Abstract
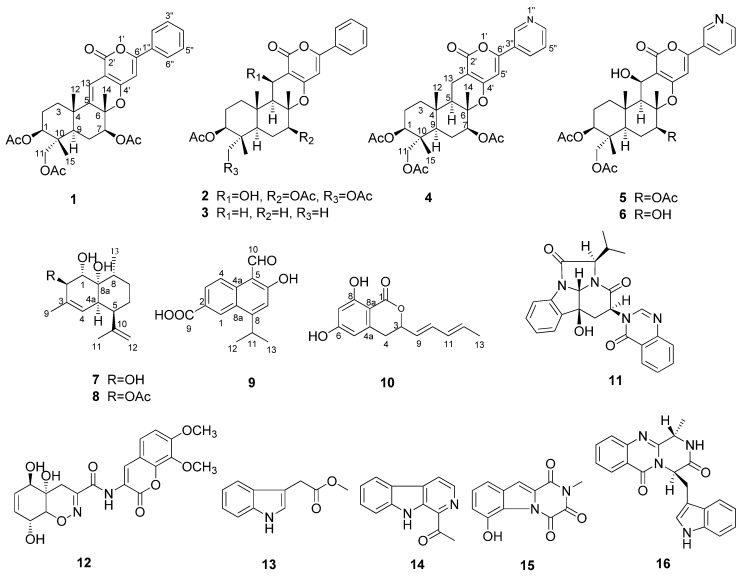
The marine fungus Neosartorya pseudofischeri was isolated from Acanthaster planci from the South China Sea. In a preliminary bioactivity screening, the crude methanol extract of the fungal mycelia showed significant inhibitory activity against the Sf9 cell line from the fall armyworm Spodoptera frugiperda. Five novel compounds, including 5-olefin phenylpyropene A (1), 13-dehydroxylpyripyropene A (4), deacetylsesquiterpene (7), 5-formyl-6-hydroxy-8-isopropyl-2- naphthoic acid (9) and 6,8-dihydroxy-3-((1E,3E)-penta-1,3-dien-1-yl)isochroman-1-one (10), together with eleven known compounds, phenylpyropene A (2) and C (3), pyripyropene A (5), 7-deacetylpyripyropene A (6), (1S,2R,4aR,5R,8R,8aR)-1,8a-dihydroxy-2-acetoxy-3,8-dimethyl-5- (prop-1-en-2-yl)-1,2,4a, 5,6,7,8,8a-octahydronaphthalene (8), isochaetominine C (11), trichodermamide A (12), indolyl-3-acetic acid methyl ester (13), 1-acetyl-β-carboline (14), 1,2,3,4-tetrahydro-6-hydroxyl-2-methyl-l,3,4-trioxopyrazino[l,2-a]-indole (15) and fumiquinazoline F (16), were obtained. The structures of these compounds were determined mainly by MS and NMR data. The absolute configuration of 9 was assigned by the single-crystal X-ray diffraction studies. Compounds 1–11 and 15 showed significant cytotoxicity against the Sf9 cells from S. frugiperda.
Keywords: marine fungus, Neosartorya pseudofischeri, phenylpyropene, pyripyropene, sesquiterpene, cytotoxic activity
1. Introduction
The fall armyworm Spodoptera frugiperda is a pest feeding on the gramineous plants occurring worldwide and causing serious damage to several economically-important crops, such as the maize, sorghum, sugarcane, as well as the cotton, cruciferous and Cucurbitaceae plants. Although other alternative methods have been investigated on the biological control, as well as the development of resistant transgenic plants, the control of S. frugiperda still relies mainly on the use of chemical pesticides, easily inducing resistance and causing severe damage to the environment and consumers. However, some natural products with higher selectivity and safety become an important potential source of new biopesticides for the control of S. frugiperda [1,2,3,4,5]. Sf9 cells are clonal isolates of S. frugiperda Sf21 cells (IPLB-SF21-AE) [6]. A cultured Sf9 cell line is commonly used for primary screening to determine insecticidal activity [7] and in biomedical research for the purpose of recombinant protein expression using insect-specific viruses called baculoviruses [8].
In recent years, we conducted research on the metabolites of marine fungi and obtained a series of novel and/or bioactive metabolites [9,10,11,12,13]. The marine fungus Neosartorya pseudofischeri was isolated from the inner tissue of a starfish Acanthaster planci collected from the South China Sea. In the previous metabolites study, we obtained three novel compounds, neosartins A–C, as well as a series of known gliotoxin analogues and diketopiperazines with potent antitumor and antibacterial activities from the culture broth extract of GlyPY (glycerol-peptone-yeast extract) and GluPY (glucose-peptone-yeast extract) media [14]. During our insecticidal activity screening against the fall armyworm S. frugiperda, we found that the crude methanol extract of the mycelia of N. pseudofischeri cultivated in GluPY showed a 65% cell growth inhibition rate against the Sf9 cell line from S. frugiperda at a concentration of 50 mg/L. The bioactivity-guided metabolites isolation afforded five novel compounds, including 5-olefin phenylpyropene A (1), 13-dehydroxyl pyripyropene A (4), deacetylsesquiterpene (7), 5-formyl-6-hydroxy-8-isopropyl-2-naphthoic acid (9) and 6,8-dihydroxy-3-((1E,3E)-penta-1,3-dien-1-yl)isochroman-1-one (10), together with eleven known compounds, phenylpyropene A (2) and C (3), pyripyropene A (5), 7-deacetylpyripyropene A (6), (1S,2R,4aR,5R,8R,8aR)-1,8a-dihydroxy-2-acetoxy-3,8-dimethyl-5-(prop-1-en-2-yl)-1,2,4a,5,6,7,8,8a-octahydronaphthalene (8), isochaetominine C (11), trichodermamide A (12), indolyl-3-acetic acid methyl ester (13), 1-acetyl-β-carboline (14), 1,2,3,4-tetrahydro-6-hydroxyl-2-methyl-l,3,4- trioxopyrazino[l,2-a]-indole (15) and fumiquinazoline F (16) (Figure 1). Compounds 1–11 and 15 showed significant cytotoxicity against the Sf9 cells from S. frugiperda. Herein, we report the isolation, structure elucidation and the bioactivity of these compounds.
Figure 1.
Structures of Compounds 1–16.
