Abstract
Objective:
Guggulu (Commiphora wightii [Arn.] Bhandari) is a well-known anti-hyperlipidaemic drug. Guggulsterones are active components of this drug which are responsible for this effect. The activity of Guggulu may depend upon its nature, fresh samples are recommended for their bṛhmaṇa (body mass increasing) effect; while lekhana (scarificant) effect is attributed to the old one. The comparative Anti-hyperlipidaemic activity of fresh and old samples has not been reported till date.
Materials and Methods:
Freshly collected and one year old samples of Guggulu were processed in gomūtra. Patients who satisfied inclusion criteria of Hyperlipidaemia were randomly distributed into two groups and the drug was administrated in a dose of 1 g with luke warm water twice a day for eight weeks.
Results:
Significant improvement was found in the symptoms of Medoroga and Lipid profile with treatment in both the groups. Fresh sample of Guggulu proved to have a better effect in lowering serum cholesterol (5.76%), triglyceride (17.17%), and very low density lipoprotein VLDL (18.36%) levels while old sample of Guggulu provided mild effect in lowering serum triglyceride (13.64%), VLDL (11.07%) and non-significant increase in serum HDL-cholesterol (0.94%). Old sample of Guggulu also provided significant decreases in body weight (7.69%) and BMI (7.82%).
Conclusions:
Old Guggulu showed better effect on body weight, BMI and cardinal symptoms along with significant lipid lowering effect whereas fresh Guggulu showed better result on lipid profile.
KEYWORDS: Commiphora wightii, fresh Guggulu, Guggulsterone, hyperlipidaemia, old Guggulu
INTRODUCTION
Raised total cholesterol level is a major cause of disease burden in both the developed and developing world as a risk factor for ischemic heart disease and stroke. Globally, a third of ischemic heart diseases are attributable to high cholesterol. 10% reduction in serum cholesterol in men aged 40 has been reported to result in a 50% reduction in heart diseases within 5 years.[1] In view of limitations of conventional drugs researches on herbal drugs are on the increase. Guggulu is such a drug which has got attention due to its various properties such as hypocholestremic,[2] lipid lowering,[3,4] anti-arthritic,[5] anti-inflammatory,[6,7,8,9] anti oxidant,[10] cardio protective,[11] antimicrobial[12] etc. There have been a number of studies that have evaluated anti-hyperlipidaemic activity of Guggulu.[13,14,15,16]
Guggulu was first introduced to the scientific world in 1966 as a lipid lowering drug[3] and soon after, the interest in using Guggulu as a remedy for treating or preventing hypercholesterolemia and related cardiovascular diseases became wide spread in the Western world.[17]
Ayurveda considers fresh Guggulu for bṅhmaṇa (body mass increasing) effect; while lekhana (scarificant) effect is attributed to the old one.[18] This signifies that therapeutic utilities of new and old Guggulu are different possibly due to certain changes which occur with time. Though studies are reported on Guggulu, comparative efficacy of navīna (fresh) and purāṇa (old) samples are not reported till date. Considering this, we attempt to evaluate the comparative effect of fresh and old Guggulu in Hyperlipidaemia.
MATERIALS AND METHODS
Drug
Guggulu wildly cultivated at Dwaraka Forest Range, Gujarat was collected from Gujarat State Forest Development Corp. Ltd., Vadodara during February 2011 (Batch no. B 05, code-148600). This sample was preserved in polyethylene bags at room temperature (28-32°C) to make it old. Though specific time period is not mentioned for Guggulu to turn old; the sample was stored for one year considering general terminology.[19] Another sample of fresh Guggulu was collected in the same manner in February 2012 (Batch no. B 01, code-128500). Both the samples were purified following classical method.[20]
Selection of patients
The study was conducted at Jamnagar. The study was started after obtaining approval from the Institutional Ethics Committee (PGT/7-A/Ethics/2011-2012/2087/26, dated-05/09/2010) and registered at Clinical Trial Registry of India, ICMR, New Delhi. (CTRI/2011/11/002117).
Inclusion criteria
Patients of either sex aged 20 to 60 years with svedādhikya (excessive sweating), daurbalya (generalized weakness), śvāsakaṣṭatā (breathlessness), nidrādhikya (excessive sleep), atikṣudhā (excessive eating), aṅgagaurava (heaviness in body), gātrāsāda (fatigue), aṃgaśaithilya (flabbiness)[21] and increased levels of any of the serum lipids such as S. cholesterol (>200 mg/dL), S. triglycerides (>150 mg/dL), S.LDL (>130 mg/dL), S. VLDL (>40 mg/dL)[22] were included in the study.
