Abstract
Human health risk assessments continue to evolve and now focus on the need for cumulative risk assessment (CRA). CRA involves assessing the combined risk from coexposure to multiple chemical and nonchemical stressors for varying health effects. CRAs are broader in scope than traditional chemical risk assessments because they allow for a more comprehensive evaluation of the interaction between different stressors and their combined impact on human health. Future directions of CRA include greater emphasis on local-level community-based assessments; integrating environmental, occupational, community, and individual risk factors; and identifying and implementing common frameworks and risk metrics for incorporating multiple stressors.
INTRODUCTION
The methodology, practice, and breadth of human health risk assessments have evolved over the last several decades and are expected to continue to advance in the future. In particular, an awareness of children’s dietary and nondietary exposures to multiple pesticides in food that have a common toxic effect1 led to the 1996 Food Quality Protection Act (FQPA), which directed the U.S. Environmental Protection Agency (EPA) to move beyond single chemical assessments and focus on the aggregate and cumulative effects of simultaneous chemical exposures. Increasingly, risk assessments must also address subtle exposures and chronic effects, requiring a more in-depth evaluation of the combined effects of multiple low-level exposures than simpler approaches that have been used historically. CRA holds promise for transforming traditional health risk assessments beyond single chemicals/stressors, exposure routes/pathways, and health end points/effects.2 Cumulative risk is defined as the combined risks from aggregate exposures to multiple chemicals and other stressors, while CRA is the analysis, characterization, and potential quantification of these combined risks.1,3 CRAs are broader in scope than the traditional health risk assessment paradigm and consist of several key components (see Table 1).
Table 1.
Key Components of CRA
|
Although CRAs have been conducted for certain chemical groupings, such as pesticides,4 dioxins,5 and phthalates,6 these assessments have not accounted for all of the factors envisioned for a complete and comprehensive CRA and much work remains to be done. The purpose of this article is to (1) provide an overview of the CRA framework developed by the EPA, (2) describe existing methods that have been used to evaluate cumulative exposures and risks in the United States and Europe, and (3) highlight efforts to extend CRA beyond traditional contexts, frameworks, and risk metrics. Along with other evolving methods and advanced risk initiatives, CRA offers potential novel opportunities for improving the risk assessment process and its application to various settings.7
CUMULATIVE RISK ASSESSMENT FRAMEWORK
The EPA8–10 framework and supporting guidance for conducting CRAs parallels the general framework for health risk assessment in the United States.3,11,12 EPA’s CRA framework consists of three main phases: (1) planning, scoping, and problem formulation; (2) analysis; and (3) interpretation and risk characterization (see Table 2). The first phase establishes the purpose, goals, and scope of the assessment and completes the conceptual model and analysis plan. The second phase integrates the hazard, exposure, and dose–response information in order to characterize the combined effects of multiple stressors, in addition to developing exposure profiles and cumulative exposure estimates. Difficult technical issues (e.g., stressor interactions, relevant analytical approaches, common metrics), vulnerable populations, and time-related aspects of exposure are addressed during the analysis phase. The final phase describes important assumptions, limitations, and uncertainties associated with the assessment and interprets the estimates of cumulative risk in the context of their significance, reliability, and overall confidence. The CRA framework is intended to support broader risk-based decision-making efforts by considering risk-management options or interventions early on in the process.3
Table 2.
| phase | steps | tasks |
|---|---|---|
| planning, scoping, and problem formulation | planning and scoping | define purpose, scope, participants, approach, resources, experience |
| problem formulation | develop conceptual model (i.e., define sources, stressors, pathways/routes, receptors, end points), develop analysis plan (i.e., describe methods, models, data gaps, uncertainties), and define appropriate risk metrics (i.e., define outcomes or benchmarks related to adversity and consequences, countervailing risks, risks and benefits) | |
| possible outcomes | discuss possible outcomes to ensure scope and methods align with needs of the assessment | |
| analysis | integration of exposure, hazard, and dose–response information | consider time-related aspects (i.e., time sequence or life-stage), vulnerability (i.e., susceptibility to harm), and subpopulations with special exposure In order of increasing complexity:
|
| interpretation and risk characterization | risk description | describe risk (i.e., describe probability of harm based on central tendency or high-end risk, individual vs population risk, risk to important subpopulations) |
| uncertainty analysis | be explicit about uncertainty (i.e., describe uncertainty vs variability, uncertainty and risk addition, sensitive parameters) |
METHODS FOR EVALUATING CUMULATIVE RISKS
Aggregate/Cumulative Exposure Models
Numerous exposure assessment models have been developed and used by the EPA for regulatory, voluntary, and research purposes.13 These include aggregate and cumulative exposure models used by the Office of Pesticide Programs in response to FQPA to predict dietary and residential exposures to pesticides for the general population or specific subgroups in support of registration and reregistration activities (see Table 3). Important and necessary features of these models include the ability to (1) assess the co-occurrence of different pesticide residues; (2) integrate exposure through food, water, and residential pathways to reflect both the probability of exposure by any given pathway and the timing of exposures through different pathways; and (3) preserve linkages between spatial, temporal, and demographic aspects of exposure for defined individuals or population members.4,14 Because modeled estimates account for the variability in human exposures they are considered more representative of population-level risks, rather than individual risks, and are considered health protective at the upper percentiles of exposure.