2. Results and Discussion
2.1. Structural Elucidation
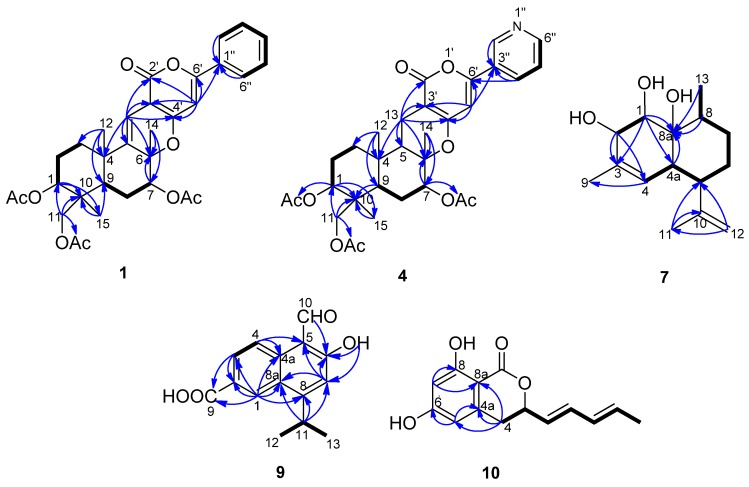
5-Olefin phenylpyropene A (1) was isolated as a yellowish oil. The molecular formula was established as C32H36O9 based on the analysis of 13C-NMR data and HR(+)ESIMS m/z 565.2448 [M + H]+ (calcd. for C32H36O9, 565.2432), implying fifteen degrees of unsaturation (Supplementary Figure S1). The IR spectrum indicated the presence of a carbonyl group (1680 cm−1) and a benzene ring (3073, 1574 and 1507 cm−1). UV maxima at 232, 278 and 322 nm supported the long conjugated system containing a benzene ring. The 13C-NMR and DEPT spectra displayed six methyls, four methylenes, ten methines and twelve quaternary carbons. The diagnostic aryl protons at δH 7.81 (m, 2H) and 7.44 (m, 3H) revealed a monosubstituted benzene ring. Two alkenyl protons at δH 6.46 (s) and 6.36 (s) indicated there were two trisubstituted double bonds in the molecule. Three methyl group singlets at δH 2.16, 2.10 and 2.04 showed HMBC correlations with the carbonyl groups at δC 169.9, 171.1 and 170.4, respectively, confirming there are three acyloxy groups. The other three methyl group singlets at δH 1.57, 1.25 and 0.87 are connected with the quaternary carbons at δC 21.2, 24.2 and 13.3, respectively. In the 1H–1H COSY spectrum, the cross peaks of H-1/H-2, H-2/H-3, H-7/H-8, H-8/H-9, H-2′′(H-6′′)/H-3′′(H-5′′) and H-3′′(H-5′′)/H-4′′ revealed the partial structure of –CHCH2CH2–, –CHCH2CH– and –CHCHCHCHCH– in the molecule (Figure 2). The key HMBC correlations of H-1/C-10, H-11/C-10, H-15/C-10, H-12/C-3, H-12/C-4, H-12/C-5, H-13/C-4, H-13/C-5, H-13/C-6, H-7/C-6, H-14/C-6, H-12/C-9 and H-15/C-9 deduced the sesquiterpene moiety. The cross peaks of H-13 to C-2′, C-3′ and C-4′, H-5′ to C-4′, C-6′ and C-1′′ in the HMBC spectrum confirmed the connectivity of the sesquiterpene fragment, α-pyrone and the monosubstituted phenyl ring (Table 1 and Supplementary Figures S2–S7).
Figure 2.
1H–1H COSY (bold lines) and the main HMBC (arrows) correlations of Compounds 1, 4, 7, 9 and 10.
Table 1.
1H and 13C-NMR data of 1, 4 and 6 at 400/100 MHz, respectively, δ in ppm.
| Position | 1 a | 4 a | 6 b | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 73.3, CH | 4.79, dd (11.6, 4.4) | 73.5, CH | 4.79, dd (11.6, 4.8) | 74.4, CH | 4.79, dd (11.2, 5.6) |
| 2 | 23.2, CH2 | 1.98, m; 1.75, m | 22.8, CH2 | 1.88, m; 1.70, m | 23.6, CH2 | 1.84, m; 1.88, m |
| 3 | 35.4, CH2 | 2.08, m; 1.62, m | 36.6, CH2 | 1.83, m; 1.20, ddd (13.2, 13.2, 3.2) | 36.8, CH2 | 1.43, td (12.8, 4.8); 2.15, td (12.8, 4.8) |
| 4 | 38.7, C | 36.6, C | 38.8, C | |||
| 5 | 143.7, C | 50.3, CH | 1.60, dd (12.4, 4.8) | 55.0, CH | 1.66, d (3.6) | |
| 6 | 83.5, C | 81.9, C | 86.2, C | |||
| 7 | 77.7, CH | 5.22, d (12.0, 5.2) | 77.5, CH | 5.02, dd (11.6, 4.8) | 77.6, CH | 4.97, dd (12.4, 5.2) |
| 8 | 24.3, CH2 | 1.81, m; 1.63, m | 24.9, CH2 | 1.78, m; 1.50, m | 29.0, CH2 | 1.60, d (12.4); 1.82, m |
| 9 | 41.0, CH | 1.73, m | 45.1, CH | 1.66, d (12.0) | 46.2, CH | 1.53, d (3.6) |
| 10 | 40.5, C | 40.2, C | 41.3, C | |||
| 11 | 64.6, CH2 | 3.78, d (12.0); 3.74, d (12.0) |
64.7, CH2 | 3.78, d (12.0); 3.73, d (12.0) |
64.6, CH2 | 3.83, d (11.6); 3.77, d (11.6) |
| 12 | 24.2, CH3 | 1.25, s | 15.5, CH3 | 1.00, s | 17.9, CH3 | 1.48, s |
| 13 | 111.4, C | 6.36, s | 16.9, CH2 | 2.57, dd (17.2, 4.8); 2.33, dd (17.2, 12.4) |
60.6, CH | 4.95, d (3.6) |
| 14 | 21.2, CH3 | 1.57, s | 15.4, CH3 | 1.32, s | 15.9, CH3 | 1.76, s |
| 15 | 13.3, CH3 | 0.87, s | 13.3, CH3 | 0.86, s | 13.3, CH3 | 0.92, s |
| 2′ | 162.1, C | 163.7, C | 163.5, C | |||
| 3′ | 100.3, C | 99.8, C | 100.1, C | |||
| 4′ | 160.2, C | 162.2, C | 157.9, C | |||
| 5′ | 97.4, CH | 6.46, s | 99.2, CH | 6.43, s | 147.6, CH | |
| 6′ | 154.9, C | 155.9, C | 128.4, C | |||
| 1′′ | 131.0, C | |||||
| 2′′ | 125.6, CH | 7.81, m | 146.7, CH | 8.99, s | 147.6, CH | 9.07, dd (2.4, 0.8) |
| 3′′ | 128.9, CH | 7.44, m | 127.3, C | 127.5, C | ||
| 4′′ | 131.0, CH | 7.44, m | 132.8, CH | 8.09, brd (8.0) | 133.6, CH | 8.22, ddd (8.4, 2.4, 1.6) |
| 5′′ | 128.9, CH | 7.44, m | 123.6, CH | 7.39, dd (8.0, 4.8) | 124.6, CH | 7.53, ddd (8.4, 4.8, 0.8) |
| 6′′ | 125.6, CH | 7.81, m | 151.2, CH | 8.66, d (4.8) | 152.3, CH | 8.69, dd (4.8, 1.6) |
| 1-OCOCH3 | 21.1, CH3 | 2.04, s | 21.1, CH3 | 2.04, s | 21.0, CH3 | 2.00, s |
| 1-OCOCH3 | 170.0, C | 170.5, C | 170.6, C | |||
| 7-OCOCH3 | 21.1, CH3 | 2.16, s | 21.3, CH3 | 2.15, s | ||
| 7-OCOCH3 | 169.9, C | 170.1, C | ||||
| 11-OCOCH3 | 20.8, CH3 | 2.10, s | 20.8, CH3 | 2.11, s | 20.7, CH3 | 1.99, s |
| 11-OCOCH3 | 171.1, C | 171.0, C | 170.8, C | |||
| 13-OH | 4.28, brs | |||||
| 7-OH | 4.10, brs | |||||
a 1H and 13C-NMR data were measured in CDCl3; b 1H and 13C-NMR data were measured in acetone-d6.
