Abstract
Women with benign heavy menstrual bleeding have the choice of a number of medical treatment options to reduce their blood loss and improve quality of life. The role of the clinician is to provide information to facilitate women in making an appropriate choice. Unfortunately, many options can be associated with hormonal side effects, prevention of fertility and lack of efficacy, leading to discontinuation and progression to surgical interventions. Herein, we discuss the various options currently available to women, including antifibrinolytics, nonsteroidal anti-inflammatory preparations, oral contraceptive pills and oral, injectable and intrauterine progestogens. In addition, we describe the more novel option of selective progesterone receptor modulators and their current benefits and limitations.
Keywords: : adenomyosis, endometrial, endometrium, estradiol, fibroid, menorrhagia, menstruation, polyps, progesterone, SPRM
Effective medical management of heavy menstrual bleeding (HMB) relies on excellent communication between a woman and her doctor. Information provision on mode of action, benefits, potential risks and alternatives of each option will allow a woman to choose the most appropriate treatment for her personal circumstances. Various medical treatment options are available, but many women proceed to surgery due to treatment failure or hormonal side effects. Surgery introduces risk of bowel, bladder and ureteric damage, as well as haemorrhage, infection and even death [1]. There is a clear unmet clinical need for better medical treatments for this benign but incapacitating condition.
Abnormal uterine bleeding may be a result of aberrations in:
Duration of bleeding;
Frequency of bleeding;
Regularity of menses or;
Volume of menstrual loss.
The clinician must carefully assess each symptomatology during the consultation to enable accurate diagnosis and management of HMB. The need for current or future fertility must also be elicited in routine history-taking to facilitate informed decision making of the women seeking treatment. After exclusion of anatomical disorders (PALM [polyps, adenomyosis, leiomyoma, malignancy]) and nonanatomical disorders (COEIN [coagulopathies, ovulatory dysfunction, endometrial, iatrogenic, not otherwise classified]) [2], managing the symptom of HMB is a priority. The clinician’s role is to provide accurate information about treatment options, allowing the woman to choose the treatment most appropriate for her. Written information is often helpful and evidence-based patient information leaflets are available online. Treatment success should be determined by improvement in the woman’s quality of life.
This review aims to provide a practical guide to well-established medical treatments for HMB. These treatments are divided into nonhormonal options and hormonal preparations and, where possible, their appropriateness for women with various causes, symptomatology and personal circumstances are highlighted. Finally, some novel treatment options for HMB are introduced and the current evidence for their use discussed.
Nonhormonal treatments for HMB
Nonpharmacological management
A careful explanation of the cause of HMB is essential in the management of women with HMB. Exclusion of pathology will often allay fears and occasionally prevent the need for pharmacological treatments.
Regular exercise and maintenance of a healthy BMI should be recommended to every woman with HMB. Although the evidence for cause and effect is limited, a high BMI will increase the risk of ovulatory dysfunction and subsequent heavy or irregular menstrual loss [3–5]. Exercise and a healthy diet will also help limit iron deficiency anaemia, raise energy levels and improve quality of life.
Antifibrinolytics
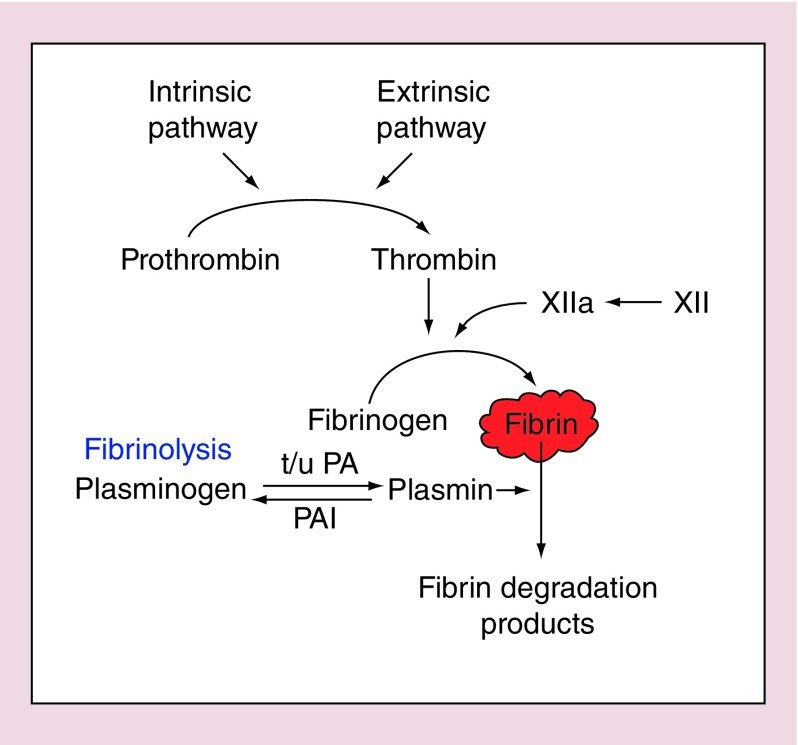
Women suffering from HMB have been shown to have over activation of the fibrinolytic system during the menstrual phase of their cycle [6]. This leads to accelerated degradation of the fibrin clot that forms to induce hemostasis (Figure 1). Therefore, an increase in fibrinolysis results in increased blood loss during endometrial shedding.