Table 3.
EPA Aggregate/Cumulative Exposure Models
| model | web site link |
|---|---|
| dietary exposure evaluation model (DEEM) | http://www.epa.gov/pesticides/science/deem/ |
| calendex | http://epa.gov/pesticides/science/calendex/ |
| cumulative and aggregate risk evaluation system (CARES) | http://www.epa.gov/scipoly/sap/tools/atozindex/cares.htm |
| lifeline | http://cfpub.epa.gov/crem/knowledge_base/crem_report.cfm?deid=152263 |
| stochastic human exposure and dose simulation model (SHEDS)-multimedia | http://www.epa.gov/heasd/products/sheds_multimedia/sheds_mm.html |
These models also share many commonalities with respect to exposure routes and pathways, model inputs and outputs, model steps and capabilities, and model evaluation efforts. For example, these models can include multiple chemicals with a common mechanism of toxicity, multiple routes of exposure (i.e., oral, dermal, inhalation), and multiple pathways of exposure (e.g., food, drinking water, air, indoor surfaces), resulting in multichemical/multiroute/multipathway assessments. Additionally, these models follow the same general steps: (1) simulate an individual and their activity patterns throughout the day; (2) combine activity information, consumption patterns, residue concentrations, and exposure factors in exposure algorithms; and (3) simulate population estimates using probabilistic sampling (i.e., the variability in population exposures is accounted for by running simulations for many individuals and then aggregating across all individuals). However, specific features of these models may differ, including the reliance on different data sources, assumptions, or algorithms (e.g., the SHEDS model is capable of simulating longitudinal activity patterns). Modeled exposures are typically expressed as absorbed or potential doses (in units of mg/kg-day) for daily and chronic scenarios, and are represented by distributions that account for regional and temporal variations among populations. Cumulative risks are estimated by comparing predicted exposures across multiple pathways to toxicity benchmarks, such as EPA’s reference doses. Because these particular models are designed to support higher-tiered (vs screening-level) assessments, they have undergone extensive peer review, including external reviews by EPA’s Federal Insecticide, Fungicide, and Rodenticide Act Science Advisory Panel. Several approaches have also been used to evaluate these models or their components including comparing modeling results to environmental monitoring or market survey data, biomonitoring data, or each other (i.e., model-to-model comparisons).
Although health-based and regulatory drivers in the United States have been a strong impetus for CRA, the concept is recognized and maturing in a global context, and the International Program for Chemical Safety (IPCS) has published guidance on cumulative risk assessment.15 In Europe, a 5-year research project called NoMiracle (NOvel Methods for Integrated Risk Assessment of CumuLative stressors in Europe) has also resulted in the development of novel exposure assessment models and tools, including methods related to evaluating the degradation, fate, sampling, pollutant pathways, and spatial variability of exposure concentrations of chemicals and mixtures in the environment.16
Cumulative Toxicity/Risk Methods
Although characterizing health risks from multiple stressors is one of the most challenging aspects of the CRA approach, advanced dose–response and risk characterization methods and tools are being developed to address cumulative health risks. However, the complexity of CRAs should follow a tiered approach, in which more refined data and sophisticated techniques are invoked only when simpler health-protective methods and assumptions indicate a concern or impact decision-making.3,15 Current risk assessment guidance on chemical mixtures utilizes a decision-tree approach where whole mixture testing data are preferred, and in the absence of such data, a component-based approach is recommended.17 For noncarcinogens, the simplest form of this approach entails calculating the ratio of the level of exposure to the safe dose for each chemical (i.e., hazard quotient, HQ) and then summing all HQs to estimate the combined risk for the entire mixture (i.e., hazard index, HI) or only the components that have the same toxic effect or affect the same target organ.18 The greater the HQ/HI is above the value of one, the greater the concern for adverse health effects. For carcinogens, rather than summing the HQs, the estimated population lifetime cancer risk for the various mixture components is added regardless of the tumor type or its origin.
