Abstract
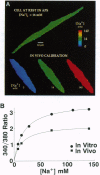
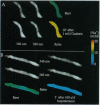
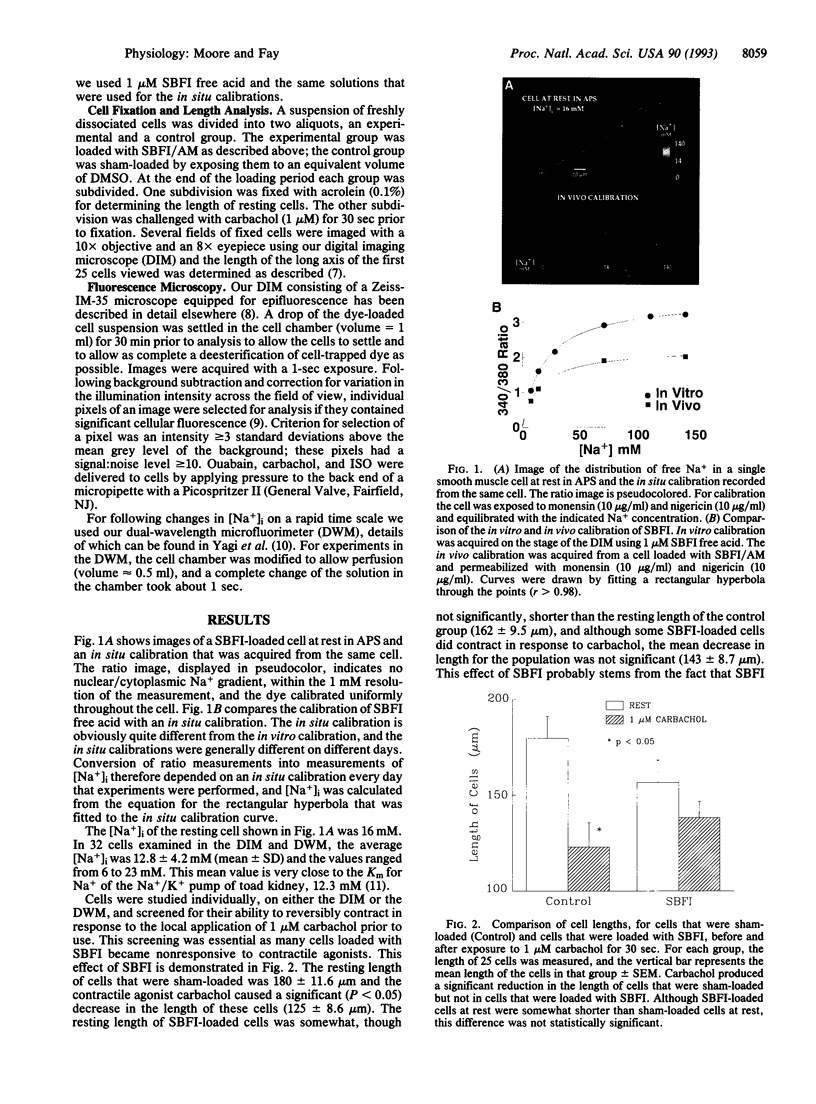
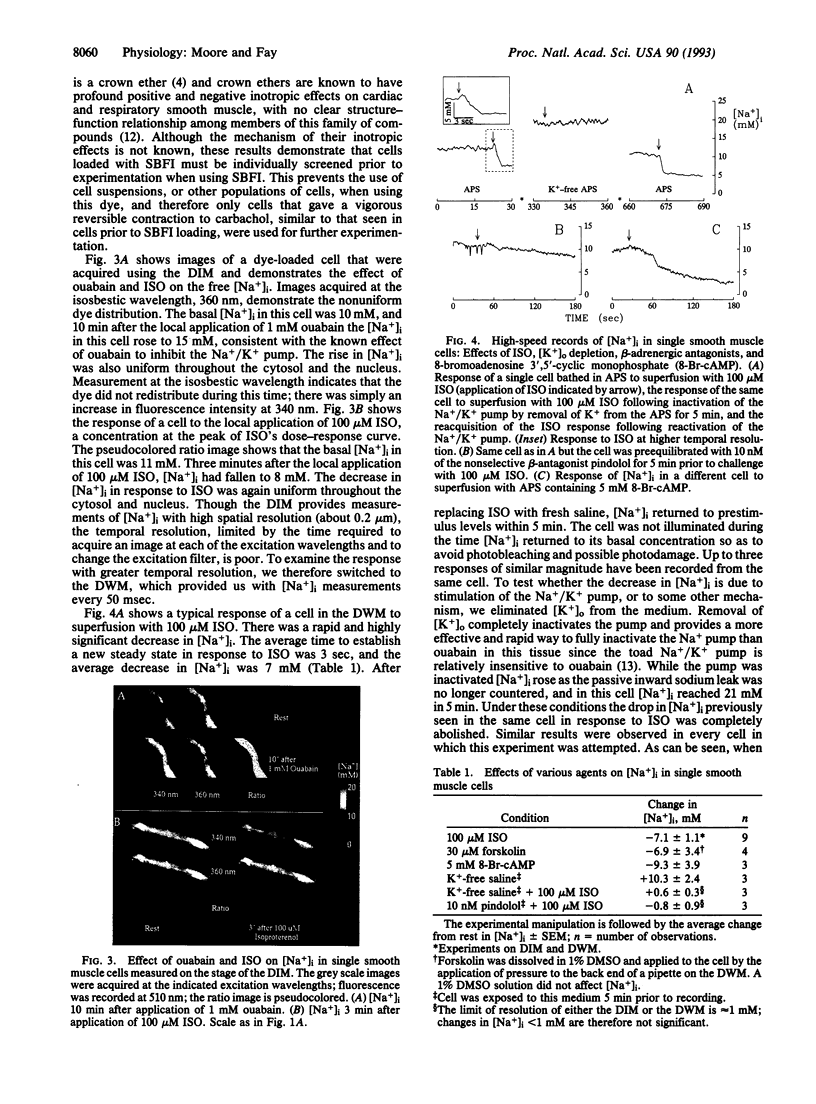
beta-Agonists cause an inhibition of contractility and a transient stimulation of Na+/K+ pumping in smooth muscle cells of the stomach from the toad Bufo marinus. To determine if the stimulation of Na+/K+ pumping causes changes in intracellular [Na+] ([Na+]i) that might link Na+ pump stimulation to decrease Ca2+ availability for contraction, [Na+]i was measured in these cells with SBFI, a Na(+)-sensitive fluorescent indicator. Basal [Na+]i was 12.8 +/- 4.2 mM (n = 32) and was uniform throughout the cell. In response to isoproterenol, [Na+]i decreased an average of 7.1 +/- 1.1 mM in 3 sec. Since this decrease in [Na+]i could be completely blocked by inhibition of the Na+ pump, or by blockade of the beta-receptor, [Na+]i reduction is the result of occupation of the beta-receptor by isoproterenol and subsequent stimulation of the Na+ pump. 8-Bromoadenosine 3',5'-cyclic monophosphate and forskolin mimicked the effect of isoproterenol, indicating that the sequence of events linking beta-receptor occupation to Na+ pump stimulation most likely includes activation of adenylate cyclase, production of cAMP, and stimulation of cAMP-dependent protein kinase. The decrease in [Na+]i is sufficiently large and fast that it is expected to stimulate turnover of the Na+/Ca2+ exchanger in the Ca2+ extrusion mode, thereby accounting for the observed linkage between stimulation of the Na+/K+ pump and inhibition of contractility in response to beta-adrenergic agonists.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C. Investigation of factors affecting the intracellular sodium activity in the smooth muscle of guinea-pig ureter. J Physiol. 1987 Apr;385:483–505. doi: 10.1113/jphysiol.1987.sp016503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aperia A., Bertorello A., Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol. 1987 Jan;252(1 Pt 2):F39–F45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- Bertorello A. M., Aperia A., Walaas S. I., Nairn A. C., Greengard P. Phosphorylation of the catalytic subunit of Na+,K(+)-ATPase inhibits the activity of the enzyme. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11359–11362. doi: 10.1073/pnas.88.24.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorello A. M., Hopfield J. F., Aperia A., Greengard P. Inhibition by dopamine of (Na(+)+K+)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism. Nature. 1990 Sep 27;347(6291):386–388. doi: 10.1038/347386a0. [DOI] [PubMed] [Google Scholar]