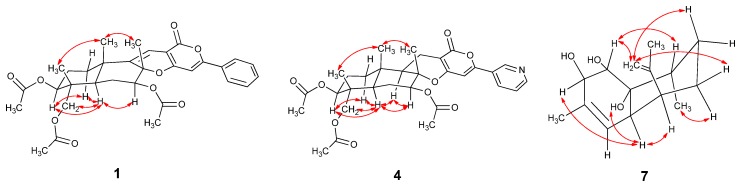
The relative configuration of 1 was determined by the analysis of the NOESY spectrum. The NOE correlations between H-12/H-14 and H-12/H-15, H-1/H-9, H-7/H-9 and H-7/H-11 were observed. Consequently, H-12, H-14 and H-15 are β-oriented, while H-1, H-7 and H-9 are α-oriented (Figure 3).
Figure 3.
Key NOESY correlations of Compounds 1, 4 and 7.
Compounds 2 and 3 were identified as phenylpyripyropene A and C [15,16], by comparing their NMR data to the reference values. The double bond between the C-5 and C-13 positions in 1 was replaced by the saturated carbon-carbon bond in 2. Compounds 1 and 2 have triacyloxy groups at the C-1, C-7 and C-11 positions; however, Compound 3 has only one acyloxy group at the C-1 position (Supplementary Figures S8–S11).
13-Dehydroxylpyripyropene A (4) was obtained as a white powder. It showed a molecular formula of C31H37NO9 determined by the 13C-NMR data and HR(+)ESIMS peak at m/z 568.2549 [M + H]+ (calcd. for C31H37NO9, 568.2541) (Supplementary Figure S12). The 13C-NMR and DEPT spectra (Table 1) displayed six methyls, five methylenes, nine methines and eleven quaternary carbons. By comparing the NMR data with pyripyropene A (5) (Supplementary Figures S13–S20) [17], a quick identification was made revealing that C-13 of 4 was a methylene (δC 16.9, δH 2.57, 2.33), corresponding to one methine group (δC 60.1, δH 4.99) connected to one hydroxyl group in pyripyropene A (Figure 2). The relative configuration of 4 was determined on the basis of NOESY data. The NOE correlations of H-12/H-15, H-14/H-15 supported these methyl groups on the β-position of the ring system; while those correlations of H-1/H-9, H-5/H-7, H-5/H-9, H-7/H-9 and H-9/H-11 were used to place these protons on the α-position (Figure 3).
Compound 6 was elucidated as 7-deacetyl pyripyropene A (Supplementary Figures S21 and S22). 6 was once obtained by hydrolysis of pyripyropene A with 1,8-diazabicyclo[5,4,0]undec-7-ene (DBU) in 80% methanol [18]. However, this is the first report including the detailed NMR data.
Deacetylsesquiterpene (7) was isolated as a yellowish oil. The molecular formula was determined as C15H24O3 from the 13C-NMR data and the HR(+)ESIMS peak at m/z 275.1603 [M + Na]+ (calcd. for C15H24O3Na, 275.1618), indicating four degrees of unsaturation (Supplementary Figure S23). The strong IR absorption at 3443 cm−1 indicated the presence of the hydroxyl groups. The 13C-NMR and DEPT spectra displayed three methyls, three methylenes, six methines and three quaternary carbons (Table 2). The quaternary carbon (δC 132.3) and the methine (δC 124.4, δH 5.28) formed a trisubstituted double bond. The quaternary carbon (δC 147.2) and the methylene (δC 111.0, δH 4.94 and 4.72) constructed a terminal double bond. Thus, Compound 7 must be bicyclic to account for the four double bond equivalents required by the molecular formula. The 1H–1H COSY cross peaks of H-1/H-2, H-4/H-4a, H-4a/H-5, H-5/H-6, H-6/H-7, H-7/H-8 and H-8/H-13 established two partial structures, –CHCH– and –CHCHCHCH2CH2CHCH3 (Figure 2). The HMBC correlations of H-1/C-8, H-2/C-3, H-5/C-10, H-8/C-8a, H-9/C-3 and H-11/C-10 established the skeleton; three hydroxyl groups were attached to C-1 (δC 74.6), C-2 (δC 74.2) and C-8a (δC 73.8). The NOESY correlations of H-1/H-12, H-7a/H-12, H-6e/H-12, H-2/H-4a, H-4a/H-5, H-4a/8a-OH and H-6a/H-13 revealing 1-OH, 8a-OH, H-5 and H-13 were α-oriented; however, 2-OH was β-oriented (Figure 3 and Supplementary Figures S24–S29).
Table 2.
1H and 13C-NMR data of 7, 9 and 10, δ in ppm.