Figure 1. . The fibrinolytic pathway.
PA: Platelet activator; PAI: Platelet activator inhibitor.
Tranexamic acid is an antifibrinolytic medication commonly used to counteract this aberration in women with heavy menstrual bleeding. It has a short half-life, necessitating regular administration of 1 g orally three- to four-times per day during menses. As it is only required during days of heavy bleeding (˜4 per month), side effects are minimal but may include gastrointestinal symptoms. Tranexamic acid is also acceptable to women who are trying to conceive, or those who experience significant side effects with hormonal preparations. There are few contraindications to tranexamic acid but it should be used with caution in women with a personal history of thromboembolism. Tranexamic acid is reported to result in approximately 50% reduction in menstrual blood loss [7,8].
NSAID preparations
Studies examining women with objectively measured heavy and normal menstrual bleeding have repeatedly demonstrated that increased local inflammation is associated with increased menstrual blood loss. The proinflammatory cytokine TNF-α was significantly elevated in menstrual effluent of women with HMB versus those with normal loss [9]. The enzyme involved in prostaglandin synthesis, COX-2, was also raised in endometrial samples from those with HMB, leading to increased prostaglandin signaling [10]. The resulting exaggerated inflammation within the endometrium may lead to increased and prolonged tissue damage at the time of menstruation. Therefore, limitation of the production of inflammatory mediators is helpful in the treatment of women with HMB.
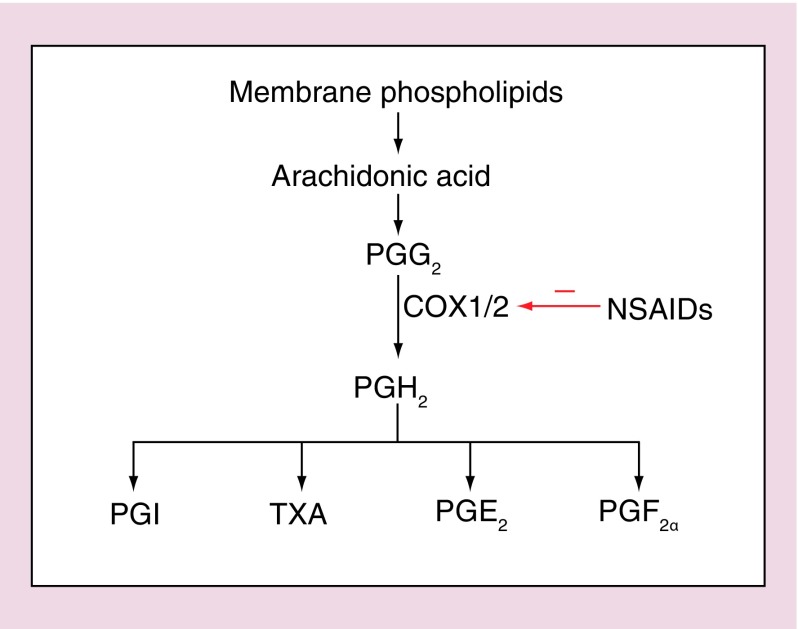
NSAIDs exert their anti-inflammatory effect through inhibition of cyclooxygenase, which is the enzyme that catalyses the transformation of arachidonic acid to prostaglandins and thromboxanes (Figure 2). Mefenamic acid is the most commonly used NSAID for treatment of HMB and results in a reported blood loss reduction of 25–50% [11]. However, other NSAIDs show similar efficacy to the more commonly prescribed mefenamic acid [7]. Like antifibrinolytic medications, NSAIDs offer a nonhormonal treatment for women wishing to conceive or avoid hormonal side effects but have the additional benefit of analgesic properties. Side effects include gastrointestinal effects and these preparations are not suitable for those women who have previously had peptic ulcer disease or who are thought to have HMB due to a coagulation disorder.