For higher-tiered assessments, the EPA recommends modifications to these approaches that incorporate mathematical interaction terms to account for additional toxicology understanding.17 This addresses a fundamental limitation of screening-level risk assessments that rely on the default assumption of additivity of dose or risk for mixed stressor exposure, because in reality, interactions that increase the risk (i.e., synergism) or decrease the risk (i.e., antagonism) are possible. For example, it is well-documented that the interaction between occupational exposure to asbestos and smoking significantly increases the lung cancer risk. Interaction profiles have recently been developed for common stressors of concern at contaminated sites in the United States, such as arsenic, cadmium, chromium, and lead.19 Methods to analyze and incorporate data on possible stressor interactions under environmentally relevant exposure scenarios continue to evolve.20,21 Because many chemical interactions are driven by interactions in toxicokinetic behavior, advances in physiologically based pharmacokinetic modeling are being used to enhance such assessments.22 Along these lines, novel approaches for evaluating the cumulative effects of chemical and nonchemical stressors have been developed under the European NoMiracle research project, including methods that assess the joint effects of chemical mixtures (including integrating all end points and combining toxicokinetics and toxicodynamics) and interactions between chemical and natural stressors.16 Major findings from this research include the importance of understanding time-dependent toxicity and mechanistic processes, and the need to focus on receptors rather than chemicals or predefined stressors.
Advances in biology are allowing for further refinements to the mixture-based approach by combining the effects of chemicals in the same toxicological class based on the potency of an index chemical or centered on a similar toxicological effect.8,9,17 These refinements require an assessment of a chemical’s toxic mode of action (MOA)—that is, the key steps in the biological process that lead from exposure to the onset of clinically relevant health effects.23,24 Chemicals that act via the same MOA are evaluated together in a CRA. One technique is to evaluate a chemical in terms of its potency relative to an index chemical. Specifically, a toxic equivalency factor (TEF) for each chemical is multiplied by the actual concentration of that chemical to derive a dose equivalent to the index chemical. Current risk assessments for certain dioxins and related chemicals use this approach.5 However, the TEF method assumes that the most sensitive adverse effects for all chemicals included in the assessment are driven by the same underlying MOA as the index chemical (e.g., arylhydrocarbon receptor activation for dioxins). Another technique is to identify a common toxic effect and estimate the relative potency inducing this effect for all chemicals that have the same MOA. This approach, which requires the calculation of a relative potency factor (RPF), has been used for organophosphate pesticides that act via inhibition of acetylcholine esterase.4 However, since the RPF method is centered on a sentinel toxic effect, chemicals included in the CRA may have other toxic effects that are more sensitive and which are not fully addressed in the cumulative risk estimate. The TEF and RPF methods are conceptually similar, but the latter is centered on the adverse effect and requires less detailed information on the underlying biological mechanisms involved. A key challenge for both methods is identifying the most appropriate end points for inclusion in a CRA, and it has been recommended that CRAs focus on chemicals or substances with a common adverse outcome regardless of mechanism or MOA.6
MOVING BEYOND TRADITIONAL CONTEXTS
Community-Based Assessments
Concerns about environmental justice issues and health inequities have led to greater interest in conducting CRAs at the local-scale community level. The intended focus of these efforts is on quantitatively assessing and prioritizing risks within individual communities using site-specific information and data on stressors most relevant for that community. For example, the EPA has initiated the Cumulative Communities Research Program in order to develop, evaluate, and apply exposure models and tools for use in community-based CRAs.25,26 In particular, the Community-Focused Exposure and Risk Screening Tool (C-FERST) is a web-based “flagship tool” under development by EPA for supporting community-level multi-media assessments. C-FERST is intended to be a one-stop shopping tool for communities that provides (1) access to relevant exposure and risk-related information and resources, (2) maps of local demographic data and environmental pollutant concentrations, (3) the ability to generate community issue profiles, and (4) links to guidance documents and best practices in other communities.27 The ultimate goal of this tool includes characterizing cumulative risks within communities and at the individual-level, identifying “hot spots” and vulnerable communities, and prioritizing community risk issues. Current priority environmental issues include diesel exhaust from traffic, selected toxic substances (e.g., benzene, lead, mercury), childhood asthma, lung cancer from radon and second-hand smoke, and early neurotoxicity effects. Although the test version of C-FERST is limited to chemical stressors, anticipated future enhancements include incorporating other risk-modifying factors. Prior to its public release, C-FERST is being pilot tested in several communities nationwide, which is expected to further refine the tool and expand its applicability and transferability.26
State environmental agencies are in the process of developing and evaluating similar types of methods for assessing cumulative impacts in communities. For example, New Jersey has developed a preliminary screening tool designed to integrate various environmental measures or indicators with demographic and socioeconomic factors in order to identify communities of concern.28 California has proposed a new screening methodology for assessing the combined effects of various pollutants in communities, particularly in situations where multiple pollution sources may be disproportionately concentrated or there is the potential for increased sensitivity to pollution in a population.29 Both tools are screening approaches intended to rank order and identify communities with the greatest cumulative impacts for priority setting purposes, but do not provide quantitative estimates of community-health risk.
