Abstract
Background and Purpose
A major obstacle to islet cell transplantation is the early loss of transplanted islets resulting from the instant blood‐mediated inflammation reaction (IBMIR). The activation of complement pathways plays a central role in IBMIR. The aim of this study was to test the inhibitory effect of “painting” human islets with APT070, a membrane‐localizing C3 convertase inhibitor, on inflammation evoked by exposure to human serum in vitro and by transplantation in vivo in a humanized diabetic mouse model.
Experimental Approach
In vitro, human islets pre‐incubated with APT070 were exposed to allogeneic whole blood. In vivo, similarly treated islets were transplanted underneath the kidney capsule of streptozotocin‐induced diabetic NOD‐SCID IL2rγ−/− mice that had been reconstituted with human CD34+ stem cells. Complement activation and islet hormone content were assayed using enzyme‐linked immunosorbent assays. Supernatants and sera were assayed for cytokines using cytometric beads array. Morphology of the islets incubated with human serum in vitro and in graft‐bearing kidney were evaluated using immunofluorescence staining.
Key Results
Pre‐incubation with APT070 decreased C‐peptide release and iC3b production in vitro, with diminished deposition of C4d and C5b‐9 in islets embedded in blood clots. In vivo, the APT070‐treated islets maintained intact structure and showed less infiltration of inflammatory cells than untreated islets. The pretreatments also significantly reduced pro‐inflammatory cytokines in supernatants and sera.
Conclusions and Implications
Pre‐treatment of islets with APT070 could reduce intra‐islet inflammation with accompanying preservation of insulin secretion by beta cells. APT070 could be as a potential therapeutic tool in islet transplantation.
Abbreviations
- CR1
complement receptor 1
- hu‐NSG
humanized NSG
- IBMIR
instant blood‐mediated inflammation reaction
- iC3b
inactivated C3b
- IEQs
islet equivalents
- LDH
lactate dehydrogenase
- NSG
non‐obese diabetic/severe combined immunodeficiency/interleukin‐2 receptor γ‐chain knockout
- sCR1
soluble complement receptor 1 (sCR1)
- UCB
umbilical cord blood
Tables of Links
| TARGETS |
| GPCRs |
| C3a receptor |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Type 1 diabetes (T1D) is characterized by permanent destruction of insulin‐producing beta cells in the pancreatic islets. Transplantation of islets of Langerhans via intra‐portal injection is a promising treatment procedure for patients with severe T1D (Robertson, 2010). However, despite recent progress, the procedure is associated with massive tissue loss caused by an inflammatory reaction, referred to as the instant blood‐mediated inflammatory reaction (IBMIR) (Nilsson et al., 2011). It is believed that complement attack is a major cause of the damage to the islets that occurs during the IBMIR (Tjernberg et al., 2008) and the reaction involves rapid activation of the coagulation and complement system, activation and binding of platelets to the islet surface, and infiltration of leukocytes into the islets (Nilsson et al., 2011). The complement cascade therefore makes a logical therapeutic target for the reduction of graft rejection (Farrar and Sacks, 2014).
The central step in the complement cascade is the cleavage of C3 to C3b by C3 convertase. This leads ultimately to the assembly of the membrane attack complex (MAC) and the release of soluble C3a and C5a, which in turn activate and recruit inflammatory cells (Sacks et al., 2009). Once C3 is deposited covalently as C3b on a membrane, it has two fates. The first is an amplification step, in which more C3 is cleaved and bound to the membrane. This process involves a further complement protein, factor B. When factor B binds to C3b, it is activated by the complement enzyme factor D and forms the C3 convertase enzyme C3bBb. This enzyme cleaves more C3, causing many more C3b molecules to be deposited on the membrane. The second possible fate of bound C3b is catabolism to iC3b (inactivated C3b) and C3dg, creating adhesive contact with corresponding receptors on leucocytes through the complement receptors, CR1‐4, particularly CR3 and CRIg (Walport, 2001a; Walport, 2001b). This step is mediated by regulators that inactivate complement binding to C3b thus promoting its removal and degradation from the enzyme complexes that convert C3 and C5 into their active forms. The regulators exist as membrane bound (e.g. CD35, CD46 and CD55) and soluble (e.g. Factor H) proteins. APT070, also known as Mirococept, is a modified fragment of the complement receptor 1 (CR1, CD35) (Mossakowska et al., 1999; Smith, 2002). It consists of the first 3 consensus domains of the human CR1 and a membrane‐interacting synthetic peptide, which mediates binding to phospholipids on the cell surface and therefore protects the cell against complement activation (Smith and Smith, 2001; Smith, 2002). APT070 showed inhibition of complement and neutrophil activation during cardiopulmonary bypass in man in vitro (De Silva et al., 2006), and of the inflammatory responses that follow intestinal (Souza et al., 2005) and renal (Patel et al., 2006) ischaemia and reperfusion injury in rat models. These studies have prompted clinical investigation of the utility of APT070 in man for ischaemia‐reperfusion injury during renal transplantation (Smith, 2007). These properties suggested the possible use of APT070 in protecting human islet transplantation.
The development of mice that are ‘humanised’ by engraftment of human tissues, haematopoietic stem cells or peripheral‐blood mononuclear cells (PBMC), provides an opportunity to study human biological processes in vivo that would otherwise not be possible (Shultz et al., 2007). We have recently described human islet allograft rejection in the CD34+ stem cell‐reconstituted humanized mouse model (Xiao et al., 2014). Although islets were placed underneath the kidney capsule, rejection was associated with C3b deposition and we suggested that complement may be involved in the recruitment and activation of innate inflammatory cells. We therefore hypothesised that inhibition of complement activation might enhance islet survival. The present study aimed to investigate whether APT070 could protect human islets from complement‐mediated destruction in vitro, and attenuate the immune response to human islet allograft in vivo in the humanized mouse model (Xiao et al., 2014).
Methods
Preparation of human islets
The procurement and use of all human tissues were with signed informed consent, in accordance with the Declaration of Helsinki and approval from the Institutional Review Board of King's College London. Human islets were obtained from King's Cell Isolation Unit, London, UK. Freshly isolated human islets were incubated with serial dilutions of membrane‐localizing complement regulator APT070 [0.4–1.6 μM; 3000–4000 human islet equivalents (IEQ) mL − 1], or its control molecule APT154 at the equivalent molar concentration, in CMRL 1066 culture medium (Invitrogen) containing 2.5% human albumin at 37 °C in an atmosphere of 95% air 5% CO2 for one hour (Figure 1). The recombinant non‐tailed CR1 fragment APT154 possesses chemical and complement inhibitory properties identical to those of APT070, but is untagged and therefore does not contain the membrane‐binding moiety (Banz et al., 2007). This control molecule was prepared using the cloning and expression strategy as previously described (Dodd et al., 1995; Mossakowska et al., 1999; Smith, 2002). To detect the bound APT070, the treated islets were incubated with a rabbit polyclonal antibody raised against recombinant SCRs (1–3) of CR1 (APT3677, 100 μg mL − 1) (Dodd et al., 1995). Antibody APT3677 was a product of Adprotech Ltd. (Saffron Walden, UK) and is available for research purposes from King's College London (Dr. Richard A Smith). After washing, tetramethylrhodamine isothiocyanante (TRITC)‐conjugated goat anti‐rabbit IgG was applied for one hour at room temperature. Images were acquired using a Cooled Mono14 Bit camera (Q IMAGING, Canada) and Micro‐Manager 1.3 software (University of California, USA).
Figure 1.
