Abstract
Three new 9,11-secosterols, pinnisterols A–C (1–3), were isolated from a gorgonian coral Pinnigorgia sp., collected off the waters of Taiwan. The structures of these compounds were elucidated on the basis of spectroscopic methods. The new sterols 1 and 3 displayed significant inhibitory effects on the generation of superoxide anions and the release of elastase by human neutrophils, and sterol 1 was found to show moderate cytotoxicity in hepatic stellate cells (HSCs).
Keywords: secosterol, gorgonian, Pinnigorgia, anti-inflammatory, superoxide anion, elastase, cytotoxicity, HSCs
1. Introduction
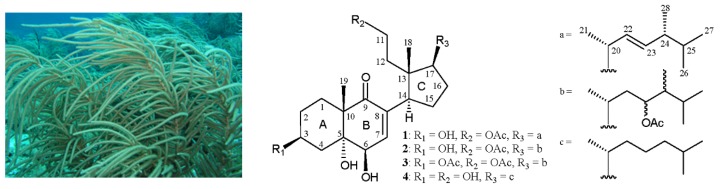
Studies on the chemical constituents of octocorals collected off the waters of Taiwan, at the intersection of the Kuroshio current and the South China Sea surface current, have led to the isolation of a series of interesting 9,11-secosterols from Cespitularia hypotentaculata [1], Cladiella hirsuta [2], Sinularia granosa [3], Sinularia leptoclados [4,5], Sinularia lochmodes [4], and Sinularia nanolobata [6]. Steroids of this type were found to possess interesting bioactivities, such as cytotoxic [1,2,3,4,5,6], anti-inflammatory [3] and antiviral [5,6] activities. In our continuing investigation of bioactive natural products obtained from Formosan soft corals, three new 9,11-secosterols, pinnisterols A–C (1–3), were obtained from a gorgonial coral identified as Pinnigorgia sp. (phylum Cinidaria, class Anthozoa, subclass Octocorallia, order Alcyonacea, family Gorgoniidae) (Figure 1). The structures of secosterols 1–3 were elucidated by spectroscopic methods and by comparison of their NMR features with those of related secosterol analogues. We report herein the isolation, structure determination and bioactivity of secosterols 1–3.
Figure 1.
Gorgonian coral Pinnigorgia sp. and the structures of 9,11-secosterols 1–4.
2. Results and Discussion
The new metabolite pinnisterol A (1) was isolated as a colorless oil, and its molecular formula was established as C30H48O6 (seven degrees of unsaturation) from a sodium adduct at m/z 527 in the electrospray ionization mass spectrum (ESIMS) and further supported by a high-resolution electrospray ionization mass spectrum (HRESIMS) at m/z 527.33440 (calcd. for C30H48O6 + Na, 527.33431). The 13C and distortionless enhancement polarization transfer (DEPT) spectroscopic data of 1 showed that this compound has 30 carbons (Table 1), including seven methyls, seven sp3 methylenes (including an oxymethylene), seven sp3 methines (including two oxymethines), three sp3 quaternary carbons (including one oxygenated quaternary carbon), three sp2 methines and three sp2 quaternary carbons (including one ketonic carbonyl and one ester carbonyl). The IR spectrum of 1 revealed the presence of hydroxy (νmax 3546 cm−1), ester (νmax 1736 cm−1) and α,β-unsaturated ketone (νmax 1683 cm−1) groups. The latter structural feature was confirmed by the presence of signals at δC 204.9 (C-9), 139.5 (CH-7) and 136.6 (C-8) in the 13C NMR spectrum. A disubstituted olefin was identified from the signals of carbons at δC 134.3 (CH-22) and 133.1 (CH-23), and was confirmed by two olefin proton signals at δH 5.24 (1H, m, H-22) and 5.22 (1H, m, H-23) (Table 1). Four doublets at δH 1.04 (3H, J = 6.8 Hz), 0.81 (3H, J = 6.8 Hz), 0.83 (3H, J = 7.2 Hz) and 0.91 (3H, J = 6.8 Hz) were due to the H3-21, H3-27, H3-26 and H3-28 methyl groups, respectively. Two sharp singlets for H3-18 and H3-19 appeared at δH 0.74 and 1.31, respectively. In the 1H NMR spectrum, one acetyl methyl signal (δH 2.00, 3H, s) was observed. Therefore, metabolite 1 must be a tricyclic compound.