Exclusion criteria
Obese patients associated with hypothyroidism, hypertension, Diabetes Mellitus, Cushing's syndrome and other concomitant serious disorders involving liver, kidneys, heart, lungs and other organs, gravid women and lactating mothers were also excluded from the study.
Posology
Patients were randomly grouped into two. Patients of Group A were administrated with śuddhanavīna (fresh) Guggulu (NG) and those in Group B were administered śuddhapurāṇa (old) Guggulu (PG). One gram of the drug with luke warm water before food was administered twice a day in both the groups for eight wks. Follow-up was done for two weeks post treatment. Patients were advised to avoid excess fatty diet such as fried spicy substances, bakery items, fast foods, cold drinks, ice creams, chocolates etc., They were also advised to not sleep during the day and to do physical exercise.
Laboratory investigations
Routine haematological tests such as TLC, RBC, Hb, WBC, ESR and urine examinations were carried out in all patients to assess the condition of disease and to exclude any underlying pathology. Biochemical investigations such aslipid profile, blood urea, RBS, S. creatinine, SGOT, SGPT, alkaline phosphatase etc., were carried out.
Assessment criteria
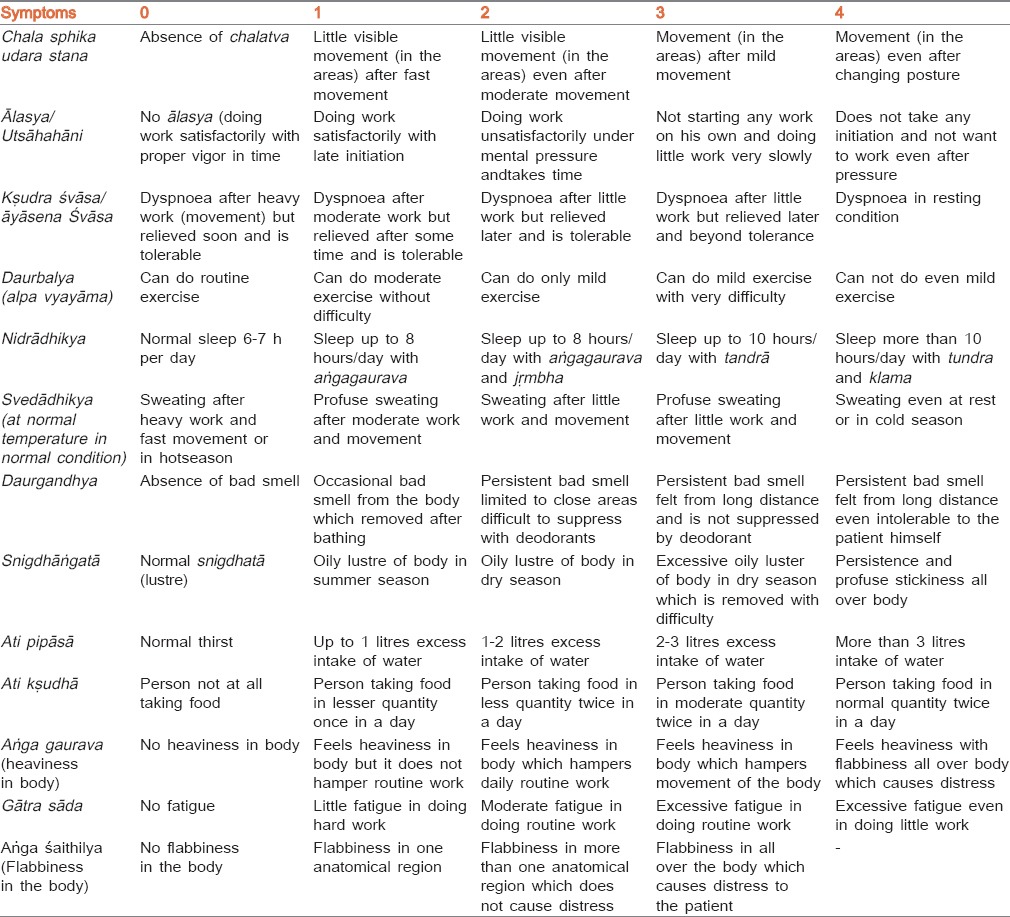
Classical signs and symptoms of Medoroga were taken as subjective criteria. Multidimensional scoring pattern [Table 1] was adopted to give results objectively and for statistical analysis. This score was obtained before and after the treatment and percent relief was determined to assess the efficacy of therapy. Objective criteria were assessed on the basis of body weight, BMI and biochemical investigations done before and after treatment in terms of percentage relief. Girth measurements of certain regions were also noted as below:[23]
Table 1.
