Abstract
Background
Diagnosing asthma in infancy is largely made on the basis of the symptoms of cough and wheeze. A similar presentation can be seen in neurologically normal infants with excessive gastroesophageal reflux (GER). There are no randomized placebo controlled studies in infants using proton pump inhibitors (PPI) alone or in addition to prokinetic agents.
Objectives
The primary objective was to confirm the presence of excessive GER in a population of infants that also had respiratory symptoms suggestive of asthma. Second, in a randomized placebo-controlled fashion, we determined whether treatment of GER with bethanacol and omeprazole could improve these respiratory symptoms.
Methods
Infants (n=22) with a history of chronic cough and wheeze were enrolled, if they had evidence of GER by history and an abnormal pH probe or gastric emptying scan. Infants were randomly allocated to four treatment groups: placebo/placebo (PP), omeprazole plus bethanacol (OB), omeprazole/placebo (OP), bethanacol/placebo (BP). Evaluations by clinic questionnaire and exam, home diary, and pH probe data were done before, after study-medication and after open label of OB.
Results
Nineteen children were studied. PP did not affect GER or respiratory symptoms, and did not decrease GER measured by pH probe. In contrast, OB decreased GER as measured by pH probe indices and parental assessment. In association, OB significantly decreased daytime coughing and improved respiratory scores. No adverse effects were reported.
Conclusions
In infants with a clinical presentation suggestive of chronic GER-related cough, the use of omeprazole and bethanacol appears to be viable therapeutic option.
Keywords: Cough, gastroesophageal reflux, pediatric asthma, esophagus
Introduction
Diagnosing asthma in infancy is a difficult process compared to that in older children or adults. Often the diagnosis of asthma is made on the basis of the symptoms of cough and wheeze alone.
Even in neurologically normal infants, a similar presentation of chronic cough, increased respiratory effort, and wheezing can be secondary to excessive gastroesophageal reflux (GER) (1-3). It is unclear whether this is due to aspiration of gastric contents into the airway, to contact of acid on or around the larynx, or from reflexes in the esophagus (2). While GER is a more common event at this age (4), some infants can still have GER more frequently and/or of larger volumes than other babies such that it may cause pathology. Gastroesophageal Reflux Disease (GERD) is defined as esophageal disease as a result of excessive GER (4,5), and studies regarding management of GERD in infants are approached from a this perspective focusing on esophageal pathology (4). Most gastroenterologists would not call GER a disease without evidence of esophageal pathology. Unfortunately, the respiratory pathology from GER does not usually fit in this definition. The issue of GER and secondary lung pathology has been examined in many studies, but consensus regarding the correct management for infants with excessive GER and lung disease remains unclear.
Gastric motility is a complex motor action of the stomach. Some infants with abnormal GER have a decreased rate of gastric emptying. Gastrointestinal (GI) prokinetic agents could improve gastric emptying decreasing available gastric residue to reflux. Acid suppressing and prokinetic GI drugs are used in children with recurrent pulmonary disorders, including wheezing, cough, and frank aspiration with the expectation that use of these drugs would decrease GER and pulmonary disease. There is reliable evidence in children to support both acid suppressing drugs and prokinetic agents i.v to decrease volume and acidity of gastric contents pre-operatively (6-8). There is only one study to support their use in infants with associated lung disease using the prokinetic agent, cisapride, and the H2 blocker, ranitidine (9). The motility agent cisapride has been completely removed from today’s market secondary to unacceptable cardiac side effects versus benefits (10), and there are newer and better acid blockers approved for pediatrics, the proton pump inhibitors.
Overall, no study with proton pump inhibitors alone or in addition to prokinetic agents has been done in a randomized placebo-controlled fashion to show an effect on GER-related respiratory symptoms. Bethanacol (Urecholine®) is a parasympathomimetic agent, which stimulates gastric motility, increases gastric tone, and affects motility (11). Omeprazole (Losec®) is established in pediatrics for its ability in reducing stomach acidity (12). We hypothesized that many infants presenting with asthma-like symptoms have excessive GER as a primary etiology. This study was designed to confirm the presence of excessive GER in a population of infants with the respiratory symptoms of asthma. Second, in a randomized placebo-controlled fashion, we determined whether treatment of these infants with bethanacol and omeprazole could improve respiratory symptoms.
Methods
Study entry criteria
Infants (3 months -2 years) were recruited in Pediatric Pulmonary and Gastroenterology clinics (Stollery Children’s Hospital, University of Alberta). All people have gastroesophageal reflux (GER) events. Gastroesophageal Reflux Disease (GERD) is defined as confirmed esophageal disease because of excessive GER. Most gastroenterologists would not call GER a disease without evidence of esophageal pathology. Unfortunately, the respiratory pathology from excessive GER does not usually fit in this definition. For our study, infants were enrolled if they were affected by chronic (>3 months) respiratory disease (recurrent cough and/or wheeze), daily GER symptoms (visible emesis and/or rumination), and objective evidence of abnormal GI motility (either an abnormal pH probe or significantly delayed gastric emptying on a nuclear medicine scan). Duel channel pH probes were placed for an 18-24 hour period (GERD Chek, Sandhill, Denver CO). While infants are more likely to also have non-acid events, unfortunately esophageal impedance measurements were not available in our centre for this study. Gastric emptying scans were performed using technetium labeled infant formula, taking one-minute images over 120 minutes to calculate an emptying half time. While there are no accepted normative guidelines available for infants, based on available childhood and adult literature (13,14) and our radiologists’ experience, most infants should have emptying times less than 90 minutes. An abnormal scan was defined as an emptying half-time greater than 90 minutes. Respiratory and GER symptoms were not necessarily temporally related for inclusion as this was not possible. Consent for a repeat pH probe and gastric emptying scan was required. Infants were excluded if they were allergic to any study medications, if they had known anatomic or neurological factors predisposing to direct pulmonary aspiration (i.e. tracheostomy, laryngeal cleft, cerebral palsy), if they had food refusal or failure to thrive, or if caregivers were unable to reliably follow the directions of the study. All parents gave written informed consent as approved by the Health Research Ethics Board, University of Alberta, prior to entry.
