Abstract
Background
Use of a rapid HIV testing algorithm (RTA) in which all tests are conducted within one client appointment could eliminate off-site confirmatory testing and reduce the number of persons not receiving confirmed results.
Methods
An RTA was implemented in 9 sites in Los Angeles and San Francisco; results of testing at these sites were compared with 23 sites conducting rapid HIV testing with off-site confirmation. RTA clients with reactive results on more than 1 rapid test were considered HIV+ and immediately referred for HIV care. The positive predictive values (PPVs) of a single rapid HIV test and the RTA were calculated compared with laboratory-based confirmatory testing. A Poisson risk regression model was used to assess the effect of RTA on the proportion of HIV+ persons linked to HIV care within 90 days of a reactive rapid test.
Results
The PPV of the RTA was 100% compared with 86.4% for a single rapid test. The time between testing and receipt of RTA results was on average 8 days shorter than laboratory-based confirmatory testing. For risk groups other than men who had sex with men, the RTA increased the probability of being in care within 90 days compared with standard testing practice.
Conclusions
The RTA increased the PPV of rapid testing to 100%, giving providers, clients, and HIV counselors timely information about a client’s HIV-positive serostatus. Use of RTA could reduce loss to follow-up between testing positive and confirmation and increase the proportion of HIV-infected persons receiving HIV care.
Keywords: HIV testing, linkage to HIV care, rapid HIV testing
INTRODUCTION
HIV testing in nonclinical settings, such as outreach or other sites that do not offer disease management or treatment services, has been shown to be effective at increasing the proportion of persons aware of their infection.1,2 In the United States, a single reactive rapid HIV test result is considered a “preliminary-positive” result.3 Although Centers for Disease Control and Prevention (CDC) and Health Resources and Services Administration (HRSA) recommend referral of eligible clients to HRSA-funded clinics after a preliminary-positive result4 to facilitate timely linkage to care, supplemental laboratory-based testing is recommended after a reactive rapid HIV test.3,5 When test sites use off-site laboratory testing to confirm a preliminary-positive result, clients must wait until their laboratory result is ready to get a definitive result. Although referral after a preliminary-positive result is permissible, many sites do not offer referrals until after supplemental laboratory test results confirm infection. HIV testing programs in nonclinical settings that do not offer immediate referrals have experienced difficulty convincing clients to provide venipuncture specimens for confirmatory testing6 and recontacting clients to deliver confirmatory test results and subsequently linking clients to medical care.1,2,6–9
CDC guidelines for HIV testing in nonclinical settings10 indicate that “if 2 or more sensitive and specific rapid HIV tests became available, one positive rapid test could be confirmed with a different rapid test,” and this was reiterated as an acceptable criteria for confirmation of diagnosis for Ryan White HIV/AIDS program eligibility by CDC and HRSA in 2013.4 Since 2001, the Food and Drug Administration (FDA) has approved 8 rapid HIV tests for use in multitest algorithms11–13 to determine the presence of HIV antibodies. Therefore, alternatives to the current testing algorithm that uses multiple rapid tests, which have been used extensively in resource-limited settings,14–18 have been proposed for use in United States.19 To date, these alternatives have principally been used to increase the positive predictive value (PPV) of the rapid HIV screening test. However, same-day referral of those with reactive rapid test results may also improve the linkage to HIV medical care.6,19–21 A goal of the President’s National HIV/AIDS Strategy is to increase the proportion of all HIV-infected clients successfully linked to HIV medical care within 90 days from 65% to 85% by 2015.22
In this study, we evaluated the PPV of a rapid HIV testing algorithm (RTA) using 3 tests in nonclinical HIV counseling and testing (HCT) sites in Los Angeles (LA) and San Francisco, CA, and the impact of testing with the RTA on receipt of confirmed HIV test results and linkage to HIV medical care.
METHODS
A total of 32 agencies funded by the collaborating local public health departments in LA and San Francisco offered rapid HCT services before study initiation in August 2007. HCT sites included mobile units, storefronts, health clinics, community-based organizations, a methadone clinic, and county jail services. Four programs in LA and 5 programs in San Francisco were selected to implement an HIV RTA as their standard method of providing HCT services for an 18-month period (intervention sites) from August 2007 through March 2009. The other 23 sites served as comparison sites. Clients testing confidentially or anonymously at both intervention and comparison sites were eligible to participate in the study. Anonymous testers could provide their name to convert to confidential testing at any point during the testing session; only those testing confidentially could be reported to the HIV surveillance system or referred for HIV medical care.
All persons tested for HIV between August 1, 2007, and February 28, 2009, at the 32 HCT sites in LA and San Francisco were included in this study. All HCT sites in both cities used the OraQuick Advance HIV-1/2 HIV antibody test (Orasure Technologies Inc., Bethlehem, PA) (OraQuick) as their rapid HIV screening test and confirmed an initial reactive test result with a Western blot or immunofluorescence assay. Therefore, we chose OraQuick as the initial screening HIV test in the RTA, so that test specificity would be consistent with the comparison sites. At the 9 sites offering an RTA, persons with a preliminary-positive OraQuick result had blood collected via venipuncture for both laboratory confirmation (using Western blot or immunofluorescence assay) and for testing with the Clearview Statpak HIV-1/2 antibody test (Alere Inc., Waltham, MA). If the Clearview test was negative, the Unigold HIV-1 Rapid test (Trinity BioTech, Bray, Ireland) was performed. If both the OraQuick and the Clearview tests were positive, the Unigold test was not used. Persons with reactive results on 2 rapid tests were considered HIV infected and received immediate counseling and referral to HIV care. Persons with negative results on both the supplemental rapid tests were considered HIV uninfected and informed of this result. At both intervention and comparison sites, anticoagulated whole-blood specimens (EDTA) were collected for laboratory confirmation of preliminary-positive rapid test results. Persons tested at comparison sites received this confirmatory result at their disclosure visit, typically scheduled for 1 week later. Additional details about the implementation of the RTA and protocols developed for this study have been described by Rurangirwa et al.23,24
Routinely collected data from 2 sources were used for our analysis. All persons seeking HIV testing at study sites provided information to the collaborating public health departments for reporting to CDC’s HIV testing evaluation program.23–25 Identifiers, including name, date of birth, test date, test identification number, site of test, and gender, which are collected and maintained in both San Francisco and LA before data are de-identified and summarized for CDC, were extracted from these testing databases and matched to the HIV/AIDS surveillance case registry, the Enhanced HIV/AIDS Reporting System (eHARS). Details of the matching procedure are included in the Supplemental Digital Content (available at http://links.lww.com/QAI/A735). For all clients whose testing and eHARS information could be matched, HIV viral load (VL) results were extracted from the eHARS and added to the data from the testing system. A VL test result after the date of testing was considered evidence of having been linked to HIV care.