Figure 2. . Synthesis and signalling of prostaglandins.
PG: Prostaglandin; PGI: Prostacyclin; TXA: Thromboxane.
Despite significant reductions in blood loss, 52% of women treated with mefenamic acid for 2 months maintained a blood loss of greater than 80 ml per cycle [11]. NSAIDs and antifibrinolytic medications can be used together but should be stopped after 3 months if there is no symptomatic improvement. If they are beneficial, they may be continued indefinitely and can also be used as adjuvant therapy with hormonal preparations.
Hormonal treatments for HMB
Human endometrial function is governed by the ovarian steroid hormones. Most research to date has focused on the role of estrogen and progesterone on the endometrium but the role of other steroids that have an impact on endometrial function (i.e., androgens and glucocorticoids) should also be considered. During the secretory phase of the menstrual cycle, progesterone is the dominant hormone and is a potent anti-inflammatory agent. In the absence of pregnancy, the corpus luteum regresses and progesterone levels sharply decline. It is this marked reduction in ovarian hormones that triggers an influx of inflammatory mediators into the endometrial environment, leading to shedding and menstruation. Maintenance of progesterone exposure limits endometrial inflammation and prevents menstruation. It is therefore unsurprising that the most effective medical treatments available for HMB are hormonal preparations. It is worth remembering that these preparations will also limit or remove fertility for the duration of their use.
Levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena®)
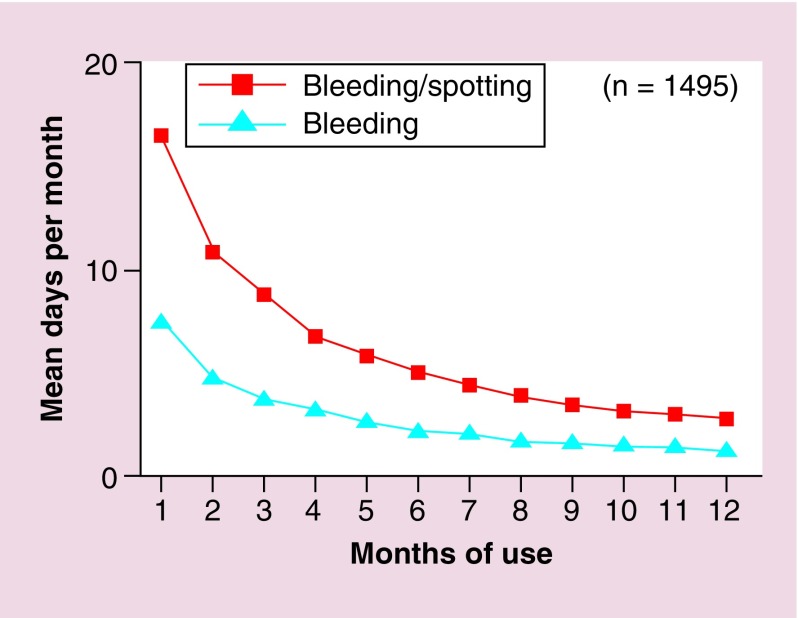
This popular intrauterine system (IUS) contains an androgenic progestogen, levonorgestrel (LNG). LNG is slowly released from the IUS to act on the local endometrial environment, preventing proliferation. It may also impact on the frequency of ovulation. The LNG-IUS can decrease menstrual loss by up to 96% after 1 year of use (Figure 3) [12] and is licensed in the UK for treatment of HMB for 5 years. After 5 years, the device should be removed and a new LNG-IUS device may be fitted immediately if desired. It is an excellent contraceptive when in situ and has the advantages of a ‘fit and forget’ method, rather than relying on patient compliance. The LNG-IUS is also associated with reduction of dysmenorrhea [13]. As its actions are local, progestogenic side effects are limited, for example, bloating, breast tenderness, mood changes.
Figure 3. . Impact of levonorgestrel-releasing intrauterine system on bleeding and spotting in the first year of use.
Reproduced with permission from [12] © Elsevier (1994).
The LNG-IUS is contraindicated in pregnancy, unexplained vaginal bleeding and uterine sepsis [1].
Risks usually outweigh benefits in women with systemic lupus erythematosus (SLE) and those with severe liver disease. Usually hormonal treatments are avoided in women with current breast cancer, but concerns about progression of the disease may be less with LNG-IUS than with oral preparations. The LNG-IUS may be considered individually, and in consultation with the woman’s breast surgeon [14]. Extra care must be taken during insertion in women with distortion of their endometrial cavity due to leiomyoma/fibroids or congenital abnormalities. In these cases, it may be safer to use an alternative hormonal treatment or to insert the IUS under hysteroscopic guidance.