Table 1.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for secosterol 1.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 1.94 m; 1.60 m | 27.6, CH2 | H2-2 | C-5 |
| 2 | 1.91 m; 1.48 m | 30.2, CH2 | H2-1, H-3 | n. o. b |
| 3 | 3.99 br s a | 67.1, CH | H2-2, H2-4 | C-5 |
| 4 | 2.07 dd (12.0, 12.0); 1.74 br d (12.0) | 38.7, CH2 | H-3 | C-2, -3, -5 |
| 5 | 76.7, C | |||
| 6 | 4.00 br s a | 71.7, CH | H-7 | C-5, -7, -8, -10 |
| 7 | 6.40 d (4.4) | 139.5, CH | H-6 | C-5, -8, -9, -14 |
| 8 | 136.6, C | |||
| 9 | 204.9, C | |||
| 10 | 48.0, C | |||
| 11 | 4.14 t (7.2) | 61.6, CH2 | H2-12 | C-12, -13, acetate carbonyl |
| 12 | 1.65 m; 1.25 m | 36.5, CH2 | H2-11 | C-11, -13, -14, -17, -18 |
| 13 | 45.9, C | |||
| 14 | 3.19 dd (9.6, 9.2) | 42.6, CH | H2-15 | C-7, -8, -9, -12, -13, -15, -18 |
| 15 | 1.60 m | 26.9, CH2 | H-14, H2-16 | n. o. |
| 16 | 1.69 m; 1.47 m | 25.5, CH2 | H2-15, H-17 | C-15 |
| 17 | 1.69 m | 50.5, CH | H2-16, H-20 | C-15, -16 |
| 18 | 0.74 s | 17.2, CH3 | C-12, -13, -14, -17 | |
| 19 | 1.31 s | 21.7, CH3 | C-1, -5, -9, -10 | |
| 20 | 2.19 m | 38.7, CH | H-17, H3-21, H-22 | C-16, -17, -22, -23 |
| 21 | 1.04 d (6.8) | 21.6, CH3 | H-20 | C-17, -20, -22 |
| 22 | 5.25 dd (15.2, 6.4) | 134.3, CH | H-20, H-23 | C-20, -23, -24 |
| 23 | 5.21 dd (15.2, 6.8) | 133.1, CH | H-22, H-24 | C-20, -22, -24, 28 |
| 24 | 1.85 q (6.4) | 43.1, CH | H-23, H-25, H3-28 | C-22, -23, -25, -26, -27, -28 |
| 25 | 1.45 m | 33.1, CH | H-24, H3-26, H3-27 | C-23, -24, -26, -27, -28 |
| 26 | 0.83 d (7.2) | 20.0, CH3 | H-25 | C-24, -25, -27 |
| 27 | 0.81 d (6.8) | 19.7, CH3 | H-25 | C-24, -25, -26 |
| 28 | 0.91 d (6.8) | 17.6, CH3 | H-24 | C-23, -24, -25 |
| 11-OAc | 171.7, C | |||
| 2.00 s | 21.2, CH3 | Acetate carbonyl |
a Signals overlapped; b n. o. = not observed.