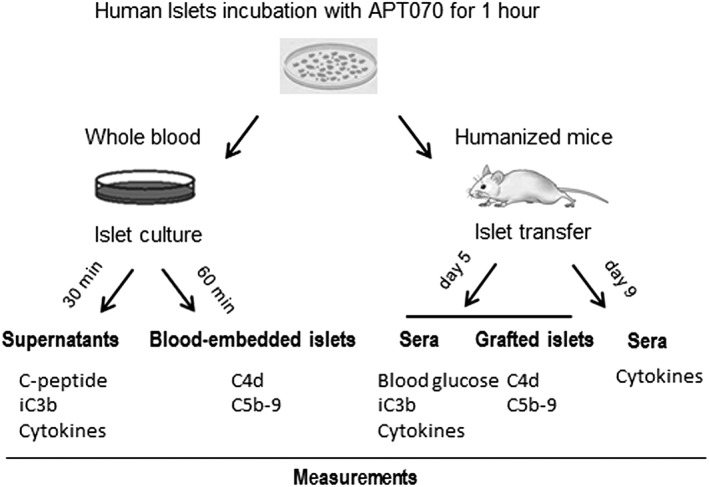
Schematic description of the in vitro model mimicking IBMIR, and in vivo model of humanized NSG mice.
In vitro viability and functional assessment of APT070‐treated islets
APT070‐treated islet viability was assessed by a fluorometric viability assay, using fluorescein diacetate and propidium iodide (Sigma, Dorset, UK) (Hansen et al., 1989; London et al., 1989), and kits of the CellTiter‐Glo Luminescent Cell Viability Assay (Promega, Southampton, UK) and lactate dehydrogenase (LDH) assay, according to the manufacturers' instruction. For measuring the dynamics of insulin release, islets were transferred into chambers (50 islets per chamber) of a temperature‐controlled multichannel perifusion system as previously described (Jones et al., 1989). The loaded islets were subsequently perifused (0.5 ml min−1) with 2 mM glucose for 10 min and 20 mM glucose for 20 min in Gey's balanced salt solution [0.05% (w/v) BSA, 2 mM CaCl2, 111 mM NaCl, 5 mM KCl, 27 mM NaHCO3, 1 mM MgCl2, 0.22 mM KH2PO4 and 0.28 mM MgSO4], followed by an additional perifusion of 500 μM carbachol for 18 min. Sampling was performed at 2 min intervals. In another experiment, the extracts of islet pellets were prepared using acid‐ethanol. Insulin content was assessed by radioimmunoassay (RIA) using in‐house assay, as described previously (Jones et al., 1988).
In vitro model of allogeneic blood‐islet combinations
Approximately 2500 IEQs APT070‐treated islets with a purity of 60–80% were re‐suspended in 1 ml of culture medium and placed in 35‐mm polystyrene petri dishes (BD). One millilitre of freshly drawn human whole blood, ABO blood group typed for compatibility with the islet donor, was added and incubated in 37 °C for 30 min. Supernatants were then collected, with the addition of EDTA (10 mM final concentration) to prevent further complement activation (Mollnes et al., 2002). These were immediately stored with protein inhibitor cocktail (Sigma) at ‐80C for future use.
Humanized mouse model with APT070‐treated islet transplantation
Reconstitution of mice with human stem cells
All animal care and experimental procedures complied with the institutional guidelines and the Home Office Animals Scientific Procedures Act (1986) (UK) and were approved by the Ethical Review Process Committee, King's College London, UK (Project no: 70/7302). These studies comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015) and the animal studies follow the ARRIVE guidelines (McGrath & Lilley, 2015; McGrath et al., 2015). All animal studies were randomized and observations were made without knowledge of the treatments.
Non‐obese diabetic/severe combined immunodeficiency/interleukin‐2 receptor γ‐chain knockout (NOD/SCID/IL2Rγ−/−, referred to here as NSG; The Jackson Laboratory) mice were bred and maintained in the Biological Services Unit of King's College London under specific pathogen‐free conditions. Umbilical cord blood (UCB) was obtained from full‐term normal deliveries. UCB‐derived CD34+ stem cells were isolated as described by Xiao et al. (2014). Briefly, mononuclear cells were isolated by Ficoll‐Paque (GE Healthcare, Hatfield, UK) gradient separation and enriched for CD34+ cells using positive isolation kit according to the manufacturer's instructions (Miltenyi Biotech, Surrey, UK). Mice (6–8 weeks old) were gamma‐irradiated (240 cGy) and then intravenously injected with 2 x 105 CD34+ stem cells (referred to as hu‐NSG mice).
Flow cytometric analysis of human haematopoietic engraftment
Tail bleeding was performed in hu‐NSG mice 12–16 weeks after injection of CD34+ cells. Blood cells were prepared for flow cytometry as previously described (Xiao et al., 2014). Fluorochrome‐coupled antibodies specific for human CD45 were used for detection of human leukocytes. Flow cytometric data were acquired using a FACS Calibur (BD Biosciences, San Jose, CA, USA) and analysed using FlowJo 7.5 software (TreeStar Inc.).
Islet transplantation into diabetic ‘humanized' NSG mice
NSG and hu‐NSG mice were rendered diabetic (blood glucose level ≥ 20 mM) by a single i.p. injection of streptozotocin (180 mg kg−1) and transplanted with human APT070‐treated islets (IEQs, 3000–4000) under the left kidney capsule as previously published (Xiao et al., 2014). During the surgery, the recipient animals were anaesthetized using 3% isoflurane in a stream of 100% O2 (2 l min−1), and were maintained using 1.5–2.0% isoflurane in a stream of 100% O2 (2 l min−1). The animals were placed on a heat pad to keep body temperature at 37 °C throughout the procedure. Blood glucose concentration of recipient mice was monitored every 24 hours at the first week and twice a week thereafter using a blood glucose sensor (Abbott Diabetes Care Ltd., Witney, Oxon, UK). Reversal of hyperglycaemia was defined as non‐fasting blood glucose concentration ≤ 13.8 mM (King et al., 2008). NSG mice with successful transplants were subjected to unilateral left nephrectomy to evaluate a return to hyperglycemic state. For islet‐transplanted diabetic hu‐NSG mice, sera were collected by tail sampling at day 5 and day 9 after transplantation, and kept at −80 °C prior to use for further test. The animals were sedated with CO2 and killed by decapitation at the end of experiments.
Enzyme‐linked immunosorbent assay (ELISA) for human insulin, C‐peptide and iC3b
Supernatant insulin and C‐peptide concentrations were assayed using ELISA kits (Millipore, Watford, UK). ELISA kit for human iC3b (Pathway Diagnostics Ltd., Surrey, UK) was used to determine levels of soluble complement activation product iC3b in supernatant and sera, according to the manufacturer's instructions. All samples were tested in triplicate in the assays.
Cytokine detection (beads array)
Human cytokines in supernatants and sera were assayed using a human Th1/Th2 11plex kit (eBiosciences) according to the manufacturer's protocol. Data acquisition was performed on a FACS Calibur (BD Biosciences) and analysed using The FlowCytomix Pro 3.0 Software from eBioscience (Xiao et al., 2014).
Histology analysis
Deposition of activated complements was examined in the islets embedded in blood clots after 60 min incubation with blood, and the harvested graft‐bearing kidney at day 5 after transplantation. The samples were fixed in 10% buffered formalin, and embedded in paraffin. Sections (5 μm) were processed with double immunofluorescence staining. After antigen retrieval by microwaving for 5 min in 0.01 M citrate buffer (pH 6.0), sections were blocked with 10% goat normal serum for 30 min and then incubated overnight at 4 °C with primary antibodies: rabbit anti‐human insulin (1:300; Bioss, polyclonal), mouse anti‐human C4d (1:25; Abcam, clone LP69), mouse anti‐human C5b‐9 (1:100; Abcam, clone aE11), mouse anti‐human CD11b (1:50; eBiosciences, clone ICRF44) and mouse anti‐human CD66b (1:50; BioLegend, clone G10F5). Secondary antibodies FITC‐conjugated goat anti‐mouse IgG and TRITC‐conjugated goat anti‐rabbit IgG (both Sigma) were applied for 2 hours at room temperature (Xiao et al., 2014). Negative controls with non‐specific IgG were processed in parallel. Images were acquired using a Nikon Eclipse Ti‐E Inverted microscopy with 4 x GaAsP Detectors and A1R Si Confocal system with a 60x objective lens and analysed by NIS‐Elements Advanced Research imaging analysis software (Nikon, Surrey, UK). Where the infiltrated cells were quantified, each sample from an individual animal (n = 5 animals) was assessed and analysed by using ImageJ software. The deposition of complement was quantified by measuring the mean fluorescence intensity (MFI) in the region of interest area.