- Blum H., Nioka S., Johnson R. G., Jr Activation of the Na+, K(+)-ATPase in Narcine brasiliensis. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1247–1251. doi: 10.1073/pnas.87.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borin M., Siffert W. Further characterization of the mechanisms mediating the rise in cytosolic free Na+ in thrombin-stimulated platelets. Evidence for inhibition of the Na+,K(+)-ATPase and for Na+ entry via a Ca2+ influx pathway. J Biol Chem. 1991 Jul 15;266(20):13153–13160. [PubMed] [Google Scholar]
- Brading A. F., Widdicombe J. H. An estimate of sodium-potassium pump activity and the number of pump sites in the smooth muscle of the guinea-pig taenia coli, using (3H)ouabain. J Physiol. 1974 Apr;238(2):235–249. doi: 10.1113/jphysiol.1974.sp010521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay F. S., Hoffmann R., Leclair S., Merriam P. Preparation of individual smooth muscle cells from the stomach of Bufo marinus. Methods Enzymol. 1982;85(Pt B):284–292. doi: 10.1016/0076-6879(82)85027-1. [DOI] [PubMed] [Google Scholar]
- Geering K., Rossier B. C. Purification and characterization of (Na+ + K+)-ATPase from toad kidney. Biochim Biophys Acta. 1979 Jan 12;566(1):157–170. doi: 10.1016/0005-2744(79)90258-4. [DOI] [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Eckert B. K., Tsien R. Y. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J Biol Chem. 1989 Nov 15;264(32):19458–19467. [PubMed] [Google Scholar]
- Ibarra F., Aperia A., Svensson L. B., Eklöf A. C., Greengard P. Bidirectional regulation of Na+,K(+)-ATPase activity by dopamine and an alpha-adrenergic agonist. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):21–24. doi: 10.1073/pnas.90.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelicks L. A., Gupta R. K. NMR measurement of cytosolic free calcium, free magnesium, and intracellular sodium in the aorta of the normal and spontaneously hypertensive rat. J Biol Chem. 1990 Jan 25;265(3):1394–1400. [PubMed] [Google Scholar]
- Kargacin G. J., Fay F. S. Physiological and structural properties of saponin-skinned single smooth muscle cells. J Gen Physiol. 1987 Jul;90(1):49–73. doi: 10.1085/jgp.90.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk K. L., Dawson D. C. Passive cation permeability of turtle colon: evidence for a negative interaction between intracellular sodium and apical sodium permeability. Pflugers Arch. 1985 Jan;403(1):82–89. doi: 10.1007/BF00583286. [DOI] [PubMed] [Google Scholar]