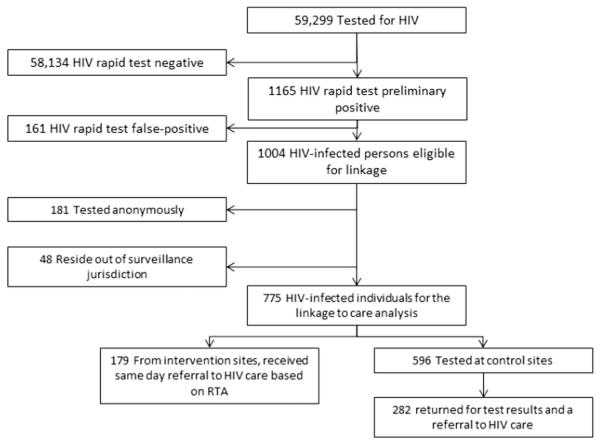
Sample sizes for analyses involving each of our primary outcomes are provided in Figure 1. Using laboratory-based results as the gold standard, we calculated the PPV for both single rapid test and RTA. Receipt of confirmatory HIV test results was based on documentation in the testing database of a date of return for posttest counseling and referral for HIV medical care. For intervention sites, counselors coded this date as the date of receipt of RTA results, which was the same day as the date of testing. Time to care was calculated as the time from the date of HIV test in the testing database to the date of the first VL result reported in the HIV surveillance registry.
FIGURE 1.
The diagram presents the sample size for each part of the analysis. Of 59,299 individuals tested, only 1165 had a preliminary-positive rapid test, including 161 false positive and 1004 results confirmed by the laboratory testing algorithm. Of 1004 persons eligible for analyses of time to care and linkage to care, 181 tested anonymously, and therefore, no information to allow for matching to the HIV case reporting system was available. An additional 48 individuals were determined to be cases who resided outside San Francisco or LA County Health Department’s jurisdiction for the purposes of tracking ongoing laboratory reporting. This left a total of 775 individuals who could be included in the linkage to care analysis, but most of these were not tested with the rapid test algorithm.
Covariates from the testing database that might affect time to care were included in this analysis. These included race and ethnicity, HIV behavioral risk group (California’s coding of presumed mode of exposure to HIV), receipt of posttest counseling and referral to care, homeless status, and HIV testing history (both self-report of previous positive test results and receipt of their most recent previous test result). Unadjusted risk ratios26 were calculated to quantify associations between these characteristics and linkage to care within 90 days of their HIV test date.
Kaplan–Meier product limit survival curves, representing the cumulative probability of care over time, based on reported VL, were calculated using standard methods.27 Differences in cumulative probabilities between strata of covariates were assessed using the log rank test.27
The probability of being linked to care within 90 days of the HIV test date was modeled using a Poisson risk model28 that accounted for effects of testing site through the use of a random effect. To investigate whether the process of RTA (both testing and associated same-day posttest counseling) could affect the probability of being linked to care within 90 days directly or only indirectly through increasing the likelihood of receipt of confirmed HIV test results and a referral for HIV care, we compared the results of 4 related models:
Models 1 and 2 contained the independent variable of primary interest (RTA vs. not) and the outcome, time to care, without an additional measure of receipt of confirmed results and referral; additionally, model 2 included the same variables as model 1 and allowed for effect modification by HIV testing history and behavioral risk group.
Model 3 was identical to model 2, except that it contained the measure of receipt of confirmed results and referral, and not the variable indicating RTA vs. not (reported in Table S1, see Supplemental Digital Content, http://links.lww.com/QAI/A735).
Model 4 contained both the variable indicating RTA vs. not and the variable measuring receipt of confirmed results and referral.
For each of these models, we calculated the ratios of the probability of being in care for those who were tested by RTA vs. received a rapid HIV test with off-site confirmatory testing while controlling for factors associated with time to care (for more discussion of the modeling strategy, see Figure S1, Supplemental Digital Content, http://links.lww.com/QAI/A735). We also assessed the possibility of effect modification by other covariates29–31 and report these results.32,33
All analyses were conducted using SAS software version 9.2 (SAS Institute, Cary, NC). This study was funded by the CDC under cooperative agreement PS06-002 and reviewed and monitored by the Institutional Review Boards at the CDC, the University of California San Francisco, and LA County Department of Public Health. All clients seeking HIV testing in study sites consented to HIV testing with either RTA or standard testing algorithm, and separate research consent was waived by the IRB under 45 CFR 46.116(d).
RESULTS
Between August 1, 2007, and February 28, 2009, a total of 59,299 HIV tests were performed at the 32 participating HCT sites (Fig. 1). Selected characteristics of the study population are included in Table 1. Most (71%) tests at both intervention and comparison sites were conducted in LA; however, proportionately more intervention site participants were recruited in San Francisco (41% vs. 24%). Participants receiving the intervention were also more likely than those who did not receive the intervention to be white, to have tested for HIV previously, and to have reported being a men who had sex with men (MSM) as their main HIV risk. There were no differences in the proportions that tested anonymously at intervention vs. comparison sites.
TABLE 1.