Data analysis
Data are presented as means ± SD. All statistical analysis was performed using the two‐tailed Student's t‐test or two‐way ANOVA analysis, followed by Bonferroni post hoc tests. P values <0.05 were considered significant.
Materials
APT070 and APT 154 were supplied by Adprotech Ltd. (Saffron Walden, UK); carbachol and streptozotocinwere supplied by Sigma (Dorset, UK).
Results
Human islets display physiological responses after treatment with APT070
To ensure that APT070‐treated islets were viable and physically identical to the freshly prepared islet, we examined the effects of APT070 on the viability of human islets. Human islets were incubated in vitro for one hour in culture medium alone (control group) or with APT070 and its control molecule APT154 (Figure 1). The data demonstrate that APT070, but not APT154 bound to the islet (Figure 2A). Dose‐titration of APT070 from 0.4 to 1.6 μM (25–100 μg mL − 1) showed that the lowest dose (0.4 μM) treatment had no effect on islet viability in the fluorescein diacetate and propidium iodide staining (Supplementary Figure S1). This was confirmed by cell viability assay based on quantitation of the APT present (Figure 2B) and the measurement of intracellular content of insulin (Figure 2C). Therefore APT070 was used at a concentration of 0.4 μM (25 μg mL − 1) in further studies. It should be noted that a concentration of 10 μg mL − 1 of APT070 has been used in perfusion experiments in human renal transplantation without apparent adverse effects (Smith et al., 2007).
Figure 2.
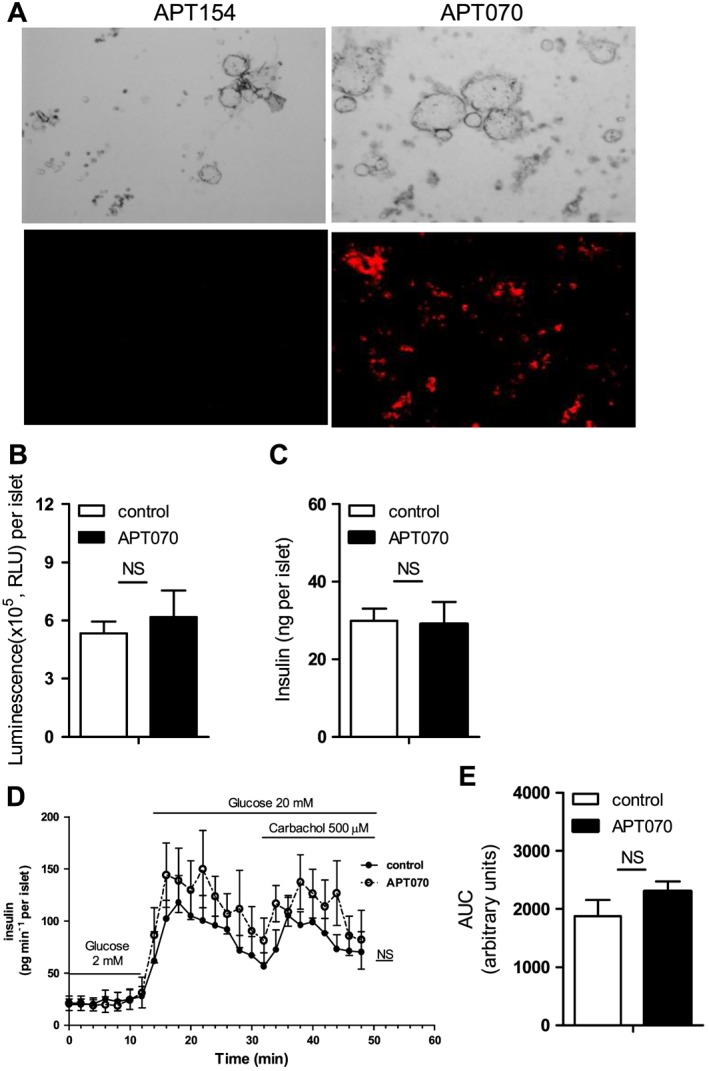
Assessment of islet viability and secretion function. (A) Binding of APT070 (0.4 μM) to human islets one hour after incubation. Positive binding is shown in red. Amplifier power: × 60. (B) Islet viability assay. Luminescent outputs, which correlated with islet cell numbers, were measured in APT070 treated and control islets. Values represent the mean ± SD, n = 6 (wells of 96‐well plate). (C) Intracellular insulin content in APT070‐treated and control islets. The data are expressed as means ± SD, n = 6. (D, E) The dynamics of insulin release. Data are shown as means ± SD, n = 8 perifusion channels. RLU: relative luminescence units. AUC: area under curve. Control islets received no treatment. NS: no significant difference.
To evaluate islet function after incubation with APT070, measurements of the rate and pattern of insulin secretion from perifused islets (Figure 2D) confirmed that APT070 pre‐treatment did not inhibit insulin secretory responses to glucose, nor to the receptor‐mediated agonist, carbachol. Expressing the cumulative total insulin secretion during the perifusion as area under the curve (AUC) showed that APT070 pre‐treatment had no significant effects on insulin secretion (Figure 2E), confirming that the β‐cells remained functionally viable after treatment.
Pre‐incubation of human islet with APT070 prevents the production of activated complement in vitro and in vivo
To test whether APT070 has an inhibitory effect on complement activation, islets were exposed to whole blood for 30 minutes. The level of iC3b was significantly decreased in the supernatant of APT070 treated‐islets, compared with control groups (n = 6 for each group) (Figure 3A).
Figure 3.
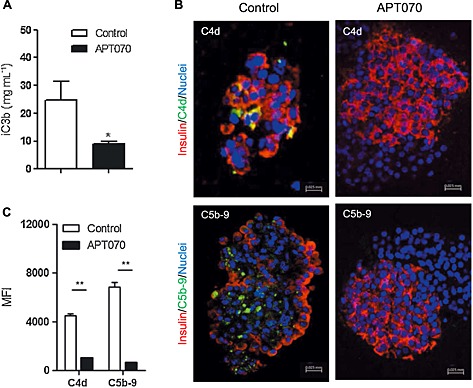
iC3b in supernatants and deposition of C4d and C5b‐9 in blood clot‐embedded islets. (A) Supernatants were collected 30 min after islet exposure to allogeneic whole blood and were assayed for iC3b level by ELISA, n = 6. (B) Freshly isolated islets were pre‐incubated with APT070 (0.4 μM) or with culture medium alone (control) for one hour, following by exposed to allogeneic whole blood for one hour. Blood clot‐embedded islets were stained with antibodies specific for insulin and C4d or C5b‐9 molecules. Images are representative from 6 sections in each group. (C) Quantitative analysis of blood‐embedded islet immunostaining. Staining for C4d and C5b‐9 is shown in green. Nuclei were stained with DAPI (blue). Yellow indicates co‐localization of insulin and C4d/C5b‐9. Data are expressed as means ± SD. *: P < 0.05; **: P < 0.01; significantly different as indicated. MFI: mean fluorescence intensity.
To further assess the in vitro inhibitory effects of APT070 on complement activation, C4d deposition, as an indicator of complement activation, and C5b‐9, which may cause cell lysis in the blood‐exposed islets, were examined. Pre‐treatment of islets with APT070 almost abolished deposition of both C4d and C5b‐9 in the islet exposed to blood for 60 minutes (Figure 3B, C).