Study design
Infants were blindly coded and allocated to one of four study-medication protocols: placebo/placebo (PP), omeprazole/bethanacol (OB), omeprazole/placebo (OP), bethanacol/placebo (BP). Patient allocation and recording of drug-related data was performed blindly in the pharmacy research unit. Based on published data, omeprazole MUPS tabs (10 mg) were given to parents to be dissolved in water or juice based on a dosing guideline of 1/2 tab BID for infants 5-7.5 kg, 1 tab BID for 7.5-12.5 kg, 1.5 tab BID for =12.5-17.5, >17.5 kg received one 20 mg tab (approximately 1 mg/kg/dose). Lactulose in a tablet served as placebo. Bethanacol was dissolved in oro-plus and ora-sweet in a 1:1 ratio, and this vehicle was also used as placebo (15). After one month, infants were restudied with pH probe and nuclear medicine gastric emptying scan. Infants continued on open label therapy, of omeprazole plus bethanacol for another month. CONSORT guidelines were followed for preparation of this manuscript (Figure 1).
Figure 1.
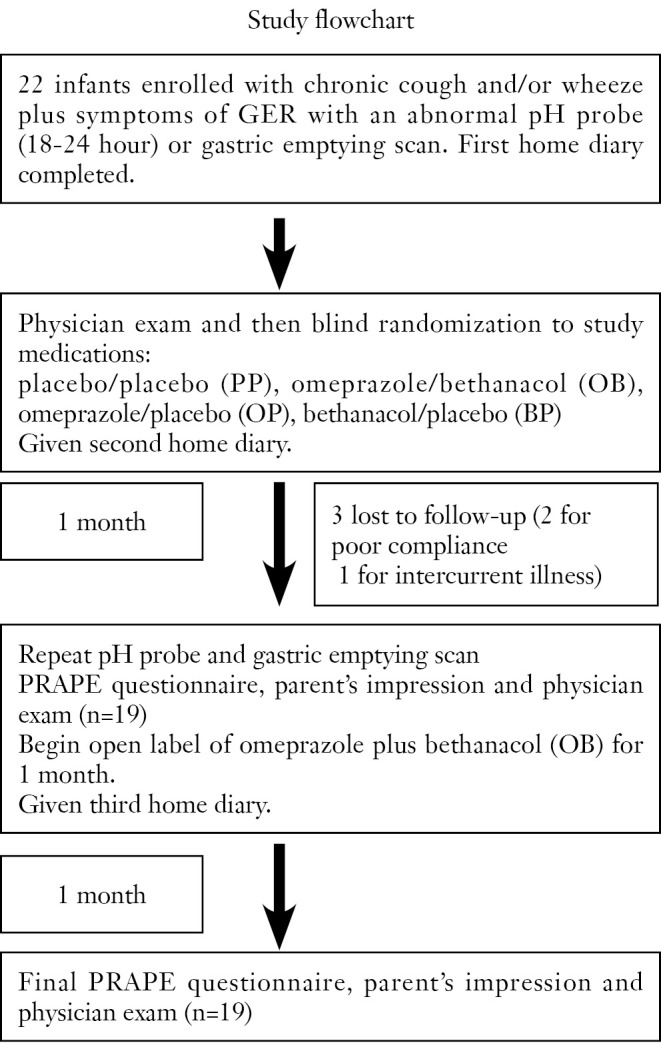
Test process.
Study outcome data
Before each visit, infants were assessed by a caregiver diary to record respiratory and GER events in the previous 7 days, including: number/day of coughing spells (not during feeds); coughing spells at night; wheezing spells; episodes of emesis/rumination; fever; and apnea. The values were averaged for the week as events/day. Emesis was defined a visible stomach contents in the mouth, while rumination was defined as the parents impression of stomach contents coming up but being swallowed down without visualization in the mouth. A Potential Reflux Associated Pulmonary Event (PRAPE) questionnaire covering similar data was completed at each visit with the parent. The parent diary was used to help parents recall their child’s past week of symptoms to generate more accurate data regarding the respiratory and GER data. Parents were also questioned regarding the use of asthma medications during the study month (less often, same, more often). At each visit, both parent and doctor gave their impression of the response to therapy. Physicians were blinded to parent’s impression. Impression was graded as: worse (–1), same (0), better (+1), and much better (+2). To objectively grade changes in pulmonary health, physicians used a standardized respiratory scoring sheet adapted from a study on infant respiratory distress (16). Infants were graded (0-4 with 4 being the most severe) for respiratory rate, wheezing, crackles, and chest retractions for a total score out of 12. Combined total score was recorded pre-study, post study and post open label. pH probe results pre and post study-medication were scored in a blinded fashion for percent time with pH<4.0 (RI%), number of episodes with pH<4.0, and a combined score. Scores were measured using the DeMeester method (17,18). Gastric emptying scans were also compared pre and post the study drug period. An abnormal scan was based or significant delays in gastric emptying time.
