The human brain is a network of nearly 100 billion neurons forming upwards of quadrillion synapses. Exactly how this massively distributed information processor produces and represents mental content remains one of the most formidable mysteries in science. A growing number of researchers consider the comprehensive, detailed map of brain synaptic connections (the “connectome”) useful, even necessary, to crack the neural code. After all, how can we hope to understand the functional organization of such complex machinery without a reliable circuit blueprint? Hence the recent surge of connectomics, accompanied by claims that many neurological and psychiatric conditions, including Alzheimer’s disease, schizophrenia, depression, and autism spectrum disorders are connectopathies.
While most progress in deciphering the human connectome has leveraged non-invasive whole-brain imaging, those approaches only capture macroscopic regional connectivity without providing information on the axonal (output) and dendritic (input) wiring of individual neurons. In contrast, basic research in animal models relies on microscopy. Optical microscopy can scan sufficiently large fields of view to encompass the substantial brain span that the axon of a single projection neuron typically traverses, but (except for super-resolution) lacks the resolving power to definitively identify synapses. Electron microscopy (EM), conversely, can detect every last neurotransmitter vesicle, but alas only in a minuscule region of interest that is inadequate to capture the extent of just one long-range axon. A study recently reported in Cell by Kasthuri and colleagues1 leverages the full power of EM to report the complete volumetric reconstruction of the local surroundings of several dendritic trees in the mouse neocortex.
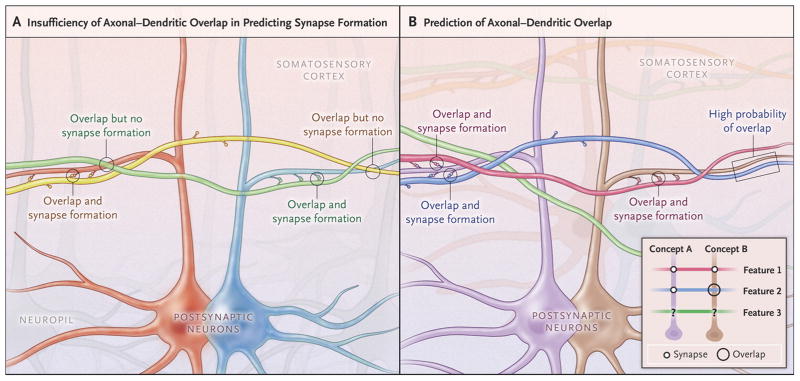
This work is noteworthy for three reasons. First, it explicitly demonstrates the massive scale of dense synaptic connectomics: notwithstanding considerable progress of enabling automation technologies both for raw data acquisition (histology and imaging) and computational analysis (tracing and annotation), the reconstructed region only amounts to a tiny proportion of a single mouse cortex. Second, the authors publicly shared the entire collection of original microscopic images as well as the analyzed subset of processed data online, providing a valuable resource for additional reconstruction and data mining. Third, quantification of the extracted circuit and its spatial embedding proved the exquisite selectivity of network connectivity: the physical proximity of axons and dendrites was not sufficient to predict synapse formation (Figure 1A). In other words, the probability of finding a synapse between two neurons is not proportional to the number of spatial overlaps between their respective axons and dendrites.
Figure 1.
Fundamental Rules of Neural Connectivity: Axonal-Dendritic Overlaps and Synaptic Selectivity. (A) The saturated reconstruction of a small volume of mouse somatosensory cortex by electron microscopy reported by Kasthuri et al. (ref. 1) demonstrated that spatial overlap of axonal and dendritic branches is strictly necessary, but not in and by itself sufficient, to predict synapse formation. Two neurons are schematically highlighted (red and blue) with their dendrites. Two axons are also colored (green and yellow) with their respective synaptic contacts, illustrating the selectivity of the circuit: although both axons are physically overlapping with both dendrites, the green axon mainly forms synapses with the blue dendrites, while the yellow axon mainly forms synapses with the red dendrite. The light grey axons and neurons/dendrites in the background illustrate that the reconstructed volume is dense with neuropil. (B) The two-way relationship between axonal-dendritic overlap and synaptic connectivity crucially constrains network circuitry. While synapses must necessarily represent a subset of axonal-dendritic overlaps, the synaptic connectome also provides information on the probability that two neurons might have an axonal-dendritic overlap. For instance, the dark blue axon is likely to overlap with the brown dendrite (arrows) because it synapses on another (purple) dendrite that is also contacted by a (green) axon also contacting the brown dendrite. The inset provides a schematic summary of this relationship (representing synapses as filled circles and axonal-dendritic overlaps as hollow black circles) under the simplifying assumption that post-synaptic neurons encode concepts and incoming axons encode related features.
What does this finding reveal about the relationship between neural structure and cognitive function? Since network connectivity constitutes the structural substrate for information transmission, the synaptic matrix determines the set of all possible activity patterns that a given brain can instantiate, that is, it determines the content of the subject’s memory. According to this logic, synaptic formation (and elimination) would then correspond to learning or forgetting2. Thus, axonal-dendritic overlaps constitute not only required conditions, but also potential opportunities to form new synapses. In Hebb’s “fire together, wire together” model of experience-dependent plasticity, two neurons form a synapse if their respective axons and dendrites are both mutually juxtaposing and consistently co-activated.