The details of the score adopted for the main signs and symptoms
Neck - Between the neck below the level of thyroid cartilage
Chest - In normal expansion at the level of nipple
Abdomen - At the level of umbilicus
Hip - At the level of highest point of distension of buttock
Mid-thigh - Mid of the thigh between pelvic and knee joints
Mid arm - Mid of the arm between shoulder joint and elbow joint.
Results and interpretation
Overall effect of therapy on each scale was calculated with reference to percentage improvement in all symptoms, the relief was assessed on the below criteria:
<25% - Poor response
26–50% - Mild improvement
51–75% - Moderate improvement
76–99% - Marked improvement
100% - Complete remission.
Statistical analysis
The data was expressed as mean ± standard error of mean for both groups. Student's t-test was used to compare the data to determine significance between groups at P < 0.05.
OBSERVATIONS AND RESULTS
The study was carried out in 72 registered patients of medoroga, out of which 13 patients dropped out and 59 completed the prescribed course of treatment. 5 patients experienced inconvenience due to the smell of the medicine, five migrated to some other place while three patients experienced excessive menstruation during treatment. In group A, 30 patients were administrated with NG whereas group B comprised of 29 patients who were administered PG.
Summary of chief complaints is recorded in Table 2. Highly significant (P < 0.001) decreases was found in ālasya (laziness), kṣudraśvāsa (dyspnea), daurbalya (weakness), aṃgagaurava (heaviness in body), gātrāsāda (fatigue) and aṅgaśaithilya (flabbiness in body) in both groups. But NG group showed highly significant (P < 0.001) result in ālasya, daurbalya, gātrāsāda and aṅgaśaithilya; whereas in kṣudraśvāsa, and aṃgagaurava, PG group found more relief than the NG group.
Table 2.
Effect of both groups in cardinal symptoms of Medoroga
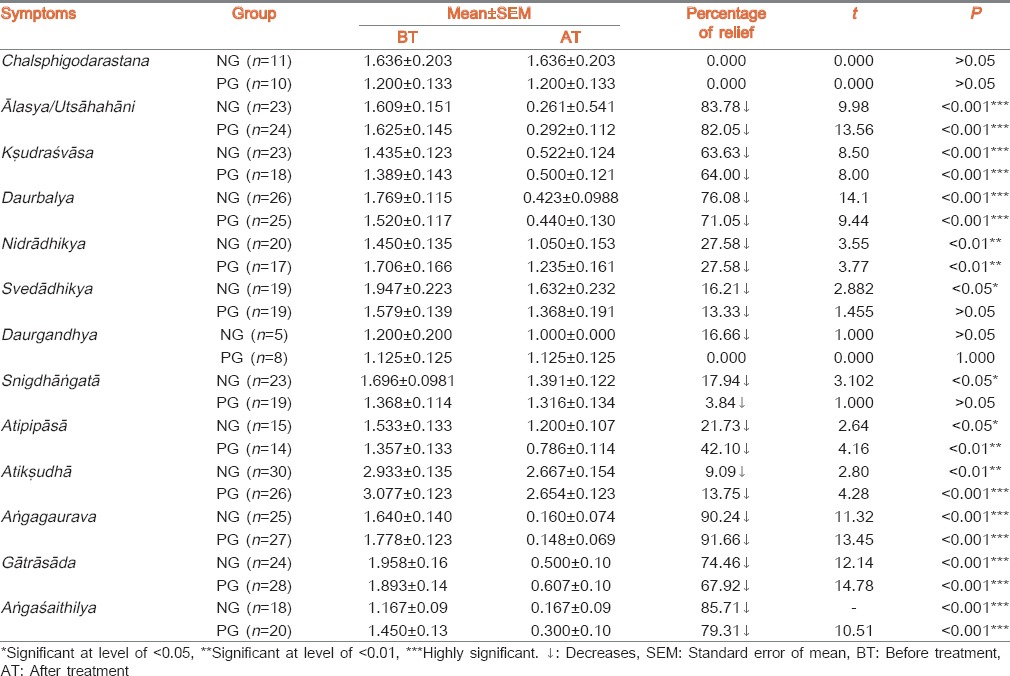
Result of lipid profile, body weight and BMI is placed in Table 3. Both groups showed non-significant decreases in S. cholesterol. NG treated group exhibited significant decrease in S. triglycerides level (17.7%) and VLDL (18.36%) while showing non-significant decreases in S.HDL. PG group also showed significant decreases in S. triglycerides level (13.64%) and VLDL (11.07%) while a non-significant increase was found in S. HDL. Both groups exhibited statistical non-significant (P > 0.05) changes in LDL level. Administration of PG showed a statistically highly significant (P < 0.001) decrease in body weight and BMI. Effect of both drugs on haematological and other biochemical parameters are shown in Table 4.