Statistical analysis
During preparation of this study, there were no studies in infants with this clinical scenario using a scoring system similar to ours. Thus, we based power calculations on published pH probe data and the assumption that therapy would have a strong impact on respiratory symptom scores. We planned for 40 infants total, but unfortunately enrolling infants for this study was difficult, given the prospect of two pH probes and the potential delay in medical therapy. As a result, the number of infants (n=19) was too low for intergroup analysis by ANOVA, yielding a maximum power to detect differences amongst the 4 treatment groups at 65.8% (comparing the pH probe RI values between the OB and PP groups). To adjust for the small numbers, each infant served as their own control, and we compared their pre, post and open label time periods using Wilcoxin Signed Rank analysis (Statview 5.0 software, SAS Institute, Cary, NC). A p-value <0.05 was considered significant. This gave acceptable power for comparing pre and post respiratory scores within the OB group (95.7% power) and pre and post daytime cough within the OB group (87.3% power). All other comparisons between the pre and post treatment values achieve less than 70.7% power to detect a statistically significant difference between the observed means. This statistical issues related to the study were reviewed and approved by an independent statistical group at the University of Alberta. All data are expressed as median and interquartile ranges.
Results
Patient characteristics
Twenty-two of 25 infants were enrolled in the study (Table 1). Most infants had been given a diagnosis of asthma previously and were having respiratory problems despite some form of asthma therapy. Three infants were unable to complete the study (2 male, 1 female), two for poor compliance, and one for intercurrent illness. There was a preponderance of boys (18 males, 4 females). There was no significant difference regarding sex in the allocation to treatment groups (one female per group). Median age was 9.0 months (interquartile range of 5.3-12.0 months), again with no difference in allocation to the treatment groups Placebo/Placebo (PP) 8.0 (6.5-9.5, n=4); omeprazole/bethanacol (OB) 7.0 (4.0-12.0, n=6); omeprazole/placebo (OP) 10.5 (7.5-12.5, n=4); bethanacol/placebo (BP) 9.0 (4.8-13.2, n=5). No parent reported any adverse side effect from any of the therapies or diagnostic tests including diarrhea.
Table 1. Patient Characteristics.
| ID | Age (Months) | Sex | Smoking in home | Known atopy | First degree relative with atopy/asthma |
|---|---|---|---|---|---|
| 1 | 9 | Male | Yes | No | Yes |
| 2 | 13 | Male | No | No | Yes |
| 3 | 5 | Male | Yes | No | Yes |
| 5 | 4 | Male | No | No | Yes |
| 7 | 10 | Male | No | No | No |
| 8 | 6 | Male | No | No | Yes |
| 9 | 10 | Male | No | No | Yes |
| 11 | 14 | Female | No | No | No |
| 12 | 7 | Male | No | No | Yes |
| 13 | 12 | Male | Yes | No | No |
| 14* | 9 | Male | Yes | No | No |
| 15 | 5 | Male | No | No | No |
| 16 | 4 | Female | No | No | Yes |
| 17 | 13 | Female | No | No | Yes |
| 18 | 9 | Male | No | No | Yes |
| 19 | 9 | Male | No | No | Yes |
| 20 | 6 | Male | No | No | Yes |
| 21 | 4 | Male | No | Yes | Yes |
| 22* | 11 | Female | No | No | No |
| 23 | 13 | Male | No | Yes | Yes |
| 24 | 12 | Male | No | No | Yes |
| 25* | 9 | Male | No | No | Yes |
*did not complete the study.
pH probe results
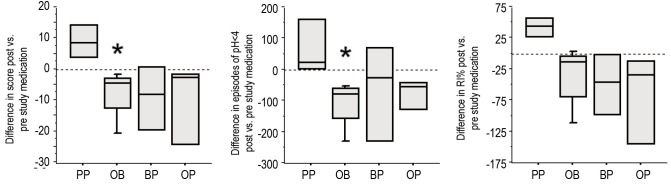
In addition to the symptoms of GER, infants were entered into the study if they had evidence of abnormalities on either a pH probe or gastric emptying scan. 15 of the 19 infants displayed increased amounts GER based on percent time with pH<4 (RI%), the number of episodes with pH<4, and a DeMeester score (Table 2). The remaining 4 infants had evidence of GER based on an abnormal gastric emptying scan with parent-observed GER. The degree of GER measured by the pH probe was not statistically different between groups at study entry. After the study period, the PP group was not improved, but tended to get worse untreated over the month as the RI, the number of episodes, and the score all increased (Figure 2). In contrast, the OB group showed a significant improvement in RI and episodes (P=0.028 each) and a trend for improvement in score after a month of therapy. The OP and BP groups also showed some improvement in all categories compared to their study entry values, but with the smaller number of patients, this did not reach statistical significance. All infants had at least one gastric emptying scan. Thirteen of the nineteen were abnormal at study entry.
Table 2. pH probe results.
| Placebo + Placebo (n=4) |
Omeprazole + Bethanacol (n=6) |
Omeprazole + Placebo (n=3) |
Bethanacol + Placebo (n=5/3) |
|||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| pH Probe Score | 39.5 (33.0-62.4) | 92.6 (67.0-114.6) | 60.0 (21.2-141.4) | 17.8 (16.1-24.9) | 46.7 (25.6-154.6) | 13.2 (10.4-14.7) | 79.1 (29.4-136.7) | 34.6 (20.7-45.3) |
| Number of Episodes (pH<4) | 87.5 (61.5-167.5) | 173.0 (112.0-285.5) | 141.5 (126-359) | 78.0* (41-121) | 98.0 (76.3-167.8) | 40.0 (34.8-44.5) | 102.0 (11.5-421.5) | 79.0 (72.3-105.3) |
| % Time pH<4 | 8.1 (5.3-11.7) | 18.1 (12.2-23.4) | 11.3 (3.8-24.9) | 2.8* (1.1-3.3) | 3.6 (3.0-24.8) | 1.3 (0.6-1.7) | 11.2 (3.5-21.8) | 3.4 (2.4-7) |
*P=0.028 after Wilcoxin signed rank analysis of pre vs. post values.
Figure 2.