- Kolbeck R. C., Hendry L. B., Bransome E. D., Speir W. A. Crown ethers which influence cardiac and respiratory muscle contractility. Experientia. 1984 Jul 15;40(7):727–731. doi: 10.1007/BF01949746. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Vassalle M. Modulation of intracellular Na+ activity and cardiac force by norepinephrine and Ca2+. Am J Physiol. 1983 Jan;244(1):C110–C114. doi: 10.1152/ajpcell.1983.244.1.C110. [DOI] [PubMed] [Google Scholar]
- Minta A., Tsien R. Y. Fluorescent indicators for cytosolic sodium. J Biol Chem. 1989 Nov 15;264(32):19449–19457. [PubMed] [Google Scholar]
- Moore E. D., Becker P. L., Fogarty K. E., Williams D. A., Fay F. S. Ca2+ imaging in single living cells: theoretical and practical issues. Cell Calcium. 1990 Feb-Mar;11(2-3):157–179. doi: 10.1016/0143-4160(90)90068-6. [DOI] [PubMed] [Google Scholar]
- Negulescu P. A., Harootunian A., Tsien R. Y., Machen T. E. Fluorescence measurements of cytosolic free Na concentration, influx and efflux in gastric cells. Cell Regul. 1990 Feb;1(3):259–268. doi: 10.1091/mbc.1.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., Ishikawa S., Saito T. Interaction between endothelin-induced Na+ and Ca2+ kinetics in cultured rat vascular smooth muscle cells. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S124–S126. doi: 10.1097/00005344-199100177-00033. [DOI] [PubMed] [Google Scholar]
- Scheid C. R., Fay F. S. Beta-adrenergic effects on transmembrane 45Ca fluxes in isolated smooth muscle cells. Am J Physiol. 1984 May;246(5 Pt 1):C431–C438. doi: 10.1152/ajpcell.1984.246.5.C431. [DOI] [PubMed] [Google Scholar]
- Scheid C. R., Fay F. S. Control of ion distribution in isolated smooth muscle cells. I. Potassium. J Gen Physiol. 1980 Feb;75(2):163–182. doi: 10.1085/jgp.75.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid C. R., Honeyman T. W., Fay F. S. Mechanism of beta-adrenergic relaxation of smooth muscle. Nature. 1979 Jan 4;277(5691):32–36. doi: 10.1038/277032a0. [DOI] [PubMed] [Google Scholar]
- Shimada K., Ohishi K., Fukunaga H., Ro J. S., Nambara T. Structure-activity relationship of bufotoxins and related compounds for the inhibition of Na+, K+ -adenosine triphosphatase. J Pharmacobiodyn. 1985 Dec;8(12):1054–1059. doi: 10.1248/bpb1978.8.1054. [DOI] [PubMed] [Google Scholar]