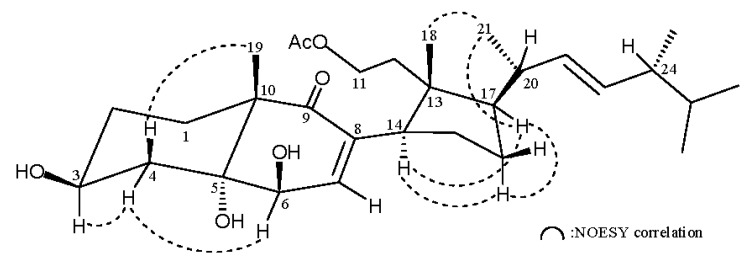
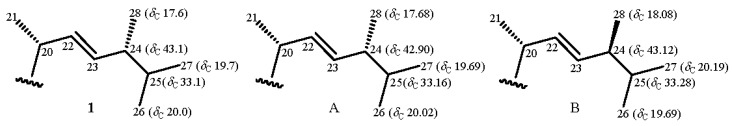
1H NMR coupling information in the 1H–1H correlation spectroscopy (COSY) spectrum of 1 enabled identification of H2-1/H2-2/H-3/H2-4, H-6/H-7, H2-11/H2-12, H-14/H2-15/H2-16/H-17/H-20/ H-22/H-23/H-24/H-25/H3-26(H3-27), H-20/H3-21 and H-24/H3-28 (Table 1). These data, together with the key HMBC correlations between protons and quaternary carbons, such as H2-1, H-3, H2-4, H-6, H-7, H3-19/C-5; H-6, H-7, H-14/C-8; H-7, H-14, H3-19/C-9; H-6, H3-19/C-10; and H2-11, H2-12, H-14, H3-18/C-13, permitted the elucidation of the main carbon skeleton of 1 (Table 1). The relative configuration of 1 was elucidated from the correlations observed in a nuclear Overhauser effect spectroscopy (NOESY) experiment and by comparison of NMR data with those of a known secosterol, aplidiasterol B (3β,5α,6β,11-tetrahydroxy-9,11-secocholest-7-en-9-one) (4) (Figure 1), isolated from a Mediterranean ascidian Aplidium conicum [7]. The relative stereochemistries at C-3, C-5, C-6, C-10, C-13, C-14 and C-17 in 1 were found to be the same as those of 4. Key NOE correlations for 1 showed interactions between H-3/H-4α (δH 1.74) and H-4α/H-6. Thus, H-3 and H-6 should be positioned on the α-face (Figure 2). A large coupling constant observed between H-22 and H-23 (J = 15.2 Hz) supported a trans relationship between H-22 and H-23. A stereogenic center (C-24) was identified in the side chain. The configuration at C-24 was suggested to be R* on the basis of the 13C NMR chemical shift of C-28 (δC 17.6). It was reported that the 13C NMR value of C-28 resonates at δC 17.68 ppm in the 24R* epimer of a known sterol, (22E,24R)-24-methylcholesta-5,22-dien-3β-ol, with the same chain, and the 24S* epimer, (22E,24S)-24-methylcholesta-5,22-dien-3β-ol, has a relative 0.4 ppm downfield chemical shift (Figure 3) [8]. Based on the above findings, the structure, including the relative configuration of 1, was suggested.
Figure 2.
Selected NOESY correlations observed for 1.
Figure 3.
The 13C NMR chemical shifts of the side-chain of pinnisterol A (1), (22E,24R)-24-methyl- cholesta-5,22-dien-3β-ol (A) and (22E,24S)-24-methylcholesta-5,22-dien-3β-ol (B) [8].
Pinnisterol B (2) was isolated as a colorless oil, and its molecular formula was established as C32H52O8 (seven degrees of unsaturation) by HRESIMS at m/z 587.35558 (calcd. for C32H52O8 + Na, 587.35544). The IR spectrum of 2 indicated the presence of hydroxy (3420 cm−1), ester (1728 cm−1) and α,β-unsaturated ketone (1678 cm−1) groups. The whole series of spectroscopic data obtained from one-dimensional (1D) and two-dimensional (2D) NMR experiments (Table 2) clearly indicated that secosterol 2 had the same core structure as secosterol 1, the differences being limited to the presence in 2 of the addition of an acetoxy group to substitute the alkene at C-23. The 1H and 13C NMR data assignments of pinnisterol B (2) were compared with the values of 1. The HMBC correlations observed fully supported the locations of the functional groups, and, hence, pinnisterol B (2) was assigned as structure 2, with the same relative configurations as secosterol 1 in the core rings A–C; the chiral carbons C-3, C-5, C-6, C-10, C-13, C-14 and C-17 of 2 were identical to those of 1, and the 1H and 13C NMR chemical shifts and proton coupling constants were also in agreement.
