Abstract
Background
Inflammation is thought to be a major contributor to post-surgical pain so nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used analgesics. However, compared to rats, considerably less is known as to how successfully these prevent pain in mice.
Methods
A fluorescent COX-2 selective probe was used for the first time to evaluate the post-surgical anti-inflammatory effects of meloxicam, and automated behaviour analyses (HomeCageScan; HCS), the Mouse Grimace Scale (MGS) and body weight changes to assess its pain preventative properties. Groups of 8-9 BALB/c mice were subcutaneously injected with saline (0.3mls) or meloxicam at (1, 5 or 20mg/kg) 1 hour before a 1.5cm midline laparotomy. The probe or a control dye (2mg/kg) was injected intravenously 3 hours later. Imaging was used to quantify inflammation at 7, 24 and 48 hours following surgery. HCS data and MGS scores were obtained from video recordings and photographs before and at 24 hours.
Results
Post-surgical inflammation was dose dependently reduced by meloxicam; with 5 or 20mg/kg being most effective compared to saline. However, all mice lost weight, MGS scores increased and behavioural activity was reduced by surgery for at least 24 hours with no perceivable beneficial effect of meloxicam on any of these potentially pain-associated changes.
Conclusions
Although meloxicam prevented inflammation, even large doses did not prevent post-laparotomy pain possibly arising due a range of factors, including, but not limited to inflammation. MGS scoring can be applied by very naïve assessors and so should be effective for cage-side use.
Introduction
Although researchers are required to prevent pain (MORI, 2010), apprehension about the side-effects of analgesic drugs often prevents their use (Stokes et al., 2009). However, unalleviated pain is also likely to impact on scientific results so establishing the lowest effective analgesic dose rates is essential to minimise potential confounds. Post-operative behaviour analysis has allowed the analgesic requirements of rats to be relatively well established (Roughan and Flecknell, 2001, 2003, 2004; Ciuffreda et al., 2014). However, being behaviourally more dynamic, detecting pain-associated behaviours is more difficult in mice, so despite advances in automated behaviour analysis (Dickinson et al., 2009; Roughan et al., 2009; Miller et al., 2011; Miller et al., 2012; Wright-Williams et al., 2013) their needs remain uncertain. In both species NSAIDs are often preferred to opiates due to fewer confounding effects. However, although these are effective in rats (Roughan and Flecknell, 2003; Brennan et al., 2009) pigs (Kluivers-Poodt et al., 2013), dogs (Mathews et al., 2001; Walton et al., 2014) and cats (Gunew et al., 2008), seemingly not in mice, or disproportionately high dose rates are required to generate any positive outcomes (Wright-Williams et al., 2007; Matsumiya et al., 2012; Miller et al., 2012). Why remains uncertain, but the recently developed ‘Mouse Grimace Scale’ (MGS) (Langford et al., 2010; Leach et al., 2012; Matsumiya et al., 2012) is providing a greater appreciation of alternative considerations in pain assessment that may be particularly relevant to mice; such as if an observer is present (Adamson et al., 2010; Sorge et al., 2014). Variations of the MGS are now available for other species (Keating et al., 2012; Dalla Costa et al., 2014) increasing its reputation as an effective method of pain assessment (NC3Rs, 2013).
Inflammation contributes to pain due to up-regulation of pro-nociceptive molecules including prostaglandins and thromboxane caused by the actions of cyclooxygenase (COX) 1 and 2 enzymes on arachidonic acid (Crofford, 1997; Ricciotti and FitzGerald, 2011). COX 1 and 2 are similar (Wuest et al., 2008), but whereas COX-1 is constitutive, COX-2 is primarily only increased in damaged tissues, thus NSAIDs preferentially inhibiting COX-2 are more effective analgesics. Evaluating COX-2 inhibition therefore provides an estimate of the analgesic potency of NSAIDs, but requires in-vitro assays. Anti-nociceptive tests provide an alternative potency estimate, but the inadequacies of these are well known and sometimes reveal a discrepancy between the anti-inflammatory versus pain-preventative properties of NSAIDs (Bianchi and Panerai, 2002). Since a similar mismatch may explain their apparent lack of efficacy in mice, we sought to determine how effectively the commonly used COX-2 preferential NSAID meloxicam prevents post-surgical inflammation and if this equates to pain prevention in mice. The recent development of a COX-2 imaging probe (Uddin et al., 2010) provided an innovative and convenient means of monitoring post-surgical inflammation, whereas the MGS, body-weight and automated behaviour analyses were used to assess pain.
Methods
Ethical approval
All work complied with the Animals (Scientific Procedures) Act 1986 (UK Home Office license PPL 60/4356) and EU Directive 2010/63, was approved by local Ethical Review, and adhered to the guidelines of the IASP.
Animal Husbandry
A group of 45 male BALB/c mice (25-30g; Charles River, Margate, Kent, UK) were housed in Macrolon Type 2 cages (North Kent Plastics, UK) in groups of 5 with free access to a pelleted diet (R&M no.3, SDS LTD., Whitham, UK) and tap water. Bedding was sawdust and wood shavings and ‘Sizzle Nest’, an aspen chew-block and a cardboard tube (B & K Universal) provided enrichment. Room temperature was 21±1°C with 15-20 air changes/hour under a 12-hour light cycle (off at 19:00h). Identification was by ear notching after 1 week of a 2 week settling period. Cages were cleaned weekly and the chew-block and cardboard tube were replaced during weekly cleaning; retaining some soiled bedding to maintain home-cage familiarity.
Study Design
Individual mice were the experimental unit. They were randomly allocated to two groups where they would be handled differently; either by the usual method of lifting by the tail, or by the ‘cupped’ handling method that reduces anxiety (Hurst and West, 2010). Although anxiety exacerbates pain in humans (Meagher, 2000) its contribution to pain in animals is uncertain. The rationale was to establish whether cupped handling, by minimising procedure-related anxiety, might also help to minimise pain. We wished to determine if this was a more refined and possibly pain-preventative method. Group 1 mice (n=20) were therefore always tail-handled, whereas group 2 (n=25) were handled daily for 30 seconds following 5 minutes of voluntary approach to gloved and ‘cupped’ hands, or following lifting using the cardboard tube provided for cage enrichment. Cupped handling began one week before surgery, whereas Group 1 mice were only ever handled by the tail (during cage cleaning). The handling groups were then further sub-divided for Saline (Sal) or Meloxicam treatment (Boehringer Ingelheim, UK) at 1 (M1), 5 (M5) or 20 (M20) mg/kg (s/c) before laparotomy (Lap), and then for injection of a fluorescent COX-2 probe (P) or a control dye (D). As there were no similar previous examples, group sizes were estimated from the results of a previous study in mice given pre-surgical doses of buprenorphine; where 8 achieved significant dose separation (Wright-Williams et al., 2013). Four mice were added to the Sal/Lap/P group to facilitate reliable inflammation quantification, and 1 to each meloxicam treated group (n=9). Group numbers and codes are summarised in TableS1.