Infants had pH probes done before and after study medications. Compared to pre-study pH probe data, the PP group (white bars, n=4) did not show any improvement in measures of percent time with pH<4 (RI%) (A), the number of episodes with pH<4 (B), or a combined score (C). In contrast, the OB group (n=6) showed a decrease in RI% (p=0.028), a decrease in episodes (P=0.028), and a decrease in score (P=0.07). The OP (n=3) and BP (n=3) groups also showed a decrease in RI%, episodes, and score, but with the smaller number of patients these improvements did not reach statistical significance.
Improvement of GI symptoms
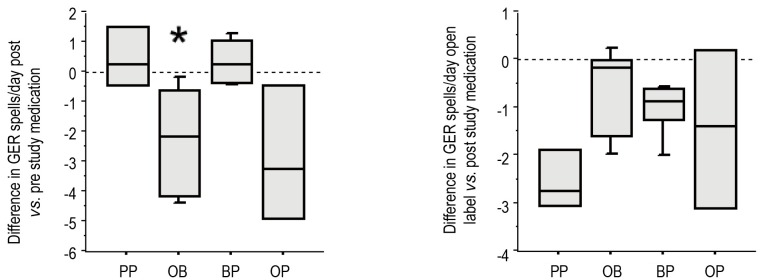
All groups displayed frequent episodes of GER symptoms (emesis and/or rumination) at study entry (Table 3). We included rumination in our symptom score as rumination can be very important from a respiratory perspective. Any stomach content coming up to the hypopharynx is potentially harmful to the larynx and lower airway. After one month of therapy, PP did not decrease the amount of GER symptoms compared to their pre-study value (Figure 3A). In contrast, treatment with either OB or OP decreased the number of GER symptoms per day, though given the smaller sample size, this decrease was only statistically significant for the OB group (P=0.028). The BP treatment showed no improvement compared to values their values at study entry. After receiving the open label therapy of bethanacol and omeprazole, the PP group demonstrated a decrease in GER symptoms similar to OB though given the small number of patients, it did not reach statistical significance (Figure 3B, P=0.06). Each study drug group also had an open period with some further improvement, but again due to small numbers statistical significance was not achieved.
Table 3. Clinical Results.
| Placebo + Placebo (n=4) |
Omeprazole + Bethanacol (n=6) |
Omeprazole + Placebo (n=4) |
Bethanacol + Placebo (n=5) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Open | Pre | Post | Open | Pre | Post | Open | Pre | Post | Open | |
| GER Episodes/Day | 2.0 (1.6-3.0) | 3.1 (2.3-3.4) | 0.2 (0.1-0.7) | 2.3 (0.6-6.7) | 0.2* (0-2.6) | 0.2 (0-1.0) | 4.9 (4.2-5.0) | 1.7 (0-3.9) | 0.5 (0.2-0.9) | 1.0 (0.8-10) | 2.0 (1.0-10.3) | 1.4 (0.2-8.5) |
| Coughing Episodes/Day | 2.9 (2.0-5.1) | 3.4 (2.5-4.9) | 1.0 (0.5-2.0) | 3.4 (1.0-6.0) | 0.4* (0-3.1) | 1.0 (0.3-1.9) | 5.6 (3.6-8.3) | 2.4 (0.5-6.6) | 0.9 (2.9-6.7) | 5.0 (4.5-8.9) | 3.0 (1.0-7.8) | 4.3 (1.0-6.6) |
| Respiratory Score (0-12) | 2.5 (2.0-4.0) | 3.0 (2.0-3.5) | 0 (0-1.0) | 3.0 (2.0-3.0) | 1.5* (0-2.0) | 0 (0-1.0) | 4.0 (3.0-5.0) | 1.5 (0.5-3.0) | 1.5 (0-4.0) | 2.0 (2.0-5.3) | 1.0 (1.0-2.5) | 1.0 (0-2.3) |
| Wheezing Episodes/Day | 1.6 (0.7-3.4) | 1.9 (0.1-4.6) | 0.5 (0-2.4) | 2.3 (0-7.5) | 0.9 (0.1-2.9) | 0.6 (0.1-1.6) | 0.9 (0.3-2.7) | 0.2 (0.7-0.4) | 0.4 (0.1-2.0) | 1.4 (0.3-3.3) | 0 (0-2.5) | 0 (0-1.7) |
| Coughing Episodes/Night | 0.6 (0.5-0.9) | 0.4 (0.4-0.6) | 0.2 (0.1-0.4) | 1.1 (0.4-2.4) | 1.1 (0.1-2.0) | 0.3 (0-1.0) | 1.3 (1.1-3.9) | 0.9 (0-2.4) | 0.1 (0-0.4) | 1.5 (0.5-2.9) | 0.1 (0-1.8) | 0 (0-0.6) |
*P=0.028 after Wilcoxin signed rank analysis of pre vs. post values.
Figure 3.
Each infant was assessed for the number of GER events/day (visible emesis and/or rumination). After one-month post-study medication, use of PP did not decrease the amount of GER episodes compared to their pre-study value. Treatment with either OB or OP decreased the number of GER episodes per day compared to their study entry, though this was statistically significant only for the OB group (P=0.028). After an open label period of bethanacol and omeprazole, the PP group demonstrated a decrease in GER episodes similar to OB though it did not reach statistical significance (P=0.06) (B).