Table 3.
Effect of therapy on lipid profile, BMI and body weight
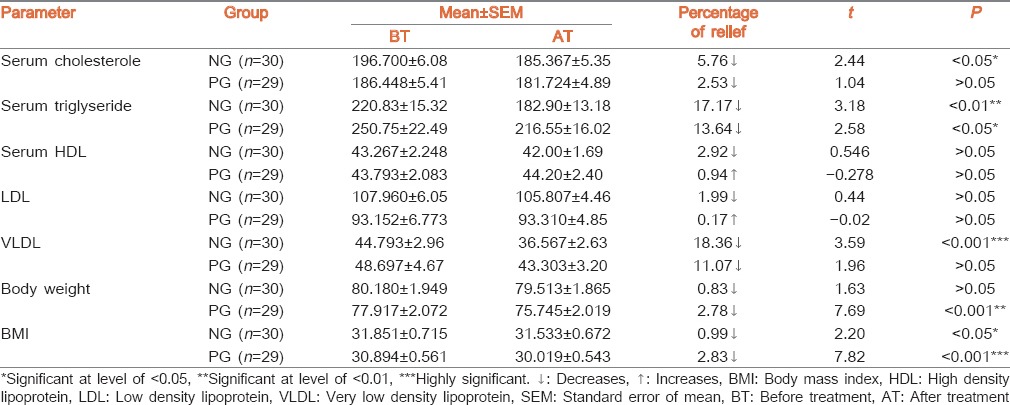
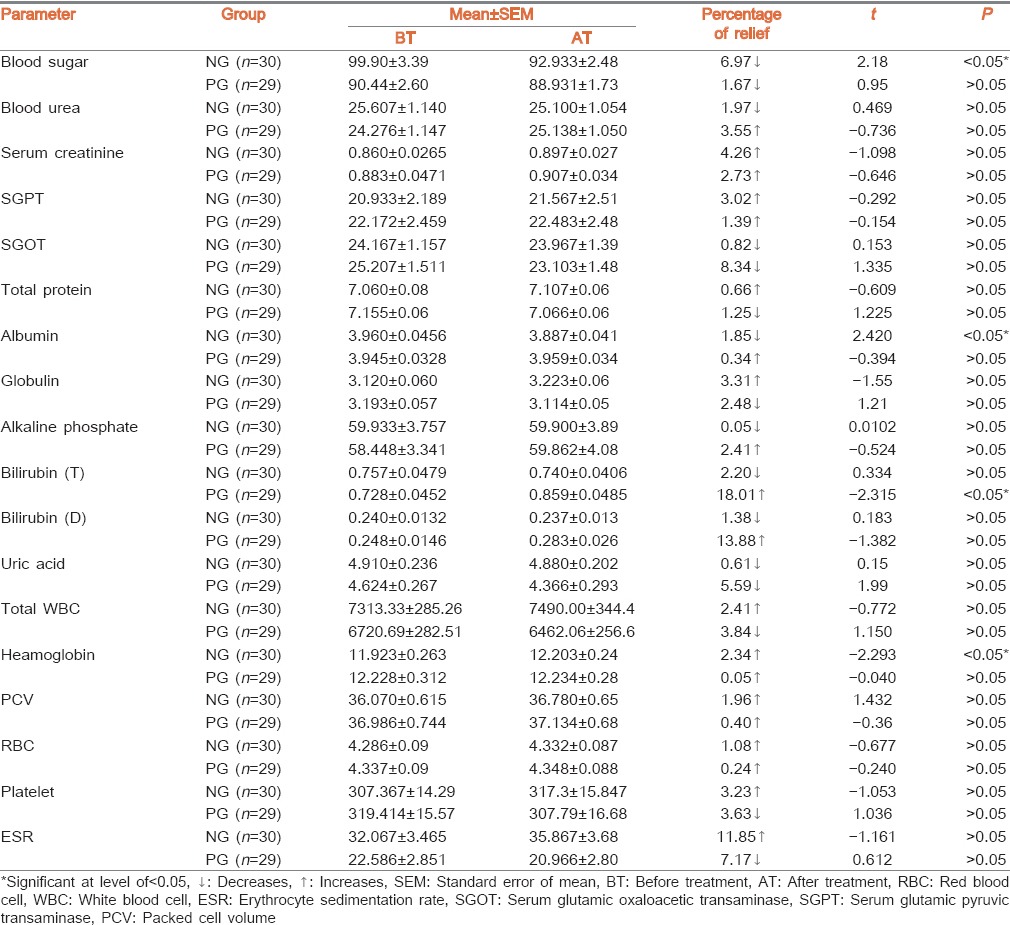
Table 4.