Table 2.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for secosterol 2.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 1.99 m; 1.70 m | 27.6, CH2 | H2-2 | C-19 |
| 2 | 1.99 m; 1.55 m | 30.5, CH2 | H2-1, H-3 | n. o. a |
| 3 | 4.07 m | 67.1, CH | H2-2, H2-4 | C-5 |
| 4 | 2.14 dd (12.4, 12.0); 1.78 dd (12.4, 3.2) | 39.2, CH2 | H-3 | C-2, -3, -5, -10 |
| 5 | 76.8, C | |||
| 6 | 4.05 m | 72.2, CH | H-7 | C-5, -7, -8, -10 |
| 7 | 6.43 d (5.2) | 138.2, CH | H-6 | C-5, -9, -14 |
| 8 | 137.5, C | |||
| 9 | 203.2, C | |||
| 10 | 48.2, C | |||
| 11 | 4.15 m | 61.2, CH2 | H2-12 | C-12, acetate carbonyl |
| 12 | 1.67 m; 1.28 m | 36.8, CH2 | H2-11 | C-11, -13, -14, -17 |
| 13 | 46.1, C | |||
| 14 | 3.27 dd (10.8, 9.2) | 42.6, CH | H2-15 | C-7, -8, -13, -15, -18 |
| 15 | 1.65 m | 27.0, CH2 | H-14, H2-16 | C-14 |
| 16 | 1.87 m; 1.47 m | 26.1, CH2 | H2-15, H-17 | C-13 |
| 17 | 1,66 m | 51.1, CH | H2-16, H-20 | C-13, -14, -20, -21, -22 |
| 18 | 0.76 s | 17.1, CH3 | C-12, -13, -14, -17, -23 | |
| 19 | 1.36 s | 21.8, CH3 | C-1, -5, -9, -10 | |
| 20 | 1.53 m | 33.4, CH | H-17, H3-21, H2-22 | C-22 |
| 21 | 1.02 d (6.8) | 20.4, CH3 | H-20 | C-17, -20 |
| 22 | 1.70 m; 1.22 m | 35.6, CH2 | H-20, H-23 | C-17, -20, -21, -23, -24 |
| 23 | 5.02 m | 76.9, CH | H2-22, H-24 | n. o. |
| 24 | 1.49 m | 42.7, CH | H-23, H-25, H3-28 | C-22, -23, -25, -26, -27, -28 |
| 25 | 1.58 m | 28.5, CH | H-24, H3-26, H3-27 | C-23, -24, 26, -27, -28 |
| 26 | 0.94 d (6.8) | 21.6, CH3 | H-25 | C-24, -25, -27 |
| 27 | 0.84 d (6.8) | 21.5, CH3 | H-25 | C-24, -25, -26 |
| 28 | 0.81 d (6.8) | 11.0, CH3 | H-24 | C-23, -24, -25 |
| 11-OAc | 171.2, C | |||
| 2.00 s | 21.1, CH3 | Acetate carbonyl | ||
| 23-OAc | 170.8, C | |||
| 2.03 s | 21.5, CH3 | Acetate carbonyl |
a n. o. = not observed.
Pinnisterol C (3) was isolated as a colorless oil. The molecular formula of 3 was established as C32H52O8 (seven degrees of unsaturation) from a [M + Na]+ ion at m/z 629.36609 in HRESIMS (calcd. for C32H52O8 + Na, 629.36600). The gross structure of 3 was established by interpretation of 1D and 2D NMR data, especially by analysis of 1H–1H COSY and HMBC correlations (Table 3). It was found that the NMR signals of 3 were similar to those of 2, except that the signals corresponding to the 3-hydroxy group in 2 were replaced by signals for an acetoxy group in 3. The correlations obtained from a NOESY experiment of 3 also showed that the configurations of chiral centers in the core rings A–C in 3 were identical to those of 2. However, the configurations of chiral carbons C-23 and C-24 of secosterols 2 and 3 were not determined at this stage.