Improvement of daytime cough
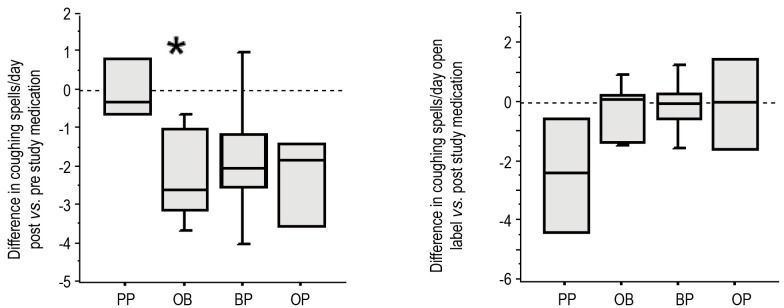
All groups displayed excessive episodes of daytime coughing at study entry, which was not statistically different between groups (Table 3). After one month of therapy, PP did not decrease the amount of coughing compared to the group’s pre-study value (Figure 4A). In contrast, the three treatments of OB, OP and BP decreased the number of coughing episodes per day, which was statistically significant for the OB group (P=0.028). After receiving the open label therapy of bethanacol and omeprazole, the PP group demonstrated a decrease in cough that was similar in magnitude to the OB study period though it did not reach statistical significance (P=0.06, Figure 4B). Each study drug group also had an open period with further minor improvement in cough.
Figure 4.
Each infant was assessed for coughing spells/day. Post-therapy, PP (n=4) did not decrease the amount of coughing compared to the group’s pre-study value (A). In contrast, the three treatments of OB (n=6), OP (n=4), and BP (n=5) did decrease the number of coughing episodes per day, but these data only reached statistical significance for the OB group (P=0.028). After open label therapy of bethanacol and omeprazole, the PP group demonstrated a decrease in cough that was similar in magnitude to the OB study period though it did not reach statistical significance (P=0.06, Figure 3B).
Improvement of respiratory scores (RS)
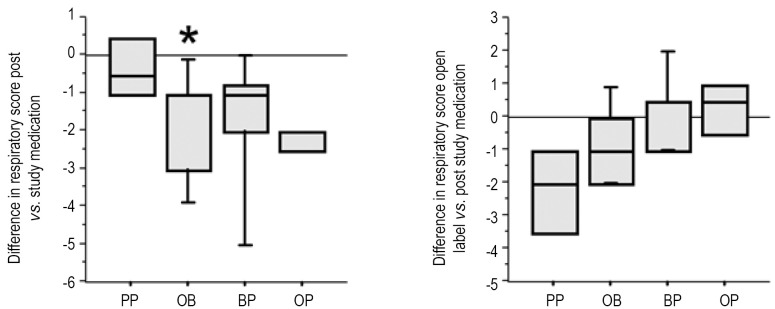
At study entry, all infants had evidence of abnormal respiratory scores, which were similar among the groups (Table 3). After the study period, infants on PP showed only a minor change in RS. In contrast, infants on study drugs showed improvement in their RS, which reached statistical significance for the OB group (P=0.04). After being on open label double therapy, PP also showed a similar degree of improvement compared to OB, though due to small sample size, it did not reach statistical significance (P=0.10). Each study drug group also had an open period with some further improvement (Figure 5).
Figure 5.
Treatment of GER improved respiratory scores (RS). At study entry, all infants had evidence of abnormal respiratory scores, which were similar among the groups (A). After the study period, PP did not improve the RS (n=4). The three treatments of OB (n=6), OP (n=4), and BP (n=5) did decrease the RS, but these data only reached statistical significance for the OB group (P=0.04). After being on open label double therapy, PP also showed an improved RS though it did not reach statistical significance (P=0.10) (B).
Improvement of wheezing and nighttime cough
All groups displayed similar degrees of wheezing and nighttime cough episodes at study entry (Table 3). After one month of therapy, PP did not decrease wheeze or nighttime cough compared to study entry data. The three treatment groups of OB, OP and BP appeared to have less complaints of wheezing or nighttime cough episodes per day, though statistical significance was not reached for any category. Combining all infants into one group and comparing the pre study values of cough and wheeze to the open label values, treatment of GER did cause a statistically significant decline in the amount of wheezing and nighttime cough (P=0.027 and P=0.002 respectively).
Parents and physicians agreement regarding study medications
At the end of each study period, both parents and physicians were asked if they thought their infant was improving on therapy. While blinded on the study medication, neither parents nor physicians saw a significant improvement for infants on PP (n=4). In contrast, despite being blinded, physicians and parents were unanimous in their impression that infants on OB were better compared to their condition at study entry (n=6). Of four infants on OP, three were unanimously improved, and one was considered better by the parent but the same by the physician (n=4). There was no clear consensus about infants on BP and one physician felt one infant was worse (n=5). After being on open label, parents and physicians of PP infants unanimously agreed that there was an improvement. Infants from the other three groups (n=15) also showed further improvement on open label, and 6 felt their infant was much better compared to study entry.
Discussion
The perception that GER can induce lung diseases in older children and adults has been well characterized (5,19-22). While the association of GER and aspiration in infants was described at least as early as 1978 (23), few studies have specifically addressed management from a pediatric lung perspective. In a study by Sheik et al., 64% of infants with persistent wheezing had excessive GER, as measured by a standard pH probe (9). Of the infants with proven GERD via pH probe, 64% improved on cisapride with ranitidine and were able to avoid inhaled corticosteroids.
The proton pump inhibitor omeprazole is superior to ranitidine in its ability to block acid secretion (6,24). The dissolvable tablet formulation also makes it reasonable for compliance in infants. Thus, the choice of omeprazole for this study was relatively clear. Since the inception of this study newer proton pump inhibitors have also come on the market for this age group (25). The choice of a motility drug in the treatment of GER was less clear. Cisapride is no longer available. It has been common practice to use prokinetic drugs, such as metaclopramide, or domperidone to “decrease GE reflux” despite no controlled studies to prove that these drugs do decrease reflux in infants, and ample studies to show that they do not (26-28). Bethanacol (Urecholine®) is a parasympathomimetic-agent, which stimulates gastric motility, increases gastric tone and affects motility (11,29). We have experienced a positive impression of its effectiveness in infants, thus we were interested to see its performance in a controlled setting. Neither bethanacol nor omeprazole had been studied in infants with GER related respiratory disease prior to this study.