| Position | 7 a | 9 b | 10 c | |||
|---|---|---|---|---|---|---|
| δC, Type | δH, Mult., (J in Hz) | δC, Type | δH, Mult., (J in Hz) | δC, Type | δH, Mult., (J in Hz) | |
| 1 | 74.6, CH | 3.98, d (1.2) | 127.9, CH | 8.92, d (1.5) | 169.1, C | |
| 2 | 74.2, CH | 4.05, s | 137.4, C | |||
| 3 | 132.3, C | 129.2, CH | 8.22, dd (9.0,1.5) | 78.4, CH | 5.13, ddd (10.0, 6.8, 4.0) | |
| 4 | 124.4, CH | 5.28, s | 121.6, CH | 8.77, d (9.0) | 32.5, CH2 | 3.00, dd (16.4, 4.0); 2.93, dd (16.4, 10.0) |
| 4a | 38.1, CH | 2.67, s | 127.3, C | 141.7, C | ||
| 5 | 41.4, CH | 2.38, brd (12.0) | 111.4, C | 107.1, CH | 6.24, d (2.0) | |
| 6 | 25.9, CH2 | 1.56, m; 1.33, m | 167.1, C | 163.4, C | ||
| 7 | 30.7, CH2 | 1.48, m; 1.40, m | 117.0, CH | 7.21, s | 101.0, CH | 6.18, d (2.0) |
| 8 | 31.2, CH | 1.99, m | 159.5, C | 164.8, C | ||
| 8a | 73.8, C | 126.3, C | 100.1, C | |||
| 9 | 20.1, CH3 | 1.78, s | 167.6, C | 127.0, CH | 5.71, dd (15.2, 6.8) | |
| 10 | 147.2, C | 195.3, CH | 11.42, brs | 133.3, CH | 6.35, dd (15.2, 10.0) | |
| 11 | 22.4, CH3 | 1.74, brs | 30.1, CH | 3.90, heptet (7.0) | 130.4, CH | 6.10, ddd (15.2, 10.0, 1.2) |
| 12 | 111.0, CH2 | 4.94, q (1.2 ); 4.72, s | 23.4, CH3 | 1.45, d (7.0) | 131.7, CH | 5.81, dq (15.2, 6.8) |
| 13 | 15.0, CH3 | 0.93, d (6.8) | 23.4, CH3 | 1.45, d (7.0) | 18.0, CH3 | 1.74, dd (6.8, 1.2) |
| 1-OH | 2.00, s | |||||
| 2-OH | 2.00, s | |||||
| 2-OCOCH3 | ||||||
| 2-OCOCH3 | ||||||
| 6-OH | 13.42, s | 11.06, brs | ||||
| 8-OH | 11.06, brs | |||||
| 8a-OH | 2.00, s | |||||
| COOH | 10.94, brs | |||||
a 1H and 13C-NMR data were measured at 400/100 MHz, in CDCl3; b 1H and 13C-NMR data were measured at 500/125 MHz, in acetone-d6; c 1H and 13C-NMR data were measured at 400/100 MHz, in DMSO-d6.
The formula of Compound 8 was identified as C17H26O4, based on the analysis of 13C-NMR data and HREIMS peak at m/z 294.1828 [M]+ (calcd. for C17H26O4, 294.1826). The NMR spectroscopic data of Compound 8 were very similar to those of Compound 7, except that the C-2 hydroxyl group in 7 was replaced by an acetoxy group in 8 (Supplementary Figures S30 and S31). Fortunately, we obtained a single crystal of 8 from the MeOH solution. In the crystal, the adjacent molecules are interlinked by a pair of strong O1-H...O4 (hydroxyl) and O4-H...O3 (carbonyl) hydrogen bonds to form a zigzag chain running parallel to the b-axis. Neighboring chains of molecules are packed closely together with the hydrophobic methyl groups pointing towards each other. The absolute configuration of 8 was determined as 1S, 2R, 4aR, 5R, 8R, 8aR with the Flack parameter value −0.16(17) by single-crystal X-ray diffraction analysis (Figure 4 and Supplementary Crystallographic Information Framework (CIF)) using Cu Kα radiation. Compound 8, named (1S,2R,4aR,5R,8R,8aR)-1,8a-dihydroxy-2-acetoxy- 3,8-dimethyl-5-(prop-1-en-2-yl)-1,2,4a,5,6,7,8,8a-octahydronaphthalene, was previously obtained as the degradation product of the natural product CJ-12662, which was obtained from the fermentation broth of Aspergillus fischeri var. thermomutatus ATCC 18,618 [19] and later also isolated from fungi Neosartorya pseudofischeri and Eurotium chevalieri [20,21]. However, the single-crystal X-ray diffraction data were never reported.
Figure 4.
ORTEP (Oak Ridge Thermal Ellipsoid Plot) drawing of Compound 8.
Compound 9 was obtained as a white powder. Its molecular formula was deduced to be C15H14O4 by analysis of the 13C-NMR data and HR(-)ESIMS ion at m/z 257.0817 [M − H]− (calcd. for C15H13O4, 257.0819), which required nine degrees of unsaturation (Supplementary Figure S32). The IR spectrum revealed the presence of a carboxyl group (3283 and 1671 cm−1) and an aldehyde group (2812 cm−1). Two methyl groups at δH 1.45 (d, J = 7.0 Hz, 6H) showed 1H–1H COSY correlations with the methine group at δH 3.90 (seven peaks, J = 7.0 Hz) and formed an isopropyl fragment (Table 2, Figure 2 and Supplementary Figures S33–S36). According to the HMBC correlations of H-1/C-2, H-1/C-8a, H-3/C-2 and H-4/C-4a, the protons at δH 8.92 (d, J = 1.5 Hz, H-1), 8.22 (dd, J = 9.0, 1.5 Hz, H-3) and 8.77 (d, J = 9.0 Hz, H-4) were located at the 1,3,4-position on a phenyl ring. HMBC correlations of H-1/C-2, H-1/C-8a, H-3/C-2, H-4/C-4a, H-4/C-5, H-7/C-5, H-7/C-6, H-11/C-7 and H-11/C-8 indicated the existence of a naphthyl ring, accounting for seven degrees of unsaturation. The remaining carboxyl group (δC 167.6, δH 10.94), the aldehyde (δC 195.3, δH 11.42), phenolic hydroxyl group (δH 13.42, and the isopropyl fragment were located on C-2, C-5, C-6 and C-8, respectively, based on the HMBC cross peaks of H-1/C-9, H-3/C-9, H-10/C-6, 6-OH/C-6, 6-OH/C-7 and H-11/C-8. The aldehyde and hydroxyl groups formed an intramolecular hydrogen bond, which accounted for the downfield resonance of the phenolic hydroxyl group. Therefore, the structure of 9 was elucidated as 5-formyl-6-hydroxy-8-isopropyl-2-naphthoic acid.
Compound 10 was isolated as a white powder. The 13C-NMR data and HR(-)ESIMS peak at m/z 245.0815 [M − H]+ (calcd. for C14H13O4, 245.0819), established the molecular formula as C14H14O4 (Supplementary Figure S37). The 13C-NMR and DEPT spectra revealed fourteen carbon resonances, including one methyl group, one methylene group, seven methine groups and five quaternary carbons. The 1H-NMR spectrum exhibited two meta-aromatic protons at δH 6.24 (d, J = 2.0 Hz) and 6.18 (d, J = 2.0 Hz), indicating the presence of a tetrasubstituted aromatic ring. Two phenolic hydroxyl groups at δH 11.06 (brs) were connected to C-6 (δC 163.4) and C-8 (δC 164.8). Additionally, two pairs of trans-oriented olefinic protons at δH 5.71 (dd, J = 15.2, 6.8 Hz), 6.35 (dd, J = 15.2, 10.0 Hz), 6.10 (ddd, J = 15.2, 10.0, 1.2 Hz), 5.81 (dq, J = 15.2, 6.8 Hz), one oxymethine at δH 5.13 (ddd, J = 10.0, 6.8, 4.0 Hz), one methylene at δH 3.00 (dd, J = 16.4, 4.0 Hz), 2.93 (dd, J = 16.4, 10.0 Hz) and one methyl group at δH 1.74 (dd, J = 6.8, 1.2 Hz) were connected in sequence and formed a pentadienyl group based on their 1H–1H COSY correlations. The remnant quaternary carbon (δC 169.1) connected with C-8a (δC 100.1) and the oxymethine and formed a lactone. The HMBC correlations of H-4/C-4a, H-4/C-8a, H-5/C-6, H-7/C-8 and H-7/C-8a revealed a 3,6,8-trisubstituted isocoumarin ring system (Table 2, Figure 2 and Supplementary Figures S38–S43). The absolute configuration at C-3 remains undetermined. Therefore, Compound 10 was elucidated as 6,8-dihydroxy-3-((1E,3E)-penta-1,3- dien-1-yl)isochroman-1-one.