Table 3.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H–1H COSY and HMBC correlations for secosterol 3.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 2.06 m; 1.71 m | 27.4, CH2 | H2-2 | C-5, -10 |
| 2 | 1.98 m; 1.61 m | 26.4, CH2 | H2-1, H-3 | C-3 |
| 3 | 5.10 m | 70.3, CH | H2-2, H2-4 | n. o. a |
| 4 | 2.21 dd (13.2, 11.6); 1.87 m | 35.5, CH2 | H-3 | C-3, -5, -10, -12 |
| 5 | 76.5, C | |||
| 6 | 4.03 d (4.8) | 72.3, CH | H-7 | C-5, -7, -8, -10 |
| 7 | 6.41 d (4.8) | 138.1, CH | H-6 | C-5, -9, -14 |
| 8 | 137.4, C | |||
| 9 | 202.8, C | |||
| 10 | 48.0, C | |||
| 11 | 4.16 m | 61.2, CH2 | H2-12 | C-12, -13, acetate carbonyl |
| 12 | 1.61 m; 1.28 m | 36.8, CH2 | H2-11 | C-11, -13, -14, -17 |
| 13 | 46.1, C | |||
| 14 | 3.27 dd (11.6, 8.0) | 42.6, CH | H2-15 | C-7, -8, -13 |
| 15 | 1.61 m | 27.0, CH2 | H-14, H2-16 | C-4, -13 |
| 16 | 1.87 m; 1.47 m | 26.1, CH2 | H2-15, H-17 | n. o. |
| 17 | 1.68 m | 51.2, CH | H2-16, H-20 | n. o. |
| 18 | 0.75 d (6.4) | 17.1, CH3 | C-12, -13, -14, -17 | |
| 19 | 1.31 s | 21.6, CH3 | C-1, -5, -10 | |
| 20 | 1.53 m | 33.4, CH | H-17, H3-21, H2-22 | n. o. |
| 21 | 1.03 d (6.4) | 20.4, CH3 | H-20 | C-17, -20, -22 |
| 22 | 1.71 m; 1.26 m | 35.5, CH2 | H-20, H-23 | C-21, -23 |
| 23 | 5.02 m | 76.9, CH | H2-22, H-24 | n. o. |
| 24 | 1.49 m | 42.8, CH | H-23, H-25, H3-28 | C-22, -23, -27, -28 |
| 25 | 1.57 m | 28.5, CH | H-24, H3-26, H3-27 | C-24, -27, -28 |
| 26 | 0.94 d (6.4) | 21.6, CH3 | H-25 | C-24, -25, -27 |
| 27 | 0.84 d (6.4) | 18.6, CH3 | H-25 | C-24, -25, -26 |
| 28 | 0.81 d (6.4) | 11.0, CH3 | H-24 | C-23, -24, -25 |
| 3-OAc | 170.8, C | |||
| 2.01 s | 21.4, CH3 | Acetate carbonyl | ||
| 11-OAc | 171.1, C | |||
| 2.00 s | 21.1, CH3 | Acetate carbonyl | ||
| 23-OAc | 170.8, C | |||
| 2.05 s | 21.5, CH3 | Acetate carbonyl |
a n. o. = not observed.
In the biological activity testing, secosterols 1 and 3 displayed significant inhibitory effects on the release of elastase (IC50 = 3.32 and 2.81 μM) and inhibitory effects on the generation of superoxide anions (IC50 = 2.33 and 2.50 μM) by human neutrophils (Table 4) [9,10]. Secosterol 2 did not show activity in the anti-inflammatory test, which indicated that an acetoxy substituent at C-3 would enhance the activity by comparison with the structure and anti-inflammatory data of 2 with those of 3.
Table 4.
Inhibitory effects of compounds 1–3 on elastase release and superoxide anion generation by human neutrophils in response to fMet-Leu-Phe/Cytochalastin B.
| Elastase Release | Superoxide Anion | |
|---|---|---|
| Compound | IC50 (μM) a | IC50 (μM) a |
| 1 | 3.32 | 2.33 |
| 2 | >10 | >10 |
| 3 | 2.81 | 2.50 |
a Concentration necessary for 50% inhibition (IC50).