In this study, we demonstrate that within a relatively brief period of time, all of our infants with increased GER showed significant improvement in their respiratory status if adequately treated for acid exposure. While it is not possible to make strong statements, given our low sample size, the best prevention of GER episodes, as measured by GI symptoms and pH probe, appears to be the combination of an acid suppressor (omeprazole) and a motility-altering agent (bethanacol). In association, both parents and physicians who were blinded to the study medications during the first month consistently agreed that there was a clinical improvement in the infants’ respiratory status on this combination therapy. In contrast, both parents and physicians observing infants treated with placebo alone saw no improvement in respiratory parameters, clinical impression, or GER events. Switching from placebo to combination therapy led to a trend in improvement of GI and respiratory outcomes, but because of the small number of patients in this subgroup, statistical significance was not reached. This is the first randomized controlled study of a proton pump inhibitor and a prokinetic agent in young children determining both the effects on GER and respiratory symptoms.
Managing infants with chronic cough and wheeze is difficult compared to that in older children. At this age, pulmonary function testing, including measurement of airway hyperresponsiveness, is more difficult and is not established in most clinical settings. The diagnosis largely relies on the symptoms of chronic cough and/or wheeze. While there are selected populations of infants at higher risk of asthma that do respond well to inhaled corticosteroids (30), most infants in the general population with chronic cough and/or wheeze do not (31). It is becoming evident that in some neurologically normal infants who have frequent chest problems the cause is not asthma, but pulmonary aspiration. Chronic pulmonary aspiration can lead to airway obstruction in the form of bronchitis and bronchiectasis (32,33).
The clinical dilemma has been in identifying this population. In the Sheikh study, 44% of infants had no visible emesis as recorded by the parents, and the diagnosis of possible GER associated pulmonary disease would not have been considered if not for the pH probe (9). This was called “silent” reflux. Thus, physician awareness of this possible diagnosis was needed as only directed diagnostic tests could clarify the etiology of the breathing difficulty. Another issue is that standards for the tests of abnormal GER are based on esophageal and not respiratory pathology (34). Standards for “normal” reflux are based on pH probe measurements in the distal esophagus usually 5 cm above the lower esophageal sphincter. The diagnosis of GERD with a standard single sensor pH probe in the lower esophagus does not consider that refluxate to the airway could be pathologic. Clearly, the squamous cell esophageal lining is better designed to handle food and acid than the columnar epithelium of the airway. In the most recent international meeting of gastroenterologists they published a consensus statement suggesting “a patient-orientated approach that is independent of endoscopic findings” when considering extra-esophageal pathology (5). Because a double sensor pH probe measures both distal and proximal esophageal pH, the double sensor pH probe has been suggested to be superior compared to the single lumen system when studying issues related to airway disease (35,36). Further, we have observed that the upper esophageal sensor may not correlate with risk of aspiration if it is placed below the upper esophageal sphincter (UES). Thus, we have been routinely placing the upper sensor in the hypopharynx. Because the current literature is so limited for standards of double sensor data, the ability to use it as a diagnostic tool has been limited (34). Work using esophageal impedance measurements would suggest that non-acidic refluxate is also important in pulmonary disease (37,38). Ideally, combined pH and impedance measurements would have been the better choice of diagnostic. Unfortunately, esophageal impedance measurements were not available at our center when this study began.
The other confounding factor in diagnosing GER-related lung disease is the quality of the swallowing mechanism. The intact airway closure mechanism of the larynx usually allows humans to eat and drink without compromising the lung (39), thus refluxed pharyngeal fluid does not necessarily equate with pulmonary aspiration. In cases of some children with apparently no neurological impairment, there can be an inability to consistently protect the airway when fluid is in the hypopharynx (33,40). Individuals with swallowing dysfunction are at higher risk of pulmonary aspiration. Ideally, we had hoped to have all our infants evaluated by speech pathology and radiology. Unfortunately, we were unable to obtain modified barium swallows to be performed in a timely manner before study entry. Thus, these data cannot be shown. We did ask parents questions about the signs of swallowing dysfunction before enrolling children, and we attempted to not enroll infants in whom the signs of direct aspiration were more obvious than the signs of indirect aspiration from GE reflux. That being said, we believe that often direct aspiration from swallowing dysfunction often improves after treatment of excessive GER.
From the Tucson Children's Respiratory Study, it has been accepted that most infants with wheeze outgrow their chest difficulties by childhood and adolescence (41). The Tucson group of infants with wheeze was divided between those with risk factors for asthma and those without. Those with no wheeze during infancy had the best lung function at 16 years old. Those with wheeze but without risk factors were called the ‘transient wheezers’ because they did not appear to have asthma in later childhood and adolescence. Despite their absence of wheeze in adolescence, the ‘transient wheezers’ continued to have significantly poorer lung function compared to those adolescents that never wheezed in infancy. The design of most infant cohorts suggests that GERD-related disease is excluded from the study. Unfortunately, pH probe or gastric emptying scans are not part of the selection criteria, nor are questions about pulmonary aspiration. Given the consistent problem of silent GER, it is likely that a significant number of these infant wheezers could have GER-related disease (42).
The transient nature of the wheeze is also suggestive of pulmonary aspiration as an underlying diagnosis. Most neurologically normal infants seem to get better over time, though there are no long-term follow-up studies to back this assertion. The reason for improvement could be from more time in an upright position, decreased feeding of a liquid diet, maturation of the swallow, or decreased respiratory rate. In the Tucson study, the deficit in lung function in ‘transient wheezers’ never recovered to that of normal or later onset wheezing infants (41). There is data that infants with GERD have persistence of esophageal disease for longer than is clinically apparent, and that these infants go on to have adult GERD (3,43). Thus, while the Tucson data is reassuring that most transient wheezers improve by age 6-16, a longitudinal study of infants with pulmonary aspiration followed for the effects on adult lung function is needed.
