Abstract
The evolution of Nephroblastoma (WT) treatment over the last decades has been one major success around the world. Despite pursuing different upfront treatment approaches the Children’s Oncology Group (COG, former National Wilms Tumor Study Group, NWTSG) and the International Society of Paediatric Oncology’s Renal Tumor Study Group (SIOP-RTSG) show the same outcome. Treatment starts with preoperative chemotherapy in SIOP-RTSG compared to initial surgery in COG. Response to chemotherapy can be used as a stratification parameter. This allows treating patients with blastemal subtype more aggressively resulting in a better event free survival (EFS). Moreover the percentage of patients with local stage III is less in SIOP-RTSG than in COG studies. Lymph node involvement, in NWTS 5 together with residual microscopic disease, results in a lower EFS in both study groups. But overall survival (OS) is not different comparing patients with or without positive lymph nodes (LN). No other reason for stage III has a significant impact on outcome. The role of radiotherapy for local tumor control in stage III is important, but the radiation dose needs to be questioned as 10.8 Gy used in COG is as efficient as 15 Gy in SIOP-RTSG protocols. In addition in part of low income countries radiotherapy can not be given due to a lack of radiation facilities. Nevertheless some patients are cured without irradiation. The analysis of local stage III patients underlines the importance of preoperative chemotherapy and the need for molecular studies to better stratify patients according to their individual risk.
Keywords: Nephroblastoma, preoperative chemotherapy, local stage III, outcome
Introduction
Nephroblastoma is the most common childhood kidney tumor. Since more than 40 years children with this tumor are treated in prospective randomized clinical trials leading to excellent outcome. The two major study groups, the Children’s Oncology Group (COG) and the Renal Tumor Study Group (RTSG) of the International Society of Paediatric Oncology (SIOP) show superimposable event free survival (EFS) and overall survival (OS) rates despite the fact that patients within COG undergo primary tumor nephrectomy whereas in SIOP they receive preoperative chemotherapy before surgery. Postoperative treatment is stratified mainly according to histology and the local stage. In COG LOH of 1p and 16q are further stratification parameters. Response to preoperative treatment did recognize remaining blastema as an independent risk factor in SIOP and thus upgrading this tumor as high risk (1,2). In both study groups response to treatment in metastatic disease is used to stratify treatment and avoiding lung irradiation in those patients with complete response under chemotherapy (3).
As outcome of patients with local stage I and II is excellent in both study groups there is still improvement possible in local stage III, especially in case of unfavorable (COG) or high risk histology (SIOP). A recent publication by Ehrlich et al. (4) addressed for the National Wilms Tumor Study 5 (NWTS) clinicopathologic findings, which are predictive of relapse in children with favorable histology stage III tumors. They evaluated the prognostic significance of positive lymph nodes (LN), peritoneal implants, residual disease and rupture as the reasons for local stage III. In their analysis they combined all local stage III patients with favorable histology (569 patients) including those with metastatic disease (109 patients). In a multivariate analysis LN involvement together with microscopic residual disease were the only factors predictive of inferior EFS. None of the analyzed factors had an influence on OS. Interestingly the rate of loss of heterozygosity was higher (6%) for those with positive LNs than for those without (2%) (4).
COG investigators argue, preoperative treatment is leading to loss of staging information, concluding SIOP stage cohorts might in reality be inconsistent by including different stage tumors and therefore their comparability to COG patients according to stage might be limited (5). It is thus intriguing whether the findings by Ehrlich and colleagues (4) are consistent with patients treated with preoperative chemotherapy according to SIOP protocols. For that purpose we have analyzed all cases of unilateral local stage III patients treated preoperatively with chemotherapy as part of the GPOH (German Society of Pediatric Oncology) subgroup of the SIOP trials and studies 9, 93-01 and 2001. Patients with stage IV are not included in this analysis.