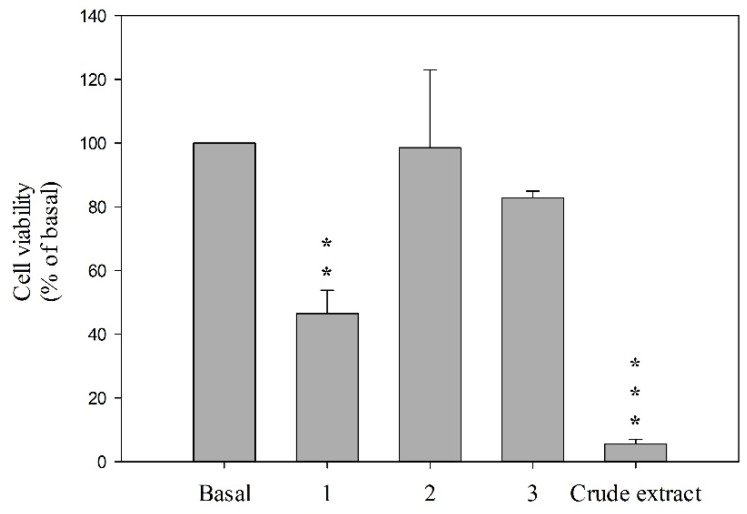
Furthermore, in the cytotoxicity testing, secosterol 1 was found to show moderate cytotoxicity towards the HSC-T6 cells at a concentration of 10 μM (inhibition rate 46.5%) after 24 h testing and secosterols 2 and 3 were not active at the highest concentration tested (Figure 4), implying that the functional groups in the side-chain of secosterols 1–3 would influence the activity.
Figure 4.
Compounds 1–3 decreased viability of HSC-T6 in 10 μM for 24 h. Cells were treated with DMSO (control) and coral crude extract at 6 μg/mL. Cytotoxicity assay was monitored spectrophotometrically at 450 nm. Quantitative data are expressed as the mean ± S.E.M. (n = 3–4). ** p < 0.01, *** p < 0.001 compared to basal.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured on a Jasco P-1010 digital polarimeter (Japan Spectroscopic Corporation, Tokyo, Japan). Infrared spectra were recorded on a Jasco FT/IR-4100 spectrometer (Japan Spectroscopic Corporation, Tokyo, Japan); peaks are reported in cm−1. The NMR spectra were recorded on a Varian Mercury Plus 400 spectrometer, using the residual CHCl3 signal (δH 7.26 ppm) as an internal standard for 1H NMR and CDCl3 (δC 77.1 ppm) for 13C NMR; coupling constants (J) are given in Hz. ESIMS and HRESIMS were recorded using a Bruker 7 Tesla solariX FTMS system (Bruker, Bremen, Germany). Column chromatography was performed on silica gel (230–400 mesh, Merck, Darmstadt, Germany). TLC was carried out on precoated Kieselgel 60 F254 (0.25 mm, Merck, Darmstade, Germany); spots were visualized by spraying with 10% H2SO4 solution followed by heating. Normal-phase HPLC (NP-HPLC) was performed using a system comprised of a Hitachi L-7110 pump (Hitachi Ltd., Tokyo, Japan) and a Rheodyne 7725 injection port (Rheodyne LLC, Rohnert Park, CA, USA). A semi-preparative normal-phase column (Supelco Ascentis Si Cat #:581515-U, 25 cm × 21.2 mm, 5 μm, Sigma-Aldrich, St. Louis, MO, USA) was used for NP-HPLC. Reversed-phase HPLC (RP-HPLC) was performed using a system comprised of a Hitachi L-2130 pump (Hitachi Ltd., Tokyo, Japan), a Hitachi L-2455 photodiode array detector (Hitachi Ltd., Tokyo, Japan), and a Rheodyne 7725 injection port (Rheodyne LLC., Rohnert Park, CA, USA). A reverse phase column (Luna® 5 μm C18(2) 100Å, AXIA Packed, 25 cm × 21.2 mm, Phenomenex Inc., Torrance, CA, USA) was used for RP-HPLC.
3.2. Animal Material
Specimens of the gorgonian corals Pinnigorgia sp. were collected by hand using scuba off the coast of Green Island, Taiwan in August 2012 and stored in a freezer until extraction. A voucher specimen (NMMBA-TW-GC-2012-130) was deposited in the National Museum of Marine Biology & Aquarium, Taiwan. This organism was identified by comparison with previous descriptions [11].