In the Sheikh study, the infants with a higher risk of asthma were less likely to have increased GERD and tended to require anti-asthma therapy despite treatment for GERD (9). In our study, infants were not selected for their atopic status or family history of asthma, and many were already failing inhaled corticosteroid therapy. Thus, we could have been biased toward a more non-atopic phenotype of infant wheezer.
Enrolling infants for this study was difficult, given the prospect of two pH probes and the potential delay in medical therapy. Despite the small number of patients in this study, we found the data compelling because of the clear and relatively rapid improvements in the respiratory status. The children in this study were referred for significant respiratory symptoms despite reasonable outpatient therapy. The parents were quite motivated to subject their children to two pH probes to determine whether GER was playing a role. Thus, while we agree with a recent publication by Khoshoo et al. that more focus should be placed on non-medical therapies for GER symptoms (44), we believe our patients did warrant therapy. There are many opinions on the management of cough and wheeze in infancy, but few good studies of anti-GER therapy at this age. Based on this randomized placebo-controlled trial, we suggest that infants with chronic symptoms of excessive GER, cough and wheeze, especially those who have failed inhaled corticosteroids, should be considered for anti-GER therapy. The combination of bethanacol and omeprazole appears to be effective.
Acknowledgements
Funding: This study was supported by the Childhood Asthma Foundation of Canada, Alberta Heritage Foundation for Medical Research (AHFMR).
We would like to thank Deb Olmstead for her tireless efforts as our research coordinator and Dr. Lesley Smith for her advice on study design.
Footnotes
Conflicts of Interest: DJ Adamko was an AHFMR Clinical Investigator. The other authors have no conflicts of interest to declare.
References
- 1.Loughlin GM. Respiratory consequences of dysfunctional swallowing and aspiration. Dysphagia 1989;3:126-30. [DOI] [PubMed] [Google Scholar]
- 2.Irwin RS, Madison JM, Fraire AE. The cough reflex and its relation to gastroesophageal reflux. Am J Med 2000;108:73S-8S. [DOI] [PubMed] [Google Scholar]
- 3.Orenstein SR, Shalaby TM, Kelsey SF, et al. Natural history of infant reflux esophagitis: symptoms and morphometric histology during one year without pharmacotherapy. Am J Gastroenterol 2006;101:628-40. [DOI] [PubMed] [Google Scholar]
- 4.Rudolph CD, Mazur LJ, Liptak GS, et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr 2001;32:S1-31. [DOI] [PubMed] [Google Scholar]
- 5.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900-20; quiz 1943. [DOI] [PubMed]
- 6.Gouda BB, Lydon AM, Badhe A, et al. A comparison of the effects of ranitidine and omeprazole on volume and pH of gastric contents in elective surgical patients. Eur J Anaesthesiol 2004;21:260-4. [DOI] [PubMed] [Google Scholar]
- 7.Kemmotsu O, Mizushima M, Morimoto Y, et al. Effect of preanesthetic intramuscular ranitidine on gastric acidity and volume in children. J Clin Anesth 1991;3:451-5. [DOI] [PubMed] [Google Scholar]
- 8.Hyman PE, Abrams C, Dubois A. Effect of metoclopramide and bethanechol on gastric emptying in infants. Pediatr Res 1985;19:1029-32. [DOI] [PubMed] [Google Scholar]
- 9.Sheikh S, Stephen T, Howell L, et al. Gastroesophageal reflux in infants with wheezing. Pediatr Pulmonol 1999;28:181-6. [DOI] [PubMed] [Google Scholar]
- 10.Maclennan S, Augood C, Cash-Gibson L, et al. Cisapride treatment for gastro-oesophageal reflux in children. Cochrane Database Syst Rev 2010;CD002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richter JE. A critical review of current medical therapy for gastroesophageal reflux disease. J Clin Gastroenterol 1986;8:72-80. [DOI] [PubMed] [Google Scholar]
- 12.Kato S, Ebina K, Fujii K, et al. Effect of omeprazole in the treatment of refractory acid-related diseases in childhood: endoscopic healing and twenty-four-hour intragastric acidity. J Pediatr 1996;128:415-21. [DOI] [PubMed] [Google Scholar]
- 13.Estevão-Costa J, Fragoso AC, Prata MJ, et al. Gastric emptying and antireflux surgery. Pediatr Surg Int 2011;27:367-71. [DOI] [PubMed] [Google Scholar]
- 14.Sachdeva P, Malhotra N, Pathikonda M, et al. Gastric emptying of solids and liquids for evaluation for gastroparesis. Dig Dis Sci 2011;56:1138-46. [DOI] [PubMed] [Google Scholar]
- 15.Nahata MCaH, T.F. Pediatric Drug Formulary. 4th ed; 2000. [Google Scholar]
- 16.Chan-Yeung M, Manfreda J, Dimich-Ward H, et al. A randomized controlled study on the effectiveness of a multifaceted intervention program in the primary prevention of asthma in high-risk infants. Arch Pediatr Adolesc Med 2000;154:657-63. [DOI] [PubMed] [Google Scholar]
- 17.Demeester TR, Johnson LF, Joseph GJ, et al. Patterns of gastroesophageal reflux in health and disease. Ann Surg 1976;184:459-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richter JE, Bradley LA, DeMeester TR, et al. Normal 24-hr ambulatory esophageal pH values. Influence of study center, pH electrode, age, and gender. Dig Dis Sci 1992;37:849-56. [DOI] [PubMed] [Google Scholar]