Study population, materials and methods
From January 1989 to the end of October 2013 1,465 patients with unilateral non-metastatic nephroblastoma have been enrolled in the above mentioned studies and trials and received preoperative chemotherapy. Of these patients 40 (2.7%) had low risk, 1,257 (85.8%) intermediate risk and 168 (11.5%) high risk histology. Altogether 211 patients were diagnosed having local stage III (14.4%) of whom 98 (46.4%) were males and 113 (53.6%) were females. A total of 122 tumors were right sided and 89 left sided. A total of 103 (48.8%) patients were treated according to SIOP 2001/GPOH and 108 (51.2%) patients according to SIOP 9 (43 patients) and SIOP 93-01 (65 patients). The difference in treatment between SIOP 2001/GPOH and SIOP 9/GPOH or 93-01/GPOH is the upgrading of blastemal subtype to high risk stratum in SIOP 2001 according to the results of Weirich et al. (6).
SIOP 2001 is registered at EMA (European Medicine Agency) with EudraCT no: 2007-004591-39. Ethical approval is given by Ärztekammer des Saarlandes’ for all studies and trials.
This is a retrospective analysis. Statistical analysis was done using IBM SPSS Statistics version 20. The EFS and OS were calculated using the Kaplan-Meier method. Log-rank tests and Breslow tests were used to compare survival among different subgroups. EFS was calculated as time from the date of diagnosis to the first recurrence or death for any reason, OS was calculated as time from diagnosis to death for any reason. Patients were censored at the time of the last follow-up record. t-test for paired samples was used to calculate differences in tumor volume before and after preoperative chemotherapy.
Results
The main reason for stage III was rupture in 57 (27.0%), open biopsy in 11 (5.2%), macroscopic or microscopic rests in 64 (30.3%) and positive LNs in 79 (37.4%) patients. Unfortunately in 61 (28.9%) patients no or incomplete LNs are sampled. Of the ruptures 21 were major and 36 minor. Nineteen (9.0%) of the ruptures occurred preoperative (9 major and 10 minor) and 38 (18.0%) intraoperative (12 major and 26 minor). Macroscopic tumor rests remained in 25 (11.8%) and microscopic rests in 39 (18.5%) patients after surgery. According to histology 175 (82.9%) tumors were low (4 tumors) or intermediate risk (171 tumors) and 36 (17.1%) were high risk tumors. Tumor volume was available in 154 (73.0%) patients at the time of diagnosis and after preoperative chemotherapy. The mean tumor volume at diagnosis was 608±436 mL and the volume-reduction after preoperative chemotherapy to 315±366 mL is highly significant (t-test for paired samples, P<0.001) (Figure 1).
Figure 1.

Tumor volume at the time of diagnosis and after preoperative chemotherapy (n=154) of patients with non-metastatic unilateral Wilms tumor and local stage III treated according to the consecutive SIOP/GPOH trials and studies 9, 93-01 and 2001. The difference is statistically highly significant (P<0.001). Mean tumor volume at diagnosis: 608±436 mL, mean tumor volume after pre-operative chemotherapy: 315±366 mL.