3.3. Extraction and Separation
Sliced bodies of Pinnigorgia sp. (wet weight 1.98 kg; dry weight 0.86 kg) were extracted with ethyl acetate (EtOAc) at room temperature. The EtOAc extract (84.9 g) was partitioned between methanol (MeOH) and n-hexane. The MeOH layer (12.6 g) was separated on Sephadex LH-20 and eluted using a mixture of dichloromethane (DCM) and MeOH (1:1) to yield seven subfractions A–F. Fraction F was separated by silica gel column chromatography and eluted using n-hexane/acetone (stepwise, 1:1–pure acetone) to afford eight subfractions F1–F8. Fraction F2 was purified by silica gel column chromatography and eluted using n-hexane/acetone (stepwise, 9:1–pure acetone) to yield 13 subfractions F2A–F2M. Fraction F2G was purified by NP-HPLC using a mixture of n-hexane/EtOAc (1:1) to afford 3 (2.4 mg). Fraction F4 was purified by NP-HPLC using a mixture of n-hexane/acetone (2:1) to yield nine subfractions F4A–F4I. Fraction F4I was repurified by NP-HPLC using n-hexane/acetone (2:1) to afford nine subfractions F4I1–F4I9. Fraction F4I6 was separated by NP-HPLC using a mixture of n-hexane and acetone (2:1) to afford five subfractions F4I6A–F4I6E. Fraction F4I6C was purified by RP-HPLC, using a mixture of MeOH/H2O (9:1) to yield 1 (16.3 mg). Fraction F4I8 was purified by RP-HPLC using MeOH/H2O (85:15) to afford 2 (3.4 mg).
Pinnisterol A (1): colorless oil; −41 (c 0.82, CHCl3); IR (neat) νmax 3546, 1736, 1683 cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 527 [M + Na]+; HRESIMS m/z 527.33440 (calcd. for C30H48O6 + Na, 527.33431).
Pinnisterol B (2): colorless oil; −31 (c 1.13, CHCl3); IR (neat) νmax 3420, 1728, 1678 cm−1; 1H and 13C NMR data, see Table 2; ESIMS m/z 587 [M + Na]+; HRESIMS m/z 587.35558 (calcd. for C32H52O8 + Na, 587.35544).
Pinnisterol C (3): colorless oil; −39 (c 0.12, CHCl3); IR (neat) νmax 3453, 1731, 1682 cm−1; 1H and 13C NMR data, see Table 3; ESIMS m/z 629 [M + Na]+; HRESIMS m/z 629.36609 (calcd. for C34H54O9 + Na, 629.36600).
3.4. Anti-Hepatofibric Assay
The anti-hepatofibric effects of tested secosterols 1–3 were assayed using a WST-1 assay method. Anti-hepatofibric assays were carried out according to the procedures described previously [12].
3.5. Generation of Superoxide Anions and Release of Elastase by Human Neutrophils
Human neutrophils were obtained by means of dextran sedimentation and Ficoll centrifugation. Measurements of superoxide anion generation and elastase release were carried out according to previously described procedures [9,10]. Briefly, superoxide anion production was assayed by monitoring the superoxide dismutase-inhibitable reduction of ferricytochrome c. Elastase release experiments were performed using MeO-Suc-Ala-Ala-Pro-Valp-nitroanilide as the elastase substrate.
4. Conclusions
Our continuing investigations demonstrated that octocorals belonging to the genus Pinnigorgia are good sources of 9,11-secosterols. Pinnisterols A (1) and B (2) are potentially anti-inflammatory, and may become lead compounds in future marine anti-inflammation drug development [13,14]. The results of this study suggested that continuing investigation of new secosterols together with examination of the potentially useful bioactivities of this marine organism is worthwhile for future drug development.
Acknowledgments
This research was supported by grants from the Asia-Pacific Ocean Research Center, National Sun Yat-sen University; the National Museum of Marine Biology & Aquarium; the National Dong Hwa University; and the Ministry of Science and Technology (Grant No. MOST 103-2325-B-291-001, MOST 104-2325-B-291-001 and MOST 104-2320-B-291-001-MY3), awarded to Jyh-Horng Sheu and Ping-Jyun Sung.
