimulatory effects of AM12 in Tet+ cells (Figure 7A and B) because Ca2+‐mediated facilitation of TRPC5 channels has been described previously in these Tet+ cells (Hui et al., 2006). Nevertheless, it remained perplexing why AM12 did not act as an inhibitor when S1P and LPC were the agonists.
Figure 7.
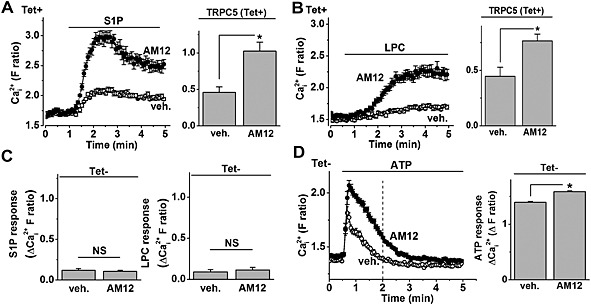
Stimulatory effects of AM12 on TRPC5. Intracellular Ca2+ was measured using fura‐2, and recordings were from TRPC5‐expressing (Tet+) HEK cells or control non‐induced (Tet−) cells. (A) Example data from Tet+ cells in a single 96‐well plate showing the response to 5 μM S1P in the presence of vehicle control (veh.) or 10 μM AM12 (left) and mean data for multiple plates of this type (n/N = 7/40). (B) Example data from Tet+ cells in a single 96‐well plate showing the response to 10 μM LPC in the presence of veh. or 10 μM AM12 (left) and mean data for multiple plates of this type (n/N = 9/56). (C) Mean data for the type of experiments shown in (A) (left; n/N = 6/31) and (B) (right; n/N = 5/20) for Tet− cells. (D) Example data from Tet− cells in a single 96‐well plate showing the response to 100 μM ATP in the presence of veh. or 10 μM AM12 (left) and mean data for multiple plates of this type (right; n/N = 5/30). *P < 0.05; NS, not significant.
Discussion and conclusions
Through a screen of a small number of natural products from traditional Chinese medicines, we found that the flavonol galangin is an inhibitor of lanthanide‐evoked activity of TRPC5 channels overexpressed in HEK 293 cells (IC50 0.45 μM against TRPC5‐mediated Ca2+ entry). Galangin also inhibited Ca2+ entry through endogenous TRPC5‐containing channels as shown by studies of differentiated 3T3‐L1 cells, although it was 5–15 times less potent in these cells. Related natural flavonols were investigated, and two, kaempferol and quercetin, were also inhibitors of overexpressed TRPC5 but with less potency than galangin. Myricetin and luteolin lacked effect. Apigenin had the reverse effect, stimulating TRPC5. Investigation of a panel of mono‐substituted flavonols led to the design of compound AM12, which inhibited lanthanide‐evoked TRPC5 activity with an IC50 of 0.28 μM and showed a degree of selectivity as demonstrated by no significant inhibitory effects at 10 μM on Ca2+ release or Ca2+ entry mediated by TRPC3, TRPV4 or TRPM2 channels. However, unlike galangin, AM12 potentiated TRPC5 activity evoked by the physiological TRPC5 stimulators S1P and LPC, apparently lacking inhibitory effect in this situation. The data suggest complex modulator effects of natural and synthetic flavonoids on TRPC5 channels.
The modulator effects of natural flavonoids depended on variations in hydroxylation pattern, with inhibition being more prominent in the flavonols compared with the flavones (X = OH vs. H) (Table 1; Figure 4A). The inhibitory potency of natural flavonols decreased with increasing hydroxylation of the phenyl ring (Table 1; Figure 4A, Z = OH), and within this series, increased hydrophobicity (higher cLogP) seems to correlate with higher potency (Table 1). To investigate structure–activity relationships of flavonols as TRPC5 modulators, we made and tested a panel of mono‐substituted flavonols (Supporting Information). Most of the compounds had weak or variable stimulatory effects, and so, we focused on inhibition (Figure 4B). Most compounds that caused >50% inhibition contained ortho‐substituted phenyl rings (Figure S3 and Figure 4B, R2 = OH, CH3, F, Cl or Br), while most compounds with R1, R3 and R4 substituents (Figure 4B and Figure S3) had weak or mixed effects. For the mono‐substituted flavonols, no correlation between hydrophobicity and potency could be detected.