Outcome in stage III in general
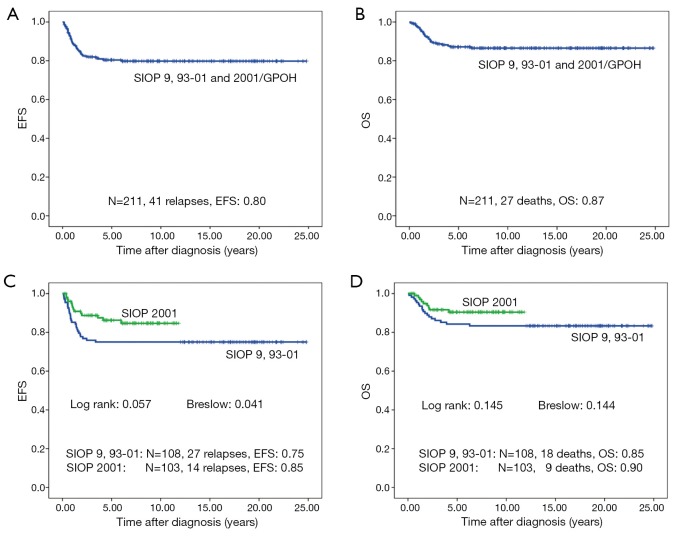
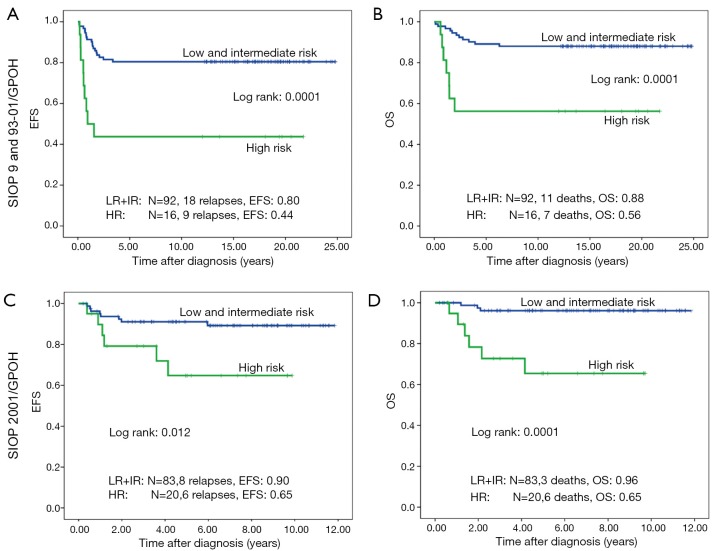
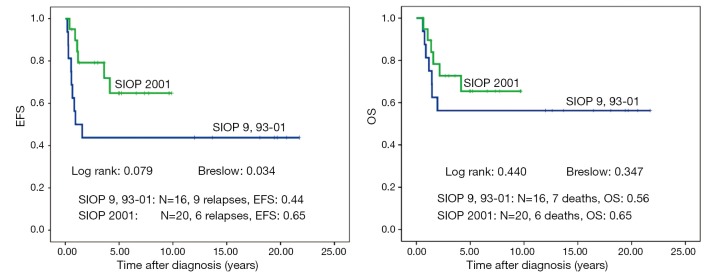
Forty-one of 211 patients (19.6%) relapsed. Nineteen of these were local or combined relapses (9.0%). Twenty seven (12.8%) patients died. The 10-year EFS rate is 80% and the OS rate 87% for the whole study group. Patients treated according to SIOP 2001/GPOH have an increased OS rate of 90% compared to 83% in SIOP9/GPOH or SIOP93-10/GPOH (EFS: 85% versus 75% in SIOP9/GPOH or 93-01/GPOH) (Figure 2). This increase in EFS is mainly attributed to the inclusion of patients with blastemal subtype to the high risk treatment group in SIOP 2001/GPOH (Figure 3). There is a highly significant difference in outcome between patients with high risk and the combined group of low and intermediate risk histology. In this respect patients of the high risk group in SIOP 2001/GPOH show a better EFS and OS (65% vs. 44% EFS and 65% vs. 56% OS) (Figure 4).
Figure 2.
Event free survival (EFS) and overall survival (OS) of patients with non-metastatic unilateral Wilms tumor and local stage III after preoperative chemotherapy treated according to the consecutive SIOP/GPOH trials and studies 9, 93-01 and 2001.
Figure 3.
Event free survival (EFS) and overall survival (OS) of patients with non-metastatic unilateral Wilms tumor and local stage III after preoperative chemotherapy treated according to the consecutive SIOP/GPOH trials and studies 9, 93-01 and 2001 according to their histology (low + intermediate and high risk).
Figure 4.
Event free survival (EFS) and overall survival (OS) of patients with non-metastatic unilateral Wilms tumor and local stage III after preoperative chemotherapy treated according to the consecutive SIOP/GPOH trials and studies 9, 93-01 and 2001. Only patients with high risk histology.