Surgery
Beginning at 8am mice received the appropriate saline or meloxicam injection subcutaneously. Injections were staggered by 15 minutes to accommodate the anticipated surgery duration of 15 minutes. Surgery began at 9am and required up to 3 hours depending on numbers, and lasted between 15 and 20 minutes for each mouse. Groups of 5-7 mice underwent laparotomy on 1 or 2 days each week by the same surgeon using aseptic techniques (www.procedureswithcare.org.uk/aseptic-technique-in-rodent-surgery-tutorial). Anaesthesia was induced in a chamber using 5% isoflurane in oxygen (2litres/ min), and was maintained using a face mask with 2.5% isoflurane (500ml/min). Eye ointment (Pliva Pharma Ltd., UK) was applied and a heat blanket maintained body temperature at 36°C. After shaving they underwent laparotomy as previously described in rats (Roughan and Flecknell, 2001) but with a shorter 1.5cm midline incision in the skin and muscle. The ileum was gently manipulated with a dry sterile swab for 60 seconds. The muscle and skin were separately closed with 4/0 polydioxanone (Ethicon, UK) using interrupted mattress sutures. Mice recovered in separate cages in a warming cabinet at 26±2°C (41% humidity). They were left for 3 hours to allow inflammation to develop and then injected in the lateral tail vein (i/v) with either 2mg/kg of the fluorescent probe (Xenolight ‘Rediject’ COX-2 probe; PerkinElmer, UK) or 2mg/kg of a control dye (‘Rediject’ COX-2 Control dye; PerkinElmer, UK) pre-warmed to room temperature. The control dye molecule had the same rhodamine-derived fluorophore tethered to indomethacin, but lacking COX-2 selectivity (Uddin et al., 2010).
Data Collection
To obtain behaviour data the mice were individually housed in clear polycarbonate cages (3 × Type 1144B, Techniplast UK Ltd, Northants., UK) without food or water. Twenty minutes of activity was recorded using HD cameras (3 × Canon Legria HFM 506) placed 30cm from each cage. The cage back and sides were lined with matte black vinyl (Wilko Retail Ltd., UK) to minimise reflections. Cardboard was used as flooring and this was renewed for each mouse. After behaviour filming each mouse was immediately placed into a clear ‘Plastiglas’ cube (H × W × D = 10cm) to obtain MGS photographs. Two still face-frontal images were obtained using a high speed camera (Casio EX-ZR1000) when not grooming. Baseline behaviour and MGS data were obtained between 3 and 5pm the day prior to surgery, approximating to the time of the first post-surgery recordings. These were at 4 hours, 1 hour after injection of the probe or dye. MGS and behaviour data collection was repeated at 24 hours. The first imaging session was at 7 hours following surgery, providing a 4 hour delay from the time of the probe or dye injection to achieve maximal COX-2 binding (Uddin et al., 2010). Imaging was repeated at 24 and 48 hours.
The mice were anaesthetised for imaging as for surgery, and were individually imaged in an IVIS Spectrum 200 (Xenogen, USA) on a heated stage at 36°C with anaesthesia maintained by face-mask delivery of 2% isoflurane in oxygen (0.5l/min). The optimal probe/dye excitation(Ex.)/emission(Em.) settings were 570/620nm (0.5sec exposure; subject depth 2cm), and spectral profiles spanning Ex.535-570nm and Em.620-640nm were obtained.
The MGS photographs were compiled into a Microsoft Excel questionnaire with 254 images in random sequence. Images were not obtained at 24 hours in the first 5 mice as this time point was added subsequently. Four scorers were recruited; one was an undergraduate laboratory animal biomedical science student and the remaining 3 were naïve of any contact with laboratory animals. Instructions included a basic description of the aim of the exercise. They each scored the images according to the 5 recognised MGS facial action units (FAU’s); Orbital tightening (Orb), Ear flattening (Ear), Nose bulge (Nose), Cheek bulge (Cheek) and Whisker Change, scoring each as either ‘0’ (not present), ‘1’ (moderately present) or 2 (severe). The sample images from the original MGS article (Langford et al., 2010) appeared on the questionnaire whilst observers decided what value to select for each FAU. As all mice underwent surgery additional blinding was not necessary. The animals were humanely killed after 48 hours by anaesthetic overdose in the IVIS machine and cervical dislocation.
Data Processing
Weight changes from baseline to 24 hours were calculated. The IVIS images were processed using the spectral-unmixing feature of ‘Living Image’ software (PerkinElmer, USA) to determine peak fluorescent signal intensity. This accounted for the auto-fluorescent background signal, so revealing the specific COX-2 bound probe (inflammation) signal intensity (Total Radiant Intensity (TR); (photons/sec)/(μW/cm2)) within identically sized (3 × 3cm) ‘regions of interest’ (ROI’s) placed over incision sites.
The HCS footage was processed on a PC installed with HomeCageScan automated behaviour analysis software (HCS: Version 3; Clever Systems Inc., USA). Initial data processing was as described by Wright-Williams et al. (2013), using discriminant analysis (DA) to identify the key parameters most significantly impacted by surgery; thus, those most likely to reveal drug or dose effects. Once identified these were combined to provide a geometric summary measure (‘Gbehave’) at each time point by calculating 10∧((Log10(fB1+1 × fB2+1 × fB3+1 × …fB(n)+1)/n) −1); where fB1,2, etc = frequency of behaviours, and ‘n’ denotes the total number of key behavioural parameters.
With the MGS data we first calculated the median of each observer’s score of the 2 photographs. For internal consistency testing, an average was calculated for each FAU (including all observers) at each time point; i.e. Ear, Nose, Cheek and Orb. An Observer Score was then computed; the mean score for each observer including 4 of the 5 FAU’s at each time-point (OS1-4). Whisker Change was excluded because all scorers were unable to reliably ascertain whisker orientation. Finally, a Global Score (GS) was determined; the grand mean OS across all observers at each time point and was used to evaluate the effects of meloxicam.