Accounting for Occupational Risk Factors
Occupational risk factors, such as workplace conditions and chemical and nonchemical stressor exposures, have long been known to compromise the health of workers.30 Although chemical risk assessments have traditionally focused on inhalation exposures, greater attention is being paid to integrating dose across exposure routes (e.g., inhalation and dermal) as part of aggregate risk assessments in the workplace. Further consideration of aggregate risks from multiple sources and pathways are addressed through the use of measured internal doses with comparison to exposure guides, such as biological exposure indices (BEIs).31
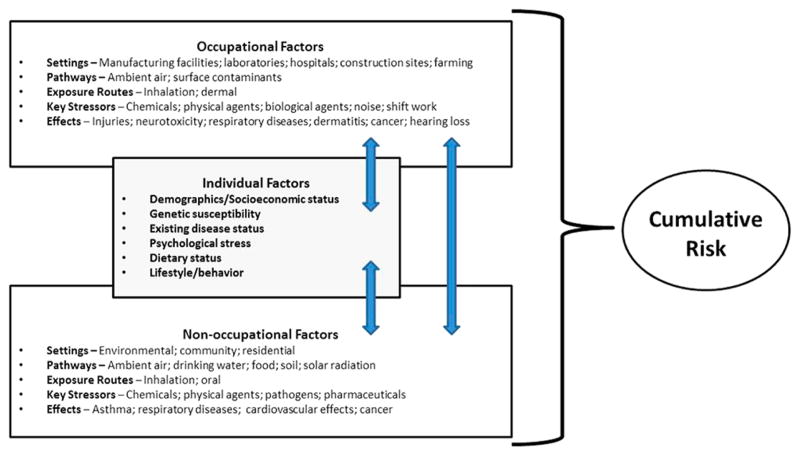
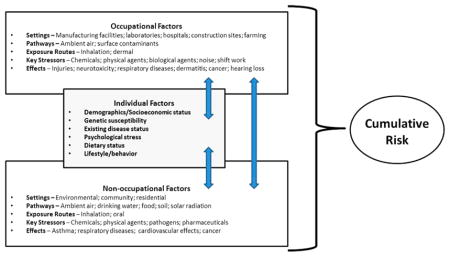
Despite the recognition that human health risks are often driven by occupational risk factors, CRAs conducted to date have focused solely on community or environmental exposures to chemical stressors, and have not attempted to integrate these contexts with occupational settings. While many models exist for evaluating either occupational or nonoccupational exposures, there are currently no well-vetted CRA models that are capable of integrating total exposure across these two domains. Depending on the scenario, the failure to include occupational risk factors in a CRA could substantially affect the utility of such an assessment. For example, hearing loss is associated with an array of factors, including genetics, age, exposure to noise, and exposure to certain ototoxicants, such as lead and toluene.32,33 Because many of these risk factors are not unique to a single setting and are encountered in the general environment, workplace, and/or community, multiple sources of exposure can contribute to the cumulative risk for hearing loss. Accounting for occupational risk factors may not be necessary for all CRAs, but these should at least be considered early on in the assessment to ensure that dominant risk factors are not overlooked or resources are not expended on noncritical risk factors. Additional considerations will be needed to address regulatory and other structures that have traditionally separated the assessment of occupational and nonoccupational health risk factors.
In an effort to more thoroughly address the role of the work environment on the overall health of individuals, the National Institute for Occupational Safety and Health has initiated the Total Worker Health program.34 This strategic initiative promotes an integrated approach to occupational safety and health that focuses on understanding the impact of the interactions between the workplace and individual lifestyle risk factors, such as age, educational level, or preexisting medical conditions. This program, which is comprised of multiple research efforts in the fields of medicine, social sciences, economics, and health sciences, complements existing approaches used to evaluate cumulative risks because it emphasizes the consideration and integration of different risk factors that have traditionally been considered separately. An example of research being conducted under this program relates to the impact of inadequate sleep on work safety and maintaining optimal health.35 Although this initiative is currently qualitative in nature, it is important because it sets the philosophical stage for linking chemical risk and lifestyle risk for workers. Another initiative currently being explored by NIOSH and others has been coined the “exposome,” which is defined as a measure of all internal and external exposures an individual receives over a lifetime, and is also intended to improve our understanding about how exposures from the environment, workplace, lifestyle, and other factors interact with individuals’ unique characteristics (e.g., genetics, physiology, epigenetic makeup, existing disease state) to cause disease.36,37
Going forward, refinements are needed to the CRA framework to allow for the identification and inclusion of the full range of relevant risk factors (including occupational risk factors) in assessments of cumulative risk (see Figure 1). Preferably, common metrics and algorithms will be developed and used to quantify cumulative risks so that different types of risks or individual risk factors can be compared and prioritized. There are many possible options for how this can be done (e.g., presentation of individual risk scores that can be rank-ordered or normalization of risk scores that can be aggregated to determine relative risk), but a hierarchy or suite of potential alternatives remains to be developed.