The other known compounds, isochaetominine C (11) [22], trichodermamide A (12) [23], indolyl-3-acetic acid methyl ester (13) [24], 1-acetyl-β-carboline (14) [25], 1,2,3,4-tetrahydro- 6-hydroxy-2-methyl-l,3,4-trioxopyrazino[l,2-a]-indole (15) [26] and fumiquinazoline F (16) [27], were identified by comparing their spectroscopic data with the literature values (Supplementary Figures S44–S55).
2.2. Proposed Biosynthetic Pathways
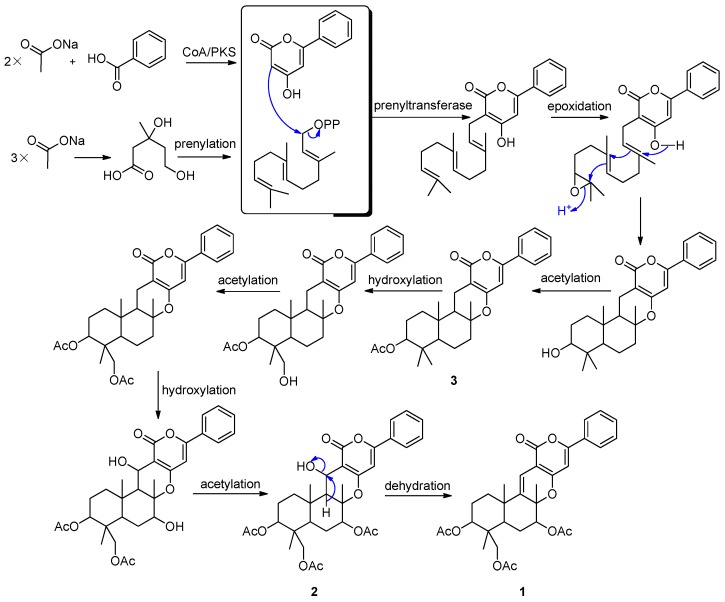
Tomoda et al. described the biosynthetic origin of pyripyropene A (5), which was established by feeding experiments using various [13C] and [14C] precursors. Pyripyropene A (5) is derived from three mevalonates, five acetates and one nicotinic acid. The pyridino-α-pyrone moiety is produced via condensation of a primer nicotinic acid with two acetates in a “head-to-tail” fashion; an all-trans farnesyl pyrophosphate is produced via the mevalonate pathway; the two parts are linked and cyclized to form the core skeleton, and then three acetyl residues from the acetates are introduced into the skeleton to yield 5 [28]. Based on the above finding, we proposed that the phenyl-α-pyrones (1–3) shared a similar biosynthetic pathway (Figure 5). Benzoic acid, instead of nicotinic acid, is one of the precursors. It is very interesting that Compound 2 was observed slowly changing into Compound 1 when stored in DMSO-d6. The change was recorded by 1H-NMR spectra. After 18 days, Compound 2 was changed into Compound 1 thoroughly. However, the pure Compounds 1 and 2 are stable at the temperature range from −20 °C–90 °C.
Figure 5.
Proposed biosynthetic pathways of 1–3.
2.3. Cytotoxicity
Compounds 1–11 and 15 showed significant cytotoxicity against the Sf9 cells (Table 3). After 48 h of treatment at the concentration of 50 mg/L, the cell growth inhibition rates of compounds 2, 3, 7–9, 11 and 15 were greater than 90%. Therefore, further in-depth studies to identify the insecticidal activities in vivo and the mechanism are warranted for their development as biorational pesticides.
Table 3.
The death rate (%) of Compounds 1–11 and 15 against insect cell line Sf9 (n = 3, p ≤ 0.05).
| Compounds | 6 h | 12 h | 24 h | 48 h |
|---|---|---|---|---|
| 1 | 65.79 | 68.26 | 76.16 | 85.24 |
| 2 | 60.75 | 68.86 | 81.21 | 95.01 |
| 3 | 72.55 | 72.15 | 73.08 | 93.73 |
| 4 | 47.53 | 57.41 | 73.21 | 85.37 |
| 5 | 41.05 | 54.60 | 58.04 | 71.77 |
| 6 | 25.58 | 29.46 | 35.30 | 56.03 |
| 7 | 79.58 | 81.89 | 90.46 | 98.68 |
| 8 | 64.52 | 69.33 | 77.98 | 91.87 |
| 9 | 57.96 | 65.32 | 69.92 | 90.97 |
| 10 | 36.47 | 38.25 | 52.36 | 61.67 |
| 11 | 63.70 | 69.16 | 71.65 | 94.53 |
| 15 | 56.97 | 61.43 | 80.33 | 92.52 |
| Rotenone | 69.21 | 79.43 | 90.93 | 98.54 |
3. Experimental Section
3.1. General Experimental Procedures
Preparative HPLC was performed using a Shimadzu LC-20AT HPLC pump (Shimadzu Corporation, Nakagyo-ku, Kyoto, Japan) equipped with an SPD-20A dual λ absorbance detector (Shimadzu Corporation, Nakagyo-ku, Kyoto, Japan) and a Shim-pack PRC-ODS HPLC column (250 mm × 20 mm, Shimadzu Corporation, Nakagyo-ku, Kyoto, Japan). Optical rotations were measured using a Schmidt and Haensch Polartronic HNQW5 optical rotation spectrometer (SCHMIDT + HAENSCH GmbH & Co., Berlin, Germany). UV spectra were recorded on a Shimadzu UV-VIS-NIR spectrophotometer (Shimadzu Corporation, Nakagyo-ku, Kyoto, Japan). IR spectra were recorded on a PerkinElmer Frontier FT-IR spectrophotometer (PerkinElmer Inc., Waltham, MA, USA). 1D and 2D NMR spectra were recorded on Bruker Avance II 400 spectrometers (Bruker BioSpin AG, Industriestrasse 26, Fällanden, Switzerland) and a Varian INOVA500NB spectrometer (Varian Medical Systems In., Salt Lake City, UT, USA). The chemical shifts are relative to the residual solvent signals (CDCl3: δH 7.26 and δC 77.0; acetone-d6: δH 2.05 and δC 29.92; DMSO-d6: δH 2.50 and δC 39.51). The low- and high-resolution EI mass spectra were obtained on Thermo DSQ and Thermo MAT95XP mass spectrometers (Thermo Fisher Scientific, Waltham, MA, USA), respectively. The low- and high-resolution ESI-MS analyses were performed with a Thermo LCQ DECA XP liquid chromatography-mass spectrometry (Thermo Fisher Scientific, Waltham, MA, USA) and a Thermo Fisher LTQ Orbitrap Elite High Resolution liquid chromatography-mass spectrometry (Thermo Fisher Scientific, Waltham, MA, USA). X-ray diffraction data were acquired on a Bruker SMART APEX CCD X-ray single-crystal diffractometer (Bruker AXS GmbH, Karlsruhe, Germany).