Even absent synapses, however, the set of axonal-dendritic overlaps in the cortex might have its own fundamental cognitive correlate. Wiring parsimony suggests that neuronal branches do not meander aimlessly: an axon passes near a dendrite only to contact another close dendrite. Similarly, two dendrites are likely to be neighbors if they receive many common inputs. Stated differently, adjacent neurons tend to encode similar content by virtue of optimal placement, consistent with the topographic organization of the cortex (Figure 1B). Therefore axonal-dendritic overlap also implies conceptual compatibility of the corresponding mental content3. For example, a hypothetical axon encoding sour-sweet taste, thanks to the multiple synapses it makes in the cortical region encoding perception of the tastes of fruit, is likely to overlap with a hypothetical taste-of-kumquats-encoding dendrite located in the same space. The prerequisite of physical proximity for synaptic formation could then explain why learning requires relevant background knowledge4. Recent computational studies show that this constraint also reduces the incidence of incorrectly learning spurious associations of randomly co-occurring events relative to real causal relations5. For instance, when eating for the first time a kumquat while listening to a song, it is easier to associate the fruit with its taste than with the melody.
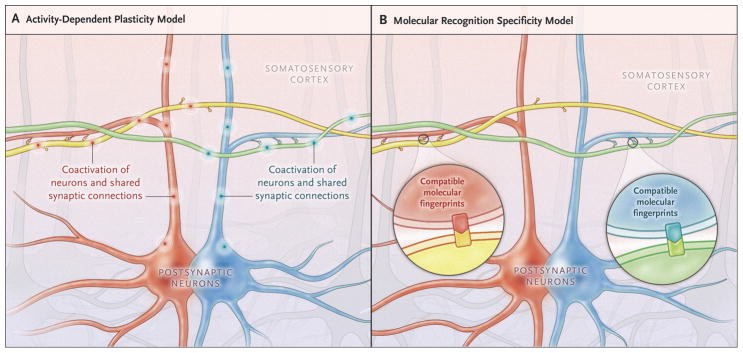
Because synaptic connectivity is also dependent on neuronal identity rather than only on location, Kasthuri and colleagues conclude that optical microscopy is insufficient for circuit mapping and EM is necessary. The two techniques could instead be viewed as addressing complementary questions: by tracking all synapses, EM might measure stored knowledge memorized from past experience. A map of axonal and dendritic arbor distributions obtained by optical microscopy, in contrast, could reveal potential future memories to be learned given appropriate experience. The observed selectivity of cortical synapses is consistent with this model of activity-dependent structural plasticity in which axonal-dendritic overlaps constitute not just probabilities, but rather capabilities, to form synapses (Figure 2A). A non-mutually-exclusive alternative could also explain synaptic selection by neuron-specific molecular recognition (Figure 2B). The distinction between these two mechanisms is clinically relevant given the promising prospects of pharmacological intervention on memory malfunction.
Figure 2. Models of Determinants of Synaptic Selectivity.
(A) Activity-dependent plasticity can explain synaptic selectivity. The animal can learn a number of associations (corresponding to all axonal-dendritic overlaps in its brain) by exposure to appropriate environmental stimuli. Of these, only those few stimuli that are in fact experienced (“fire together”) are reflected in actual synapses (“wire together”). In this example, the synaptic connections are consistent with past co-activation of the yellow axon with the red dendrite (pink discharges), but not with the blue dendrite, and vice versa for the green axon (turquoise discharges). (B) Alternatively, synaptic selectivity could rely on molecular recognition specificity. In this model, individual neurons would express a unique combination of genes and proteins allowing them to select their synaptic partners based on compatible molecular fingerprints.
Paradoxically, although EM was instrumental in providing evidence that synaptic formation is not just a random consequence of axonal-dendritic overlaps, optical microscopy currently appears better equipped both to record neuronal activity in vivo and to characterize intracellular biochemical content. Thus fully understanding the links between brain circuitry and computational function will likely require continuous parallel advancement of both approaches.
References
- 1.Kasthuri N, Hayworth KJ, Berger DR, et al. Saturated reconstruction of a volume of neocortex. Cell. 2015;162(3):648–61. doi: 10.1016/j.cell.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 2.Bailey CH, Kandel ER, Harris KM. Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb Perspect Biol. 2015;7(7) doi: 10.1101/cshperspect.a021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascoli GA. Trees of the brain, roots of the mind. Cambridge, MA: MIT Press; 2015. p. 248. [Google Scholar]
- 4.Sadtler PT, Quick KM, Golub MD, Chase SM, Ryu SI, Tyler-Kabara EC, Yu BM, Batista AP. Neural constraints on learning. Nature. 2014;512(7515):423–6. doi: 10.1038/nature13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mainetti M, Ascoli GA. A neural mechanism for background information-gated learning based on axonal-dendritic overlaps. PLoS Comput Biol. 2015;11(3):e1004155. doi: 10.1371/journal.pcbi.1004155. [DOI] [PMC free article] [PubMed] [Google Scholar]