To evaluate the in vivo effects of APT070 on human allograft, humanized mice were generated (hu‐NSG mice) as previously described (Xiao et al., 2014). Immunodeficient mice (NSG) of 4–6 weeks old were engrafted with UCB‐derived CD34+ stem cells and, after 16 weeks, a significant percentage of human CD45+ cells was detectable in the blood of these mice (Supplementary Figure 2). APT070‐treated islets were transplanted under the kidney capsule of hu‐NSG mice. Although the kidney capsule is not a site where classical IBMIR occurs, deposition of human C3 on human islets implanted beneath the kidney capsule of hu‐NSG mice, associated with circulating human C3, has previously been shown (Xiao et al., 2014), therefore it is appropriate to assess the effect of APT070 on these parameters. The level of human iC3b was found to be decreased in the sera of mice receiving APT070 treated‐islet at day 5 after transplantation, compared with controls (Figure 4A). Furthermore, there was very little deposition of C4d and C5b‐9 in the islet allografts in the APT070 treated group. In contrast, allografts from hu‐NSG mice that had received untreated‐islets exhibited an abundant staining for C4d and C5b‐9 (Figure 4B). There was a significant difference between the two groups (Figure 4C).
Figure 4.
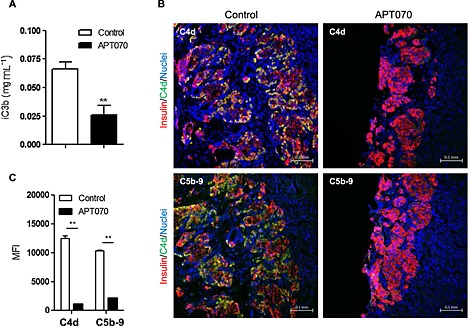
Serum iC3b levels (A) and deposition of C4d and C5b‐9 in islet graft bearing kidney. Sera (A) and islet graft‐bearing kidneys (B) were collected from humanized NSG mice 5 days after islet transplantation. (C) Quantification of the deposition of C4d and C5b‐9 in the graft‐bearing kidney. Islets were pre‐incubated with APT070 for one hour before surgery. Staining for C4d and C5b‐9 are shown in green. Nuclei were stained with DAPI (blue). n = 5 mice for each group. Data are expressed as means ± SD. Control islets were incubated with culture medium alone. **: P < 0.01; significantly different as indicated. MFI: mean fluorescence intensity.
APT070 reduces human islet destruction in human blood in vitro and in islet allografts in humanized NSG mice
To evaluate the damage in islets exposed to blood in vitro, the release of C‐peptide and of LDH were assessed as indicators of beta cell damage. Measurements of C‐peptide and LDH in supernatants 30 min after incubation of untreated islets with human blood showed significant increases, consistent with islet cell damage (Figure 5). However, the release of C‐peptide and LDH was completely inhibited in the APT070‐treated group, demonstrating protection from blood‐mediated islet cell damage (Figure 5).
Figure 5.
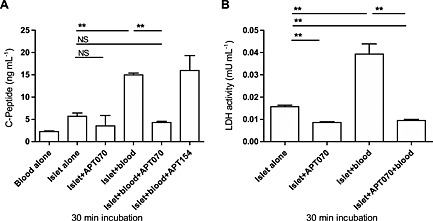
C‐peptide and LDH levels in supernatant from co‐culture of human islet and allogeneic whole blood. The supernatants were sampled 30 min after exposure of human islets to allogeneic whole blood. Data are means ± SD of 6 Eppendorf tubes from two independent experiments. **: P < 0.01; significantly different as indicated. NS: no significant difference. Control islets had no treatment.
Activated complement proteins C3a and C5a act as chemoattractants to recruit human leukocytes (Li and Zhou, 2013). To evaluate the inhibitory effect of APT070 on the production of these molecules, infiltration of human CD45+ cells, macrophages (CD11b+) and neutrophils (CD66b+) into graft‐bearing kidneys, 5 days after transplantation was examined. APT070‐treated islets displayed intact structure with decreased infiltration of these inflammatory cells, compared with controls (Figure 6).
Figure 6.
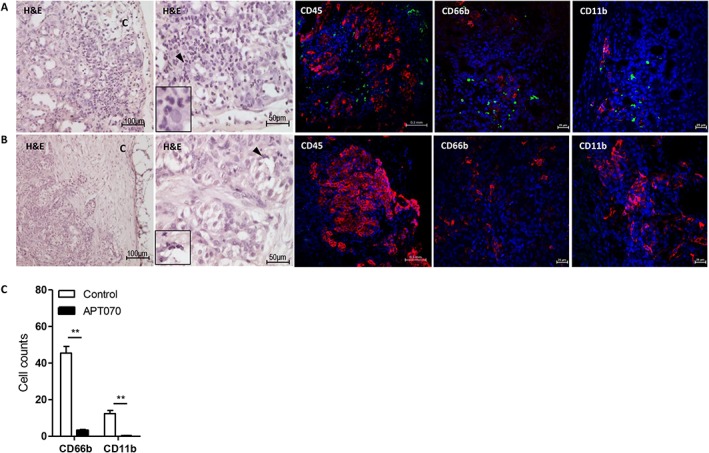
Infiltration of human leukocytes into human islet allografts from humanized NSG mice. Human islet grafts were harvested 5 days after transplantation and were fixed, embedded and sectioned. Samples were stained with H&E or with antibodies specific for human antigen: insulin (red), CD45 (green), CD66b (green), and CD11b (green). Nuclei were stained with DAPI (blue). HE staining shows massive infiltration of leukocytes in control grafts (A, H&E) compared to APT070 treated grafts (B, H&E). Images are representatives for control group (A) and APT070‐treated group (B). (C) Quantification of infiltration of CD66b and CD11b positive cells. n = 5 slides for each group. Data were represented as means ± SD of cell number in the field in amplifier power × 60. Cell numbers were counted in 3–5 fields in each slide and average was determined. H&E: haematoxylin and eosin staining. C: kidney capsule. Insets in the H&E staining images show enlarged area indicated by a black arrow.
Pre‐incubation of islets with APT070 suppresses cytokine production in vitro and at early stages in vivo
To further demonstrate the protective effect of APT070 on islet graft, human cytokine profiles were evaluated both in vitro and in vivo. A bead based Analyte Detection System was used for quantitative detection of human IFN‐γ, IL‐1β, IL‐2, IL‐4, IL‐5, IL‐6, IL‐8, IL‐10, TNF‐α and TNF‐β in the supernatants taken from the incubation of islets with blood, and in sera from islet‐transplanted humanized mice. Analysis revealed that treatment with APT070 significantly reduced levels of IFN‐γ, IL‐1β, IL‐2, IL‐4, IL‐5, IL‐10, TNF‐α and TNF‐β, with no effect on IL‐12, IL‐8 and IL‐6 (n = 6) (Figure 7A), following 30 minutes incubation.
Figure 7.