Demographic Characteristics of Clients Tested in LA and San Francisco Publically Funded HCT Sites, August 2007–March 2009
| Total |
Intervention Sites (n = 9) |
Comparison Sites (n = 23) |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Characteristic | 59,299 | 100.0 | 17,386 | 100.0 | 41,913 | 100.0 |
| Project area | ||||||
| LA | 42,108 | 71.0 | 10,243 | 58.9 | 31,865 | 76.0 |
| SF | 17,191 | 29.0 | 7143 | 41.1 | 10,048 | 24.0 |
| Risk group | ||||||
| MSM | 25,474 | 43.0 | 9878 | 56.8 | 15,596 | 37.2 |
| IDU | 3577 | 6.0 | 607 | 3.5 | 2970 | 7.1 |
| Other | 30,248 | 51.0 | 6901 | 39.7 | 23,347 | 55.7 |
| Race/ethnicity | ||||||
| White | 20,839 | 35.1 | 7067 | 40.7 | 13,772 | 32.9 |
| Black | 10,819 | 18.2 | 2709 | 15.6 | 8110 | 19.4 |
| Hispanic | 19,555 | 33.0 | 4180 | 24.0 | 15,375 | 36.7 |
| Other | 8086 | 13.6 | 3430 | 19.7 | 4656 | 11.1 |
| Homeless | 3340 | 5.6 | 804 | 4.6 | 2536 | 6.1 |
| Anonymous tests | 14,768 | 24.9 | 4510 | 25.9 | 10,258 | 24.5 |
| Previously tested | 45,163 | 76.2 | 14,437 | 83.0 | 30,726 | 73.3 |
| Known positive | 224 | 0.4 | 22 | 0.1 | 202 | 0.5 |
| Did not get most recent result | 729 | 1.2 | 140 | 0.8 | 589 | 1.4 |
IDU, Injection drug user; SF, city of San Francisco.
Table 2 describes the RTA results and the conventional testing results for 1165 clients with a preliminary-positive rapid test. Most of these tests occurred at comparison sites (79%), but the false-positive rates were similar at intervention and comparison sites. By design, all clients at the intervention sites with a preliminary-positive rapid test received results of additional rapid tests performed on site in the same visit and therefore also received same-day referral to HIV care. All 213 clients with positive results on 2 rapid tests were confirmed positive by the laboratory algorithm (PPV = 100%); likewise, 37 clients with false-positive OraQuick results were negative by both the laboratory algorithm and the RTA.
TABLE 2.
HIV Test Results and Time to Receipt of Confirmed Results and Referral Among Clients With Preliminary-Positive Rapid Test Results in LA and San Francisco, CA, August 2007–March 2009
| Intervention Sites |
Comparison Sites |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Total tested | 17,386 | 100.0 | 41,913 | 100.0 |
| Positive on first rapid | 250 | 1.40 | 915 | 2.20 |
| False positive on first rapid test* | 37 | 14.8 | 124 | 13.6 |
| Positive by RTA | 213 | NA | ||
| Confirmed positive* | 213 | 85.2 | 791 | 86.4 |
| PPV† | 100 | 86.4 | ||
| Received results | 250 | 100.0 | 430 | 47.0 |
| Days between initial and confirmed results,‡ median (range) |
0 | 8 | (1–137) | |
Denominator for percentage is those with a positive result on the first rapid test.
For intervention sites, the PPV reported is that of the rapid test algorithm, compared with laboratory-based confirmatory testing. For comparison sites, the PPV is of the single initial rapid test, again compared with laboratory-based confirmatory testing.
The intervention included same-day referral for all persons with a positive rapid test algorithm result.
NA, not applicable because the RTA was not performed in Comparison sites.
The PPV of the single rapid test at the comparison sites was 86.4%. More than half (53%) of the clients at comparison sites failed to return for laboratory-based confirmatory test results, including 76 clients with false-positive and 409 with confirmed positive results. For clients tested at comparison sites to receive the results of their laboratory-based confirmatory test took an average of 8 days (range, 1–137 d).
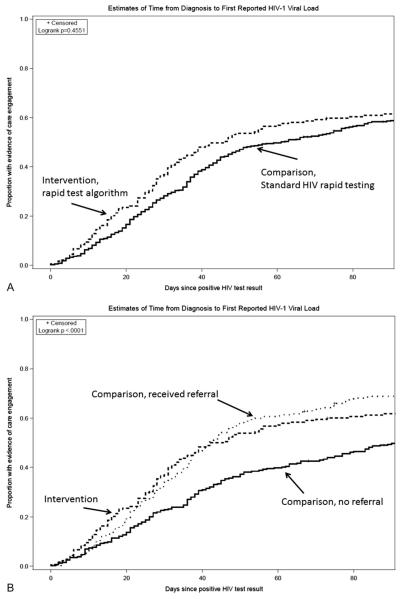
Of the 1004 persons determined to be HIV infected, 775 could be linked to the eHARS data registry (Fig. 1). Of these, 179 (23%) tested at intervention sites. Overall, there was a shorter time (statistically nonsignificant) from testing to first laboratory evidence of linkage to HIV care among those tested at the intervention sites, compared with those tested at the comparison sites (Fig. 2A). Clients who received their test results and referral, either by returning for confirmatory results at a comparison site or by testing at an intervention site, had a significantly shorter time from HIV test result to HIV care compared with those who did not come back to receive their laboratory-based confirmatory results and therefore did not receive a referral to HIV care (Fig. 2B).
FIGURE 2.
A, Kaplan–Meier failure time curves representing the estimated time from diagnosis to first laboratory evidence of HIV care (defined as the first HIV-1 VL result reported in the HIV surveillance registry), stratified by whether a client tested at an intervention or comparison site. Intervention sites included 9 sites in LA and San Francisco, CA, that offered a rapid test algorithm (RTA) and same-day referral to HIV care to persons who tested positive by RTA. Comparison sites offered the standard rapid testing protocol for the United States, a single rapid test, which, if reactive, would require laboratory-based confirmation. Standard HIV rapid testing sites provided posttest counseling and referral after the confirmatory test results were available from the laboratory, on average 8 days after the initial HIV rapid test. Although clients tested at intervention sites had a slightly shorter time to laboratory evidence of care, this difference did not reach statistical significance, P = 0.4551, log rank test (assuming no difference between the 2 curves). B, The same Kaplan–Meier failure time curve for intervention sites, but here those testing positive at comparison sites were further divided based on whether they returned for their test results. Clients who received their test results and referral because they returned for confirmatory results at a comparison site and clients who received a result by testing at an intervention site had a very similar distribution of time to laboratory evidence of HIV care, although the initial difference between immediate referral and delayed referral based on the need to wait for a laboratory result is apparent up through day 20 after the positive rapid test result. Those who received a result and a referral (whether at intervention or comparison sites) had a significantly shorter time from HIV test result to HIV care compared with those who did not come back to receive their laboratory-based confirmatory results and therefore did not receive a referral to HIV care, P < 0.001, log rank test (assuming no difference between the curves).