Effect of therapy in haematological and biochemical parameter
PG treated group showed significant reduction in circumferences of chest (1.89%), abdomen (1.6%), hip (1.52%) and mid thigh (3.49%); whereas the NG treated group reduced circumferences of mid arm (1.92%), chest (1.51%), hip (1.4%) and mid thighs (4.3%). All of these results were significant or highly significant and are tabulated in Table 5.
Table 5.
Effect of both trial drugs on girth measurement

Overall results are summarized in Figure 1. One patient (3.44%) from PG group showed marked improvement. Two patients (6.66%) of NG group and six patients (20.68%) of PG group showed moderate improvement. Seventeen patients (56.67%) of NG group and twelve patients (41.37%) of PG group showed mild improvement. Improvement was found in eleven patients (36.67%) of NG group and ten patients (34.48%) of PG group.
Figure 1.
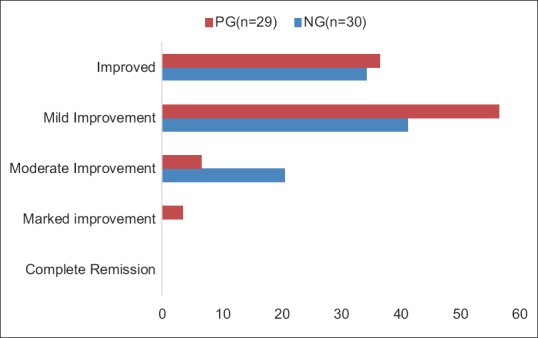
Overall effect of Navīna Guggulu (NG) and Purāṇa Guggulu (PG)
DISCUSSION
In NG group, all closely related biochemical markers i.e. S. cholesterol, LDL, direct and indirect bilirubin, S. alkaline phosphatase have shown statistically non-significant decrease. The common site of synthesis, regulation and or excretion of these products is the liver. Simultaneously VLDL shows highly significant decrease. S. cholesterol is necessary for synthesis of all steroidal hormones. It is noted that plant sterols decrease absorption of cholesterol. Liver is responsible for 80% of endogenous cholesterol synthesis. The liver has a major role in controlling plasma levels of LDL cholesterol, because most of the LDL receptors are located in the liver.[24] Liver synthesizes cholesterol and removes cholesterol from lipoprotein remnants, it also controls body cholesterol pool by converting cholesterol to bile acids. It is an established and published fact that, Guggulsterone enhances transcription of bile salt export pump.[25] In the current study, the NG group shows decrease in many lipid parameters like S. cholesterol, S. LDL, S. VLDL as well as S. bilirubin and S. alkaline phosphatase. In this group, comparatively more quantity of Guggulostereone-E and Guggulsterone-Z have been found in HPLC study which supports the fact that plant sterols decreases absorption of cholesterol.[26] In PG group, there was also a decrease in similar components of lipid profile but there wasn’t a decrease in S. Bilirubin, SGPT and Alkaline Phosphatase. It may be considered that the above observations favours the theory of enhancement of transcription of bile salt export pump by Guggulsterone. Similar kind of results were also found in an animal experimental study.[27]
NG group shows statistically non-significant increase in S. globuline. When albumin level is decreased, body tries to compensate it by increasing the production of globulins from reticulo-endothelial system.[28] Blood is a part of reticulo-endothelial system. Red blood cells, platelets are basically reticulocytes which are produced and released from bone marrow.[29] Thus non significant increase in packed cell volume (PCV), RBC, platelet supports stimulation of stem cell production from bone marrow leading to proliferation of these cells and red cell indices i.e. PCV.
NG group exhibits statistically significant decrease in blood sugar and statistically non-significant increase in S. creatinine after treatment. Liver and kidneys are the two organs that synthesize and secrete glucose into the blood stream for utilization by other organs. In the fasting state, glucose is released into the bloodstream by hepatic glycogenolysis and gluconeogenesis which is termed as hepatic glucose output. The kidney also produces glucose by gluconeogenesis. The process is facilitated by excess of glucagon and lowered insulin level.[30] Supressibility of hepatic glucose output contributes significantly to both fasting as well as post prandial hyperglycemia. Restoration of inhibition of hepatic gluco neogenesis to normal is one among the goals of ideal initial therapeutic agent for diabetes. increased glomerular permeability causes albuminuria hence hypoalbuminemia, a similar condition was observed in this treatment group.