Figure 1.
Accounting for Occupational and Non-Occupational Risk Factors in CRAs.
MOVING BEYOND TRADITIONAL FRAMEWORKS AND RISK METRICS
Integrating Chemical and Non-Chemical Stressors
Despite being a key feature of the CRA approach, nonchemical stressors have not been routinely incorporated in quantitative CRAs. One important challenge is identifying which non-chemical stressors are most relevant for the populations and effects of interest and obtaining sufficient data on these stressors. For example, there are many different types of nonchemical stressors that can potentially enhance or attenuate the toxic effects of chemical or other nonchemical stressors including psychological stress, noise, sociodemographic factors and socioeconomic status, residential crowding, violence and crime, behavior and lifestyle characteristics, and occupational exposures and risk factors. There is currently no generally accepted list of nonchemical stressors or associated health outcomes that should be included in CRAs, and various stressors have been mentioned inconsistently in the literature.38
A major reason for this lack of consensus is the difficulties associated with quantifying dose and response metrics for this class of hazards. Simplified risk-assessment tools are needed to address the complexity of considering multiple factors simultaneously.3 For diverse stressors, an approach that integrates adverse health response across all stressors at a common end point or risk measure is needed. Such metrics might be at the community level (e.g., number of hospital visits) or at the individual or biological level (e.g., total level of serum inflammatory markers). Whatever approach is used, efforts to validate the quantitative relationship between stressors and health metrics will require significant research and several research efforts are underway that explore these issues. For example, a community-scale CRA of radon in the presence of smoking was conducted in order to establish a screening-level approach for well-known stressor interactions that could be generalized to other multiple stressor scenarios.39 Theoretical frameworks or “families” of conceptual models that have an established theoretical basis or have been empirically verified have also been proposed to support more realistic and reliable CRAs in the future.40,41 These include (1) social determinant models (i.e., health is regarded as a product of social factors), (2) health disparity models (i.e., health is regarded as a product of biological and contextual interactions), and (3) multiple stressor models (i.e., health is regarded as a product of exposure to environmental stressors).
Regardless of which stressors are identified or what type of framework is used to evaluate cumulative risks, common metrics will be needed to integrate exposure and effects data for chemical and nonchemical stressors. Resolution of such issues will require ongoing dialogue among stakeholders, development of cases studies, and guidance for application of novel techniques. Recognition of this need has led to various research alliances and collaborations that may serve as a useful model for how to solve the complexities associated with the CRA approach.42
Biomarker-Based Risk Assessment
One way to gain a better understanding of the cumulative impacts of disparate stressors is to identify common exposure and effect metrics as an integration point for the analysis, such as using biomarkers. Developments are well underway to address all three basic prongs of the biomarker spectrum: exposure, susceptibility, and effect.43 Perhaps most advanced is our progress related to integrating multiple sources and exposure pathways through increased access to measures of chemicals in biological tissues. Specifically, biological monitoring (or biomonitoring) is a method for assessing human exposure to chemicals by measuring the chemicals or their metabolites in human biological media (e.g., blood, urine, expelled air, hair, nails). Biomonitoring is therefore considered a “biomarker of exposure” in that chemicals that have entered the human body leave biologic indicators (markers) reffecting this exposure.44 Biomonitoring data provide a direct measure of how much of a chemical has been absorbed into the body from all potential sources, and many population-based biomonitoring efforts are underway.44,45 Biomonitoring data can be compared to toxicity benchmarks on the basis of internal doses (biological equivalents) derived from traditional safe doses for general population risk assessments.46 This type of approach is needed to put measured concentrations of chemicals in biological media in the general population into a risk context.
There has also been an increased interest in understanding the basis for human variability, including efforts to evaluate biomarkers of human susceptibility. For example, that National Institutes of Health is leading a program focused on developing innovative tools and technologies to determine how environ-mental exposures (including diet, physical activity, stress, drug use) contribute to human disease. While many individual factors contribute to human variability in susceptibility, in the context of biomarkers, significant attention has been paid to genetic determinants of variable response. For example, differences in drug metabolism due to polymorphism of xenobiotic metabolizing enzymes have a robust history of study (i.e., pharmacogenetics). The same enzyme systems are active in metabolizing environmental pollutants, and quantitative methods to address such polymorphisms in environmental settings have been demonstrated.47 Future advancements related to biomarkers of susceptibility will elucidate epigenetic effects (i.e., heritable changes in gene expression that are not due to changes in the underlying DNA sequence).