Table 3 summarizes results of 3 mixed-effects Poisson risk regression models (models 1, 2, and 4, see Methods) assessing factors associated with linkage to HIV care within 90 days of HIV testing. Being tested at an intervention site with the RTA was not significantly associated with linkage to HIV care within 90 days after testing positive in either the unadjusted model (relative risk = 1.04, 95% confidence interval: 0.91 to 1.19) or model 1 that adjusted for other factors associated with linkage to care (relative risk = 1.09; 95% confidence interval: 0.98 to 1.23). However, the unadjusted risk ratios suggested significant variation in the effect of RTA by risk group (MSM vs. other) and a previous HIV-positive test result. In a model that allowed for different RTA effects in different levels of these 2 covariates (Table 3, model 2), the RTA had a significant effect on linkage to care for non-MSM (for further description of the modification of the effect of RTA by risk group, see Table S2 and Figure S2, Supplemental Digital Content, http://links.lww.com/QAI/A735). In model 4 (Table 3), which also controlled for the effect of receiving posttest counseling and referral, the effect of the RTA was reduced and of similar magnitude to the effect of receiving posttest counseling and referral in a model that did not control for exposure to RTA (see Table S1, model 3, Supplemental Digital Content, http://links.lww.com/QAI/A735). All models included a random intercept term for test site, representing the variation in baseline probability of linking clients to care within 90 days for each site. Heterogeneity in the baseline probability of linkage to care was spread across both intervention and comparison sites but was reduced significantly when receipt of results and referral were accounted for in the main effects of the models, such that the random effect term was not significant in model 3 (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A735) or 4 (Table 3).
TABLE 3.
Factors Associated With Having Laboratory Evidence of HIV Care Within 90 Days of a Positive HIV Test in Poisson Risk Models, for 775 Clients Tested in LA and San Francisco, CA, August 2007–March 2009
| Model 1 |
Model 2 |
Model 4 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total |
Linked < 90 d |
|||||||||||
| n | % | n | % | RR* | 95% CI | aRR1† | 95% CI | aRR2‡ | 95% CI | aRR4§ | 95% CI | |
| Exposure variables | ||||||||||||
| Received result and referral (model 4) |
||||||||||||
| Yes | 461 | 59.5 | 307 | 66.7 | 1.33 | 1.16 to 1.51 | 1.35 | 1.20 to 1.51 | ||||
| No | 314 | 40.5 | 158 | 50.3 | ||||||||
| Tested with the rapid test algorithm (RTA) (models 2 and 4) |
||||||||||||
| Overall | 179 | 23.1 | 111 | 62.0 | 1.04 | 0.91 to 1.19 | 1.09 | 0.98 to 1.23 | ||||
| 596 | 76.9 | 354 | 59.4 | |||||||||
| Model interaction terms, strata of exposure and covariates |
||||||||||||
| Neither exposed to the RTA nor MSM |
177 | 22.8 | 106 | 59.9 | Ref | Ref | ||||||
| MSM but not exposed to the RTA |
419 | 54.1 | 248 | 59.2 | 0.99 | 0.79 to 1.24 | 1.02 | 0.89 to 1.16 | 1.01 | 0.89 to 1.15 | ||
| Exposed to the RTA but not MSM |
43 | 5.5 | 35 | 81.4 | 1.36 | 0.93 to 1.99 | 1.43 | 1.11 to 1.84 | 1.25 | .97 to 1.62 | ||
| Both exposed to the RTA and MSM |
136 | 17.5 | 76 | 55.9 | 0.93 | 0.69 to 1.25 | 0.98 | 0.80 to 1.21 | 0.87 | 0.70 to 1.07 | ||
| Not exposed to the RTA and not previously diagnosed |
476 | 61.4 | 280 | 58.8 | Ref | Ref | ||||||
| Not exposed to the RTA and previously diagnosed |
120 | 15.4 | 74 | 61.7 | 1.05 | 0.81 to 1.36 | 1.03 | 0.89 to 1.20 | 1.08 | 0.95 to 1.22 | ||
| Exposed to the RTA and not previously diagnosed |
173 | 22.3 | 106 | 61.3 | 1.04 | 0.83 to 1.30 | 1.43 | 1.11 to 1.84 | 1.25 | 0.97 to 1.62 | ||
| Exposed to the RTA and previously diagnosed |
6 | 0.8 | 5 | 88.3 | 1.42 | 0.58 to 3.44 | 1.81 | 1.00 to 3.28 | 1.57 | 0.85 to 2.89 | ||
| Random effect for study site mixture test P value∥ |
0.0075 | 0.0154 | 0.2213 | |||||||||
RR, unadjusted ratio of the probability of having laboratory evidence (at least one HIV-1 VL reported to HIV surveillance) within 90 days of the study HIV test date, relative to the same probability in the reference category for each characteristic listed in the first column.
aRR1 obtained from a Poisson risk model23 that included a random intercept for study site; model 1 includes the main effect of the intervention (rapid test algorithm with same-day referral) overall and indicator variables for race, ethnicity, homeless status, history of any HIV test, and whether the participant received the result of their most recent test. Neither the interaction terms nor an indicator for receipt of results and referral (hypothesized to be an intermediate effect of the intervention on the probability of having laboratory evidence of HIV care within 90 days of the date of HIV testing) were included in this model.
aRR2 for model 2. The model was exactly the same as model 1 except it also included multiplicative interaction terms for the effect of RTA by risk group (categorized as MSM and non-MSM) and a multiplicative interaction term for the effect of RTA across categories of client self-report of a positive HIV test result before the current study HIV test date.
aRR4 for model 4. Same as model 2, with the addition of the hypothesized intermediate variable indicating receipt of results and referral.