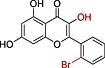
Table 1.
Relationships between substituent pattern, hydrophobicity and potency of natural flavonoids and AM12a
| Compound name | Compound structure | IC50 (μM) | cLogPb |
|---|---|---|---|
| AM12c |
|
0.28 | 3.53 |
| Galangin |
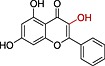
|
0.45 | 2.76 |
| Kaempferol |

|
3.9 | 2.46 |
| Quercetin |
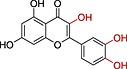
|
6.5 | 2.16 |
| Myricetin |
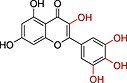
|
>>10 | 1.85 |
| Apigenind |
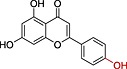
|
>>10 | 2.71 |
| Luteolin |
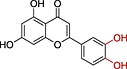
|
>>10 | 2.40 |
TRPC5 inhibition was measured in TRPC5‐expressing (Tet+) HEK 293 cells treated with ethanolic solutions of flavonoids (10 mM stock solutions). TRPC5 was stimulated with 50 μM Gd3+, and Ca2+ entry was measured using the calcium‐reactive dye fluo‐4 unless stated otherwise. The positive controls consisted of HEK 293 cells overexpressing TRPC5 and stimulated with 50 μM Gd3+, treated with vehicle (ethanol) only. The negative controls consisted of recordings from (Tet−) cells, which do not overexpress TRPC5.
cLogPs were calculated using chemicalize.org; ChemAxon: http://www.chemaxon.com; accessed October 2015.
The calcium‐reactive dye fura‐2 was used in experiments with AM12.
Apigenin acted as a stimulator of TRPC5 instead (Figure 3C–E).
The pronounced effect of ortho‐substituents (R2) as compared with meta‐substituents (R3) and para‐substituents (R4) on the flavonol phenyl ring might be attributed to their influence on the dihedral angle between the chromone and phenyl rings of the flavonol scaffold. Ortho‐substituents may cause restrictions on the conformational freedom of such biaryl scaffolds, favouring non‐planar conformers. Therefore, if TRPC5 inhibition is favoured by twisted flavonol conformers, ortho‐substituents may offer an energetic advantage by providing a conformational lock. To test this hypothesis, we designed and synthesized AM12 (Figure 4C), a galangin analogue with an ortho‐bromine substituent on the phenyl ring (see the Supporting Information for synthesis). AM12 was slightly more potent than galangin as an inhibitor of Gd3+‐evoked activity (Figure 4E cf. Figure 1B), which could be attributed to restriction of conformational freedom, but also fits the tentative correlation between hydrophobicity and potency as observed for natural flavonols (Table 1). However, the surprising stimulatory effect (Figure 7) and the more rapid reversibility of the inhibitory effect (Figure 5) suggested that subtle changes of flavonol substituents have a major impact on activity, mode of action and/or interaction with other calcium responses in the cell.
Based on simulations of molecular dynamics and functional membrane protein assays, it has been hypothesized that amphiphilic polyphenol phytochemicals – including the polyphenol resveratrol – localize to the membrane/solution interface, thereby reducing the energy required for bilayer adaptations perpendicular to the plane of the bilayer (Ingolfsson et al., 2014). Such an effect could alter conformational equilibria of membrane proteins whose function depends on conformational changes that are associated with bilayer perturbations. Quercetin and its metabolites have also been proposed to localize to the membrane/solution interface, enhancing their local concentrations and thereby their antioxidant effect on nearby lipids and membrane proteins (Kosinova et al., 2012). In addition, membrane penetration, and therefore local antioxidant effect, of quercetin derivatives was predicted to correlate with polarity of substituents (Kosinova et al., 2012). TRPC5 channels are susceptible to perturbations of the lipid bilayer as suggested by effects on channel activity of lipids depending on chain length and general anesthetics (Bahnasi et al., 2008; Flemming et al., 2006). Therefore, a plausible mechanism of action for flavonoids on TRPC5 is local perturbation of the bilayer, which then modulates channel activity.