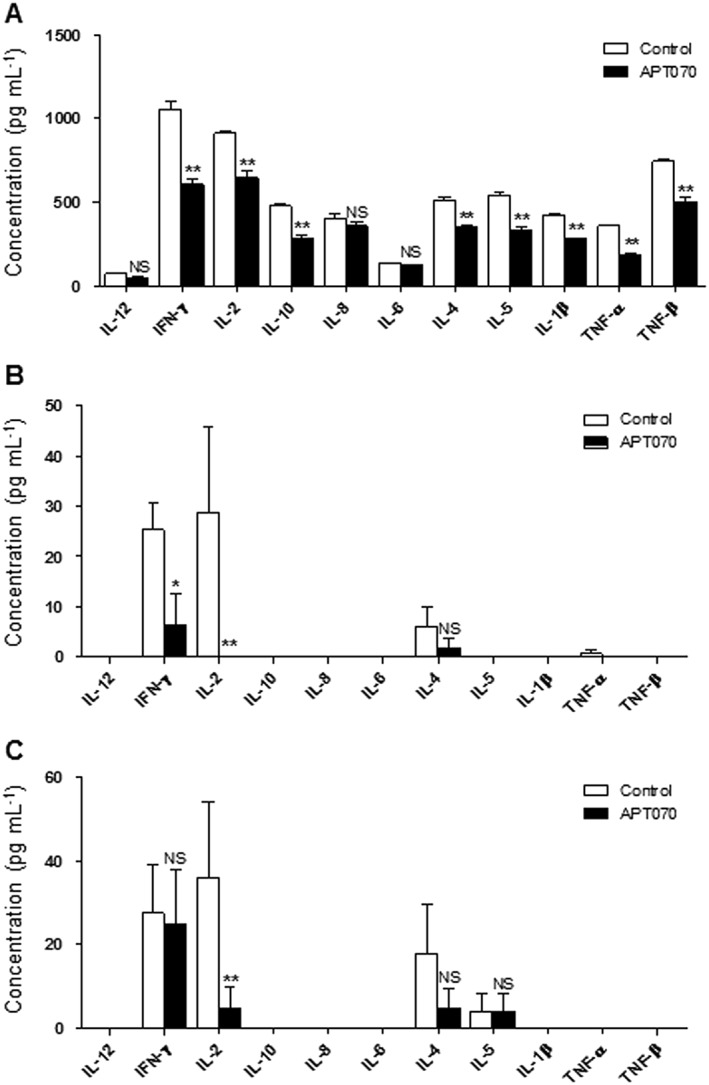
Supernatant and serum levels of cytokines. Supernatants were collected 30 min after exposure of islet to allogeneic whole blood (A). Sera were obtained from islet‐transplanted humanized NSG mice 5 (B) and 9 (C) days after transplantation. For supernatants, n = 6; for sera, n = 5 animals for each group. Values are mean ± SD. *P < 0.05. ** P < 0.01; significantly different as indicated. NS: no significant difference. Control islets had no treatment.
In vivo, serum levels of human IFN‐γ and IL‐2 were significantly reduced in humanized mice 5 days after APT070 treated‐islet grafting, compared with untreated‐islet controls (n = 5 animals for each group) (Figure 7B), although IFN‐γ values were the same after 9 days. IL‐1β, IL‐6, IL‐8, IL‐10 and TNF‐α were undetectable (Figure 7C).
Pre‐treatment of islets with APT070 induces earlier reversal of hyperglycaemia in vivo
To correlate in vitro insulin secretion measurements with the ability of islets to restore normoglycemia in vivo, APT070‐treated islets were transplanted under the kidney capsule of diabetic hu‐NSG mice and NSG mice that did not receive CD34+ cells. Hyperglycaemia in both diabetic NSG (Figure 8A) and hu‐NSG mice (Figure 8B) was reversed earlier with APT070 treated islets, compared with the control islets, although normoglycaemia was not attained in hu‐NSG mice by the end of the experiment (5 days) (Xiao et al., 2014). To determine the function of the transplant, the NSG mice underwent unilateral left nephrectomy at 60 days after transplantation. The data showed that a hyperglycemic state was restored within 24–48 hours after nephrectomy (Supplementary Figure 3).
Figure 8.
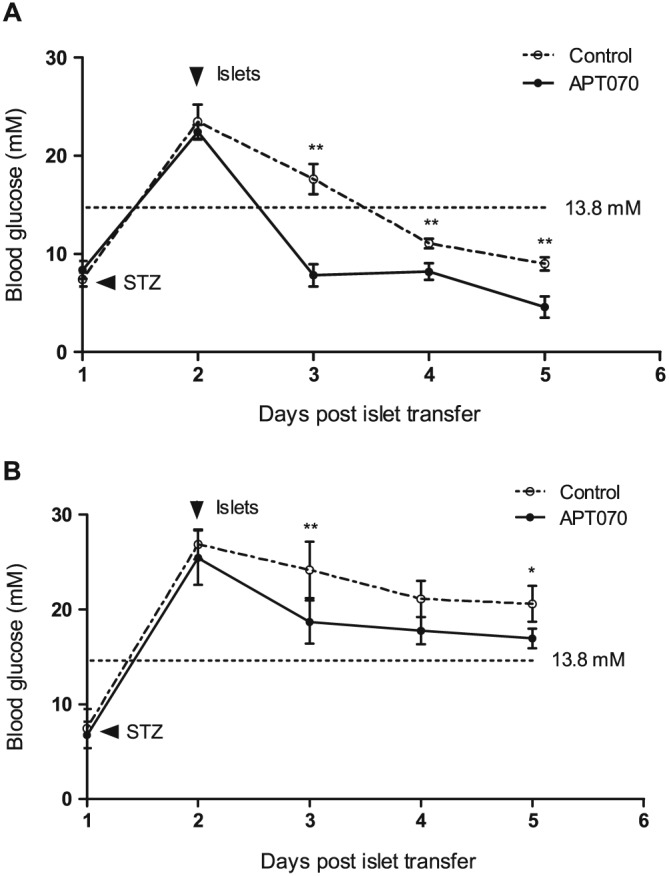
The plasma glucose level in the APT070‐treated and untreated human islet transferred into NSG (A) and hu‐NSG (B) mice rendered diabetic with streptozotocin. Tail blood was assessed for glucose after islet transplantation. n = 5 animals for each group. Data are expressed as means ± SD. *P < 0.05. **P < 0.01; significantly different as indicated. STZ: streptozotocin. Control islets had no treatment.
Discussion
In this study, it was found that a membrane‐localizing C3 convertase inhibitor (APT070) inhibited complement activation in vitro in a model mimicking IBMIR, and in vivo in humanized mice transplanted with human islets. The data demonstrated that APT070 protects human islet allografts from complement‐mediated damage through inhibition of infiltrates into grafts and regulation of cytokine production.
IBMIR is one of the main challenges faced by clinical islet transplantation and results in significant destruction of islets (Bennet et al., 2000a; van der Windt et al., 2007). IBMIR is believed to be a non‐specific inflammatory response related to the transplantation of islets directly to the blood microenvironment of the portal vein. It is probable that implanted islets express tissue factor (CD142) that activates the coagulation system. As a result, complement is activated and the islets are destroyed by innate factors. In the present study, the pathophysiological events triggered during direct exposure of human islets to an allogenic blood in vitro, mimicking clinical transplantation of human islets into the portal vein, have been characterised. Notably, the non‐physiological release of C‐peptide, which indicates massive beta cell death, was observed in vitro (Figure 5). This is consistent with the in vivo reports of auto‐, allo‐, and xeno‐islet transplantation (Moberg et al., 2002; Mollnes et al., 2002). Thus, incubation of human islets with allogenic blood has been proved to be a valuable model to investigate the events, as previously shown in a similar approach by van der Windt et al. (2012).
Incubation with anti‐coagulation agents leads to the surface modification of islets, which can inhibit IBMIR (Chen et al., 2011; Teramura and Iwata, 2011). Different compounds targeting the complement system have been tested in vitro and/or in vivo to improve early islet survival in experimental models, such as the C5a inhibitory peptide (Tokodai et al., 2010) and soluble complement receptor 1 (TP10) (Bennet et al., 2000b; Lundgren et al., 2001). Although a high‐dose, systemic i.v. infusion of soluble complement receptor 1 (sCR1) (40 mg kg−1) has been shown to improve hyperacute xenograft rejection (Bennet, et al., 2000b), the immobilization of sCR1 on islets was more effective than systemic infusion (Luan et al., 2011). Like the immobilized sCR1, APT070 is a membrane‐bound protein, which inhibits complement activation at appropriate sites. When freshly isolated human islets were pretreated with APT070 (25 μg mL−1) for one hour, there was no a deleterious effect on islet secretory function (Figure 2). Glucose‐induced insulin secretion requires a depolarisation‐dependent influx of extracellular Ca2 + via voltage‐dependent calcium channels (VDCC) so the maintenance of normal secretory responses to glucose demonstrates that using the membrane bound APT070 did not adversely affect the beta cell membrane potential, nor the normal operation of VDCC. Similarly, the ability of pre‐treated islet to respond to the muscarinic cholineric agonist carbachol confirms that coating the islets with APT70 did not interfere with receptor expression or intracellular signalling pathways.