Statistically non-significant increase in SGPT may further support hepatic functional impairment along with decreased S. albumin and blood sugar. Although SGOT is found significantly decreased in NG group, still SGPT/SGOT ratio was found to be normal (<2) and there is statistically non-significant increment in S. globulin but ratio of albumin/globulin is found to be normal (<1.4). Hence, from these values, hepatic impairment cannot be established.
The group which received PG had shown statistically significant increment in total S. biliribin and statistically non-significant increment in direct bilirubin, SGPT, S. alkaline phosphatase but A:G ratio and SGPT: SGOT ratio was not crossing its normal limits - a similar finding as NG group. Hence, the changes are less likely due to hepatic impairment.
The possibility of hypolipidemic effect exclusively due to restriction of excessively fatty diet and recommendation of exercise, is excluded by evidence of significant alteration in biological markers of hepatic function (major site of lipid metabolism). Increased transformation of lipids into bile salts (compensatory change in hepatic function) is probably a significant cause for hypolipidemic effect of trial drugs as evidenced by elevation of SGPT, decrement in total bilirubin, direct bilirubin. Reduction in fasting plasma sugars (which is mainly dependent on hepatic glucose output) and S. albumin was also noted. This suggests that hypolipidemic action of both trial drugs is probably due to increased excretion through Liver.
Reduction in weight is also due to anti-obesity effect of Guggulu.[31] Gomūtra, the media used for śodhana process, is a proven bio-enhancer.[32] It may also enhance anti-obsesity activity. Suppression of appetite probably has major role in reduction of body weight by both drugs. This is because weight loss along with reduction in circumferences of body parts was found comparatively more in PG group along with comparatively greater reduction of Kṣudhādhikya. Alterations in biogenic amines anddopamine ß-hydroxylase activity may be one of the possible mechanisms for the anti-lipidaemiceffect of guggulsterone.[33,34] Hence it may be postulated that PG maybe working primarily by appetite suppression through change in hormones responsible for appetite.
Vitiated kapha and vāta are crucial in the manifestation of Medoroga. Hence, drugs that pacify Vāta and Kapha should be selected in treating the condition. As Guggulu is having all pharmacodynamic actions against the kapha and medodhātu such as kaṭu-tikta rasa (pungent-bittertaste); laghu (light), tīkṣṇa (~sharpness), sūkṣma (~subtle), guṇa (quality); uṣṇa (hot) vīrya and kaṭu vipaka,[35] it enhances metabolic activity in the body, which digest the produced āma. Gomūtra is dominated by kṣārīya (alkaline) substances, kaṭu-tikta rasa, tīkṣṇa and laghu guṇa, uṣṇa vīrya and kaṭu vipāka.[36] By virtue of these properties, it enhances āma-medohara action of Guggulu and reduces various lipid contents in the body. These elevated lipids can be inferred to be āma medodhātu. Old (purāṇa) Guggulu is attributed with atilekhana property which manifests as reduced body weight and BMI in treated patients, when compared to those treated with fresh (navīna) Guggulu. Both Guggulu and Gomūtra have the action of kaphavātaśāmaka. Due to synergistic effect, Guggulu processed in Gomūtra may enhance this action hence may be beneficial in reducing the symptoms of Medoroga. These qualities are also beneficial in lowering the lipid profile in the patients.
CONCLUSION
Lipid lowering property and weight reducing effect of Guggulu gets altered with aging of Guggulu, in that, old Guggulu is comparatively better in the management of hyperlipidemia (Medoroga). The active principles behind this specific activity may be further evaluated through well designed experimental trials.
Financial support and sponsorship
Institute for Post Graduate Teaching and Research in Ayurveda, Gujarat Ayurved University.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Authors are thankful to the Mr. N. J. Vaishnav (Sub-divisional Manager, Junagadh) and Mr. Bhatt (Forest supervisor), Gujarat State Forest Development Corp. Ltd., Vadodara for their help in procurement of Guggulu samples.