The flavonols tested in this study all have a predicted pKa1 (first deprotonation) of ~6.4, which means that at physiological pH, their amphiphilic mono‐anions are the most prevalent species (Figure S4). In addition, the predicted octanol/water partition coefficients (cLogP) of galangin and AM12 (2.76 and 3.53 respectively) are in the same range as those of other polyphenol phytochemicals predicted to localize to the membrane/solution interface (Ingolfsson et al., 2014), and the apparent correlation between hydrophobicity within a subset of TRPC5‐inhibiting flavonols – but not flavones – (Table 1) is consistent with localization of the (substituted) phenyl ring of these compounds to a hydrophobic environment. However, the observation that subtle changes of flavonol substituent patterns can turn a poorly reversible inhibitor into a readily reversible inhibitor (galangin cf. AM12) and a TRPC5 inhibitor into a TRPC5 activator (galangin cf. apigenin) suggests that the mechanism of action of flavonols is more complex: activities may depend on membrane affinity, membrane localization and perturbation, and redox potential. In addition, distinct TRPC5 binding sites and/or flavonoid‐mediated modulation of calcium responses (including calcium release) through other proteins cannot be excluded. Moreover, the discovery of inhibitory and stimulatory effects of AM12 suggests a combination of effects.
Apigenin activated TRPC5, and it has previously been reported as a TRP activator, stimulating TRPV4 channels (Ma et al., 2012). A related isoflavone genistein also stimulated TRPC5 (Wong et al., 2010). Genistein has effects consistent with perturbation of the lipid bilayer (Ingolfsson et al., 2014).
The failure of AM12 to inhibit S1P‐evoked or LPC‐evoked TRPC5 activity is perplexing. It appears to be the case that AM12 had a separate stimulatory effect on another Ca2+ entry mechanism, which might then have facilitated TRPC5 activity. Nevertheless, despite such a possibility for facilitation, Gd3+‐evoked TRPC5 activity was inhibited by AM12, whereas S1P‐evoked and LPC‐evoked activities were not. These observations suggest that AM12 is not a direct inhibitor of the TRPC5 ion pore but a modulator that allosterically affects TRPC5 gating or the ion pore – by binding to TRPC5 or by perturbing the plasma membrane around TRPC5‐containing channels, or by acting more indirectly via a molecule closely associated with TRPC5. The data suggest the possibility for differential modulation of TRPC5 depending on its activation state; for example, it seems possible to inhibit lanthanide‐evoked and constitutive channel activity without affecting lipid‐evoked activity. We suspect that this complication is also involved in (−)‐Englerin A‐evoked activity. Although 5 μM AM12 inhibited (−)‐Englerin A‐evoked TRPC5 activity, the effect was on average less than that observed against Gd3+‐evoked activity, and in some recordings, the effect against (−)‐Englerin A‐evoked activity was surprisingly small, suggesting that a mixture of inhibitory and stimulatory actions of AM12 can affect the (−)‐Englerin A response.