Outcome in stage III as a result of the different reasons for stage III
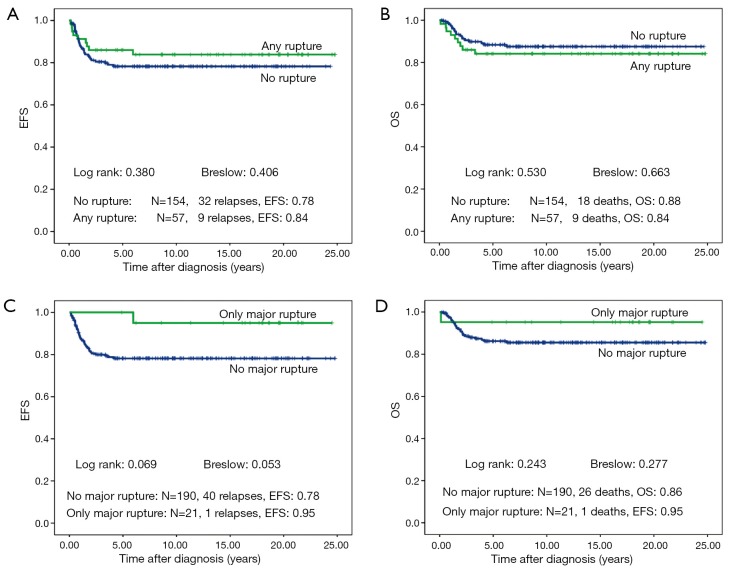
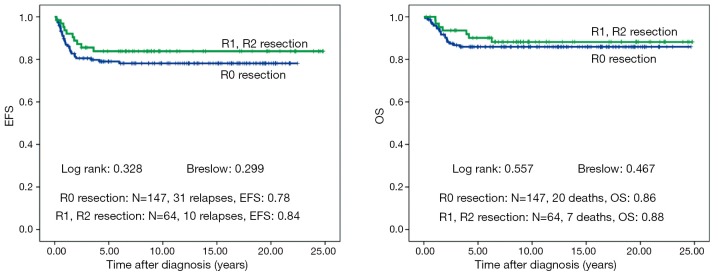
There is no difference in outcome between patients with rupture or no rupture (Figure 5). Interestingly patients with major ruptures seem to do better than those with minor ruptures or no rupture (statistically not significant). Patients with tumor rest after surgery (R1: microscopic rest, R2: macroscopic rest) show the same outcome as those patients with no remaining tumor (R0) (Figure 6). The only difference in outcome is seen in patients with positive LNs (Figure 7). The difference is highly significant in EFS being 70% for patients with positive LNs compared to 86% for patients with negative LNs. But there is no difference found in OS. Patients with positive LNs have an OS of 84% compared to 88% for those with negative LNs.
Figure 5.
Event free survival (EFS) and overall survival (OS) of patients with non-metastatic unilateral Wilms tumor and local stage III after preoperative chemotherapy treated according to the consecutive SIOP/GPOH trials and studies 9, 93-01 and 2001. Influence of rupture is shown.
Figure 6.
Event free survival (EFS) and overall survival (OS) of patients with non-metastatic unilateral Wilms tumor and local stage III after preoperative chemotherapy treated according to the consecutive SIOP/GPOH trials and studies 9, 93-01 and 2001. Influence of resection R0 compared to R1 and R2 is shown.
Figure 7.
Event free survival (EFS) and overall survival (OS) of patients with non-metastatic unilateral Wilms tumor and local stage III after preoperative chemotherapy treated according to the consecutive SIOP/GPOH trials and studies 9, 93-01 and 2001. Influence of lymph node involvement is shown.