Statistical Analyses
All analyses were performed using SPSS software (IBM, Version 21). Body weight changes from before to after surgery were compared between groups using one-way ANOVA with multiple comparisons to determine any treatment effects (Bonferroni). Repeated measures ANOVA was used to examine the behaviour changes (Gbehave) from baseline to the postoperative recordings; thus ‘Time’ was the subject’s factor and had 3 levels, and ‘Treatment’ (meloxicam or saline) had 4 levels and was the between subject’s factor. Bonferroni comparisons determined individual treatment differences at each time point. TR underwent the analysis procedure but one-way ANOVA was also used to evaluate individual group differences at 7 hours post-surgery. The OS MGS data at baseline, 4 and 24 hours following surgery were first subjected to reliability analysis using a simplified version of that described for the Rat Grimace Scale (RGS) (Oliver et al., 2014). Internal consistency was tested (Cronbach’s alpha) using the pooled score for individual FAU’s (across all observers) at each separate time-point. Alpha values were determined for the overall scale and with individual FAU’s dropped; to determine if any of the 4 FAU’s were poorly representative of the overall scale. The analysis was performed at each separate time to reveal whether any one or a combination of FAU’s was most relevant in detecting pain. Intra-observer reliability was then determined as the intra-class correlation coefficient (ICC) using the overall OS scores (OS1-4). This was important as the consistency of these would reflect overall MGS utility, and if it could be used successfully by relatively naïve assessors, so the effect of excluding individual scores was determined as in the internal consistency test. The Global MGS scores (GS) underwent repeated measures ANOVA to determine overall time and treatment effects. Multiple regression was used to determine whether inflammation severity (IVIS signal intensity) at 7 hours following surgery was predicted by body weight change, or GS and Gbehave changes from before to 4 hours after surgery (using stepwise entry with an independent variable removal set at p>0.1). Lastly, the effects of handling technique on pre- to post-surgery body weight, Gbehave, GS and inflammation (TR) were determined using probability corrected paired t-tests. All results in the text are expressed as means ±1SD, and all figures show mean values ±1SEM.
Results
There were no intra-operative complications, but for no apparent reason 2 mice in the Sal/Lap/P group died at the 24 hour imaging time.
Body Weight
There was no significant difference in the mean body weights at baseline, indicating equivalent initial group weights; 28.5±1.4g (n=45). There were no significant treatment-related weight effects. A paired samples t-test showed surgery caused a significant average loss of 1.58g±1.1g (t(1,44)=9.4, p<0.001). The greatest losses were in the untreated groups (Sal/Lap/P, 2±1.6g; Sal/Lap/D, 1.9±1.5g), whilst groups M1, M5 and M20 lost slightly less (1.1±0.5, 1.1±0.8 and 1.7±0.8g, respectively). There was no overall effect of handling method on weight loss. However, in line with our prediction that cupped handling, by minimising anxiety, might also minimise pain, an independent samples t-test found tail-handled mice in group Sal/Lap/P lost more weight than those than were cup-handed (3.4±1.3 vs. 1.1±0.8g; p=0.03). This group and Sal/Lap/D received only saline prior to surgery, so in the context of the relationship between weight loss (possibly indicating pain) and handling were the most relevant to examine. Unexpectedly however, mice in the Sal/Lap/D group that were cup handled lost equivalent weight to the tail handled mice, negating the possibility that cupped handling reduced pain.
Imaging
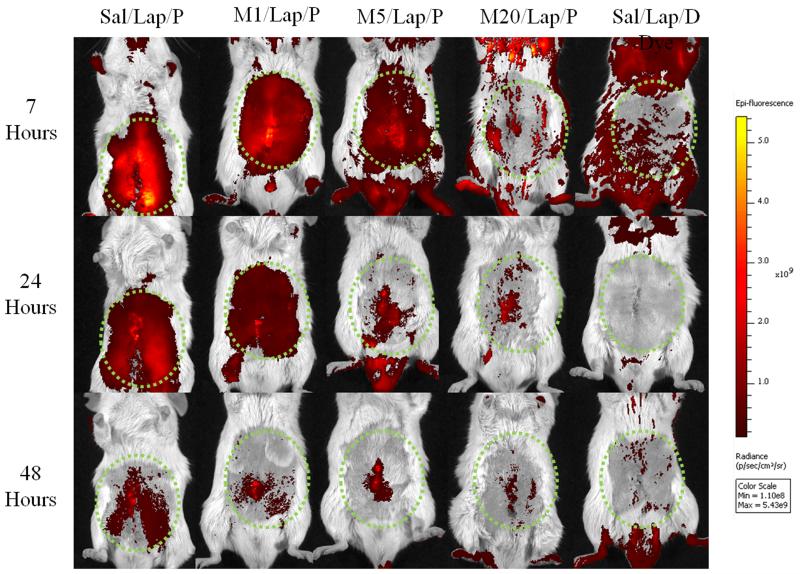
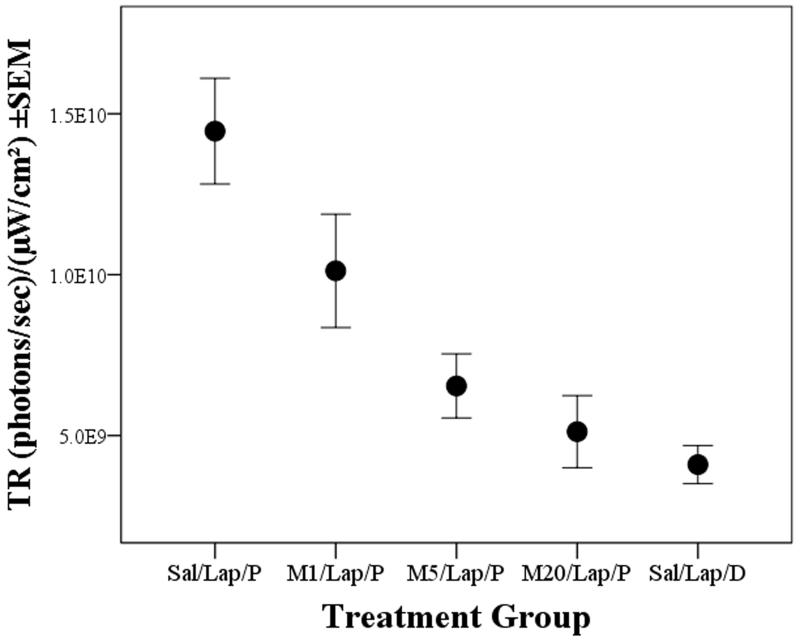
Fig. 1 shows the imaging results from a randomly selected mouse from each treatment group at each imaging time. Inflammation was most intense around the site of surgery (e.g. Fig. 1; 7 hours), but differed according to meloxicam pre-treatment. Ignoring treatment, FigureS1 shows TR declined from 7 to 48 hours (significant ‘Time’ effect (f(2,70)=19.4, p<0.001). Because it was unknown if this resulted from probe metabolism, reduced inflammation or both, only the 7 hour imaging results were used to assess the effect of meloxicam. As Fig. 2 shows, TR in the saline group (Sal/Lap/P; 1.45×1010) significantly exceeded that in either the 5 or 20mg/kg groups (M5/Lap/P, 6.5×109; M20/Lap/P, 5.1×109: p=0.002, p<0.001, respectively). TR following 1mg/kg meloxicam (M1/Lap/P; 1×1010) was less than in the Sal/Lap/P group but was not significant. Unsurprisingly, TR was lowest in the control (Sal/Lap/D) group (4×109); less than in either the Sal/Lap/P or M1/Lap/P groups (p<0.001; p=0.037, respectively) and was similar to the 5 or 20mg/kg meloxicam groups.