Although CRAs have been limited to common MOA categories from mixed chemical exposures or empirical effect measurements for binary chemical/nonchemical stressor interactions, this limitation may be partially addressed through the use of biomarkers of effect in the risk assessment process. The maturation of computational and systems biology approaches that use biomarkers of effect centered on common disease pathways has been touted as the future direction of risk assessments.48 Biomarkers of effect focus on early events in the sequence of biological events that lead from exposure to adverse health effects. Examples of such measures include activation of cellular receptors or the initial changes in gene expression patterns that arise from such interactions. Efforts to validate markers for early perturbations of normal cell or tissue homeostasis are the focus of many research efforts.49 The reliance on biomarkers to determine cumulative exposures and risks from community and occupational settings has many ethical considerations, and may prove controversial for employers and the public. For example, there will likely be concerns about maintaining the privacy of study participants and preventing the improper use of personal data, such as using biological specimens as a means of pre-employment screening (e.g., to identify or discriminate against individuals with pre-existing health conditions or genetic susceptibilities to work-related diseases).50
CONCLUSIONS
Human health may be negatively affected by an array of stressors arising from environmental, occupational, and community settings, in addition to lifestyle or behavioral risk factors and those unique to each individual. Assessing the risk associated with the combinations of and interactions between these stressors has not been possible using traditional health risk assessment approaches. Where beneficial, CRA has the potential to overcome these shortcomings, but the successful application of this innovative approach will likely require significant research and multidisciplinary expertise in public and occupational health, toxicology, epidemiology, environmental science, mathematics, and the social sciences. Many scientific and technical challenges must also be overcome to advance the principals and practice of CRA. These include (1) identifying relevant risk modifying factors and common effects, (2) integrating nonoccupational and occupational exposures, and (3) developing and implementing a cohesive common metric or framework for combining chemical and nonchemical stressors. Although much work is still needed, future enhancements to CRA may enable risk assessors and risk managers to identify the primary contributors to public health risk, thereby leading to better informed decisions and more effective risk reduction strategies. Moving forward on this initiative is timely given the significant emphasis on harmonization of risk assessment methods.51
Acknowledgments
We thank T. J. Lentz (NIOSH), Sudha Pandalai (NIOSH), Donna Heidel (NIOSH), Fred Boelter (ENVIRON), and Nancy Beck (American Chemistry Council) for their informal review of and insightful comments on this article. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health. No outside funding was received for this work.
Biographies
Pamela R. D. Williams, MS, Sc.D. is a Principal at E Risk Sciences, LLP, a small, women-owned business whose mission is to provide sound scientific analyses and tools to support risk-based decision-making related to human health and the environment. She is also a Clinical Assistant Professor in the Department of Environmental and Occupational Health at the Colorado School of Public Health, and a Fellow with the nonprofit organization Toxicology Excellence for Risk Assessment. Her primary areas of research and expertise relate to characterizing human exposures and health risks to chemicals in community and occupational settings.
G. Scott Dotson, Ph.D., CIH is an industrial hygienist with the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. His research interests focus on the development and implementation of scientific-decision making strategies designed to assess the health risks of occupational exposures to chemicals.
Andrew Maier, Ph.D., CIH, DABT is the Director of Toxicology Excellence for Risk Assessment. His research focuses on risk assessment methods for occupational and environmental chemical exposures. He serves as an Adjunct Associate Professor of Environ-mental Health at the University of Cincinnati and is a Toxicology Fellow with the National Institute for Occupational Safety and Health.
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Pesticides in the Diets of Infants & Children. National Research Council; The National Academies Press; Washington, DC: 1993. [PubMed] [Google Scholar]