Women should be counseled about potential complications of LNG-IUS use including:
Unscheduled bleeding: this occurs in the majority of women during the first 3–6 months of use. Women should be advised that they may experience daily spotting but that this usually settles after 6 months. Perseverance for a minimum of 6 months is required for benefits to be appreciated and for unscheduled, usually light, bleeding to subside (Figure 3). Approximately one in five women will experience on-going problems with persistent bleeding [15]. This is thought to be due to endometrial vascular fragility, secondary to sustained progestogen exposure, a decrease in steroid hormone receptors and a lack of local estrogen effects [16]. A proportion of women will benefit from adjuvant tranexamic/mefenamic acid treatment or, if no contraindications, a 3-month course of a combined oral contraceptive pill. Unfortunately, those with persistent problems or intolerable side effects will require alternative management of their HMB [17];
Infection: women have an increased risk of infection for the first 3 weeks after insertion. Some clinicians recommend that women should not use tampons in this time to minimize this risk. If they notice an offensive discharge they must seek medical advice and may require antibiotic treatment. After 3 weeks post-insertion, their risk of infection returns to the same as women without an IUS;
Expulsion of IUS: up to one in five LNG-IUS devices can be expelled from the uterine cavity after insertion, with the greatest risk of this during the first 6 weeks post-insertion. The rate of expulsion is higher in nulliparous women [14]. Women should be advised to check the threads by digital self-examination on a regular basis, particularly if relying on the IUS for contraception. If they are not happy to do so, they should have a speculum examination 6 weeks after insertion to ensure the IUS is in place before relying on it for contraception;
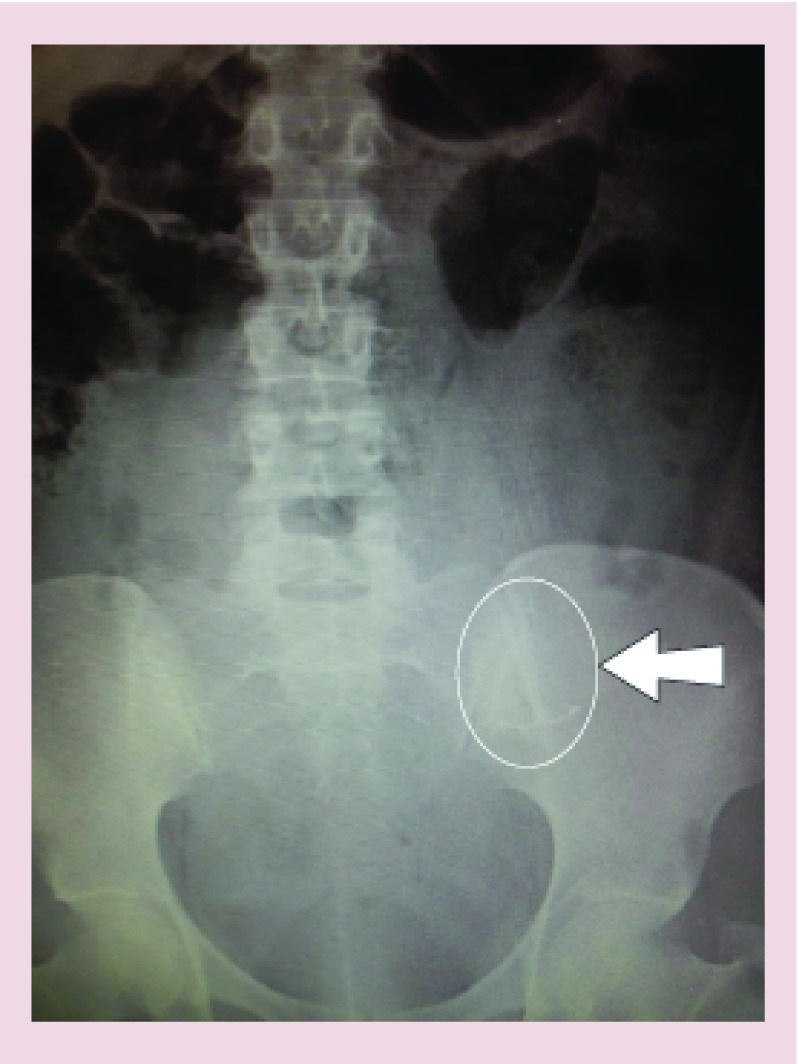
Perforation: a rare but serious complication of LNG-IUS insertion is uterine perforation, occurring in 1:1000 cases [14]. Distortion of the endometrial cavity, uterine infection or being less than 4 weeks postpartum will increase the risk of perforation substantially. Suspicion of perforation at the time of insertion warrants ultrasonic assessment. A woman should be advised to seek medical help if post insertion cramps are not eased with routine analgesics. Should the IUS threads not be visible, ultrasound assessment ± abdominal x-ray is indicated to exclude perforation (Figure 4).