- 19.Nordenstedt H, Nilsson M, Johansson S, et al. The relation between gastroesophageal reflux and respiratory symptoms in a population-based study: the Nord-Trøndelag health survey. Chest 2006;129:1051-6. [DOI] [PubMed] [Google Scholar]
- 20.Debley JS, Carter ER, Redding GJ. Prevalence and impact of gastroesophageal reflux in adolescents with asthma: a population-based study. Pediatr Pulmonol 2006;41:475-81. [DOI] [PubMed] [Google Scholar]
- 21.Wong BC, Kinoshita Y. Systematic review on epidemiology of gastroesophageal reflux disease in Asia. Clin Gastroenterol Hepatol 2006;4:398-407. [DOI] [PubMed] [Google Scholar]
- 22.Saglani S, Nicholson AG, Scallan M, et al. Investigation of young children with severe recurrent wheeze: any clinical benefit? Eur Respir J 2006;27:29-35. [DOI] [PubMed] [Google Scholar]
- 23.Christie DL, O’Grady LR, Mack DV. Incompetent lower esophageal sphincter and gastroesophageal reflux in recurrent acute pulmonary disease of infancy and childhood. J Pediatr 1978;93:23-7. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein JL, Johanson JF, Suchower LJ, et al. Healing of gastric ulcers with esomeprazole versus ranitidine in patients who continued to receive NSAID therapy: a randomized trial. Am J Gastroenterol 2005;100:2650-7. [DOI] [PubMed] [Google Scholar]
- 25.Khoshoo V, Dhume P. Clinical response to 2 dosing regimens of lansoprazole in infants with gastroesophageal reflux. J Pediatr Gastroenterol Nutr 2008;46:352-4. [DOI] [PubMed] [Google Scholar]
- 26.Chicella MF, Batres LA, Heesters MS, et al. Prokinetic drug therapy in children: a review of current options. Ann Pharmacother 2005;39:706-11. [DOI] [PubMed] [Google Scholar]
- 27.Machida HM, Forbes DA, Gall DG, et al. Metoclopramide in gastroesophageal reflux of infancy. J Pediatr 1988;112:483-7. [DOI] [PubMed] [Google Scholar]
- 28.Carroccio A, Iacono G, Montalto G, et al. Domperidone plus magnesium hydroxide and aluminum hydroxide: a valid therapy in children with gastroesophageal reflux. A double-blind randomized study versus placebo. Scand J Gastroenterol 1994;29:300-4. [DOI] [PubMed] [Google Scholar]
- 29.Farrell RL, Roling GT, Castell DO. Stimulation of the incompetent lower esophageal sphincter. A possible advance in therapy of heartburn. Am J Dig Dis 1973;18:646-50. [DOI] [PubMed] [Google Scholar]
- 30.Teper AM, Kofman CD, Szulman GA, et al. Fluticasone improves pulmonary function in children under 2 years old with risk factors for asthma. Am J Respir Crit Care Med 2005;171:587-90. [DOI] [PubMed] [Google Scholar]
- 31.Merkus PJ, de Jongste JC. Inhaled corticosteroids in wheezy infants. Am J Respir Crit Care Med 2005;172:1058-9; author reply 1059. [DOI] [PubMed] [Google Scholar]
- 32.El-Serag HB, Gilger M, Kuebeler M, et al. Extraesophageal associations of gastroesophageal reflux disease in children without neurologic defects. Gastroenterology 2001;121:1294-9. [DOI] [PubMed] [Google Scholar]
- 33.Loughlin GM, Lefton-Greif MA. Dysfunctional swallowing and respiratory disease in children. Adv Pediatr 1994;41:135-62. [PubMed] [Google Scholar]
- 34.Sifrim D, Castell D, Dent J, et al. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut 2004;53:1024-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conley SF, Werlin SL, Beste DJ. Proximal pH-metry for diagnosis of upper airway complications of gastroesophageal reflux. J Otolaryngol 1995;24:295-8. [PubMed] [Google Scholar]
- 36.Little JP, Matthews BL, Glock MS, et al. Extraesophageal pediatric reflux: 24-hour double-probe pH monitoring of 222 children. Ann Otol Rhinol Laryngol Suppl 1997;169:1-16. [PubMed] [Google Scholar]
- 37.Rosen R, Nurko S. The importance of multichannel intraluminal impedance in the evaluation of children with persistent respiratory symptoms. Am J Gastroenterol 2004;99:2452-8. [DOI] [PubMed] [Google Scholar]
- 38.López-Alonso M, Moya MJ, Cabo JA, et al. Twenty-four-hour esophageal impedance-pH monitoring in healthy preterm neonates: rate and characteristics of acid, weakly acidic, and weakly alkaline gastroesophageal reflux. Pediatrics 2006;118:e299-308. [DOI] [PubMed] [Google Scholar]
- 39.Wilson SL, Thach BT, Brouillette RT, et al. Coordination of breathing and swallowing in human infants. J Appl Physiol 1981;50:851-8. [DOI] [PubMed] [Google Scholar]
- 40.Sheikh S, Allen E, Shell R, et al. Chronic aspiration without gastroesophageal reflux as a cause of chronic respiratory symptoms in neurologically normal infants. Chest 2001;120:1190-5. [DOI] [PubMed] [Google Scholar]
- 41.Morgan WJ, Stern DA, Sherrill DL, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med 2005;172:1253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eid NS, Morton RL. Rational approach to the wheezy infant. Paediatr Respir Rev 2004;5:S77-9. [DOI] [PubMed] [Google Scholar]
- 43.Gold BD. Is gastroesophageal reflux disease really a life-long disease: do babies who regurgitate grow up to be adults with GERD complications? Am J Gastroenterol 2006;101:641-4. [DOI] [PubMed] [Google Scholar]
- 44.Khoshoo V, Edell D, Thompson A, et al. Are we overprescribing antireflux medications for infants with regurgitation? Pediatrics 2007;120:946-9. [DOI] [PubMed] [Google Scholar]