3.2. Fungal Material
The marine fungus N. pseudofischeri was isolated from the inner tissue of the starfish A. planci collected from the Hainan Sanya National Coral Reef Reserve, China. The fungus was identified on the basis of the DNA sequences of the ITS1-5.8S-ITS2 (Internal Transcribed Spacer, ITS) regions of their rRNA gene. The ITS gene sequence showed 100% homology with that of the fungus N. pseudofischeri in GenBank (Accession Number KF999816).
3.3. Fermentation, Extraction and Isolation
The 20 L scale-up fermentation of N. pseudofischeri was carried out in 1000 mL Erlenmeyer flasks containing 500 mL liquid culture media, which was composed of glucose 10 g, peptone 5 g, yeast extract 2 g, CaCO3 1 g and sea water 1 L. After 30 days of growth, the mycelia of N. pseudofischeri were extracted with methanol, and the organic solvent was evaporated to dryness under vacuum to afford the crude extract (5.36 g). Then, the extract was separated by flash silica gel column chromatography using petroleum ether-ethyl acetate in a gradient elution (100:0–0:100, v/v), followed by ethyl acetate-methanol in a gradient elution (100:0–0:100, v/v) to give 10 fractions (F1–F10). Subfractions F4.1–F4.6 were obtained from F4 (610 mg) by reverse phase silica gel using methanol-water (30:70–100:0, v/v) gradient elution. Then, F4.3 (44 mg) was further separated by RP-HPLC using methanol-water (65:35, v/v) as the eluent to obtain Compounds 1 (6.2 mg), 4 (4.1 mg) and 5 (3.4 mg). F4.4 (32 mg) was further separated by RP-HPLC eluted with methanol-water (68:32, v/v) to obtain Compounds 2 (10.1 mg), 3 (3.5 mg), 6 (1.7 mg) and 12 (2.4 mg). F5 (157 mg) was further separated by RP-HPLC eluted with methanol-water (70:30, v/v) to obtain Compound 8 (32.6 mg). F6 (113 mg) was further separated by RP-HPLC eluted with methanol-water (60:40, v/v) to obtain 7 (12.1 mg) and 9 (2.8 mg). F7 (201 mg) was separated by RP-HPLC eluted with MeCN-water (60:40, v/v) to get Compounds 10 (4.5 mg), 11 (3.1 mg) and 16 (2.3 mg). F8 (97 mg) was further separated by RP-HPLC eluted with MeCN-water (50:50, v/v) to obtain Compounds 13 (2.0 mg), 14 (2.9 mg) and 15 (23.2 mg).
5-Olefin phenylpyropene A (1): yellowish oil; : + 129° (c = 0.1, CHCl3); UV (MeOH) λmax (ɛ) 234 (13,810), 322 (10,108) nm; IR νmax 2960, 2925, 1735, 1602, 1566, 1451, 1378, 1241, 1043, 758 cm−1; for 1H and 13C-NMR data, see Table 1; LR(+)ESIMS m/z 565.3 [M + H]+. HR(+)ESIMS m/z 565.2448 [M + H]+ (calcd. for C32H37O9, 565.2432).
13-Dehydroxylpyripyropene A (4): white powder; : +20° (c = 0.1, CHCl3); white powder; UV (MeOH) λmax (ɛ) 216 (18,120), 246 (12,977), 278 (4038) nm; IR νmax 2960, 2921, 2851, 1713, 1464, 1377, 1260, 1035, 810, 721 cm−1. For 1H and 13C-NMR data, see Table 1; LR(+)ESIMS m/z 568.2 [M + H]+. HR(+)ESIMS m/z 568.2549 [M + H]+ (calcd. for C31H38NO9, 568.2541).
7-deacetylpyripyropene A (6): white powder; for 1H and 13C-NMR data, see Table 1.
Deacetylsesquiterpene (7): yellowish oil; : +38° (c = 0.058, MeOH); UV (MeOH) λmax (ɛ) 212 (136) nm; IR νmax 3443, 2959, 2926, 2856 1721, 1675, 1609, 1455, 1376, 1260, 1095, 1029, 992, 887, 756 cm−1. For 1H and 13C-NMR data, see Table 2; HR(+)ESIMS: m/z 275.1603 [M + Na]+ (calcd. for C15H24O3Na, 275.1618).
(1S,2R,4aR,5R,8R,8aR)-1,8a-dihydroxy-2-acetoxy-3,8-dimethyl-5-(prop-1-en-2-yl)-1,2,4a,5,6,7,8, 8a-octahydronaphthalene (8): white crystal. : −68° (c = 0.079, MeOH); IR (MeOH) νmax 3677, 3436, 3317, 2928, 2862, 1731, 1642, 1444, 1405, 1369, 1238, 1030, 967, 945, 927, 885, 720, 609 cm−1. For 1H and 13C-NMR spectra, see Supplementary Figures S30 and S31; HREIMS: m/z 294.1828 [M]+ (calcd. for C17H26O4, 294.1826). The crystal of 8 was obtained from MeOH solution. C17H26O4, M = 294.38, colorless block, Orthorhombic system, P 21 21 21 (19), a = 8.3364 (1), b = 9.3709 (2), c = 21.3150 (5) Å. V = 1665.12 (6) Å3, Z = 4. Dcalcd = 1.174 g/cm3, crystal size 0.41 mm × 0.45 mm × 0.43 mm, F(000) = 640, T = 173(2) K.
5-formyl-6-hydroxy-8-isopropyl-2-naphthoic acid (9): white powder; IR νmax 3283, 3020, 2953, 2928, 2867, 2812, 2720, 1671, 1616, 1601, 1512, 1458, 1422, 1393, 1289, 1026, 1208, 1148, 1109, 1080, 1008, 985, 921, 796 cm−1; for 1H and 13C-NMR data, see Table 2; HR(-)ESIMS m/z 257.0817 [M − H]− (calcd. for C15H13O4, 257.0819).