Mixture test P value for the effect of the site random intercept term in each model. Models with P <0.05 indicate significant unexplained heterogeneity in the baseline probability of being in HIV care within 90 days of the HIV test date across study sites.
aRR, adjusted relative risk; CI, confidence interval; RR, relative risk.
DISCUSSION
Our study found that adding a second rapid test to confirm a preliminary-positive rapid test had significant advantages compared with use of a single rapid test confirmed by standard laboratory testing. First, the predictive value of the rapid test algorithm was 100% compared with 86.4% for the single rapid test. Furthermore, being tested at an RTA site allowed for same-day receipt of a confirmed test result4 and referral to care compared with sites offering standard laboratory confirmation, where less than half returned for confirmatory results and it took an average of 8 days between testing and return of these results with referral to HIV care. And, for non-MSM, being tested at an RTA site increased the probability of being in care within 90 days compared with standard testing practice.
Assuming independence and that the reported specificities11–13,34 for each test are accurate, we would expect a false-positive rapid test algorithm result to occur only once in every 500,000 HIV tests of uninfected individuals. Although a prospective study large enough to confirm this is cost prohibitive, the only other large-scale evaluation of the RTA in the United States reported one false-positive result in 51,413 tests.20 Modeling35 based on studies in which all rapid tests were performed on the same individuals11 suggests that although complete independence cannot be assumed, the number of specimens with concordant false-positive results on 2 different rapid HIV tests is less than 1/5000 for all possible combinations of FDA-approved rapid tests. In the present study, using an algorithm with tests with relatively lower individual specificity among the FDA-approved rapid tests11–13,34–37 suggests that it is even lower than this value. The excellent performance of FDA-approved tests in multitest algorithms had been anticipated based on extensive use of this strategy in developing countries where it is used routinely even in high-prevalence settings to improve the predictive value of rapid HIV testing.14–18 If study sites had referred all clients with a single reactive rapid test to HIV care, 161 such clients who were determined to be uninfected would have required at least one follow-up visit with the HIV care provider to figure out that they were uninfected. The ability to increase the predictive value of HIV testing at the point of contact is one key advantage of a rapid test algorithm, allowing both clients and counselors to have sufficient confidence in the test results to move to same-day HIV care referral.
This same-day referral seems to be the main advantage of the rapid test algorithm. Receiving confirmed results (whether via the RTA or not) during posttest counseling and referral was the strongest predictor of laboratory evidence of linkage to HIV care within 90 days of testing, with a 35% increase in the probability of being linked to care for those who received a referral compared with those who did not (Table 3, model 4). Because clients who received the RTA received a referral during the same visit, most of the effect of the RTA on linkage to care within 90 days of testing resulted from the same-day receipt of a referral. However, there seems to be some additional benefit to the RTA particularly for risk groups (injecting drug users and high-risk heterosexuals) who have previously been reported to have difficulty in successfully linking to HIV care.38–40 This result is surprising as the RTA in our study was not coupled with any other interventions41–48 that have already been shown to improve linkage to HIV care. Future studies could combine the RTA with initiation of one or more of these linkage interventions at the first visit to maximize the impact of testing on linkage to care48 to meet the objectives of the National HIV/AIDS Strategy.22
In our study, MSM not tested with the RTA were more likely (59.2% vs. 55.9%) to be linked to care within 90 days than MSM tested with the RTA. In contrast, we found that RTA testing clients in other risk groups were more likely to be in care within 90 days of their HIV test. We attempted to control for differences in underlying client characteristics (eg, drug use, poverty, health insurance status) and site characteristics (eg, experience of counselors, established linkages between testing sites and clinical care sites) by including an effect for random variation by study site, assuming that clients testing at a particular site were similar on these unmeasured potential confounders of our effect of interest. However, there were a small number (n = 43) of non-MSM tested via RTA, and reasons for their higher rate of linkage to care within 90 days could not be explored further in this analysis. It is possible that including a random effect variable for site did not adequately account for within-site differences in linkage to care across risk behavior groups, particularly for RTA sites that served primarily MSM populations.
The study has several limitations, including the lack of a randomized design. We were unable to randomize intervention or comparison sites because of the need for existing staff capacity and experience conducting rapid testing and limited resources with which to implement a research protocol with a large number of sites with a small volume of tests performed annually. Although we attempted to control for factors that might contribute to both testing at an intervention site and linkage to HIV care, multivariate models that included effects for individual sites and even study area (LA and San Francisco) did not converge, likely because of the small number of HIV-infected persons tested with the RTA (n = 179) for whom linkage data were available (data not shown). The small sample size and possibility of residual confounding make separation of the effect of RTA into effects due to receipt of referral and other direct effects on linkage to care unwise.49 Furthermore, even if it is true that most of the effect of RTA on linkage to care was because of an increase in the number of clients who received confirmed results and a referral, we did not assess whether immediate referral to care after a single rapid test could have a similar impact on our outcome measure or what the impact of referral of persons who would ultimately be determined to be uninfected (161 persons with a false-positive rapid test in our study) might be. Nevertheless, having a comparison group and controlling for factors that might affect both receiving the RTA and accessing HIV care are improvements over previously reported evaluations of the RTA.20
The outcomes considered in this study (PPV, receipt of results, and linkage to HIV care within 90 d of testing) do not evaluate the relative sensitivity of rapid testing compared with laboratory testing. In high-incidence settings, false-negative rapid tests may occur and other testing methods may be preferred.50–52 However, data suggest that although laboratory-based confirmatory testing may exhibit high sensitivity required to reduce the possibility of false-negative results, delays inherent in these testing methods still lead to loss to follow-up and delays in receipt of test results and referral to HIV care.53,54 The “realized sensitivity” of laboratory testing, in terms of number of persons who receive timely diagnosis of their HIV infection and linkage to HIV care, will be lower than that of currently available rapid tests if these clients are lost to follow-up without receiving their test results. Newly approved rapid tests,10,11 including one that detects HIV-1 antigen directly,11 have been reported by some55,56 but not all52 to be more sensitive than the tests used in this study, and their evaluation in RTA in a setting with high HIV incidence is warranted.