Discussion
Preoperative chemotherapy results in downstaging of nephroblastoma with a lower percentage of stage III patients in SIOP compared to COG (7). Out of 2,397 patients with favorable histology enrolled in NWTS 5 569 patients had local stage III disease resulting in 23.7% (4) compared to only 14.4% in this analysis including also patients with high risk histology. Stage IV patients, who had been included in the Ehrlich report different to our analysis, might have added to this but hardly explain this remarkable proportional difference. Therefore local irradiation with its long term late effects can be avoided by applying preoperative chemotherapy to all patients with nephroblastoma. To automatically upgrade patients receiving preoperative chemotherapy to local stage III as done by COG for primarily inoperable tumors (8) is arbitrary and refuted by the SIOP experience. In SIOP postoperative treatment is based on local staging after preoperative chemotherapy with excellent results for ‘downstaged’ local stage I and II with less treatment intensity compared to stage III (1,2,7,9).
Despite the difference in the percentage of local stage III in both series LN involvement is the most frequent criterion for stage III in 38% of patients. This indicates that preoperative chemotherapy is also effective in eliminating LN metastasis as already shown by Grundy et al. (10). As the combination of LN positivity and microscopic residual disease had a significant negative impact on EFS and OS in NWTS 5 (4) a reduction in the number of patients with positive LNs might have an impact on outcome in patients with nephroblastoma.
Independent of the treatment approach (primary surgery in COG and preoperative chemotherapy in SIOP) the outcome of patients with local stage III is excellent with around 90% OS (87% in SIOP/GPOH group including high risk histology and 91% in NWTS 5 including only favorable histology but stage IV patients) (4). In the SIOP/GPOH series only patients with positive LNs have a significant worse EFS compared to patients with negative LNs. But their OS is not different, showing that patients with LN involvement might have a second chance to be cured after relapse. The same is also true for NWTS 5 (4). Even the combination of LN involvement and microscopic residual showing the poorest EFS in their analysis is without statistical impact on OS. Nevertheless they conclude that reduction of treatment in patients with negative LNs and no residual microscopic disease is not justified as the good outcome maybe relate to the use of doxorubicin and radiotherapy. In addition the small number and the retrospective nature of both analyses are further arguments for not changing treatment based on these results.
In our series tumor rupture and microscopic or macroscopic residual disease is without influence on outcome. This might be attributed to efficient treatment protocols including radiotherapy and indicates a principle biological difference between remaining primary tumor after incomplete surgery or tumor rupture and LN involvement. The spread of tumor cells to the LN should be separated from all other reasons for local stage III if molecular biology analyses are done to find risk factors for outcome in nephroblastoma. Therefore it is also of utmost importance to have a complete sampling of LNs in all patients with nephroblastoma. Unfortunately in nearly 30% (61/211) of our patients with local stage III no or incomplete LN sampling was done. This will most likely cause unnecessary relapses in lower stages if positive LNs are the only criterion for stage III as it has already been discussed by Shamberger et al. in 1999 (11). Such protocol violations need to be avoided.
We included also patients with high risk histology in our series, because treatment for blastemal subtype was different in SIOP 9/GPOH and 93-01/GPOH compared to SIOP 2001/GPOH protocol. In the later study blastemal subtype was included in the high risk treatment, since the earlier studies had shown an inferior outcome of patients with this histological subtype than all other subtypes of intermediate histology (1,6). The poorer outcome is related to resistant blastemal cells to vincristine and actinomycin after preoperative chemotherapy. Response to preoperative chemotherapy is thus recognized as an important marker for treatment stratification after surgery in SIOP. Intensification of postoperative treatment in SIOP 2001/GPOH results in a better EFS for stage III compared to the preceding studies as shown in Figure 2. Around 40% of primary operated nephroblastomas show blastemal predominant tumors whereas the percentage decreases to less than 10% after preoperative chemotherapy (7). Unfortunately there are no molecular markers available up to now to distinguish between resistant and non-resistant blastema to vincristine and actinomycin. For that reason molecular studies are of utmost importance to analyze the blastemal phenotype with all available technologies in molecular biology including next generation sequencing. Such new markers will help to stratify patients much better than today. This is not only important for stage III but for all patients with nephroblastoma.