The present data also show that the pretreatment with APT070 resulted in a significant decrease in the release of C‐peptide and LDH from islets after incubation with human blood. Consistent with this, a peak serum level of C‐peptide can be associated with early islet cell death and/or islet destruction in patients with allo‐transplantation (Moberg et al., 2002). The strategy of ‘painting’ APT070 on islets was also effective in preventing complement activation, as shown by the decreased production of soluble iC3b in vitro (Figure 3). Analysis of islet morphology revealed damaged islets embedded in blood clots with C4d and C5b‐9 deposited on the islet surface in untreated islets, but not in the APT070 pre‐treated group. This finding reflects clinical studies that suggest that C4d‐positive interacinar capillaries correlate with donor‐specific antibody‐mediated rejection in pancreas allografts (Torrealba et al., 2008). Similar results have been obtained showing that APT070 completely prevented complement membrane attack complex formation at the nerve terminals in Miller Fisher Syndrome (Halstead et al., 2005).
Expression of inflammatory mediators in isolated islets with immune modulating capacity could markedly influence the outcome of clinical islet transplantation (Johansson et al., 2003). Use of APT070 has the potential to attenuate hyperinflammatory responses through reduction of local damage caused by the membrane attack complex (MAC) as well as by reduction of neutrophil activation directly through inhibition of C3a/C5a release and indirectly through inhibition of the complement‐mediated release of cytokines (Souza et al., 2005). Our data showed that inhibition of the complement cascade by APT070 resulted in significantly decreased concentration of a number of pro‐inflammatory cytokines, including IFN‐γ, IL‐2, IL‐10, IL‐4, IL‐5, IL‐1β, TNF‐α and TNF‐β (Figure 7), consistent with a protective effect of APT070 against cytokine‐mediated damage to islet cells in the immediate post‐transplantation period. IL‐10 is a major protective endogenous cytokine in an islet xenograft rejection model (Yi et al., 2012) and during intestinal reperfusion injury in rats (Souza et al., 2003). Thus, it is clear that APT070 inhibits complement activation and islet damage by a mechanism other than by IL‐10 production. Interestingly, complement inhibition did not have an effect on IL‐8, which is the most relevant pro‐inflammatory cytokine found both in transplanted patients and in the murine counterpart after experimental islet transplant (Citro et al., 2013). An explanation for this effect may lie in the time of sampling. Polymorphonuclear leukocytes (PMNs) and monocytes are the dominant cell types infiltrating the islet and producing cytokines in vitro, appearing in large numbers, 2 hours after incubation with ABO‐compatible blood (Moberg et al., 2005), which is much later than our sampling time at 30 min. Equally beneficially, as IL‐6 protects pancreatic islet beta cells from pro‐inflammatory cytokine‐induced cell death and functional impairment in vitro and in vivo (Choi et al., 2004), it is remarkable that APT070 had no effect on the production of IL‐6.
Immunodeficient mice that are engrafted with human functional cells (termed as humanized mice) are ideal preclinical models to investigate human immune responses in an in vivo setting (Brehm, et al., 2010a; Shultz et al., 2007; King et al., 2008). Non‐obese diabetic/severe combined immunodeficient mice harbouring a complete null mutation of IL‐2 receptor γ chain, NOD/SCID IL2r γnull (NSG), are characterised by impairment in murine T‐, B‐ and NK‐cell development and function(Cao et al., 1995; DiSanto et al., 1995; Ohbo et al., 1996), and can efficiently support the development of a functional human hemato‐lymphopoiesis (Brehm, et al., 2010b; Ishikawa et al., 2005; Watanabe et al., 2009). Using the diabetic humanized NSG mouse model (Xiao et al., 2014), we found that inhibition of the complement cascade by APT070 significantly decreased concentration of at least two pro‐inflammatory cytokines, IL‐2 and IFN‐γ (Figure 7). Whether APT070 had the same effects on cytokine profile in vivo are not known, because some human cytokines are undetectable in the humanized mice system (Xiao et al., 2014). Nevertheless, in support of this concept, there was, at an early stage in the APT070‐treated animals, a marked inhibition of other cytokines, including TNF‐α, TNF‐b and IL‐6 (Souza et al., 2005). The mechanisms underlying the inhibitory effects of APT070 on cytokines need to be further investigated. There are a number of limitations that prevent full use of our model system. In NSG mice, the human T cells are restricted to mouse major histocompatibility complex (MHC) and fail to interact productively with human antigen presenting cell within the host, leading to lack of class switching and immunoglobulin G (IgG) antibody production (Watanabe et al., 2009; Xiao et al., 2014). The generation of memory T cells in the model can also be problematic (Brehm et al., 2013). This insufficient development of antigen‐specific immunity might explain why some of human cytokines could not be identified in our model system. To overcome these limitations, investigators have developed HLA‐transgenic NSG mice (Shultz et al., 2010; Covassin et al., 2011; Serra‐Hassoun et al., 2014).
Using the hu‐NSG mice, we have recently shown a close relationship between complement C3d deposition and the damage of human islet allografts (Xiao et al., 2014). The complement detected in this model system was human. The results suggested that complement might be involved in the destruction of islets even in the context of being placed under the kidney capsule, a site at which classical IBMIR does not occur (Xiao et al., 2014). Now, using the same mouse model (Supplementary Figure S2), we have found that ATP070 markedly decreased complement deposition and infiltration of inflammatory cells at the early stage (Figure 4, 6), consistent with the in vitro data presented here. Consistently, APT070 treatment decreased serum levels of iC3b. These data indicate an inhibitory effect of APT070 on local production of complement. Indeed, within the graft, local production of complement contributes to non‐specific inflammation and parenchymal destruction (Brown et al., 2006). It has been known for several years that innate immune cells, including macrophages and dendritic cells, can express and secrete a variety of complement components (Sacks et al., 2009). These appear to be sufficient for the cleavage of C3 in the local inflammatory environment (Zhou et al., 2006; Peng et al., 2008). The data also suggested that pre‐incubation of human islet with APT070 may have partly affected reversal of hyperglycaemia in vivo in humanized NSG mice (Figure 8). Complete restoration of normoglycemia in APT070‐treated group was not observed, within the experimental period (5 days), whereas other studies (King et al., 2008; Xiao et al., 2014) showed that normoglycemia (blood glucose <13.8 mmol/l) was achieved after 5 days. Furthermore, the return of hyperglycemic state following removal of graft suggested the functionality of APT070‐treated islet in vivo (Supplementary Figure S3).
Given that the majority of the membrane‐localizing complement regulator was internalized by 40 h after intragraft delivery of APT070 (Patel et al., 2006), the stability of APT070 in islets is a major obstacle that remains to be overcome. This may explain why the inhibitory action of APT070 on IFN‐γ production was lost after 9 days of transplantation (Figure 7). Ideally, APT070 should persist and control complement activation indefinitely (Patel et al., 2006). Consequently, the possibility that combination therapy with APT070 can contribute to the permanent survival of human islet grafts is being explored.
In conclusion, this study demonstrates that APT070 prevented early islet damage involved in membrane leakage, activation of complement and deposition of the membrane attack complex in vitro and in vivo. Although the benefit did not translate into a statistically significant improvement of human islet allograft survival, the fact that both cytokine levels and tissue damage were considerably improved indicates the importance of APT070 as a potential therapeutic tool. These data might provide a rationale for considering clinical trials of APT070 in human islet transplantation, and other complement dependent disorders.
Conflict of interest
The authors declare no conflicts‐of‐interest.
Authorship contribution statement
F. X. and L. M. performed the research; M. Z. and A. P. helped in in vitro viability and functional assessment of the islets; F. X., L. M., R. L. and G. L. designed the research study; R. A. S., G. C. H., P. M. J. and S. P. contributed essential reagents and tools; F. X. and L. M. analysed the data; L. M., A. D., R. A. S., P. M. J, R. L. and G. L. edited the manuscript; F. X. wrote the paper.
Supporting information
Figure 1 Assessment of APT070‐treated islet viability. Human islets were treated with APT070 of serial dilutions of 0.4 µM to 0.16 µM, and then stained with fluorescein diacetate (green) and propidium iodide (red). Control islets received no treatment. Inset images show enlarged area indicated by a white arrow. Green and red colours indicate live and dead cells respectively.