For analyses with time to evidence of linkage to HIV care and probability of being linked to care within 90 days as the outcomes of interest, we are limited by the completeness of surveillance reporting of HIV laboratory results. Since July 2002, licensed laboratories in California have been required to report all HIV VL test results to the local health department. Evaluations of the HIV/AIDS surveillance system have found that the reporting of laboratory results for HIV/AIDS cases was over 95% complete.57–59 Although it is possible that our estimates of both time to care and the proportion in care within 90 days may be biased because of incomplete reporting, it seems unlikely that those tested by the RTA would have a systematic difference in the probability of having a laboratory report collected by the eHARS system. Likewise, some persons without documentation of receipt of posttest counseling and referral may have in fact received their results. These incomplete data would only affect comparison sites and would argue for considering the model with only receipt of RTA and not receipt of posttest counseling and referral (Table 3, model 2) as our primary model because it does not include this potentially biased covariate. Both LA and San Francisco allow for anonymous HIV testing at several of their HIV test sites. Those testing anonymously could neither be linked to surveillance data nor actively referred for HIV care because a name must be associated with the test result for these activities to occur. Using the RTA with clients who test anonymously might provide an additional opportunity to discuss switching to confidential testing to facilitate immediate referral.60
Recent CDC guidance for HIV testing program managers includes instructions for use of multiple rapid tests in nonclinical settings,61 and the recently revised surveillance case definition for HIV infection allows for reporting of HIV cases to the CDC’s National HIV Surveillance System based on reactive results on 2 different rapid tests.62 Both jurisdictions participating in this study have continued to use RTA since the study ended, although they now use an algorithm that includes 2 finger stick rapid tests.23,24 Other jurisdictions have also implemented RTA and found it to be cost saving compared with requiring laboratory confirmation of all preliminary-positive rapid test results.20,21 Use of RTA involves some additional logistical complexities; these implementation challenges are discussed in detail elsewhere.23,24 However, this study demonstrated the benefits of an improved PPV and immediate referral to HIV care without opportunity for loss during follow-up. These advantages, coupled with other interventions designed to improve linkage to HIV care and treatment, make implementation of RTA an appealing addition to the complement of strategies for HIV testing and linkage to care in nonclinical settings in the United States.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge all the individuals who sought testing in San Francisco and Los Angeles during the study at both RTA implementation and comparison sites. The authors would also like to thank Grant Colfax, Sophia Rumanes, Duncan Mackellar, and James Heffelfinger for their support during the development and implementation of this project and Chun-Mai Kuo and other staff in the HIV epidemiology sections of both health departments for maintaining and providing complete and accurate data for the evaluation. Most importantly, the authors would like to thank the HIV counselors and other staff at the HIV testing sites for their support of the project. The findings and conclusion in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
This study was funded by the CDC under cooperative agreement PS06-002.
Footnotes
Preliminary findings from this study were presented in part at the 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, February 28 to March 3, 2011.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
REFERENCES
- 1.CDC Rapid HIV testing in outreach and other community settings—United States, 2004–2006. MMWR Morb Mortal Wkly Rep. 2007;56:1233–1237. [PubMed] [Google Scholar]
- 2.Bowles KE, Clark HA, Tai E, et al. Implementing rapid HIV testing in outreach and community settings: results from an advancing HIV prevention demonstration project conducted in seven US cities. Public Health Rep. 2008;123(suppl 3):78–85. doi: 10.1177/00333549081230S310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC Notice to readers: protocols for confirmation of reactive rapid HIV tests. MMWR Morb Mortal Wkly Rep. 2004;53:221–222. [Google Scholar]
- 4.CDC. HRSA [Accessed May 27, 2015];Dear Colleague Letter 2-25-2013. Available from: http://www.cdc.gov/hiv/pdf/testing_dcl_hrsa_cdc_2013.pdf.
- 5.Centers for Disease Control and Prevention. Association of Public Health Laboratories [Accessed May 27, 2015];Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. Available at: http://stacks.cdc.gov/view/cdc/23447.
- 6.Piatek AS, Paul SM, Ibrahim AR, et al. Single rapid HIV testing and entry into care: experience in NJ, 2005-2006. Presented at: The 2010 HIV Diagnostics Conference; Orlando, FL. March 24–26, 2010; [Accessed May 27, 2015]. Available from: http://www.hivtestingconference.org/hivtesting2010/pdf/posters/piatek.pdf. [Google Scholar]
- 7.Clark HA, Bowles KE, Song B, et al. Implementation of rapid HIV testing programs in community and outreach settings: perspectives from staff at eight community-based organizations in seven US cities. Public Health Rep. 2008;123(suppl 3):86–93. doi: 10.1177/00333549081230S311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torian LV, Wiewel EW, Liu KL, et al. Risk factors for delayed initiation of medical care after diagnosis of human immunodeficiency virus. Arch Intern Med. 2008;168:1181–1187. doi: 10.1001/archinte.168.11.1181. [DOI] [PubMed] [Google Scholar]