Figure 4. . x-ray confirming levonorgestrel intrauterine system perforation at time of insertion.
Combined oral contraceptive pill
The combined oral contraceptive (COCP) contains estrogen and progestogen and is usually given for 3 weeks followed by a ‘pill free’ week in which the woman experiences a hormone withdrawal bleed. The COCP produces an estimated reduction in blood loss of 50% and has the additional benefit of regulation of bleeding [18]. Therefore, it is a particularly attractive option for women experiencing frequent or irregular heavy bleeding, once pathology has been excluded. The COCP can be ‘tri-cycled', in other words, three packets taken consecutively without ‘pill-free’ weeks. This will reduce the number of menses experienced as well as the volume of blood loss and is an attraction option for many women [19].
The risks of the COCP are mainly due to its estrogen content and include increased risk of thromboembolism, stroke, cardiovascular disease or breast cancer. Therefore, it is contraindicated in women with a BMI >35, smokers over 35 years, women with hypertension, vascular disease, migraine with aura, current/recent breast cancer, those with a personal or strong family history of venous thromboembolism or with a known thrombogenic mutation [14]. The COCP also has a detrimental effect on breast milk production and is contraindicated in breastfeeding women [14]. In the absence of risk factors, women can use the COCP until menopause if desired.
Progesterone only pill
In contrast to the combined pill, the progesterone only pill (POP) is associated with irregular and unpredictable blood loss. Therefore, it is not usually recommended as a treatment for HMB. However, if no other options are acceptable or safe for a woman to use, a trial of a POP may be appropriate. As these pills do not contain estrogen, they are a safer alternative to the COCP. Some POPs induce amenorrhea in up to 20% of users, for example, desogestrel containing POPs, and are effective treatments for a small proportion of women [20].
Injectable progestogens
Intramuscular or subcutaneous injection of high dose progestogens (e.g., depot medroxyprogesterone acetate [DMPA]) can induce amenorrhea in up to 50% of users [21]. This method of administration offers women an alternative to tablets or intrauterine devices. Injections are usually given every 12 weeks to maintain progestogen exposure and ensure contraceptive efficacy. The principle mechanism of action of injectable progestogens is inhibition of follicle-stimulating hormone (FSH) release from the anterior pituitary. Therefore, follicle development in the ovary is inhibited and ovulation is prevented. This ovulatory suppression has the additional benefit of reducing dysmenorrhea. However, as a consequence of follicular suppression, there is also a reduction in estradiol production. Therefore, women may have a transient reduction in their bone mineral density with long-term use. The clinical impact of this loss of bone mineral density remains uncertain; a retrospective cohort study of general practice records showed that users of DMPA had a fracture rate of 9.1 per 1000 person-years compared with a rate of 7.3 for nonusers [22]. However, DMPA users had an increased incidence of fractures before they had even commenced DMPA use and there was no increase in fracture incidence with increased duration of use. Considering these inconclusive data and the complete restoration of bone mineral density on cessation of use, it is a suitable preparation for most women. Side effects can limit compliance and include weight gain, greasy skin and hair, acne and bloating [14].
Oral progestogens
Norethisterone is the most commonly used oral progestogen in the treatment of HMB. This should be prescribed as a 5 mg tablet, to be taken three-times per day from day 5 to 26 of the menstrual cycle. This regimen has been shown to reduce blood loss by >80% [23]. By contrast, norethisterone administration in the luteal phase only was of no benefit to women with HMB and is not recommended [24]. Despite a significant reduction in HMB with norethisterone treatment from day 5–26, patient satisfaction may limit long term use due to a high incidence of progestogenic side effects. Therefore, it is more commonly prescribed as a short term measure, for example, to terminate a heavy bleed or regulate menstruation for a holiday or an important life event.