- 2.Callahan MA, Sexton K. If cumulative risk assessment is the answer, what is the question? Environ Health Perspect. 2007;115:799–806. doi: 10.1289/ehp.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Science and Decisions: Advancing Risk Assessment. National Research Council; The National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- 4.Organophosphorus Cumulative Risk Assessment—2006 Update. United States Environmental Protection Agency; Washington, DC: 2006. [Google Scholar]
- 5.Recommended Toxicity Equivalence Factors (TEFs) for Human Health Risk Assessments of 2,3,7,8-Tetrachlorodibenzo-p-dioxin and Dioxin-Like Compounds. United States Environ-mental Protection Agency; Washington, DC: 2010. EPA/100/R 10/005. [Google Scholar]
- 6.Phthalates and Cumulative Risk Assessment: The Task Ahead. National Research Council; The National Academies Press; Washington, DC: 2008. [PubMed] [Google Scholar]
- 7.Williams PRD, Dotson GS, Maier A. Risk assessment’s new era. Part 2: Evolving methods and future directions. Synergist. 2012;23(5):46–48. [PMC free article] [PubMed] [Google Scholar]
- 8.Guidance on Cumulative Risk Assessment of Pesticide Chemicals That Have a Common Mechanism of Toxicity. United States Environmental Protection Agency; Washington, DC: 2002. [Google Scholar]
- 9.Framework for Cumulative Risk Assessment. United States Environmental Protection Agency; Washington, DC: 2003. EPA/630/P-02/001F. [Google Scholar]
- 10.Concepts, Methods and Data Sources for Cumulative Health Risk Assessment of Multiple Chemicals, Exposures and Effects: A Resource Document. United States Environmental Protection Agency; Washington, DC: 2007. EPA/600/R-06/013F. [Google Scholar]
- 11.Risk Assessment in the Federal Government: Managing the Process. National Research Council; The National Academies Press; Washington, DC: 1983. [PubMed] [Google Scholar]
- 12.Science and Judgment in Risk Assessment. National Research Council; The National Academies Press; Washington, DC: 1994. [PubMed] [Google Scholar]
- 13.Williams PRD, et al. An overview of exposure assessment models used by the U.S. Environmental Protection Agency. In: Hanrahan G, editor. Modelling of Pollutants in Complex Environmental Systems. Vol. 2. ILM Publications; United Kingdom: 2010. pp. 61–131. [Google Scholar]
- 14.Revised Organophosphorous Pesticide Cumulative Risk Assessment. United States Environmental Protection Agency; Washington, DC: 2002. [Google Scholar]
- 15.Meek ME, Boobis AR, Crofton KM, Heinemeyer G, Raaij MV, Vickers C. Risk assessment of combined exposure to multiple chemicals: A WHO/IPCS framework. Regul Toxicol Pharmacol. 2011;60:S1–S14. doi: 10.1016/j.yrtph.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 16.LØkke H. Novel methods for integrated risk assessment of cumulative stressors Results from the NoMiracle project. Sci Total Environ. 2010;408:3719–3724. doi: 10.1016/j.scitotenv.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. United States Environmental Protection Agency; Washington, DC: 2000. EPA/630/R-00/002. [Google Scholar]
- 18.Haber LT, et al. Noncancer risk assessment: principles and practice in environmental and occupational Settings. In: Bingham E, Cohrssen B, editors. Patty’s Toxicology. John Wiley & Sons, Inc; New York: 2012. pp. 89–132. [Google Scholar]
- 19. [accessed September 11, 2012];Interaction Profiles for Toxic Substances. www.atsdr.cdc.gov/interactionprofiles/index.asp.
- 20.Boobis A, Budinsky R, Collie S, Crofton K, Embry M, Felter S, Hertzberg R, Kopp D, Mihlan G, Mumtaz M, Price P, Solomon K, Teuschler L, Yang R, Zaleski R. Critical analysis of literature on low-dose synergy for use in screening chemical mixtures for risk assessment. Crit Rev Toxicol. 2011;41(5):369–383. doi: 10.3109/10408444.2010.543655. [DOI] [PubMed] [Google Scholar]
- 21.Rider CV, Dourson ML, Hertzberg RC, Mumtaz MM, Price PS, Simmons JE. Incorporating nonchemical stressors into cumulative risk assessments. Toxicol Sci. 2012;127:10–17. doi: 10.1093/toxsci/kfs088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haddad S, Beliveau M, Tardif R, Krishnan K. A PBPK modeling-based approach to account for interactions in the health risk assessment of chemical mixtures. Toxicol Sci. 2001;63:125–131. doi: 10.1093/toxsci/63.1.125. [DOI] [PubMed] [Google Scholar]
- 23.Boobis AR, Cohen SM, Dellarco V, McGregor D, Meek ME, Vickers C, Willcocks D, Farland W. IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol. 2006;36:781–792. doi: 10.1080/10408440600977677. [DOI] [PubMed] [Google Scholar]
- 24.Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Munn S, Ruchirawat M, Schlatter J, Seed J, Vickers C. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol. 2008;38:87–96. doi: 10.1080/10408440701749421. [DOI] [PubMed] [Google Scholar]
- 25.Barzyk TM, Conlon KC, Chahine T, Hammond DM, Zartarian V, Schultz BD. Tools available to communities for conducting cumulative exposure and risk assessments. J Exposure Sci Environ Epidemiol. 2010;20:371–384. doi: 10.1038/jes.2009.25. [DOI] [PubMed] [Google Scholar]