- 9.Bucher JB, Thomas KM, Guzman D, et al. Community-based rapid HIV testing in homeless and marginally housed adults in San Francisco. HIV Med. 2007;8:28–31. doi: 10.1111/j.1468-1293.2007.00423.x. [DOI] [PubMed] [Google Scholar]
- 10.CDC Revised guidelines for HIV counseling, testing, and referral. MMWR Recomm Rep. 2001;50:1–58. [PubMed] [Google Scholar]
- 11.Delaney KP, Branson BM, Uniyal A, et al. Evaluation of the performance characteristics of 6 rapid HIV antibody tests. Clin Infect Dis. 2011;52:257–263. doi: 10.1093/cid/ciq068. [DOI] [PubMed] [Google Scholar]
- 12.Approval Letter: Insti HIV-1 Antibody Test Kit. Department of Health and Human Services, Food and Drug Administration; [Accessed September 11, 2015]. 2010. Available at: http://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/ucm235239.htm. [Google Scholar]
- 13.Approval Letter: Alere Determine HIV-1/2 Ag/Ab Combo. Department of Health and Human Services, Food and Drug Administration; [Accessed September 11, 2015]. 2013. Available at: http://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/ucm364653.htm. [Google Scholar]
- 14.Plate DK. Evaluation and implementation of rapid HIV tests: the experience in 11 African countries. AIDS Res Hum Retroviruses. 2007;23:1491–1498. doi: 10.1089/aid.2007.0020. [DOI] [PubMed] [Google Scholar]
- 15.Rouet F, Ekouevi DK, Inwoley A, et al. Field evaluation of a rapid human immunodeficiency virus (HIV) serial serologic testing algorithm for diagnosis and differentiation of HIV type 1 (HIV-1), HIV-2, and dual HIV-1-HIV-2 infections in West African pregnant women. J Clin Microbiol. 2004;42:4147–4153. doi: 10.1128/JCM.42.9.4147-4153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato PA, Maskill WJ, Tamashiro H, et al. Strategies for laboratory HIV testing: an examination of alternative approaches not requiring Western blot. Bull World Health Organ. 1994;72:129–134. [PMC free article] [PubMed] [Google Scholar]
- 17.Stetler HC, Granade TC, Nunez CA, et al. Field evaluation of rapid HIV serologic tests for screening and confirming HIV-1 infection in Honduras. AIDS. 1997;11:369–375. doi: 10.1097/00002030-199703110-00015. [DOI] [PubMed] [Google Scholar]
- 18.WHO-UNAIDS Joint United Nations Programme on HIV/AIDS (UNAIDS)-WHO Revised recommendations for the selection and use of HIV antibody tests. Wkly Epidemiol Rec. 1997;72:81–87. [PubMed] [Google Scholar]
- 19.Association of Public Health Laboratories. Centers for Disease Control and Prevention [Accessed September 11, 2015];HIV Testing Algorithms: A Status Report. 2009 Available at: http://www.aphl.org/aphlprograms/infectious/hiv/Documents/ID_2009April_HIV-Testing-Algorithms-Status-Report.pdf.
- 20.Martin EG, Salaru G, Paul SM, et al. Use of a rapid HIV testing algorithm to improve linkage to care. J Clin Virol. 2011;52(suppl 1):S11–S15. doi: 10.1016/j.jcv.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Stevinson K, Martin EG, Marcella S, et al. Cost effectiveness analysis of the New Jersey rapid testing algorithm for HIV testing in publicly funded testing sites. J Clin Virol. 2011;52(suppl 1):S29–S33. doi: 10.1016/j.jcv.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 22. [Accessed September 11, 2015];National HIV/AIDS Strategy. Available from: http://whitehouse.gov/sites/default/files/uploads/NHAS.pdf.
- 23.Rurangirwa J, Janson M, Kerndt PR, King J. Use of a Three Rapid HIV Test Algorithm at Point-of-care Settings: County of Los Angeles, Department of Public Health Experience. Presented at The 2010 HIV Diagnostics Conference; Orlando, FL. March 24–26, 2010; [Accessed September 11, 2015]. Available from: http://www.hivtestingconference.org/hivtesting2010/PDF/Presentations/Rurangirwa.pdf. [Google Scholar]
- 24.Knoble T, Dowling T, Underwood N, Colfax G. San Francisco’s Experience with Rapid Testing Algorithms (RTAs). Presented at The 2010 HIV Diagnostics Conference; Orlando, FL. March 24–26, 2010; [Accessed September 11, 2015]. Available from: http://www.hivtestingconference.org/hivtesting2010/PDF/Presentations/Knoble.pdf. [Google Scholar]
- 25. [Accessed September 11, 2015];San Francisco Department of Public Health. HIV Counseling Information Form. 2010 Available at: http://www.sfhiv.org/documents/SANFRANCISCOHIVCOUNSELINGINFORMATIONFORM2010.pdf.
- 26.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed Lippincott Williams and Wilkins; Philadelphia, PA: 2008. [Google Scholar]
- 27.Kleinbaum DG, Klein M. Survival Analysis: A Self-learning Text. 3rd ed Springer Science and Business Media; New York, NY: 2012. [Google Scholar]
- 28.McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 29.Kleinbaum DG, Klein M. Logistic Regression: A Self-learning Text. 3rd ed Springer Science and Business Media; New York, NY: 2010. [Google Scholar]
- 30.Vanderweele TJ. Sufficient cause interactions and statistical interactions. Epidemiology. 2009;20:6–13. doi: 10.1097/EDE.0b013e31818f69e7. [DOI] [PubMed] [Google Scholar]
- 31.Vanderweele TJ. On the distinction between interaction and effect modification. Epidemiology. 2009;20:863–871. doi: 10.1097/EDE.0b013e3181ba333c. [DOI] [PubMed] [Google Scholar]
- 32.Knol MJ, Vanderweele TJ, Groenwold RH, et al. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol. 2011;26:433–438. doi: 10.1007/s10654-011-9554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knol MJ, Vanderweele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41:514–520. doi: 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CDC [Accessed September 11, 2015];FDA-approved Rapid HIV Antibody Screening Tests. 2008 Available at: http://www.cdc.gov/hiv/pdf/testing_listnonclinicalsettings.pdf.