Figure 2 Flow cytometry analysis of human cell grafts three months after CD34+ stem cell reconstitution in NSG mice. Each plot represents one mouse. (A) The percentage of human cell engraftment in the peripheral blood 16 weeks after CD34+ stem cell injection. (B) Representative plot of flow cytometry analysis.
Figure 3 Non‐fasted plasma glucose level over time in islet transplanted NSG mice rendered diabetic with streptozotocin. Values are shown as means ± SD. N = 3 animals for each group. Nephrectomy: graft‐bearing kidney was removed. Normoglycemia was defined as ≤ 13.8 mM glucose in plasma.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgements
We thank Professor Gavin P Vinson (The School of Biological and Chemical Sciences, Queen Mary University of London, UK) for help in the preparation of the manuscript. This work was supported by the Medical Research Council, UK (MR/J006742/1).
Xiao, F. , Ma, L. , Zhao, M. , Smith, R. A. , Huang, G. , Jones, P. M. , Persaud, S. , Pingitore, A. , Dorling, A. , Lechler, R. , and Lombardi, G. (2016) APT070 (mirococept), a membrane‐localizing C3 convertase inhibitor, attenuates early human islet allograft damage in vitro and in vivo in a humanized mouse model. British Journal of Pharmacology, 173: 575–587. doi: 10.1111/bph.13388.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. (2013). The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol 170: 1797–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banz Y, Hess OM, Robson SC, Csizmadia E, Mettler D, Meier P, et al. (2007). Attenuation of myocardial reperfusion injury in pigs by Mirococept, a membrane‐targeted complement inhibitor derived from human CR1. Cardiovasc Res 76: 482–493. [DOI] [PubMed] [Google Scholar]
- Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O (2000a). Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci 105: 125–133. [DOI] [PubMed] [Google Scholar]
- Bennet W, Sundberg B, Lundgren T, Tibell A, Groth CG, Richards A, et al. (2000b). Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: protective effects of sCR1 and heparin. Transplantation 69: 711–719. [DOI] [PubMed] [Google Scholar]
- Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J , et al. (2010a). Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol 135: 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm MA, Shultz LD, Greiner DL (2010b) Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes 17: 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm MA, Shultz LD, Luban J, Greiner DL (2013). Overcoming current limitations in humanized mouse research. J Infect Dis 208 (Suppl 2): S125–S130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KM, Kondeatis E, Vaughan RW, Kon SP, Farmer CK, Taylor JD, et al. (2006). Influence of donor C3 allotype on late renal‐transplantation outcome. N Engl J Med 354: 2014–2023. [DOI] [PubMed] [Google Scholar]
- Cao X, Shores EW, Hu‐Li J, Anver MR, Kelsall BL, Russell SM, et al. (1995). Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 2: 223–238. [DOI] [PubMed] [Google Scholar]
- Chen H, Teramura Y, Iwata H (2011). Co‐immobilization of urokinase and thrombomodulin on islet surfaces by poly(ethylene glycol)‐conjugated phospholipid. Journal of controlled release: official journal of the Controlled Release Society 150: 229–234. [DOI] [PubMed] [Google Scholar]
- Choi SE, Choi KM, Yoon IH, Shin JY, Kim JS, Park WY, et al. (2004). IL‐6 protects pancreatic islet beta cells from pro‐inflammatory cytokines‐induced cell death and functional impairment in vitro and in vivo. Transpl Immunol 13: 43–53. [DOI] [PubMed] [Google Scholar]
- Citro A, Cantarelli E, Piemonti L (2013). Anti‐inflammatory strategies to enhance islet engraftment and survival. Curr Diab Rep 13: 733–744. [DOI] [PubMed] [Google Scholar]
- Covassin L, Laning J, Abdi R, Langevin DL, Phillips NE, Shultz LD, et al. (2011). Human peripheral blood CD4 T cell‐engrafted non‐obese diabetic‐scid IL2rgamma(null) H2‐Ab1 (tm1Gru) Tg (human leucocyte antigen D‐related 4) mice: a mouse model of human allogeneic graft‐versus‐host disease. Clin Exp Immunol 166: 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Bond, R. A. , Spina, D. , Ahluwalia, A. , Alexander, S. P. A. , Giembycz, M. A et al, (2015), Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva RJ, Vuylsteke A, Fritchley SJ, Trull AK, Dunning JJ, Wallwork J (2006). APT070 inhibits complement activation during in vitro cardiopulmonary bypass. European journal of cardio‐thoracic surgery: official journal of the European Association for Cardio‐thoracic Surgery 30: 72–76. [DOI] [PubMed] [Google Scholar]
- DiSanto JP, Muller W, Guy‐Grand D, Fischer A, Rajewsky K (1995). Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci U S A 92: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd I, Mossakowska DE, Camilleri P, Haran M, Hensley P, Lawlor EJ, et al. (1995). Overexpression in Escherichia coli, folding, purification, and characterization of the first three short consensus repeat modules of human complement receptor type 1. Protein Expr Purif 6: 727–736. [DOI] [PubMed] [Google Scholar]
- Farrar CA, Sacks SH (2014). Mechanisms of rejection: role of complement. Curr Opin Organ Transplant 19: 8–13. [DOI] [PubMed] [Google Scholar]
- Halstead SK, Humphreys PD, Goodfellow JA, Wagner ER, Smith RA, Willison HJ (2005). Complement inhibition abrogates nerve terminal injury in Miller Fisher syndrome. Ann Neurol 58: 203–210. [DOI] [PubMed] [Google Scholar]
- Hansen WA, Christie MR, Kahn R, Norgaard A, Abel I, Petersen AM, et al. (1989). Supravital dithizone staining in the isolation of human and rat pancreatic islets. Diabetes Res 10: 53–57. [PubMed] [Google Scholar]
- Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, et al. (2005). Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 106: 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgren O (2003). Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem Biophys Res Commun 308: 474–479. [DOI] [PubMed] [Google Scholar]
- Jones PM, Persaud SJ, Howell SL (1989). Time‐course of Ca2 + −induced insulin secretion from perifused, electrically permeabilised islets of Langerhans: effects of cAMP and a phorbol ester. Biochem Biophys Res Commun 162: 998–1003. [DOI] [PubMed] [Google Scholar]
- Jones PM, Salmon DM, Howell SL (1988). Protein phosphorylation in electrically permeabilized islets of Langerhans. Effects of Ca2+, cyclic AMP, a phorbol ester and noradrenaline. Biochem J 254: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M, Pearson T, Shultz LD, Leif J, Bottino R, Trucco M, et al. (2008). A new Hu‐PBL model for the study of human islet alloreactivity based on NOD‐scid mice bearing a targeted mutation in the IL‐2 receptor gamma chain gene. Clin Immunol 126: 303–314. [DOI] [PubMed] [Google Scholar]
- Li K, Zhou W (2013). Anaphylatoxins in organ transplantation. Semin Immunol 25: 20–28. [DOI] [PubMed] [Google Scholar]
- London NJ, Contractor H, Lake SP, Aucott GC, Bell PR, James RF (1989). A microfluorometric viability assay for isolated human and rat islets of Langerhans. Diabetes Res 12: 141–149. [PubMed] [Google Scholar]
- Luan NM, Teramura Y, Iwata H (2011). Immobilization of soluble complement receptor 1 on islets. Biomaterials 32: 4539–4545. [DOI] [PubMed] [Google Scholar]
- Lundgren T, Bennet W, Tibell A, Soderlund J, Sundberg B, Song Z, et al. (2001). Soluble complement receptor 1 (TP10) preserves adult porcine islet morphology after intraportal transplantation into cynomolgus monkeys. Transplant Proc 33 (1–2): 725. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, McLachlan EM, Zeller R (2015). Transparency in Research involving Animals: The Basel Declaration and new principles for reporting research in BJP manuscripts. Br J Pharmacol 172: 2427–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Kallen R, et al. (2002). Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet 360: 2039–2045. [DOI] [PubMed] [Google Scholar]