Figure 1.
Post-operative images of one randomly selected mouse from each pre-treatment group at 7, 24 and 48 hours, illustrating the time dependent (top to bottom) and meloxicam dose dependent (left to right) decrease in COX-2 Total Radiance (TR; ((photons/sec)/(μW/cm2))). Dotted circles are regions of interest (ROI’s) used to measure TR.
Figure 2.
Mean Total Radiance (TR±1SEM) emitted by the COX-2 probe (P) or control dye (D) at the 7 hour IVIS imaging time following laparotomy (Lap) in mice pre-treated with saline (Sal) or meloxicam at 1, 5 or 20mg/kg (M1, M5, M20); illustrating significantly reduced inflammation in groups M5 and M20 compared to group Sal/Lap/P (p=0.002, p<0.001, respectively).
Behaviour (HCS)
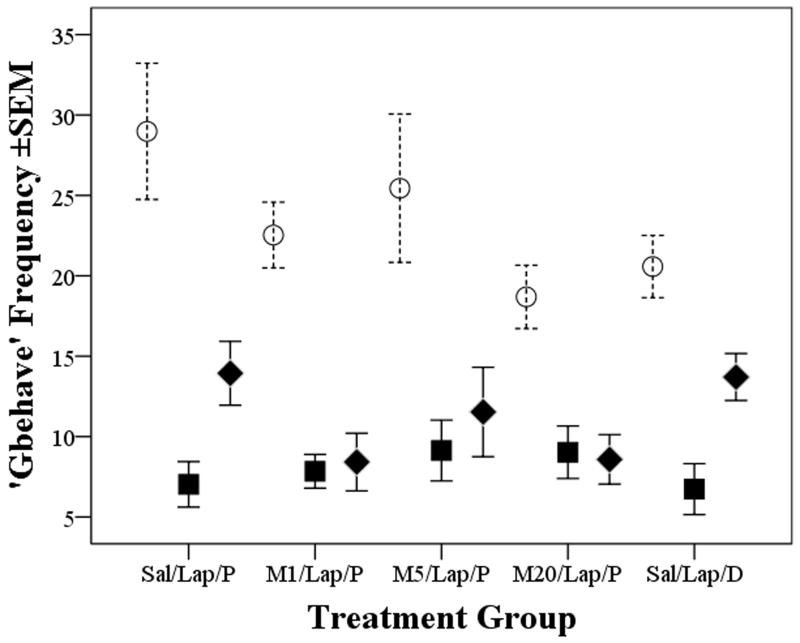
HCS scored 32 of the 38 behaviours it should recognise (Roughan et al., 2009), and DA identified five that were most susceptible to surgery. Walking, rearing, stretching and distance travelled declined, whereas grooming increased. These four parameters and the inverse of grooming were combined to compute the summary measure Gbehave. Fig. 3 shows surgery caused a significant overall reduction in Gbehave (‘Time’ significant; f(2,72)=74.3, p<0.001). Individual contrasts showed reduced behaviour frequency at both 4 and 24 hours compared to baseline (f(1,36)=103, p<0.001; f(1,36)=70, p<0.001, respectively). There were indications of recovery at the 24 hour time-point, but not significantly so. There were no other individual treatment differences and no significant interactions. Tail handled mice in group Sal/Lap/P were less active (Gbehave reduced) than those that were cup-handled at 4 hours (f(1,8)=9, p=0.017), but as in the weight analysis, this did not provide evidence of less pain as a consequence of cupped handling because all mice in the other untreated group (Sal/Lap/D) were equally inactive.
Figure 3.
Mean frequency (±1SEM) of the Gbehave summary behavioural parameter at baseline (open circles) and then 4 and 24 hours (closed squares or diamonds, respectively) in groups pre-treteated with saline (Sal) or 1,5, or 20mg/kg meloxicam (M1; M5; M20) before laparotomy (Lap) and COX-2 probe (P) or control dye injection (D); indicates a significant reduction in active behaviours at both post-surgery recording times (p<0.001 for each time relative to baseline).