The suppressive effect of galangin on lanthanum‐evoked Ca2+ entry in differentiated 3T3‐L1 cells suggests that it inhibits native TRPC5‐containing channels, which include other TRPC proteins such as TRPC1 (Sukumar et al., 2012). Consistent with the weaker effect of galangin on native channels (as compared with TRPC5 homomers), 5 μM AM12 had a small inhibitory effect on TRPC1–TRPC5 heteromeric channels (Figure 5D–F). Activity of galangin and other flavonoids in adipocytes has been previously reported. Ethanolic A. officinarum extract has been reported to inhibit adipocyte differentiation and high‐fat diet‐induced obesity in mice, and galangin (its major component), and had anti‐adipogenic effects in 3T3‐L1 cells (Jung et al., 2012). Genistein inhibited proliferation and subsequent differentiation of 3T3‐L1 cells (Harmon and Harp, 2001). Quercetin inhibited 3T3‐L1 cell growth and apoptosis (Hsu and Yen, 2006). TRPC5‐containing endogenous channels were up‐regulated in differentiated 3T3‐L1 cells, and inhibition of channel function in vivo by a dominant‐negative mutant TRPC5 raised circulating adiponectin levels, which is expected to have a cardioprotective effect (Sukumar et al., 2012). Therefore, flavonoids may act as natural regulators of adipocyte biology at least in part via modulation of Ca2+ entry through TRPC5‐containing channels, conferring a mechanism for integration with the environment via dietary intake. It should be noted, however, that flavonoids are not specific for TRPC5 channels. Galangin inhibited Cav1.2 channels in smooth muscle, while quercetin, myricetin and kaempferol were stimulators (Saponara et al., 2011). Kaempferol and quercetin stimulated BKCa channels (Cogolludo et al., 2007; Xu et al., 2008b). Apigenin and quercetin inhibited GABA‐evoked ionic currents (Goutman et al., 2003).
In summary, the study suggests that naturally occurring flavonoids can modulate TRPC5 channels and that one consequence in vivo might be modulation of adiponectin secretion. The effects of synthetic flavonols on TRPC5 activity show that potency and mode of action of flavonols on TRPC5 channels can be tuned by subtle changes of substituent patterns and suggest future directions for the development of more potent flavonol‐based TRPC5 inhibitors. Nevertheless, effects of flavonols are difficult to predict, and their numerous biological activities render them potentially problematic for drug discovery efforts (Baell and Walters, 2014).
Conflict of interest
The authors state no conflict of interest.
Author contributions
J. N., A. M., H. J. G., M. S. A., L. A. W., M. M., S. Y. C., H. N. R., N. M. B., K. E. M., M. J. L., W. D. E., B. L. G., Y. Y., J. L. and K. M. performed the research. H. Y., C. W. G. F., D. J. B. and R. S. B. designed the research study. J. N., A. M., H. J. G., M. S. A., L. A. W., S. Y. C., H. N. R., B. L. G., J. L., K. M., D. J. B. and R. S. B. analysed the data. D. J. B. and R. S. B. wrote the paper.
Supporting information
Figure S1 Overview of chemicals from traditional Chinese medicines screened against Ca2+entry in HEK 293 cells overexpressing human TRPC5.
Figure S2 Screen of natural flavonols against Ca2+ entry in HEK 293 cells over‐expressing human TRPC5. Intracellular Ca2+ was measured using XRhod‐1. Mean data comparing responses to 50 μM Gd3+ in the presence of 10 μM galangin, kaempferol, quercetin, myricetin,apigenin, luteolin or vehicle control (veh.) (n/N=3/12 each). Data were normalized to the Gd3+ response in vehicle and Tet+ cells.
Figure S3 Overview of syntheticmono‐substituted flavonols that were screened for TRPC5 inhibition at 10 μM. Compounds that inhibited Gd3+‐evoked calcium entry in TRPC5‐expressing HEK293 cells by > 50% are highlighted in red.
Figure S4 pKa1 values and structures of major microspecies (mm) of tested natural flavonoids and AM12 (predicted using Marvin Beans; downloaded from ChemAxon: http://www.chemaxon.com).
Scheme S1 General synthetic route towards a library of mono‐substituted flavonols 4. Aldol condensation of 2‐hydroxyacetophenones 1 with benzaldehydes 2 was followed by oxidative cyclisation of the intermediate chalcones 3 by use of an Algar‐Flynn‐Oyamada reaction.
Scheme S2 Synthesis of synthetic flavonol AM12. Benzoic anhydride 6 was prepared from its corresponding benzoic acid 5. Friedel‐Crafts acylation of phloroglucinol with acyl chloride 8 gave intermediate 9. Combination of building blocks 6 and 9 in an Allan‐Robinson reaction followed by boron tribromide‐mediated demethylation afforded AM12. THF: tetrahydrofuran.