- Sims S. M., Singer J. J., Walsh J. V., Jr Antagonistic adrenergic-muscarinic regulation of M current in smooth muscle cells. Science. 1988 Jan 8;239(4836):190–193. doi: 10.1126/science.2827305. [DOI] [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V., Jr Passive properties of the membrane of single freshly isolated smooth muscle cells. Am J Physiol. 1980 Nov;239(5):C153–C161. doi: 10.1152/ajpcell.1980.239.5.C153. [DOI] [PubMed] [Google Scholar]
- Vasilets L. A., Schwarz W. Regulation of endogenous and expressed Na+/K+ pumps in Xenopus oocytes by membrane potential and stimulation of protein kinases. J Membr Biol. 1992 Jan;125(2):119–132. doi: 10.1007/BF00233352. [DOI] [PubMed] [Google Scholar]
- Vasilets L. A., Schwarz W. Regulation of endogenous and expressed Na+/K+ pumps in Xenopus oocytes by membrane potential and stimulation of protein kinases. J Membr Biol. 1992 Jan;125(2):119–132. doi: 10.1007/BF00233352. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fay F. S. Calcium transients and resting levels in isolated smooth muscle cells as monitored with quin 2. Am J Physiol. 1986 May;250(5 Pt 1):C779–C791. doi: 10.1152/ajpcell.1986.250.5.C779. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]
- Yagi S., Becker P. L., Fay F. S. Relationship between force and Ca2+ concentration in smooth muscle as revealed by measurements on single cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4109–4113. doi: 10.1073/pnas.85.11.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Honeyman T. W., Fay F. S. Beta-adrenergic actions on membrane electrical properties of dissociated smooth muscle cells. Am J Physiol. 1988 Mar;254(3 Pt 1):C423–C431. doi: 10.1152/ajpcell.1988.254.3.C423. [DOI] [PubMed] [Google Scholar]
- Yeh L. A., Ling L., English L., Cantley L. Phosphorylation of the (Na,K)-ATPase by a plasma membrane-bound protein kinase in friend erythroleukemia cells. J Biol Chem. 1983 May 25;258(10):6567–6574. [PubMed] [Google Scholar]
- Zeidel M. L., Brady H. R., Kone B. C., Gullans S. R., Brenner B. M. Endothelin, a peptide inhibitor of Na(+)-K(+)-ATPase in intact renaltubular epithelial cells. Am J Physiol. 1989 Dec;257(6 Pt 1):C1101–C1107. doi: 10.1152/ajpcell.1989.257.6.C1101. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Somlyo A. V., Somlyo A. P. Beta-adrenergic effects on cellular Na, Mg, Ca, K and Cl in vascular smooth muscle: electron probe analysis of rabbit pulmonary artery. Cell Calcium. 1992 Oct;13(9):593–602. doi: 10.1016/0143-4160(92)90039-u. [DOI] [PubMed] [Google Scholar]