MGS
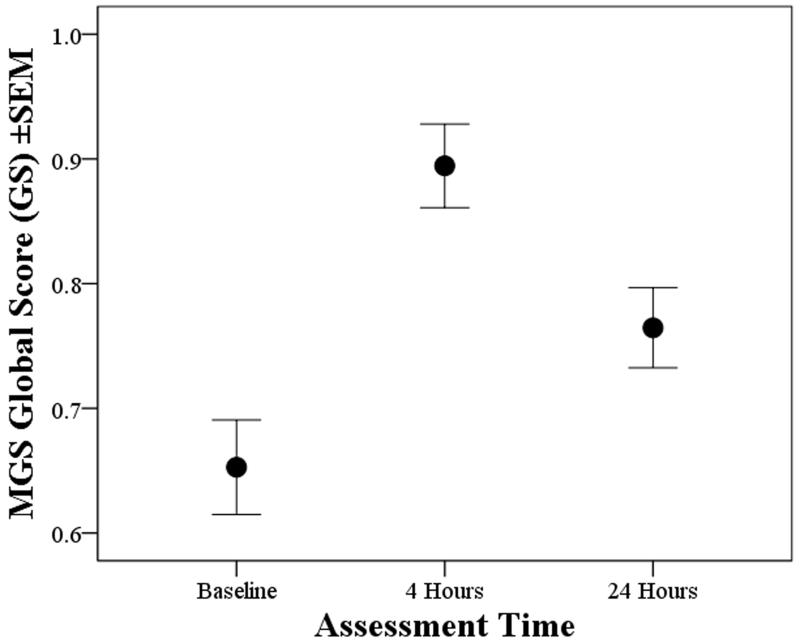
The initial reliability analyses were to determine the internal validity of the individual FAU’s. With the inclusion of all 4 FAU’s, Cronbach’s alpha values at baseline, 4 and 24 hours were 0.71, 0.66 and 0.69, respectively. The effect of dropping individual FAU’s was similar at each time-point and for each FAU, causing little change in alpha. Depending on the FAU dropped these varied from 0.6 to 0.71 at baseline, from 0.53 to 0.68 at 4 hours and 0.57 to 0.73 at 24 hours; meaning all FAU’s were relevant. Excluding ear flattening marginally increased alpha from 0.66 to 0.68 at 4 hours, and from 0.69 to 0.73 at 24 hours. There was also a high level of inter-observer consistency, and ICC coefficient values for the OS scores were 0.82 at baseline, 0.84 at 4 hours and 0.75 at 24 hours. As in the internal consistency test, the individual ICC values remained high irrespective of the exclusion of any individual score; thus all of the observers were able to apply the scale effectively. Fig. 4 shows the Global Scores (GS) at each assessment time; GSB, GS1 and GS24 (respectively baseline, 4 and 24 hours following surgery). The mice ‘Grimaced’ more at 4 hours compared to baseline (f(1,44)=33, p<0.001), and although this lessened at 24 hours (f(1,44)=15.4, p<0.001), they still appeared worse than before surgery (f(1,44)=12.5,p=0.001). There were no meloxicam dose effects, and no difference between the probe and control dye groups. Handling method had no bearing on either the individual OS or GS values.
Figure 4.
Mean MGS global scores (GS±1SEM) at baseline and 4 and 24 hours post-laparotomy (all treatment groups); indicating increased grimacing at 4 and 24 hours (p<0.001 for each time compared to baseline).
Regression
Inflammation severity was not related to body weight change or grimacing (GS), however, there was a modestly significant negative association between Gbehave at 4 hours and TR at 7 hours following surgery; i.e. active behaviours declined and grooming increased in more inflamed mice (r=0.35; (F(1,43)=5.8, p=0.02; not shown).
Refinement outcomes
Contrary to guidelines the imaging reagents were given intravenously not intraperitoneally (i/p), partly because i/p injections fail in up to 14% of animals (Arioli and Rossi, 1970), and also because this may have resulted in additional inflammation or pain. Injection of the probe or control dye caused signs of discomfort (writhing), and although this rapidly dissipated (within 30 seconds) it caused a conspicuous tail-spot whose COX-2 reactivity lasted as long as the effects or surgery; and was unresponsive to meloxicam. Subcutaneous probe injection may therefore be preferable; although the response profile would need to be determined. Although ‘cupped’ handling minimised weight loss and abnormal behaviour, not uniformly, so this was not confirmed to be a procedural refinement. However, tail handled mice were clearly more agitated during weighing, so ‘cupping’ mice may reduce stress in some situations. COX-2 reactivity was detected at regions remote from the incision site; joints and testes. These were possibly fighting injuries, due to restraint for i/v injections, surgical preparation (e.g. shaving) or caused during anaesthesia induction. Using depilatory creams might minimise this but at a cost to behaviour results (e.g. abnormal grooming). Extremely careful shaving and padding induction chambers are therefore recommended.
Discussion
This study investigated the apparent lack of effectiveness of NSAIDs such as meloxicam in alleviating pain in mice, and to determine the contribution of inflammation to post-laparotomy pain. A novel COX-2 probe was used to quantify post-surgical inflammation that if prevented by meloxicam could imply effective pain prevention. However, this would depend on obtaining supplementary evidence of pain, and ideally, by generating a meloxicam dose-response curve. Three dose rates were tested, and three methods of pain assessment were applied to try to negate the possibility that prior negative or inconclusive findings have been due to assessment shortfalls (Matsumiya et al., 2012). In addition to imaging inflammation we therefore also assessed body weight, spontaneous behaviour changes and facial grimacing via the MGS. Surgery caused significant COX-2 up-regulation at both 7 and 24 hours following surgery (Figs. 1 and S1) and this was reduced by meloxicam at either 5 or 20mg/kg (Fig. 2). Following this signal intensities waned (FigureS1), but determining whether this indicated probe clearance or lessened inflammation would have meant re-injecting the probe. We preferred not to do in this initial study, but as more inflamed mice would conceivably need at least 48 hours to recover, probe metabolism seems more likely. Meloxicam therefore minimised inflammation, the COX-2 probe was effective and these are the first data on its use in assessing inflammation post-surgically. Although these were both positive results, with regard to the study’s refinement goals the subsequent findings were less encouraging.
The ‘Gbehave’ parameter summarised the impact of surgery on behaviour, and was similar to one we previously used to demonstrate the effectiveness of buprenorphine in vasectomised mice (Wright-Williams et al., 2013). As then, we supposed that if this was adversely affected by surgery, but was normalised following meloxicam, then indirect conclusions could be drawn both on the presence of pain and its prevention. However, all groups lost weight and behaviour was suppressed for at least 24 hours. This led to an initial proposition that meloxicam might simply be ineffective; as was later supported by the MGS results. These took some time to collect, initially in designing the score-sheet, but also to obtain naïve volunteers that were willing to score the large number of photographs. In fact, it was this retrospective nature of MGS scoring that gave us some initial concerns about its likely effectiveness. Nevertheless, to apply the MGS according to the established methodology we used photographs also. The high inter- and intra-observer consistency reported for the MGS and RGS (Langford et al., 2010; Sotocinal et al., 2011; Oliver et al., 2014) was almost matched by our 4 naïve scorers. Thus, in principle the MGS should be effective for cage-side use and these post-laparotomy results support its particular relevance for assessing pain of moderate intensity/duration due to visceral manipulations (Langford et al., 2010).