Supporting info item
Acknowledgements
This work was supported by the Wellcome Trust, the Leeds Teaching Hospitals Trust Charitable Foundation, BBSRC PhD Studentships to A. M. and L. A. W., a Daniel Turnberg Travel Fellowship from the Academy of Medical Sciences to M. S. A., a BHF PhD Studentship to H. J. G. and a UK–China BBSRC Partnership Award to Y. Y.
Naylor, J. , Minard, A. , Gaunt, H. J. , Amer, M. S. , Wilson, L. A. , Migliore, M. , Cheung, S. Y. , Rubaiy, H. N. , Blythe, N. M. , Musialowski, K. E. , Ludlow, M. J. , Evans, W. D. , Green, B. L. , Yang, H. , You, Y. , Li, J. , Fishwick, C. WG. , Muraki, K. , Beech, D. J. , and Bon, R. S. (2016) Natural and synthetic flavonoid modulation of TRPC5 channels. British Journal of Pharmacology, 173: 562–574. doi: 10.1111/bph.13387.
References
Zhu Y, Lu Y, Qu C, Miller M, Tian J, Thakur DP, et al (2015). Identification and optimization of 2‐aminobenzimidazole derivatives as novel inhibitors of TRPC4 and TRPC5 channels. Br J Pharmacol 172: 3495–3509.
- Abramowitz J, Birnbaumer L (2009). Physiology and pathophysiology of canonical transient receptor potential channels. FASEB journal 23: 297–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, Bruns A, et al (2015). (−)‐Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl 54: 3787–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al (2013). The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol 170: 1607–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer MS, McKeown L, Tumova S, Liu R, Seymour VA, Wilson LA, et al (2013). Inhibition of endothelial cell Ca2+ entry and transient receptor potential channels by sigma‐1 receptor ligands. Br J Pharmacol 168: 1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baell J, Walters MA (2014). Chemical con artists foil drug discovery. Nature 513: 481–483. [DOI] [PubMed] [Google Scholar]
- Bahnasi YM, Wright HM, Milligan CJ, Dedman AM, Zeng F, Hopkins PM, et al (2008). Modulation of TRPC5 cation channels by halothane, chloroform and propofol. Br J Pharmacol 153: 1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ (2007). Canonical transient receptor potential 5. Handb Exp Pharmacol 179: 109–123. [DOI] [PubMed] [Google Scholar]
- Beech DJ (2013). Characteristics of transient receptor potential canonical calcium‐permeable channels and their relevance to vascular physiology and disease. Circ J 77: 570–579. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L (2009). The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu Rev Pharmacol Toxicol 49: 395–426. [DOI] [PubMed] [Google Scholar]
- Bon RS, Beech DJ (2013). In pursuit of small molecule chemistry for calcium‐permeable non‐selective TRPC channels – mirage or pot of gold? Br J Pharmacol 170: 459–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogolludo A, Frazziano G, Briones AM, Cobeno L, Moreno L, Lodi F, et al (2007). The dietary flavonoid quercetin activates BKCa currents in coronary arteries via production of H2O2. Role in vasodilatation. Cardiovasc Res 73: 424–431. [DOI] [PubMed] [Google Scholar]
- Damann N, Voets T, Nilius B (2008). TRPs in our senses. Curr Biol 18: R880–R889. [DOI] [PubMed] [Google Scholar]
- Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, et al (2006). Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem 281: 4977–4982. [DOI] [PubMed] [Google Scholar]
- Goedhart J, van Weeren L, Hink MA, Vischer NO, Jalink K, Gadella TW Jr (2010). Bright cyan fluorescent protein variants identified by fluorescence lifetime screening. Nat Methods 7: 137–139. [DOI] [PubMed] [Google Scholar]
- Goutman JD, Waxemberg MD, Donate‐Oliver F, Pomata PE, Calvo DJ (2003). Flavonoid modulation of ionic currents mediated by GABA(A) and GABA(C) receptors. Eur J Pharmacol 461: 79–87. [DOI] [PubMed] [Google Scholar]
- Greka A, Navarro B, Oancea E, Duggan A, Clapham DE (2003). TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci 6: 837–845. [DOI] [PubMed] [Google Scholar]