6,8-dihydroxy-3-((1E,3E)-penta-1,3-dien-1-yl)isochroman-1-one (10): white powder; : +40° (c = 0.1, MeOH); UV (MeOH) λmax (ɛ) 232 (6312), 268 (3427), 302 (2060) nm; IR νmax 3528, 3400, 2953, 2926, 2855, 1656, 1622, 1460, 1374, 1247, 1110, 1003, 772 cm−1. For 1H and 13C-NMR data, see Table 2; HR(-)ESIMS m/z 245.0815 [M − H]− (calcd. for C14H13O4, 245.0819).
3.4. Bioassay
To evaluate the biological activities of these compounds, cytotoxic assays were carried out with insect cultured cell line Sf9 from S. frugiperda. Sf9 cells were maintained at 27 °C in TC-199-MK medium supplemented with 10% FCS (v/v), 1% L-glutamine 200 mmol/L and penicillin-streptomycin-neomycin solutions (v/v). The cell growth inhibition was measured by the MTT method. Cells were seeded in 96-well microtitration plates at the exponential growth phase. Different concentrations of compounds diluted with medium were added at their log-phase growth stage. Additionally, 0.2 μL of solvent (DMSO) only was added as the control (CK). The final concentration of solvent in the cultures assayed was 1%. Compounds were solubilized with DMSO at concentrations of 10 and 50 mg/L. The rotenone was used as the positive control. After 6 h, 12 h, 24 h and 48 h of treatment, 5 mg/mL MTT were dissolved in PBS, and 20 μL of this stock solution were added to the culture cells. After an additional 3 h of incubation, the medium was discarded, and the 96-well plates were dried in the air. Then, 100 μL of DMSO were added to dissolve the formazan crystals, and the absorbance was measured at 570 nm by a microplate reader (Spectramex, 190 Molecular Devices Inc., Sunnyvale, CA, USA).
The cytotoxic effect was expressed as the relative percentage of inhibition calculated as follows: cell growth inhibition rate (%) = [(A control − A treatment)/A control] × 100 [29].
4. Conclusions
In summary, sixteen compounds, including five new compounds (1, 4, 7, 9 and 10), were obtained from the mycelium of marine fungus N. pseudofischeri. Their structure types include phenylpyripyropenes, pyripyropenes, sesquiterpenoids, isocoumarin and alkaloids. Our findings provide further evidence that the marine fungus N. pseudofischeri has the tremendous potential of biosynthesis. The potent cytotoxicity of these compounds against the Sf9 cells revealed the prospect to develop biorational pesticides.
Acknowledgments
This project is financially supported by Guangdong Provincial Science and Technology Research Program (Nos. 2013B021100010, 2013B021100012, 2014A020217004 and 2015A020216007), Guangzhou Science and Technology Research Program (No. 2014J4100059), the Fundamental Research Funds for the Central Universities (Nos. 15ykpy05 and 14yksh01), the instrumental analysis fund of Sun Yat-sen University (No. 0504015080000) and the special financial fund of innovative development of marine economic demonstration project GD2012-D01-001.
Supplementary Files
Author Contributions
Conceived of and designed the experiments: Wen-Jian Lan, Hou-Jin Li. Performed the experiments: Wen-Jian Lan, Sheng-Jiao Fu, Meng-Yang Xu, Wan-Ling Liang, Chi-Keung Lam, Guo-Hua Zhong, Jun Xu, De-Po Yang. Wrote the paper: Wen-Jian Lan, Hou-Jin Li.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ansante T.F., Ribeiro L.D., Bicalho K.U., Fernandes J.B., da Silva M.F.D.F., Vieira P.C., Vendramim J.D. Secondary metabolites from Neotropical Annonaceae: Screening, bioguided fractionation, and toxicity to Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) Ind. Crops Prod. 2015;74:969–976. doi: 10.1016/j.indcrop.2015.05.058. [DOI] [Google Scholar]
- 2.Munoz E., Escalona D., Salazar J.R., Alarcon J., Cespedes C.L. Insect growth regulatory effects by diterpenes from Calceolaria talcana Grau & Ehrhart (Calceolariaceae: Scrophulariaceae) against Spodoptera frugiperda and Drosophila melanogaster. Ind. Crop. Prod. 2013;45:283–292. [Google Scholar]
- 3.Devappa R.K., Angulo-Escalante M.A., Makkar H.P.S., Becker K. Potential of using phorbol esters as an insecticide against Spodoptera frugiperda. Ind. Crop. Prod. 2012;38:50–53. doi: 10.1016/j.indcrop.2012.01.009. [DOI] [Google Scholar]
- 4.Sarria A.L.F., Soares M.S., Matos A.P., Fernandes J.B., Vieira P.C., da Silva M.F.G.F. Effect of triterpenoids and limonoids isolated from Cabralea canjerana and Carapa guianensis (Meliaceae) against Spodoptera frugiperda (J.E. Smith) Z. Naturforschung C. 2011;66:245–250. doi: 10.5560/ZNC.2011.66c0245. [DOI] [PubMed] [Google Scholar]
- 5.Nihei K.I., Asaka Y., Mine Y., Ito C., Furukawa H., Motoharu J.I., Kubo I. Insect antifeedants from tropical plants: Structures of dumnin and dumsenin. J. Agric. Food Chem. 2004;52:3325–3328. doi: 10.1021/jf049819c. [DOI] [PubMed] [Google Scholar]
- 6.Vaughn J.L., Goodwin R.H., Tompkins G.J., McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae) In Vitro. 1977;13:213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- 7.Zeng Y., Zhang Y.M., Weng Q.F., Hu M.Y., Zhong G.H. Cytotoxic and insecticidal activities of derivatives of harmine, a natural insecticidal component isolated from Peganum harmala. Molecules. 2010;15:7775–7791. doi: 10.3390/molecules15117775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider E.H., Seifert R. Sf9 cells: A versatile model system to investigate the pharmacological properties of G protein-coupled receptors. Pharmacol. Ther. 2010;128:387–418. doi: 10.1016/j.pharmthera.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Li H.J., Jiang W.H., Liang W.L., Huang J.X., Mo Y.F., Ding Y.Q., Lam C.K., Qian X.J., Zhu X.F., Lan W.J. Induced marine fungus Chondrostereum sp. as a means of producing new sesquiterpenoids chondrosterins I and J by using glycerol as the carbon source. Mar. Drugs. 