- Moberg L, Korsgren O, Nilsson B (2005). Neutrophilic granulocytes are the predominant cell type infiltrating pancreatic islets in contact with ABO‐compatible blood. Clin Exp Immunol 142: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, et al. (2002). Essential role of the C5a receptor in E coli‐induced oxidative burst and phagocytosis revealed by a novel lepirudin‐based human whole blood model of inflammation. Blood 100: 1869–1877. [PubMed] [Google Scholar]
- Mossakowska D, Dodd I, Pindar W, Smith RA (1999). Structure‐activity relationships within the N‐terminal short consensus repeats (SCR) of human CR1 (C3b/C4b receptor, CD35): SCR 3 plays a critical role in inhibition of the classical and alternative pathways of complement activation. Eur J Immunol 29: 1955–1965. [DOI] [PubMed] [Google Scholar]
- Nilsson B, Ekdahl KN, Korsgren O (2011). Control of instant blood‐mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant 16: 620–626. [DOI] [PubMed] [Google Scholar]
- Ohbo K, Suda T, Hashiyama M, Mantani A, Ikebe M, Miyakawa K, et al. (1996). Modulation of hematopoiesis in mice with a truncated mutant of the interleukin‐2 receptor gamma chain. Blood 87: 956–967. [PubMed] [Google Scholar]
- Patel H, Smith RA, Sacks SH, Zhou W (2006). Therapeutic strategy with a membrane‐localizing complement regulator to increase the number of usable donor organs after prolonged cold storage. Journal of the American Society of Nephrology: JASN 17: 1102–1111. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, Davenport AP, McGrath JC, Peters JA, Southan C, Spedding M, Yu W, Harmar AJ; NC‐IUPHAR. (2014) The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 42 (Database Issue): D1098‐1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q, Li K, Anderson K, Farrar CA, Lu B, Smith RA, et al. (2008). Local production and activation of complement up‐regulates the allostimulatory function of dendritic cells through C3a‐C3aR interaction. Blood 111: 2452–2461. [DOI] [PubMed] [Google Scholar]
- Robertson RP (2010). Islet transplantation a decade later and strategies for filling a half‐full glass. Diabetes 59: 1285–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks S, Lee Q, Wong W, Zhou W (2009). The role of complement in regulating the alloresponse. Curr Opin Organ Transplant 14: 10–15. [DOI] [PubMed] [Google Scholar]
- Serra‐Hassoun M, Bourgine M, Boniotto M, Berges J, Langa F, Michel ML, et al. (2014). Human hematopoietic reconstitution and HLA‐restricted responses in nonpermissive alymphoid mice. J Immunol 193: 1504–1511. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Ishikawa F, Greiner DL (2007). Humanized mice in translational biomedical research. Nature reviews. Immunology 7: 118–130. [DOI] [PubMed]
- Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, et al. (2010). Generation of functional human T‐cell subsets with HLA‐restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A 107: 13022–13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GP, Smith RA (2001). Membrane‐targeted complement inhibitors. Mol Immunol 38 (2–3): 249–255. [DOI] [PubMed] [Google Scholar]
- Smith RA (2002). Targeting anticomplement agents. Biochem Soc Trans 30 (Pt 6): 1037–1041. [DOI] [PubMed] [Google Scholar]
- Smith RAGKG, Chowdhury P, Smith KKC, Watson CJ, Nicholson ML, Zhou WD, et al. (2007). Membrane‐localising complement inhibitors‐clinical progress. Mol Immunol 44: 1. [Google Scholar]
- Souza DG, Esser D, Bradford R, Vieira AT, Teixeira MM (2005). APT070 (Mirococept), a membrane‐localised complement inhibitor, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol 145: 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza DG, Guabiraba R, Pinho V, Bristow A, Poole S, Teixeira MM (2003). IL‐1‐driven endogenous IL‐10 production protects against the systemic and local acute inflammatory response following intestinal reperfusion injury. J Immunol 170: 4759–4766. [DOI] [PubMed] [Google Scholar]
- Teramura Y, Iwata H (2011). Improvement of graft survival by surface modification with poly(ethylene glycol)‐lipid and urokinase in intraportal islet transplantation. Transplantation 91: 271–278. [DOI] [PubMed] [Google Scholar]
- Tjernberg J, Ekdahl KN, Lambris JD, Korsgren O, Nilsson B (2008). Acute antibody‐mediated complement activation mediates lysis of pancreatic islets cells and may cause tissue loss in clinical islet transplantation. Transplantation 85: 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokodai K, Goto M, Inagaki A, Nakanishi W, Ogawa N, Satoh K, et al. (2010). Attenuation of cross‐talk between the complement and coagulation cascades by C5a blockade improves early outcomes after intraportal islet transplantation. Transplantation 90: 1358–1365. [DOI] [PubMed] [Google Scholar]
- Torrealba JR, Samaniego M, Pascual J, Becker Y, Pirsch J, Sollinger H, et al. (2008). C4d‐positive interacinar capillaries correlates with donor‐specific antibody‐mediated rejection in pancreas allografts. Transplantation 86: 1849–1856. [DOI] [PubMed] [Google Scholar]
- van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK (2007). Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation 14: 288–297. [DOI] [PubMed] [Google Scholar]
- van der Windt DJ, Marigliano M, He J, Votyakova TV, Echeverri GJ, Ekser B, et al. (2012). Early islet damage after direct exposure of pig islets to blood: has humoral immunity been underestimated? Cell Transplant 21: 1791–1802. [DOI] [PubMed] [Google Scholar]
- Walport MJ (2001a). Complement. First of two parts. N Engl J Med 344: 1058–1066. [DOI] [PubMed] [Google Scholar]
- Walport MJ (2001b). Complement. Second of two parts. N Engl J Med 344: 1140–1144. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Takahashi T, Okajima A, Shiokawa M, Ishii N, Katano I, et al. (2009). The analysis of the functions of human B and T cells in humanized NOD/shi‐scid/gammac(null) (NOG) mice (hu‐HSC NOG mice). Int Immunol 21: 843–858. [DOI] [PubMed] [Google Scholar]
- Xiao F, Ma L, Zhao M, Huang G, Mirenda V, Dorling A, et al. (2014). Ex vivo expanded human regulatory T cells delay islet allograft rejection via inhibiting islet‐derived monocyte chemoattractant protein‐1 production in CD34+ stem cells‐reconstituted NOD‐scid IL2rgammanull mice. PLoS One 9: e90387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Ji M, Wu J, Ma X, Phillips P, Hawthorne WJ, et al. (2012). Adoptive transfer with in vitro expanded human regulatory T cells protects against porcine islet xenograft rejection via interleukin‐10 in humanized mice. Diabetes 61: 1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Patel H, Li K, Peng Q, Villiers MB, Sacks SH (2006). Macrophages from C3‐deficient mice have impaired potency to stimulate alloreactive T cells. Blood 107: 2461–2469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1 Assessment of APT070‐treated islet viability. Human islets were treated with APT070 of serial dilutions of 0.4 µM to 0.16 µM, and then stained with fluorescein diacetate (green) and propidium iodide (red). Control islets received no treatment. Inset images show enlarged area indicated by a white arrow. Green and red colours indicate live and dead cells respectively.
Figure 2 Flow cytometry analysis of human cell grafts three months after CD34+ stem cell reconstitution in NSG mice. Each plot represents one mouse. (A) The percentage of human cell engraftment in the peripheral blood 16 weeks after CD34+ stem cell injection. (B) Representative plot of flow cytometry analysis.
Figure 3 Non‐fasted plasma glucose level over time in islet transplanted NSG mice rendered diabetic with streptozotocin. Values are shown as means ± SD. N = 3 animals for each group. Nephrectomy: graft‐bearing kidney was removed. Normoglycemia was defined as ≤ 13.8 mM glucose in plasma.
Supporting info item
Supporting info item
Supporting info item
Supporting info item