Author Contributions
Chan-Shing Lin, Jyh-Horng Sheu and Ping-Jyun Sung designed the whole experiment and contributed to manuscript preparation. Yu-Chia Chang researched data. Liang-Mou Kuo, Tsong-Long Hwang, Jessica Yeh, Zhi-Hong Wen, Lee-Shing Fang and Ya-Chang Wu analyzed the data and performed data acquisition.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Duh C.-Y., Li C.-H., Wang S.-K., Dai C.-F. Diterpenoids, norditerpenoids, and secosteroids from the Formosan soft coral Cespitularia hypotentaculata. J. Nat. Prod. 2006;69:1188–1192. doi: 10.1021/np0505465. [DOI] [PubMed] [Google Scholar]
- 2.Chen B.-W., Chang S.-M., Huang C.-Y., Su J.-H., Wen Z.-H., Wu Y.-C., Sheu J.-H. Hirsutosterols A–G, polyoxygenated steroids from a Formosan soft coral Cladiella hirsuta. Org. Biomol. Chem. 2011;9:3272–3278. doi: 10.1039/c1ob05106g. [DOI] [PubMed] [Google Scholar]
- 3.Huang C.-Y., Su J.-H., Duh C.-Y., Chen B.-W., Wen Z.-H., Kuo Y.-H., Sheu J.-H. A new 9,11-secosterol from the soft coral Sinularia granosa. Bioorg. Med. Chem. Lett. 2012;22:4373–4376. doi: 10.1016/j.bmcl.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Su J.-H., Tseng Y.-J., Huang H.-H., Ahmed A.F., Lu C.-K., Wu Y.-C., Sheu J.-H. 9,11-Secosterols from the soft corals Sinularia lochmodes and Sinularia leptoclados. J. Nat. Prod. 2006;69:850–852. doi: 10.1021/np060031t. [DOI] [PubMed] [Google Scholar]
- 5.Cheng S.-Y., Chen H.-P., Wang S.-K., Duh C.-Y. Three new 9,11-secosterols from the Formosan soft coral Sinularia leptoclados. Bull. Chem. Soc. Jpn. 2011;84:648–652. doi: 10.1246/bcsj.20110046. [DOI] [Google Scholar]
- 6.Tseng Y.-J., Wang S.-K., Duh C.-Y. Secosteroids and norcembranoids from the soft coral Sinularia nanolobata. Mar. Drugs. 2013;11:3288–3296. doi: 10.3390/md11093288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aiello A., Esposito G., Fattorusso E., Iuvone T., Luciano P., Menna M. Aplidiasterols A and B, two new cytotoxic 9,11-secosterols from the mediterranean ascidian Aplidium conicum. Steroids. 2003;68:719–723. doi: 10.1016/S0039-128X(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 8.Wright J.L.C., McInnes A.G., Shimizu S., Smith D.G., Walter J.A., Idler D., Khalil W. Identification of C-24 alkyl epimers of marine sterols by 13C nuclear magnetic resonances spectroscopy. Can. J. Chem. 1978;56:1898–1903. [Google Scholar]
- 9.Yu H.-P., Yang S.-C., Chung P.-J., Ho C.-M., Kuo C.-Y., Hung M.-F., Huang Y.-T., Chang W.-Y., Chang Y.-W., Chan K.-H., et al. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013;190:6511–6519. doi: 10.4049/jimmunol.1202215. [DOI] [PubMed] [Google Scholar]
- 10.Yu H.-P., Hsieh P.-W., Chang Y.-J., Chung P.-J., Kuo L.-M., Hwang T.-L. 2-(2-Fluorobenzamido) benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011;50:1737–1748. doi: 10.1016/j.freeradbiomed.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 11.Fabricius K., Alderslade P. Soft Corals and Sea Fans—A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea. 1st ed. Australian Institute of Marine Science; Queensland, Australia: 2001. pp. 218–219. [Google Scholar]
- 12.Kuo L.-M., Kuo C.-Y., Lin C.-Y., Hung M.-F., Shen J.-J., Hwang T.-L. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules. 2014;19:3327–3344. doi: 10.3390/molecules19033327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei W.-C., Sung P.-J., Duh C.-Y., Chen B.-W., Sheu J.-H., Yang N.-S. Anti-inflammatory activities of natural products isolated from soft corals of Taiwan between 2008 and 2012. Mar. Drugs. 2013;11:4083–4126. doi: 10.3390/md11104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senthilkumar K., Kim S.-K. Marine invertebrate natural products for anti-inflammatory and chronic diseases. Evid. Based Complement. Alternat. Med. 2013;2013 doi: 10.1155/2013/572859. [DOI] [PMC free article] [PubMed] [Google Scholar]