2014;12:167–175. doi: 10.3390/md12010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan W.J., Liu W., Liang W.L., Xu Z., Le X., Xu J., Lam C.K., Yang D.P., Li H.J., Wang L.Y. Pseudaboydins A and B: Novel isobenzofuranone derivatives from marine fungus Pseudallescheria boydii associated with starfish Acanthaster planci. Mar. Drugs. 2014;12:4188–4199. doi: 10.3390/md12074188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H.J., Chen T., Xie Y.L., Chen W.D., Zhu X.F., Lan W.J. Isolation and structural elucidation of chondrosterins F–H from the marine fungus Chondrostereum sp. Mar. Drugs. 2013;11:551–558. doi: 10.3390/md11020551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H.J., Xie Y.L., Xie Z.L., Chen Y., Lam C.K., Lan W.J. Chondrosterins A–E, triquinane-type sesquiterpenoids from soft coral-associated fungus Chondrostereum sp. Mar. Drugs. 2012;10:627–638. doi: 10.3390/md10030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H.J., Lan W.J., Lam C.K., Yang F., Zhu X.F. Hirsutane sesquiterpenoids from the marine-derived fungus Chondrostereum sp. Chem. Biodivers. 2011;8:317–324. doi: 10.1002/cbdv.201000036. [DOI] [PubMed] [Google Scholar]
- 14.Liang W.L., Le X., Li H.J., Yang X.L., Chen J.X., Xu J., Liu H.L., Wang L.Y., Wang K.T., Hu K.C., et al. Exploring the chemodiversity and biological activities of the secondary metabolites from the marine fungus Neosartorya pseudofischeri. Mar. Drugs. 2014;12:5657–5676. doi: 10.3390/md12115657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon O.E., Rho M.C., Song H.Y., Lee S.W., Chung M.Y., Lee J.H., Kim Y.H., Lee H.S., Kim Y.K. Phenylpyropene A and B, new inhibitors of acyl-CoA: Cholesterol acyltransferase produced by Penicillium griseofulvum F1959. J. Antibiot. 2002;55:1004–1008. doi: 10.7164/antibiotics.55.1004. [DOI] [PubMed] [Google Scholar]
- 16.Rho M.C., Lee H.S., Chang K.T., Song H.Y., Kwon O.E., Lee S.W., Ko J.S., Hong S.G., Kim Y.K. Phenylpyropene C, a new inhibitor of acyl-CoA: Cholesterol acyltransferase produced by Penicillium griseofulvum F1959. J. Antibiot. 2002;55:211–214. doi: 10.7164/antibiotics.55.211. [DOI] [PubMed] [Google Scholar]
- 17.Choi J.H., Rho M.C., Lee S.W., Choi J.N., Lee H.J., Bae K.S., Kim K., Kim Y.K. Penicillium griseofulvum F1959, high-production strain of pyripyropene A, specific inhibitor of Acyl-CoA: Cholesterol acyltransferase 2. J. Microbiol. Biotechnol. 2008;18:1663–1665. [PubMed] [Google Scholar]
- 18.Obata R., Sunazuka T., Li Z., Tian Z., Harigaya Y., Tabata N., Tomoda H., Omura S. Chemical modification and structure-activity relationships of pyripyropenes. 1. Modification at the four hydroxyl groups. J. Antibiot. 1996;49:1133–1148. doi: 10.7164/antibiotics.49.1133. [DOI] [PubMed] [Google Scholar]
- 19.Didier C., Critcher D.J., Walshe N.D., Kojima Y., Yamauchi Y., Barrett A.G.M. Full stereochemical assignment and synthesis of the potent anthelmintic pyrrolobenzoxazine natural product CJ-12662. J. Org. Chem. 2004;69:7875–7879. doi: 10.1021/jo048711t. [DOI] [PubMed] [Google Scholar]
- 20.Masi M., Andolfi A., Mathieu V., Boari A., Cimmino A., Moreno Y Banuls L., Vurro M., Kornienko A., Kiss R., Evidente A. Fischerindoline, a pyrroloindole sesquiterpenoid isolated from Neosartorya pseudofischeri, with in vitro growth inhibitory activity in human cancer cell lines. Tetrahedron. 2013;69:7466–7470. doi: 10.1016/j.tet.2013.06.031. [DOI] [Google Scholar]
- 21.Kanokmedhakul K., Kanokmedhakul S., Suwannatrai R., Soytong K., Prabpai S., Kongsaeree P. Bioactive meroterpenoids and alkaloids from the fungus Eurotium chevalieri. Tetrahedron. 2011;67:5461–5468. doi: 10.1016/j.tet.2011.05.066. [DOI] [Google Scholar]
- 22.Liao L.J., You M.J., Chung B.K., Oh D.C., Oh K.B., Shin J. Alkaloidal metabolites from a marine-derived Aspergillus sp. fungus. J. Nat. Prod. 2015;78:349–354. doi: 10.1021/np500683u. [DOI] [PubMed] [Google Scholar]
- 23.Garo E.C., Starks M., Jensen P.R., Fenical W., Lobkovsky E., Clardy J. Trichodermamides A and B, cytotoxic modified dipeptides from the marine-derived fungus Trichoderma virens. J. Nat. Prod. 2003;66:423–426. doi: 10.1021/np0204390. [DOI] [PubMed] [Google Scholar]
- 24.Zheng D., Jiang Y., Han L., Lou K., Chen Y., Xu L., Huang X. Chemical constituents from the mycelium of a new streptomycete. Chin. J. Med. Chem. 2010;20:201–205. [Google Scholar]
- 25.Lee D.S., Eom S.H., Jeong S.Y., Shin H.J., Je J.Y., Lee E.W., Chung Y.H., Kim Y.M., Kang C.K., Lee M.S. Anti-methicillin-resistant Staphylococcus aureus (MRSA) substance from the marine bacterium Pseudomonas sp. UJ-6. Environ. Toxicol. Phar. 2013;35:171–177. doi: 10.1016/j.etap.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Lowe G., Taylor A., Vining L.C. Sporidesmins. VI. Isolation and structure of dehydrogliotoxin a metabolite of Penicillium terlikowskii. J. Chem. Soc. Perkin Trans.1. 1966;20:1799–1803. doi: 10.1039/j39660001799. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi C., Matsushita T., Doi M., Minoura K., Shingu T., Kumeda Y., Numata A. Fumiquinazolines A–G, novel metabolites of a fungus separated from a Pseudolabrus marine fish. J. Chem. Soc. Perkin Trans. 1995;18:2345–2353. doi: 10.1039/p19950002345. [DOI] [Google Scholar]
- 28.Tomoda H., Tabata N., Nakata Y., Nishida H., Kaneko T., Obata R., Sunazuka T., Omura S. Biosynthesis of pyripyropene A. J. Org. Chem. 1996;61:882–886. doi: 10.1021/jo951424s. [DOI] [Google Scholar]
- 29.Huang J.F., Tian M., Lv C.J., Li H.Y., Muhammad R.H., Zhong G.H. Preliminary studies on induction of apoptosis by abamectin in Spodoptera frugiperda (Sf9) cell line. Pestic. Biochem. Physiol. 2011;100:256–263. doi: 10.1016/j.pestbp.2011.04.010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.