Enhanced COX-2 selectivity underpins the tolerability and analgesic effectiveness of meloxicam and similar NSAIDS in humans (Del Tacca et al., 2002; Schwartz et al., 2008). Here, although it prevented inflammation, the behaviour and MGS findings suggested it did not prevent pain. Although the behaviour changes could have been attributed to stress or malaise (Roughan et al., 2014), probably not according to their agreement with the MGS findings in both time-course and magnitude; a 50% activity reduction accompanied a 40% rise in MGS scores lasting at least 24 hours. Based on lesions to brain regions associated with pain ‘affect’, and the finding that acid-induced writhing then continues without a ‘pain face’ Langford et al. proposed that the MGS might reflect the emotional component of pain (Langford et al., 2010). If so, the current behaviour and MGS similarities suggest they were not artefacts caused by peripheral nociceptive stimulation per se, rather, they shared pain as a common origin. The problem is that this lack of beneficial effects of meloxicam contrasts with findings in several other species (Mathews et al., 2001; Gunew et al., 2008; Kluivers-Poodt et al., 2013; Walton et al., 2014). The mouse ‘pain-hiding’ proposition could explain this, but is one that until very recently we viewed rather sceptically. However, the MGS has been shown to be sensitive enough to detect pain suppression due to stress-induced analgesia caused by the presence of an observer; especially if they are male (Sorge et al., 2014). Both sexes were involved at all stages here, except that a female technician collected all the HCS data (but left the room during filming). These variable husbandry arrangements made it impossible to determine if stress influenced our findings, but if so we have more likely underestimated rather than inflated our appraisal of pain, thus our inability to detect analgesia was probably not due to any stress-induced dampening of pain susceptibility.
The finding of superior anti-inflammatory versus pain relieving properties of meloxicam is not new (Bianchi and Panerai, 2002). A lack of centralised analgesic properties of NSAIDS including meloxicam was originally concluded from negative results in heat and mechanical tests in mice and the writhing assay in rats (reviewed by Engelhardt, 1996). However, these testing methods have limited value for estimating clinical drug potency, and probably model only transitory pain (Dennis and Melzack, 1979; Mogil and Crager, 2004; Sufka, 2011; Mao, 2012). Understanding is growing as to how modulation of CNS COX-2 up-regulation contributes to pain sensitivity (Samad et al., 2001), and meloxicam may well have a role in this as evidenced by its COX-2 mediated protective effects against ischemic brain damage (Llorente et al., 2013) and traumatic brain or spinal cord injury in rats (Hakan et al., 2010; Hakan et al., 2011). Spinal COX inhibition alters pain perception (McCormack, 1994), and other inflammatory conditions up-regulate COX-2 in the ventral mid-brain, hypothalamus and thalamus (Samad et al., 2001). The CNS is generally permeable to NSAIDS (Parepally et al., 2006), and COX-2 inhibition following intrathecal injection also diminishes pain sensitivity (Samad et al., 2001). The parent compound of the probe substance (indomethacin) also crosses the blood brain barrier (Parepally et al., 2006), so why meloxicam was not effective remains uncertain. However, these results align with other studies where mice seem to need large doses of meloxicam or other NSAIDs (Wright-Williams et al., 2007; Matsumiya et al., 2012). Thus, although not well understood, the balance between the centralised and peripheral actions of NSAIDs probably underlies their differential abilities to prevent pain. What is most disconcerting, however, is that these drugs are used prophylactically in our facility (and presumably others) at dose rates of between 1 and 10mg/kg for various surgery types in mice but are probably not effective.
There were 2 untreated groups (Sal/Lap/P and Sal/Lap/D), and these offered the best chance of detecting whether cupped handling, by minimising anxiety, could also reduce pain. Although cupped handling minimised weight loss and improved mobility in group Sal/Lap/D, not in group Sal/Lap/D. We therefore could not establish the refinement potential of cupped handling, but where anxiety is unwanted it seems a sensible alternative to tail handling.
To conclude, we assessed if meloxicam prevented post-surgical pain in mice. Inflammation severity, body weight, behaviour analyses and one of the latest techniques for assessing mouse pain were applied; the Mouse Grimace Scale. Meloxicam at 5 and 20 mg/kg significantly reduced abdominal inflammation; however, all other parameters indicated pain or another negatively affective state remained. Whether the mice experienced this as a centralised phenomenon is uncertain. We cannot say why pain persisted other than to speculate that movement elicited activation of muscle and skin mechanoreceptors may have contributed. The alternative is that neither the MGS nor behaviour findings were linked to pain, but this appears unlikely. This suggests that meloxicam should not be used to treat post-laparotomy pain in BALB/c mice, but perhaps not in other strains. In CD1 mice for example, 20mg/kg seems to have positive consequences in vasectomised mice (Leach et al., 2012).
A causal relationship between inflammation and pain generally holds true, but in this study we found pain continued despite effective COX-2 inhibition; at least in the case of BALB/c mice treated with meloxicam. Assuming our pain assessment methods were effective, confirming this separation would require COX-2 imaging in other mouse strains and using less COX-2 specific NSAIDs. Alternatively opioids could be used, in which case we would expect pain suppression despite inflammation. Although these tend to be avoided due to their confounding effects on behaviour (Hayes et al., 2000; Roughan and Flecknell, 2000; Wright-Williams et al., 2013), the MGS is apparently less susceptible to these; as demonstrated with morphine in rats (Sotocinal et al., 2011) and buprenorphine in mice (Matsumiya et al., 2012). The importance of this is that it offers the potential for assessing pain relief following joint administration of an NSAID and an opioid; and simultaneously assessing anti-inflammatory and pain preventative outcomes. Indeed, this may be what is actually required to completely eliminate signs of post-surgical pain in mice. Our demonstration of real time quantification of COX-2-mediated inflammation in-vivo makes it feasible for future investigations to assess this synergistic approach to preventing laparotomy pain in mice and possibly in other models or species.
Supplementary Material
What’s already known about this topic?