REFERENCES
- 1.Global Health Observatory (GHO); WHO Global InfoBase. [Last retrieved on 2014 Dec 29]. Available from: http://www.who.int/gho/ncd/risk_factors/cholesterol_text/en/
- 2.Malhotra SC, Ahuja MM. Comparative hypolipidaemic effectiveness of gum guggulu (Commiphora mukul) fraction ‘A’, ethyl-P-chlorophenoxyisobutyrate and Ciba-13437-Su. Indian J Med Res. 1971;59:1621–32. [PubMed] [Google Scholar]
- 3.Satyavati GV. MD Thesis (Doctor of Ayurvedic Medicine) Varanasi: Banaras Hindu University; 1966. Effect of an Indigenous Drug on Disorders of Lipid Metabolism with Special Reference to Atherosclerosis and Obesity (Medoroga) M.D. Thesis (Doctor of Ayurvedic Medicine) [Google Scholar]
- 4.Nityanand S, Kapoor NK. Hypocholesterolemic effect of Commiphora mukul resin (guggal) Indian J Exp Biol. 1971;9:376–7. [PubMed] [Google Scholar]
- 5.Gujral ML, Sareen K, Tangri KK, Amma MK, Roy AK. Antiarthritic and anti-inflammatory activity of gum guggul (Balsamodendron mukul Hook) Indian J Physiol Pharmacol. 1960;4:267–73. [PubMed] [Google Scholar]
- 6.Shantakumari G, Gujral ML, Sareen K. Further studies on the anti-arthritic and anti-inflammatory activites of gum guggul [Letter] Indian J Physiol Pharmacol. 1964;8:36. [Google Scholar]
- 7.Sosa S, Tubaro R, Della Loggia R, Bombardelli E. Anti-imflammatory activity of commiphora mukul extracts. Pharmacol Res. 1993;27(Suppl 1):89–90. [Google Scholar]
- 8.Sharma JN, Sharma JN. Comparison of the anti-inflammatory activity of Commiphora mukul (an indigenous drug) with those of phenylbutazone and ibuprofen in experimental arthritis induced by mycobacterial adjuvant. Arzneimittelforschung. 1977;27:1455–7. [PubMed] [Google Scholar]
- 9.Pandit MM, Shukla CP. Study of shuddha guggulu on rheumatoid arthritis. Rheumatism. 1981;16:54–67. [Google Scholar]
- 10.Bordia A, Chuttani SK. Effect of gum guggulu on fibrinolysis and platelet adhesiveness in coronary heart disease. Indian J Med Res. 1979;70:992–6. [PubMed] [Google Scholar]
- 11.Chander R, Rizvi F, Khanna AK, Pratap R. Cardioprotective activity of synthetic guggulsterone (E and Z-isomers) in isoproterenol induced myocardial ischemia in rats: A comparative study. Indian J Clin Biochem. 2003;18:71–9. doi: 10.1007/BF02867370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saeed MA, Sabir AW. Antibacterial activities of some constituents from oleo-gum-resin of Commiphora mukul. Fitoterapia. 2004;75:204–8. doi: 10.1016/j.fitote.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Deng R. Therapeutic effects of guggul and its constituent guggulsterone: Cardiovascular benefits. Cardiovasc Drug Rev. 2007;25:375–90. doi: 10.1111/j.1527-3466.2007.00023.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuppurajan K, Rajagopalan SS, Rao TK, Sitaraman R. Effect of guggulu (Commiphora mukul – Engl.) on serum lipids in obese, hypercholesterolemic and hyperlipemic cases. J Assoc Physicians India. 1978;26:367–73. [PubMed] [Google Scholar]
- 15.Kotiyal JP, Bisht DB, Singh DS. Double cross-over trial of gum guggulu (Commiphora mukul) fraction A in hypercholesterolemia. J Res Indian Med Yoga Hom. 1979;14:11–6. [Google Scholar]
- 16.Agarwal RC, Singh SP, Saran RK, Das SK, Sinha N, Asthana OP, et al. Clinical trial of gugulipid – A new hypolipidemic agent of plant origin in primary hyperlipidemia. Indian J Med Res. 1986;84:626–34. [PubMed] [Google Scholar]
- 17.Szapary PO, Wolfe ML, Bloedon LT, Cucchiara AJ, DerMarderosian AH, Cirigliano MD, et al. Guggulipid for the treatment of hypercholesterolemia: A randomized controlled trial. JAMA. 2003;290:765–72. doi: 10.1001/jama.290.6.765. [DOI] [PubMed] [Google Scholar]
- 18.9th ed. Varanasi: Chaukhambha Sanskita Bhavana; 2002. Bhavaprakasha, Bhavamishra, Vidhyodini Hindi Commentary, Purva Khanda, Karpuradi Varga (42) p. 205. [Google Scholar]
- 19.6th ed. Varanasi: Chaukhamba Surbharati Prakashana; 2005. Sarngadhara Samhita, Sarngadharacarya, Dipika and Gudhartha Dipika Commentary, Pt Kashiram and Adhamalla, Prathama Khanda (1:44) p. 11. [Google Scholar]
- 20.2nd ed. Ch. 5. II. New Delhi: Dept. of ISM and H, Ministry of Health and Family Welfare, Govt. of India; 2003. Anonymous, Ayurvedic Formulary of India, Guggulu. Part-1; p. 58. [Google Scholar]
- 21.Varanasi: Chokhambha Surbharti Prakashan; 2009. Charaka Samhita, Agnivesha, Ayurveda Dipika Commentary, Chakrapani Datt, Sutrasthana (21:4). Reprint. [Google Scholar]
- 22.Braunwald E, Fauci AS, Hausar SL, Kasper DL, et al. Harrison's Principles of Internal Medicine. 15th ed. Appendix-7. Vol. 2. Columbus: The McGraw Hill Companies, Medical Publishing Division; 2001. p. A-7. [Google Scholar]
- 23. [Last retrieved on 2014 Dec 12]. Available from: http://www.ptdirect.com/training.delivery/client-assessment/taking-body-dimension-girth- measurements .