- Harmon AW, Harp JB (2001). Differential effects of flavonoids on 3T3‐L1 adipogenesis and lipolysis. Am J Physiol Cell Physiol 280: C807–C813. [DOI] [PubMed] [Google Scholar]
- Hsu CL, Yen GC (2006). Induction of cell apoptosis in 3T3‐L1 pre‐adipocytes by flavonoids is associated with their antioxidant activity. Mol Nutr Food Res 50: 1072–1079. [DOI] [PubMed] [Google Scholar]
- Hui H, McHugh D, Hannan M, Zeng F, Xu SZ, Khan SU, et al (2006). Calcium‐sensing mechanism in TRPC5 channels contributing to retardation of neurite outgrowth. J Physiol 572: 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolfsson HI, Thakur P, Herold KF, Hobart EA, Ramsey NB, Periole X, et al (2014). Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chem Biol 9: 1788–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH, Gamper N, Beech DJ (2011). Properties and therapeutic potential of transient receptor potential channels with putative roles in adversity: focus on TRPC5, TRPM2 and TRPA1. Curr Drug Targets 12: 724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Jang SJ, Ahn J, Gwon SY, Jeon TI, Kim TW, et al (2012). Alpinia officinarum inhibits adipocyte differentiation and high‐fat diet‐induced obesity in mice through regulation of adipogenesis and lipogenesis. J Med Food 15: 959–967. [DOI] [PubMed] [Google Scholar]
- Jung S, Muhle A, Schaefer M, Strotmann R, Schultz G, Plant TD (2003). Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem 278: 3562–3571. [DOI] [PubMed] [Google Scholar]
- Kosinova P, Berka K, Wykes M, Otyepka M, Trouillas P (2012). Positioning of antioxidant quercetin and its metabolites in lipid bilayer membranes: implication for their lipid‐peroxidation inhibition. J Phys Chem B 116: 1309–1318. [DOI] [PubMed] [Google Scholar]
- Kremers GJ, Goedhart J, van Munster EB, Gadella TW (2006). Cyan and yellow super fluorescent proteins with improved brightness, protein folding, and FRET Foerster radius. Biochemistry 45: 6570–6580. [DOI] [PubMed] [Google Scholar]
- Ma X, Chen Z, Hua D, He D, Wang L, Zhang P, et al (2014). Essential role for TrpC5‐containing extracellular vesicles in breast cancer with chemotherapeutic resistance. Proc Natl Acad Sci U S A 111: 6389–6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, He D, Ru X, Chen Y, Cai Y, Bruce IC, et al (2012). Apigenin, a plant‐derived flavone, activates transient receptor potential vanilloid 4 cation channel. Br J Pharmacol 166: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ (2003). Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem 278: 11002–11006. [DOI] [PubMed] [Google Scholar]
- Miller M, Shi J, Zhu Y, Kustov M, Tian JB, Stevens A, et al (2011). Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J Biol Chem 286: 33436–33446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor J, Al‐Shawaf E, McKeown L, Manna PT, Porter KE, O'Regan D, et al (2011). TRPC5 channel sensitivities to antioxidants and hydroxylated stilbenes. J Biol Chem 286: 5078–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, Davenport AP, McGrath JC, Peters JA, Southan C, Spedding M, Yu W, Harmar AJ; NC‐IUPHAR. (2014) The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 42 (Database Issue): D1098‐1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan KD, Shwe UT, Abramowitz J, Wu H, Rhee SW, Howell MD, et al (2013). Canonical transient receptor channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Mol Pharmacol 83: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, et al (2009). Essential role for TRPC5 in amygdala function and fear‐related behavior. Cell 137: 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JM, Schaefer M, Hill K (2014a). Clemizole hydrochloride is a novel and potent inhibitor of transient receptor potential channel TRPC5. Mol Pharmacol 86: 514–521. [DOI] [PubMed] [Google Scholar]