Today all patients with stage III are locally irradiated. In many low resource countries irradiation is not available for patients with stage III nephroblastoma (12). Nevertheless some of these patients do not relapse and survive. Therefore the question can be asked, when radiotherapy is needed in stage III? In addition the dose for local irradiation differs between COG and SIOP with 10.8 Gy in COG compared to 15 Gy in SIOP (13). As the results of both study groups are not different it can be discussed if in the SIOP-RTSG approach 15 Gy are needed to treat patients with stage III. Investigating local stage III nephroblastoma patients treated without any irradiation might add knowledge to our understanding about, who needs local radiotherapy. Collaborating with study groups of countries with low income is a solution to get this answer.
In conclusion the analysis of local stage III patients underlines the importance of preoperative chemotherapy and the need for molecular studies to better stratify patients according to their individual risk.
Acknowledgements
Funding: The SIOP 2001/GPOH Study was funded by ‘Deutsche Krebshilfe, project number: 70-1899 and 50-2709-Gr 2’.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Reinhard H, Semler O, Bürger D, et al. Results of the SIOP 93-01/GPOH trial and study for the treatment of patients with unilateral nonmetastatic Wilms Tumor. Klin Padiatr 2004;216:132-40. [DOI] [PubMed] [Google Scholar]
- 2.Graf N, van Tinteren H, Bergeron C, et al. Characteristics and outcome of stage II and III non-anaplastic Wilms’ tumour treated according to the SIOP trial and study 93-01. Eur J Cancer 2012;48:3240-8. [DOI] [PubMed] [Google Scholar]
- 3.Verschuur A, Van Tinteren H, Graf N, et al. Treatment of pulmonary metastases in children with stage IV nephroblastoma with risk-based use of pulmonary radiotherapy. J Clin Oncol 2012;30:3533-9. [DOI] [PubMed] [Google Scholar]
- 4.Ehrlich PF, Anderson JR, Ritchey ML, et al. Clinicopathologic findings predictive of relapse in children with stage III favorable-histology Wilms tumor. J Clin Oncol 2013;31:1196-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green DM. Controversies in the management of Wilms tumour - immediate nephrectomy or delayed nephrectomy? Eur J Cancer 2007;43:2453-6. [DOI] [PubMed] [Google Scholar]
- 6.Weirich A, Ludwig R, Graf N, et al. Survival in nephroblastoma treated according to the trial and study SIOP-9/GPOH with respect to relapse and morbidity. Ann Oncol 2004;15:808-20. [DOI] [PubMed] [Google Scholar]
- 7.Graf N, Tournade MF, de Kraker J. The role of preoperative chemotherapy in the management of Wilms’ tumor. The SIOP studies. International Society of Pediatric Oncology. Urol Clin North Am 2000;27:443-54. [DOI] [PubMed] [Google Scholar]
- 8.Ritchey ML, Pringle KC, Breslow NE, et al. Management and outcome of inoperable Wilms tumor. A report of National Wilms Tumor Study-3. Ann Surg 1994;220:683-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Kraker J, Graf N, van Tinteren H, et al. Reduction of postoperative chemotherapy in children with stage I intermediate-risk and anaplastic Wilms’ tumour (SIOP 93-01 trial): a randomised controlled trial. Lancet 2004;364:1229-35. [DOI] [PubMed] [Google Scholar]
- 10.Grundy P, van Tinteren H, Anderson JR, et al. Significance of lymph node involvement in nephroblastoma. Pediatr Blood Cancer 2011;55:822-3. [Google Scholar]
- 11.Shamberger RC, Guthrie KA, Ritchey ML, et al. Surgery-related factors and local recurrence of Wilms tumor in National Wilms Tumor Study 4. Ann Surg 1999;229:292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magrath I, Steliarova-Foucher E, Epelman S, et al. Paediatric cancer in low-income and middle-income countries. Lancet Oncol 2013;14:e104-16. [DOI] [PubMed] [Google Scholar]
- 13.Spreafico F, Bellani FF. Wilms’ tumor: past, present and (possibly) future. Expert Rev Anticancer Ther 2006;6:249-58. [DOI] [PubMed] [Google Scholar]