- 24.Vasudevan DM, Sreekumari S. Textbook of Biochemistry. 4th ed. Ch. 13. New Delhi: Jaypee Brother Medical Publishers (P) Ltd; 2005. Cholesterol, lipoproteins and cardiovascular disease; p. 147. [Google Scholar]
- 25.Cui J, Huang L, Zhao A, Lew JL, Yu J, Sahoo S, et al. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J Biol Chem. 2003;278:10214–20. doi: 10.1074/jbc.M209323200. [DOI] [PubMed] [Google Scholar]
- 26.Vyas KY. Jamnagar: Institute of Post Graduate Teaching and Research in Ayurveda, Gujarat Ayurved University; 2013. Evaluation of fresh (Naveena) and old (Purana) Guggulu w.s.r. to their anti hyperlipidaemic (medohara) effect. MD Ayudissertation. [Google Scholar]
- 27.Vyas KY, Nariya M, Galib R, Prajapati PK. Anti-hyperlipidaemic activity of fresh and old guggulu (Commiphora wightii (Arn.) Bhandari) in experimental animals. Int J Green Pharma. 2014;8:257–62. [Google Scholar]
- 28.Vasudevan DM, Sreekumari S. Textbook of Biochemistry. 4th ed. Ch 13. New Delhi: Jaypee Brother Medical Publishers (P) Ltd; 2005. Cholesterol, lipoproteins and cardiovascular disease; p. 223. [Google Scholar]
- 29.John WA, Dan L. Harrison's Principle of Internal Medicines. 15th ed. Ch 61. I. Columbus: The McGraw Hill Companies, Medical Publishing Division; 2001. Anemia and polycythemia; p. 350. [Google Scholar]
- 30.Kapur A. Prodial Glucose Regulation – Implications in Management of Type-2 Diabetes, Novo Nordisk Diabetes Updates-1998 Proceedings, Novo Nordisk Pharma India Ltd.; 20-22 February. 1998:107. [Google Scholar]
- 31.Bhatt AD, Dalal DG, Shah SJ, Joshi BA, Gajjar MN, Vaidya RA, et al. Conceptual and methodologic challenges of assessing the short-term efficacy of guggulu in obesity: Data emergent from a naturalistic clinical trial. J Postgrad Med. 1995;41:5–7. [PubMed] [Google Scholar]
- 32.Randhawa GK. Cow urine distillate as bioenhancer. J Ayurveda Integr Med. 2010;1:240–1. doi: 10.4103/0975-9476.74089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shrivastava M, Kapoor NK. Guggulsterone induced changes in the levels of biogenic monoamines and dopamine ß-hydroxylase activity of rat tissues. J Biosci. 1986;10:15–9. [Google Scholar]
- 34.Mithila MV, Khanum F. The appetite regulatory effect of guggulsterones in rats: A repertoire of plasma hormones and neurotransmitters. J Diet Suppl. 2014;11:262–71. doi: 10.3109/19390211.2014.937045. [DOI] [PubMed] [Google Scholar]
- 35.Sushruta Samhita, Sushruta, Nibandha Smgraha, Dalhanacharya . Varanasi: Chaukhambha Surbharti Prakashan; 2003. Commentary, Chikitsa Sthana (5:40-42). Reprint; p. 430. [Google Scholar]
- 36.4th ed. Varanasi: Chowkhambha Krishnadas Academy; 2008. Siddha Bhaishajya Manimala, Krishnaraman Bhatta, Vaishwanara’ Hindi Commentary (2:276) [Google Scholar]