- Richter JM, Schaefer M, Hill K (2014b). Riluzole activates TRPC5 channels independently of PLC activity. Br J Pharmacol 171: 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saponara S, Carosati E, Mugnai P, Sgaragli G, Fusi F (2011). The flavonoid scaffold as a template for the design of modulators of the vascular Ca(v) 1.2 channels. Br J Pharmacol 164: 1684–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaldecker T, Kim S, Tarabanis C, Tian D, Hakroush S, Castonguay P, et al (2013). Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest 123: 5298–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar P, Sedo A, Li J, Wilson LA, O'Regan D, Lippiat JD, et al (2012). Constitutively active TRPC channels of adipocytes confer a mechanism for sensing dietary fatty acids and regulating adiponectin. Circ Res 111: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Nilius B, Vennekens R (2008). Herbal compounds and toxins modulating TRP channels. Curr Neuropharmacol 6: 79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CO, Huang Y, Yao X (2010). Genistein potentiates activity of the cation channel TRPC5 independently of tyrosine kinases. Br J Pharmacol 159: 1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, et al (2006). A sphingosine‐1‐phosphate‐activated calcium channel controlling vascular smooth muscle cell motility. Circ Res 98: 1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, et al (2008a). TRPC channel activation by extracellular thioredoxin. Nature 451: 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ (2005). Block of TRPC5 channels by 2‐aminoethoxydiphenyl borate: a differential, extracellular and voltage‐dependent effect. Br J Pharmacol 145: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YC, Leung GP, Wong PY, Vanhoutte PM, Man RY (2008b). Kaempferol stimulates large conductance Ca2+‐activated K+ (BKCa) channels in human umbilical vein endothelial cells via a cAMP/PKA‐dependent pathway. Br J Pharmacol 154: 1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Xu SZ, Jackson PK, McHugh D, Kumar B, Fountain SJ, et al (2004). Human TRPC5 channel activated by a multiplicity of signals in a single cell. J Physiol 559 (Pt 3): 739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos AV (2014). Trpc5. Handb Exp Pharmacol 222: 129–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Overview of chemicals from traditional Chinese medicines screened against Ca2+entry in HEK 293 cells overexpressing human TRPC5.
Figure S2 Screen of natural flavonols against Ca2+ entry in HEK 293 cells over‐expressing human TRPC5. Intracellular Ca2+ was measured using XRhod‐1. Mean data comparing responses to 50 μM Gd3+ in the presence of 10 μM galangin, kaempferol, quercetin, myricetin,apigenin, luteolin or vehicle control (veh.) (n/N=3/12 each). Data were normalized to the Gd3+ response in vehicle and Tet+ cells.
Figure S3 Overview of syntheticmono‐substituted flavonols that were screened for TRPC5 inhibition at 10 μM. Compounds that inhibited Gd3+‐evoked calcium entry in TRPC5‐expressing HEK293 cells by > 50% are highlighted in red.
Figure S4 pKa1 values and structures of major microspecies (mm) of tested natural flavonoids and AM12 (predicted using Marvin Beans; downloaded from ChemAxon: http://www.chemaxon.com).
Scheme S1 General synthetic route towards a library of mono‐substituted flavonols 4. Aldol condensation of 2‐hydroxyacetophenones 1 with benzaldehydes 2 was followed by oxidative cyclisation of the intermediate chalcones 3 by use of an Algar‐Flynn‐Oyamada reaction.
Scheme S2 Synthesis of synthetic flavonol AM12. Benzoic anhydride 6 was prepared from its corresponding benzoic acid 5. Friedel‐Crafts acylation of phloroglucinol with acyl chloride 8 gave intermediate 9. Combination of building blocks 6 and 9 in an Allan‐Robinson reaction followed by boron tribromide‐mediated demethylation afforded AM12. THF: tetrahydrofuran.
Supporting info item