Inflammation probably contributes to post-surgical pain so NSAIDs including meloxicam are often used in mice, but have rarely been found to effectively prevent pain.
What does this study add?
We used a COX-2 probe to quantify the real-time anti-inflammatory effects of meloxicam in a clinically relevant setting for the first time. Although meloxicam prevented inflammation, persistent behavioural abnormalities and elevated Mouse Grimace Scores confirmed it is probably not an effective analgesic for BALB/c mice.
Acknowledgements
The mice were provided free of charge by Charles River UK. The COX-2 reagents were administered by Mr Christopher Huggins or Mr Robert Stewart.
Funding: The COX-2 imaging reagents were provided by the Centre for Behaviour and Evolution, Newcastle University. The UK National Centre for the 3Rs provided the imaging time (G0900763/1), and the Wellcome Trust provided the IVIS machine (Grant number 087961).
Footnotes
Conflicts of Interest: None arising.
References
- Adamson TW, Kendall LV, Goss S, Grayson K, Touma C, Palme R, Chen JQ, Borowsky AD. Assessment of carprofen and buprenorphine on recovery of mice after surgical removal of the mammary fat pad. J Am Assoc Lab Anim Sci. 2010;49:610–616. [PMC free article] [PubMed] [Google Scholar]
- Arioli V, Rossi E. Errors related to different techniques of intraperitoneal injection in mice. Appl Microbiol. 1970;19:704–705. doi: 10.1128/am.19.4.704-705.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M, Panerai AE. Effects of lornoxicam, piroxicam, and meloxicam in a model of thermal hindpaw hyperalgesia induced by formalin injection in rat tail. Pharmacol Res. 2002;45:101–105. doi: 10.1006/phrs.2001.0921. [DOI] [PubMed] [Google Scholar]
- Brennan MP, Sinusas AJ, Horvath TL, Collins JG, Harding MJ. Correlation between body weight changes and postoperative pain in rats treated with meloxicam or buprenorphine. Lab Animal. 2009;38:87–93. doi: 10.1038/laban0309-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda MC, Tolva V, Casana R, Gnecchi M, Vanoli E, Spazzolini C, Roughan J, Calvillo L. Rat experimental model of myocardial ischemia/reperfusion injury: an ethical approach to set up the analgesic management of acute post-surgical pain. PLoS ONE [Electronic Resource] 2014;9:e95913. doi: 10.1371/journal.pone.0095913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol Suppl. 1997;49:15–19. [PubMed] [Google Scholar]
- Dalla Costa E, Minero M, Lebelt D, Stucke D, Canali E, Leach MC. Development of the Horse Grimace Scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS ONE [Electronic Resource] 2014;9:e92281. doi: 10.1371/journal.pone.0092281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Tacca M, Colucci R, Fornai M, Blandizzi C. Efficacy and Tolerability of Meloxicam, a COX-2 Preferential Nonsteroidal Anti-Inflammatory Drug. Clin Drug Investig. 2002;22:799–818. [Google Scholar]
- Dennis S, Melzack R. Comparison of phasic and tonic pain in animals. Raven Press; New York: 1979. [Google Scholar]
- Dickinson AL, Leach MC, Flecknell PA. The analgesic effects of oral paracetamol in two strains of mice undergoing vasectomy. Lab Anim. 2009;43:357–361. doi: 10.1258/la.2009.009005. [DOI] [PubMed] [Google Scholar]
- Engelhardt G. Pharmacology of meloxicam, a new non-steroidal anti-inflammatory drug with an improved safety profile through preferential inhibition of COX-2. Br J Rheumatol. 1996;35(Suppl 1):4–12. doi: 10.1093/rheumatology/35.suppl_1.4. [DOI] [PubMed] [Google Scholar]
- Gunew MN, Menrath VH, Marshall RD. Long-term safety, efficacy and palatability of oral meloxicam at 0.01-0.03 mg/kg for treatment of osteoarthritic pain in cats. J Feline Med Surg. 2008;10:235–241. doi: 10.1016/j.jfms.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakan T, Toklu HZ, Biber N, Celik H, Erzik C, Ogunc AV, Cetinel S, Sener G. Meloxicam exerts neuroprotection on spinal cord trauma in rats. Int J Neurosci. 2011;121:142–148. doi: 10.3109/00207454.2010.537415. [DOI] [PubMed] [Google Scholar]
- Hakan T, Toklu HZ, Biber N, Ozevren H, Solakoglu S, Demirturk P, Aker FV. Effect of COX-2 inhibitor meloxicam against traumatic brain injury-induced biochemical, histopathological changes and blood-brain barrier permeability. Neurol Res. 2010;32:629–635. doi: 10.1179/016164109X12464612122731. [DOI] [PubMed] [Google Scholar]
- Hayes KE, Raucci JA, Jr., Gades NM, Toth LA. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp Topics Lab Anim Sci. 2000;39:18–23. [PubMed] [Google Scholar]
- Hurst JL, West RS. Taming anxiety in laboratory mice. Nat Methods. 2010;7:825–826. doi: 10.1038/nmeth.1500. [DOI] [PubMed] [Google Scholar]
- Keating SCJ, Thomas AA, Flecknell PA, Leach MC. Evaluation of EMLA cream for preventing pain during tattooing of rabbits: changes in physiological, behavioural and facial expression responses. PLoS ONE [Electronic Resource] 2012;7:e44437. doi: 10.1371/journal.pone.0044437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluivers-Poodt M, Zonderland JJ, Verbraak J, Lambooij E, Hellebrekers LJ. Pain behaviour after castration of piglets; effect of pain relief with lidocaine and/or meloxicam. Animal. 2013;7:1158–1162. doi: 10.1017/S1751731113000086. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- Leach MC, Klaus K, Miller AL, Scotto di Perrotolo M, Sotocinal SG, Flecknell PA. The assessment of post-vasectomy pain in mice using behaviour and the Mouse Grimace Scale. PLoS ONE [Electronic Resource] 2012;7:e35656. doi: 10.1371/journal.pone.0035656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente IL, Perez-Rodriguez D, Burgin TC, Gonzalo-Orden JM, Martinez-Villayandre B, Fernandez-Lopez A. Age and meloxicam modify the response of the glutamate vesicular transporters (VGLUTs) after transient global cerebral ischemia in the rat brain. Brain Res Bull. 2013;94:90–97. doi: 10.1016/j.brainresbull.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Mao J. Current challenges in translational pain research. Trends Pharmacol Sci. 2012;33:568–573. doi: 10.1016/j.tips.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews KA, Pettifer G, Foster R, McDonell W. Safety and efficacy of preoperative administration of meloxicam, compared with that of ketoprofen and butorphanol in dogs undergoing abdominal surgery. Am J Vet Res. 2001;62:882–888. doi: 10.2460/ajvr.2001.62.882. [DOI] [PubMed] [Google Scholar]
- Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, Zaloum A, King OD, Mogil JS. Using the Mouse Grimace Scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci. 2012;51:42–49. [PMC free article] [PubMed] [Google Scholar]
- McCormack K. The spinal actions of nonsteroidal anti-inflammatory drugs and the dissociation between their anti-inflammatory and analgesic effects. Drugs. 1994;47(Suppl 5) doi: 10.2165/00003495-199400475-00006. [DOI] [PubMed] [Google Scholar]
- Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84:65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- Miller AL, Flecknell PA, Leach MC, Roughan JV. A comparison of a manual and an automated behavioural analysis method for assessing post-operative pain in mice. Appl Anim Behav Sci. 2011;131:138–144. [Google Scholar]
- Miller AL, Wright-Williams SL, Flecknell PA, Roughan JV. A comparison of abdominal and scrotal approach methods of vasectomy and the influence of analgesic treatment in laboratory mice. Lab Anim. 2012;46:304–310. doi: 10.1258/la.2012.012078. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals. Pain. 2004;112:12–15. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- MORI I. Views On Animal Testing. 2010.