Gonadotropin-releasing hormone agonists
These are synthetic peptides administered by an intramuscular, subcutaneous or intranasal route and utility should really be for short-term use. These continuous delivery preparations have a much longer half-life than the natural gonadotropin-releasing hormone (GnRH) released in a pulsatile manner from the hypothalamus. This sustained presence of GnRH results in low FSH and luteinizing hormone (LH) production and GnRH agonists induce a profound hypogonadal state, in other words, a medical menopause. As there is no stimulation of the endometrium from the resulting low ovarian hormone levels, menstruation does not take place. GnRH agonists are particularly useful in the treatment of uterine fibroids (leiomyoma), which can reduce considerably in size when ovarian hormone levels are suppressed. GnRH agonists may be used prior to surgical intervention in women with fibroids, or for those in whom surgery is not suitable or desirable [25].
Studies have demonstrated excellent efficacy, with an amenorrhea rate of up to 90% with GnRH agonist use [26,27]. However, these compounds are associated with very significant side effects secondary to estrogen deficiency that limit use; namely flushing, vaginal dryness, headaches and decreased libido. Most of the side effects can be attributed to low estrogen levels and limitation of these menopausal symptoms can be achieved with ‘add-back’ hormone replacement therapy (HRT). This is necessary after 6 months of use to protect bone mineral density. Usually the GnRH agonist is commenced alone, to achieve maximal effects on menstrual blood loss and fibroid shrinkage and discontinued after 6 months. ‘Add-back’ HRT is introduced if the woman continues treatment for greater than 6 months, or sooner if warranted by symptomatology.
Novel treatments for HMB
Selective progesterone receptor modulators
An exciting new group of pharmacological agents is in development and has the future potential to provide effective oral treatment for HMB. These selective progesterone receptor modulators (SPRMs) impart a tissue-specific partial progesterone antagonist effect and act upon progesterone receptors in the endometrium and the underlying myometrial tissue. They have the additional benefit of maintenance of estradiol levels, meaning hypoestrogenic side effects are not an issue.
The mechanism by which these SPRMs reduce menstrual blood loss is still to be fully defined but distinct histological morphology has been identified with their use (progesterone receptor modulator associated endometrial changes [PAEC]). Ulipristal Acetate (UPA) is the only SPRM to have been licensed for use in clinical practice, albeit restricted to 3 months pretreatment of fibroids prior to surgical removal. Study of the endometrium of women taking this treatment regimen showed altered architectural features including extensive cystic dilatation of the epithelial glands, inactivity or features of abortive subnuclear vacuolization, occasional mitoses and apoptosis. Histology returned to normal after discontinuation of treatment [28,29]. Study of a different SPRM, asoprisnil, revealed a decreased uterine artery blood flow after 3 months of treatment, which may contribute to their efficacy [30].
The recent introduction of UPA followed evaluation in two concurrent randomized controlled trials [31,32]. ‘PEARL I’ assessed the efficacy of UPA 5 mg and 10 mg daily on uterine bleeding and fibroid volume when compared with placebo. ‘PEARL II’ assessed UPA versus the gonadotropin-releasing hormone analogue leuprolide acetate in the treatment of symptomatic uterine fibroids prior to surgery. Both trials demonstrated control of HMB in over 90% of women and amenorrhea in over 70% women. Control of HMB was achieved significantly more quickly in the UPA group versus GnRH agonist. There was a statistically significant reduction in the size of fibroids (12–21% decrease). Compliance with treatment over 3 months was high in both studies (96 and 98%) and reported side effects were limited to minor complaints. Headache (4%) and breast complaints (4%) were the most common side effects reported but there was no difference between active drug and placebo groups. There are no publications to date on the clinical utility of SPRMs in the management of women with HMB who do not have fibroids or who have other conditions associated with HMB, such as adenomyosis.
These studies have concluded that short term use of UPA is effective in treating HMB associated with uterine fibroids (3–10 cm in size). However, UPA also has the potential to provide a safe, fertility preserving, rapidly effective and convenient oral medical treatment for women with HMB whether associated with fibroids or not. Clinical trials are currently in progress to assess SPRMs in this group of women. This further research is required to fully understand their mechanism of action, longer-term safety and effectiveness prior to recommending their use as a long-term medical treatment option for women with HMB with and without fibroids.
Conclusion & future perspective
A number of medical options and routes of administration exist for the hundreds of thousands of women in the UK who suffer from HMB. Unfortunately, most are associated with hormonal side effects and limited efficacy. SPRMs offer hope of a new, fertility sparing class of medical therapies for these women that may provide a long-term treatment option. Continued research into the causes of HMB will yield new medical therapies for this common, debilitating disorder to improve the quality of life of many women.
Executive summary.
Heavy menstrual bleeding is a common and debilitating condition that has a significant impact on a woman’s quality of life, her family and a more widespread effect on society as a whole.