- 35.Delaney KP. Comparing the performance of the APHL/CDC proposed POC testing strategies and other potential options using data from the CDC’s evaluation of FDA approved rapid tests. Presented at: The 2007 HIV Diagnostics Conference; Atlanta, GA. 2007. [Google Scholar]
- 36.CDC False-positive oral fluid rapid HIV tests—New York City, 2005-2008. MMWR Morb Mortal Wkly Rep. 2008;57:1–5. [PubMed] [Google Scholar]
- 37.Delaney KP, Branson BM, Uniyal A, et al. Performance of an oral fluid rapid HIV-1/2 test: experience from four CDC studies. AIDS. 2006;20:1655–1660. doi: 10.1097/01.aids.0000238412.75324.82. [DOI] [PubMed] [Google Scholar]
- 38.Catz SL, McClure JB, Jones GN, et al. Predictors of outpatient medical appointment attendance among persons with HIV. AIDS Care. 1999;11:361–373. doi: 10.1080/09540129947983. [DOI] [PubMed] [Google Scholar]
- 39.Tobias CR, Cunningham W, Cabral HD, et al. Living with HIV but without medical care: barriers to engagement. AIDS Patient Care STDS. 2007;21:426–434. doi: 10.1089/apc.2006.0138. [DOI] [PubMed] [Google Scholar]
- 40.Torian LV, Wiewel EW. Continuity of HIV-related medical care, New York City, 2005-2009: do patients who initiate care stay in care? AIDS Patient Care STDS. 2011;25:79–88. doi: 10.1089/apc.2010.0151. [DOI] [PubMed] [Google Scholar]
- 41.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19:423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 42.Gardner LI, Marks G, Craw J, et al. Demographic, psychological, and behavioral modifiers of the Antiretroviral Treatment Access Study (ARTAS) intervention. AIDS Patient Care STDS. 2009;23:735–742. doi: 10.1089/apc.2008.0262. [DOI] [PubMed] [Google Scholar]
- 43.Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr. 2008;47:597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 44.Bradford JB. The promise for outreach engaging and retaining out-of-care persons in HIV medical care. AIDS Patient Care STDS. 2007;21(suppl 1):85–91. doi: 10.1089/apc.2007.9983. [DOI] [PubMed] [Google Scholar]
- 45.Bradford JB, Coleman S, Cunningham W. HIV System Navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS. 2007;21(suppl 1):49–58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- 46.Rajabiun S, Mallinson RK, McCoy K, et al. “Getting me back on track”: the role of outreach interventions in engaging and retaining people living with HIV/AIDS in medical care. AIDS Patient Care STDS. 2007;21(suppl 1):S20–S29. doi: 10.1089/apc.2007.9990. [DOI] [PubMed] [Google Scholar]
- 47.Cabral HJ, Tobias C, Rajabiun S, et al. Outreach program contacts: do they increase the likelihood of engagement and retention in HIV primary care for hard-to-reach patients? AIDS Patient Care STDS. 2007;21(suppl 1):59–67. doi: 10.1089/apc.2007.9986. [DOI] [PubMed] [Google Scholar]
- 48.Mugavero MJ, Norton WE, Saag MS. Health care system and policy factors influencing engagement in HIV medical care: piecing together the fragments of a fractured health care delivery system. Clin Infect Dis. 2011;52(suppl 2):S238–S246. doi: 10.1093/cid/ciq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. doi: 10.1037/a0031034. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stekler JD, Swenson PD, Coombs RW, et al. HIV testing in a high-incidence population: is antibody testing alone good enough? Clin Infect Dis. 2009;49:444–453. doi: 10.1086/600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel P, Mackellar D, Simmons P, et al. Detecting acute human immunodeficiency virus infection using 3 different screening immunoassays and nucleic acid amplification testing for human immunodeficiency virus RNA, 2006-2008. Arch Intern Med. 2010;170:66–74. doi: 10.1001/archinternmed.2009.445. [DOI] [PubMed] [Google Scholar]
- 52.Stekler JD, O’Neal JD, Lane A, et al. Relative accuracy of serum, whole blood, and oral fluid HIV tests among Seattle men who have sex with men. J Clin Virol. 2013;58(suppl 1):e119–e122. doi: 10.1016/j.jcv.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hutchinson AB, Patel P, Sansom SL, et al. Cost-effectiveness of pooled nucleic acid amplification testing for acute HIV infection after third-generation HIV antibody screening and rapid testing in the United States: a comparison of three public health settings. PLoS Med. 2010;7:e1000342. doi: 10.1371/journal.pmed.1000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wesolowski LG, Wroblewski K, Bennett SB, et al. Nucleic acid testing by public health referral laboratories for public health laboratories using the U.S. HIV diagnostic testing algorithm. J Clin Virol. 2015;65:6–10. doi: 10.1016/j.jcv.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pilcher CD, Louie B, Facente S, et al. Performance of rapid point-of-care and laboratory tests for acute and established HIV infection in San Francisco. PLoS One. 2013;8:e80629. doi: 10.1371/journal.pone.0080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masciotra S, Luo W, Youngpairoj AS, et al. Performance of the Alere Determine HIV-1/2 Ag/Ab Combo Rapid Test with specimens from HIV-1 seroconverters from the US and HIV-2 infected individuals from Ivory Coast. J Clin Virol. 2013;58(suppl 1):e54–e58. doi: 10.1016/j.jcv.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartz S, Hsu L, Chu PL, et al. Evaluation of a non-name-based HIV reporting system in San Francisco. J Acquir Immune Defic Syndr. 2002;29:504–510. doi: 10.1097/00042560-200204150-00011. [DOI] [PubMed] [Google Scholar]
- 58.Chu PL, Hsu LC, Schwarcz S. Comparison of methods to measure completeness of HIV/AIDS reporting in San Francisco. Presented at the XVII International AIDS Conference; Mexico City, Mexico. August 3–8, 2008. [Google Scholar]
- 59.Hsu L, Schwarcz S. Laboratory testing among persons diagnosed with HIV/AIDS in San Francisco, United States, 2002-2004. Presented at the XVI International AIDS Conference; Toronto, Canada. August 13–18, 2006. [Google Scholar]
- 60.Richardson-Moore A. Two rapid test strategy in anonymous HIV counseling and testing (ACT) sites in New York state 2008-2009. Presented at The 2010 HIV Diagnostics Conference; Orlando, FL. March 24–26, 2010; [Accessed May 27, 2015]. Available from: http://www.hivtestingconference.org/hivtesting2010/PDF/Presentations/Richardson-Moore.pdf. [Google Scholar]
- 61.CDC [Accessed September 11, 2015];Planning and Implementing HIV Testing and Linkage Programs in Non-clinical Settings. 2012 Available from: https://effectiveinterventions.cdc.gov/docs/default-source/public-health-strategies-docs/HIVTestingImplementationGuide_Final.pdf.
- 62.CDC Revised surveillance case definition for HIV infection–United States, 2014. MMWR Recomm Rep. 2014;63:1–10. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.