- 26.Zartarian VG, Schultz BD. The EPA’s human exposure research program for assessing cumulative risk in communities. J Exposure Sci Environ Epidemiol. 2010;20:351–358. doi: 10.1038/jes.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zartarian VG, Schultz BD, Barzyk TM, Smuts MB, Hammond DM, Medina-Vera M, Geller AM. The Environmental Protection Agency’s community-focused exposure and risk screening tool (C-FERST) and its potential use for environmental justice efforts. Am J Public Health. 2011;101(S1):S286–S294. doi: 10.2105/AJPH.2010.300087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.A Preliminary Screening Method to Estimate Cumulative Environmental Impacts. New Jersey Department of Environmental Protection; Trenton, NJ: 2009. [Google Scholar]
- 29.Cumulative Impacts: Building a Scientific Foundation. Office of Environmental Health Hazard Assessment, California Environmental Protection Agency; Sacramento, CA: 2010. [Google Scholar]
- 30.Schulte PA, Pandalai S, Wulsin V, Chun H. Interaction of occupational and personal risk factors in workforce health and safety. Am J Public Health. 2012;102(3):434–448. doi: 10.2105/AJPH.2011.300249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Documentation of the Threshold Limit Values and Biological Exposure Indices. American Conference of Governmental Industrial Hygienists; Cincinnati, OH: 2012. [Google Scholar]
- 32.Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol. 2009;30(2):139–145. doi: 10.1097/MAO.0b013e318192483c. [DOI] [PubMed] [Google Scholar]
- 33.Vyskocil A, Truchon G, Leroux T, Lemay F, Gendron M, Gagnon F, El Majidi N, Boudjerida A, Lim S, Emond C, Viau C. A weight of evidence for the assessment of the ototoxic potential of industrial chemicals. Toxicol Ind Health. 2011 doi: 10.1177/0748233711425067. [DOI] [PubMed] [Google Scholar]
- 34. [accessed September 11, 2012];NIOSH: Total Worker Health. www.cdc.gov/niosh/twh/
- 35. [accessed September 11, 2012];NIOSH: Sleep and Work. http://blogs.cdc.gov/niosh-science-blog/2012/03/sleep-and-work/
- 36.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 37. [accessed September 11, 2012];Exposome and Exposomics. www.cdc.gov/niosh/topics/exposome/
- 38.Lewis AS, Sas SN, Wason SC, Campleman SL. Non-chemical stressors and cumulative risk assessment: an overview of current initiatives and potential air pollutant interactions. Int J Environ Res Public Health. 2011;8:2020–2073. doi: 10.3390/ijerph8062020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chahine T, Schultz BD, Zartarian VG, Xue J, Subramanian SV, Levy JI. Modeling joint exposures and health outcomes for cumulative risk assessment: the case of radon and smoking. Int J Environ Res Public Health. 2011;8:3688–3711. doi: 10.3390/ijerph8093688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linder SH, Sexton K. Conceptual models for cumulative risk assessment. Am J Public Health. 2011;101(S1):S74–S81. doi: 10.2105/AJPH.2011.300318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sexton K, Linder SH. Cumulative risk assessment for combined health effects from chemical and nonchemical stressors. Am J Public Health. 2011;101(S1):S81–S88. doi: 10.2105/AJPH.2011.300118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. [accessed September 11, 2012];Beyond Science and Decisions: From Problem Formulation to Dose-Response Assessment. www.allianceforrisk.org/ARA_Dose-Response.htm.
- 43.Schulte PA, Hauser JE. The use of biomarkers in occupational health research, practice, and policy. Toxicol Lett. 2011;213(1):91–99. doi: 10.1016/j.toxlet.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 44.Human Biomonitoring for Environmental Chemicals. National Research Council; The National Academies Press; Washington, DC: 2006. [Google Scholar]
- 45. [accessed September 11, 2012];National Biomonitoring Program. www.cdc.gov/biomonitoring.
- 46.Hays SM, Aylward LL, LaKind JS, Bartels MJ, Barton HA, Boogaard PJ, Brunk C, DiZio S, Dourson M, Goldstein DA, Lipscomb J, Kilpatrick ME, Krewski D, Krishnan K, Nordberg M, Okino M, Tan YM, Viau C, Yager JW. Guidelines for the derivation of biomonitoring equivalents: report from the biomonitoring equivalents expert workshop. Regul Toxicol Pharmacol. 2011;51:S4–S15. doi: 10.1016/j.yrtph.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Gentry PR, Hack CE, Haber L, Maier A, Clewell HJ., 3rd An approach for the quantitative consideration of genetic polymorphism data in chemical risk assessment: examples with warfarin and parathion. Toxicol Sci. 2002;70:120–139. doi: 10.1093/toxsci/70.1.120. [DOI] [PubMed] [Google Scholar]
- 48.Toxicity Testing in the 21st Century: A Vision and a Strategy. National Research Council; The National Academies Press; Washington, DC: 2007. [Google Scholar]
- 49. [accessed September 11, 2012];Advancing the Next Generation of Risk Assessment. www.epa.gov/risk/nexgen/
- 50.Schulte PA, Hunter D, Rothman N. Ethical and social issues in the use of biomarkers in epidemiological research. IARC Sci Publ. 1997;142:313–318. [PubMed] [Google Scholar]
- 51. [accessed September 11, 2012];IPCS Harmonization Project. www.who.int/ipcs/methods/harmonization/en/