Various medical treatment options are available but side effects often limit compliance and efficacy.
Nonhormonal options are limited to tranexamic or mefenamic acid.
Hormonal options include the levonorgestrel-releasing intrauterine system, the combined oral contraceptive pill or progestogen preparations.
Gonadotropin Releasing Hormone analogues can be a useful short-term option, particularly for women with fibroids.
There is a clear unmet need for effective, acceptable medical treatments for HMB. Selective progesterone receptor modulators may provide a novel therapeutic option for these women in the future.
Acknowledgements
The authors thank S Milne for her help with manuscript preparation and R Grant for support with illustrations.
Footnotes
Financial & competing interests disclosure
HOD Critchley has received collaborative Research Grant Support for research staff and consumables in context of AUB treatment from TAP Pharmaceuticals and Bayer Pharma AG; and provided Consultancy/Advisory Board contributions on AUB Treatment (Bayer Pharma AG, Preglem/Gedeon-Richter, Vifor Pharma). HOD Critchley and JA Maybin have received research grant support from the Medical Research Council (G1002033 (HODC, JAM), MR/J003611/1 (HODC) and The Wellcome Trust (100646/Z/12/Z [JAM]). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-No Derivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.RCOG. Abdominal Hysterectomy for Benign Conditions. 2009. www.rcog.org.uk/globalassets/documents/guidelines/consent-advice/ca4-15072010.pdf
- 2.Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int. J. Gynaecol. Obstet. 2011;113(1):3–13. doi: 10.1016/j.ijgo.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Hartz AJ, Barboriak PN, Wong A, Katayama KP, Rimm AA. The association of obesity with infertility and related menstural abnormalities in women. Int. J. Obes. 1979;3(1):57–73. [PubMed] [Google Scholar]
- 4.Rowland AS, Baird DD, Long S, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13(6):668–674. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Critchley HO, Duncan WC, Brito-Mutunayagam S, Reynolds RM. Obesity and menstrual disorders. In: Mahmood TA, Arulkumaran S, editors. Obesity: A Ticking Time Bomb for Fertility (Elsevier Insights) Elsevier; Oxford, UK: 2013. pp. 525–535. [Google Scholar]
- 6.Gleeson N, Devitt M, Sheppard BL, Bonnar J. Endometrial fibrinolytic enzymes in women with normal menstruation and dysfunctional uterine bleeding. Br. J. Obstet. Gynaecol. 1993;100(8):768–771. doi: 10.1111/j.1471-0528.1993.tb14272.x. [DOI] [PubMed] [Google Scholar]
- 7.Andersch B, Milsom I, Rybo G. An objective evaluation of flurbiprofen and tranexamic acid in the treatment of idiopathic menorrhagia. Acta Obstet. Gynecol. Scand. 1988;67(7):645–648. doi: 10.3109/00016348809004279. [DOI] [PubMed] [Google Scholar]
- 8.Gleeson NC, Buggy F, Sheppard BL, Bonnar J. The effect of tranexamic acid on measured menstrual loss and endometrial fibrinolytic enzymes in dysfunctional uterine bleeding. Acta Obstet. Gynecol. Scand. 1994;73(3):274–277. doi: 10.3109/00016349409023453. [DOI] [PubMed] [Google Scholar]
- 9.Malik S, Day K, Perrault I, Charnock-Jones DS, Smith SK. Reduced levels of VEGF-A and MMP-2 and MMP-9 activity and increased TNF-alpha in menstrual endometrium and effluent in women with menorrhagia. Hum. Reprod. 2006;21(8):2158–2166. doi: 10.1093/humrep/del089. [DOI] [PubMed] [Google Scholar]
- 10.Smith OP, Jabbour HN, Critchley HO. Cyclooxygenase enzyme expression and E series prostaglandin receptor signalling are enhanced in heavy menstruation. Hum. Reprod. 2007;22(5):1450–1456. doi: 10.1093/humrep/del503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron IT, Haining R, Lumsden MA, Thomas VR, Smith SK. The effects of mefenamic acid and norethisterone on measured menstrual blood loss. Obstet. Gynecol. 1990;76(1):85–88. [PubMed] [Google Scholar]
- 12.Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49(1):56–72. doi: 10.1016/0010-7824(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 13.Sheng J, Zhang WY, Zhang JP, Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception. 2009;79(3):189–193. doi: 10.1016/j.contraception.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 14.FSRH. UK Medical Eligiblility Criteria for Contraceptive Use. 2009 www.fsrh.org/pdfs/UKMEC2009.pdf [Google Scholar]