- NC3Rs The untold story: what statistics don’t tell you about animal use in science. 2013.
- Oliver V, De Rantere D, Ritchie R, Chisholm J, Hecker KG, Pang DSJ. Psychometric assessment of the Rat Grimace Scale and development of an analgesic intervention score. PLoS ONE [Electronic Resource] 2014;9:e97882. doi: 10.1371/journal.pone.0097882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parepally JMR, Mandula H, Smith QR. Brain uptake of nonsteroidal anti-inflammatory drugs: ibuprofen, flurbiprofen, and indomethacin. Pharm Res. 2006;23:873–881. doi: 10.1007/s11095-006-9905-5. [DOI] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscl Throm Vas. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan JV, Coulter CA, Flecknell PA, Thomas HD, Sufka KJ. The conditioned place preference test for assessing welfare consequences and potential refinements in a mouse bladder cancer model. PLoS ONE [Electronic Resource] 2014;9:e103362. doi: 10.1371/journal.pone.0103362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan JV, Flecknell PA. Effects of surgery and analgesic administration on spontaneous behaviour in singly housed rats. Res Vet Sci. 2000;69:283–288. doi: 10.1053/rvsc.2000.0430. [DOI] [PubMed] [Google Scholar]
- Roughan JV, Flecknell PA. Behavioural effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain. 2001;90:65–74. doi: 10.1016/s0304-3959(00)00387-0. [DOI] [PubMed] [Google Scholar]
- Roughan JV, Flecknell PA. Evaluation of a short duration behaviour-based post operative pain scoring system in rats. Eur J Pain. 2003;7:397–406. doi: 10.1016/S1090-3801(02)00140-4. [DOI] [PubMed] [Google Scholar]
- Roughan JV, Flecknell PA. Behaviour-based assessment of the duration of laparotomy-induced abdominal pain and the analgesic effects of carprofen and buprenorphine in rats. Behav Pharmacol. 2004;15:461–472. doi: 10.1097/00008877-200411000-00002. [DOI] [PubMed] [Google Scholar]
- Roughan JV, Wright-Williams SL, Flecknell PA. Automated analysis of postoperative behaviour: assessment of HomeCageScan as a novel method to rapidly identify pain and analgesic effects in mice. Lab Anim. 2009;43:17–26. doi: 10.1258/la.2008.007156. [DOI] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Schwartz JI, Dallob AL, Larson PJ, Laterza OF, Miller J, Royalty J, Snyder KM, Chappell DL, Hilliard DA, Flynn ME, et al. Comparative inhibitory activity of etoricoxib, celecoxib, and diclofenac on COX-2 versus COX-1 in healthy subjects. J Clin Pharmacol. 2008;48:745–754. doi: 10.1177/0091270008317590. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11:629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, Mapplebeck JCS, Wei P, Zhan S, Zhang S, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes E, Flecknell P, Richardson C. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim. 2009;43:149–154. doi: 10.1258/la.2008.008020. [DOI] [PubMed] [Google Scholar]
- Sufka K. Translational challenges and analgesic screening assays. Pain. 2011;152:1942–1943. doi: 10.1016/j.pain.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Uddin MJ, Crews BC, Blobaum AL, Kingsley PJ, Gorden DL, McIntyre JO, Matrisian LM, Subbaramaiah K, Dannenberg AJ, Piston DW, Marnett LJ. Selective visualization of cyclooxygenase-2 in inflammation and cancer by targeted fluorescent imaging agents. Cancer Res. 2010;70:3618–3627. doi: 10.1158/0008-5472.CAN-09-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton MB, Cowderoy EC, Wustefeld-Janssens B, Lascelles BDX, Innes JF. Mavacoxib and meloxicam for canine osteoarthritis: a randomised clinical comparator trial. Vet Rec. 2014;175:280. doi: 10.1136/vr.102435. [DOI] [PubMed] [Google Scholar]
- Wright-Williams S, Flecknell PA, Roughan JV. Comparative effects of vasectomy surgery and buprenorphine treatment on faecal corticosterone concentrations and behaviour assessed by manual and automated analysis methods in C57 and C3H mice. PLoS ONE [Electronic Resource] 2013;8:e75948. doi: 10.1371/journal.pone.0075948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright-Williams SL, Courade J-P, Richardson CA, Roughan JV, Flecknell PA. Effects of vasectomy surgery and meloxicam treatment on faecal corticosterone levels and behaviour in two strains of laboratory mouse. Pain. 2007;130:108–118. doi: 10.1016/j.pain.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Wuest F, Kniess T, Bergmann R, Pietzsch J. Synthesis and evaluation in vitro and in vivo of a 11C-labeled cyclooxygenase-2 (COX-2) inhibitor. Bioorg Med Chem. 2008;16:7662–7670. doi: 10.1016/j.bmc.2008.07.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.