- 15.Abdel-Aleem H, D’arcangues C, Vogelsong K, Gulmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin only contraceptives. Cochrane Database Syst. Rev. 2007;(2) doi: 10.1002/14651858.CD003449.pub2. CD003449. [DOI] [PubMed] [Google Scholar]
- 16.Guttinger A, Critchley HO. Endometrial effects of intrauterine levonorgestrel. Contraception. 2007;75(Suppl. 6):S93–S98. doi: 10.1016/j.contraception.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 17.FSRH. CEU Guidance on Unscheduled Bleeding. 2015 www.fsrh.org/pdfs/CEUGuidanceProblematicBleedingHormonalContraception.pdf [Google Scholar]
- 18.Fraser IS, Kovacs GT. The efficacy of non-contraceptive uses for hormonal contraceptives. Med. J. Aust. 2003;178(12):621–623. doi: 10.5694/j.1326-5377.2003.tb05387.x. [DOI] [PubMed] [Google Scholar]
- 19.Loudon NB, Foxwell M, Potts DM, Guild AL, Short RV. Acceptability of an oral contraceptive that reduces the frequency of menstruation: the tri-cycle pill regimen. Br. Med. J. 1977;2(6085):487–490. doi: 10.1136/bmj.2.6085.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benagiano G, Primiero FM. Seventy-five microgram desogestrel minipill, a new perspective in estrogen-free contraception. Ann. NY Acad. Sci. 2003;997:163–173. doi: 10.1196/annals.1290.019. [DOI] [PubMed] [Google Scholar]
- 21.Said S, Omar K, Koetsawang S, et al. A multicentered Phase III comparative clinical trial of depot-medroxyprogesterone acetate given three-monthly at doses of 100 mg or 150 mg: II. The comparison of bleeding patterns. World Health Organization. Task force on long-acting systemic agents for fertility regulation special programme of research, development and research training in human reproduction. Contraception. 1987;35(6):591–610. doi: 10.1016/s0010-7824(87)80019-7. [DOI] [PubMed] [Google Scholar]
- 22.Lanza LL, Mcquay LJ, Rothman KJ, et al. Use of depot medroxyprogesterone acetate contraception and incidence of bone fracture. Obstet. Gynecol. 2013;121(3):593–600. doi: 10.1097/AOG.0b013e318283d1a1. [DOI] [PubMed] [Google Scholar]
- 23.Irvine GA, Campbell-Brown MB, Lumsden MA, Heikkila A, Walker JJ, Cameron IT. Randomised comparative trial of the levonorgestrel intrauterine system and norethisterone for treatment of idiopathic menorrhagia. Br. J. Obstet. Gynaecol. 1998;105(6):592–598. doi: 10.1111/j.1471-0528.1998.tb10172.x. [DOI] [PubMed] [Google Scholar]
- 24.Lethaby A, Irvine G, Cameron I. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst. Rev. 2008;(1) doi: 10.1002/14651858.CD001016.pub2. CD001016. [DOI] [PubMed] [Google Scholar]
- 25.Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst. Rev. 2001;(2) doi: 10.1002/14651858.CD000547. CD000547. [DOI] [PubMed] [Google Scholar]
- 26.Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The leuprolide study group. Obstet. Gynecol. 1991;77(5):720–725. [PubMed] [Google Scholar]
- 27.Takeuchi H, Kobori H, Kikuchi I, Sato Y, Mitsuhashi N. A prospective randomized study comparing endocrinological and clinical effects of two types of GnRH agonists in cases of uterine leiomyomas or endometriosis. J. Obstet. Gynaecol. Res. 2000;26(5):325–331. doi: 10.1111/j.1447-0756.2000.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 28.Mutter GL, Bergeron C, Deligdisch L, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod. Pathol. 2008;21(5):591–598. doi: 10.1038/modpathol.2008.19. [DOI] [PubMed] [Google Scholar]
- 29.Williams AR, Bergeron C, Barlow DH, Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int. J. Gynecol. Pathol. 2012;31(6):556–569. doi: 10.1097/PGP.0b013e318251035b. [DOI] [PubMed] [Google Scholar]
- 30.Wilkens J, Chwalisz K, Han C, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. J. Clin. Endocrinol. Metab. 2008;93(12):4664–4671. doi: 10.1210/jc.2008-1104. [DOI] [PubMed] [Google Scholar]
- 31.Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N. Engl. J. Med. 2012;366(5):409–420. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- 32.Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N. Engl. J. Med. 2012;366(5):421–432. doi: 10.1056/NEJMoa1103180. [DOI] [PubMed] [Google Scholar]