Abstract
Background:
Śilājatu (Shilajit; SJ) is claimed in traditional Indian medical practice to be useful in the treatment of nervous disorders, epilepsy and as antistress.
Aim:
To investigate whether SJ possesses antiepileptic and antipsychotic activities in rodents.
Materials and Methods:
Isonicotinyl hydrazine (INH), pentylenetetrazole (PTZ), apomorphine, phenytoin, diazepam, haloperidol and other chemicals of analytical grade were procured from standard companies. The antiepileptic activity of SJ was assessed using maximal electro shock (MES)-induced seizures in rats, INH and PTZ-induced seizures in mice. The antipsychotic effect of SJ was evaluated using apomorphine-induced climbing and stereotyped behaviours respectively, in mice and rats.
Settings and Designs:
SJ (25 and 50 mg/kg, p.o.) was given orally once daily for 15 days in all the rodent models. On the test day, SJ was administered 1 h prior to electric shock or chemical inducers (INH/PTZ/apomorphine) in experimental animals; the animals were then observed for different phases of seizures and psychotic behaviours. In addition, gamma-aminobutyric acid (GABA) content in the brain of rats and mice was estimated in seizure models.
Statistical Analysis:
The data were expressed as mean ± standard error of mean. Statistical comparisons were performed by one-way ANOVA followed by Tukey's post-test using Graph Pad Prism version 5.0, USA. A P < 0.05 was considered significant.
Results and Conclusions:
SJ pretreatment significantly inhibited the seizures induced by MES, INH and PTZ in a dose dependent manner. Further, SJ augmented brain GABA levels to normal, decreased by INH and PTZ in mice brain. SJ pretreatment also significantly inhibited the climbing and stereotyped behaviours induced by apomorphine. The present data seems to confirm the antiepileptic activity of SJ which may be because of enhancing the GABAergic system. The antipsychotic activity observed may be due to anti-dopaminergic and/or GABA-mimetic actions.
KEYWORDS: Dopamine, fulvic acid, GABA, humic acid, seizures
INTRODUCTION
Epilepsy and psychosis are the most common chronic noncommunicable neurological disorders of the brain.[1,2] About 50 million people worldwide have epilepsy. In developing countries, the incidence of epilepsy in the general population is approximately estimated to be 80 per 100,000 per year.[2] The lifetime prevalence of all psychotic disorders is 5-8% in the general population and becomes more common as people age.[3] The currently available drugs for these chronic neurological disorders are synthetic molecules associated with side effects such as ataxia, sedation, depression, hepatic failure, dizziness, and behavioural disturbances.[1] Moreover, approximately 30% of the patients continue to have seizures with synthetic drug treatment.[4] Owing to this, there is a demand for complementary medications and remedies because of lesser toxicity and cost effectiveness compared to synthetic drugs. The aim also seems to enhance long-term therapy compliance of epileptic and psychotic patients.[5]
Śilājatu (Shilajit; SJ) is a brown to blackish naturally occurring herbo-mineral sticky exudate found in steep rocks in mountainous Himalayan regions of the Indian subcontinent in the peak summer months. Molecularly, SJ contains mainly humic substances, which are the products of degradation of plants by microorganisms. This leads to the generation of a phytocomplex rich in humic substances, fulvic acid, resins, amino acids, and selenium, among others. SJ is reported to contain fatty acids, benzcoumarin derivatives, triterpenes, several phenolic compounds such as shilajitol, shilanthralin, naphsilajitone and shilaxanthone.[6,7] Pharmacologically reported central nervous system (CNS) actions of SJ are nootropic, antianxiety,[8] neuroprotective,[9] antioxidant, hypnosedative,[7] glycine, and GABA-mimetic.[10]
However, SJ has not been screened so far for antiepileptic and antipsychotic properties, though in traditional Indian medicine, it is used for the treatment of epilepsy, neurobehavioural disorders and as an antistress agent.[7] Śāstric formulations containing SJ such as Śiva guṭikā and Mānasamitra vaṭaka are mainly used for mental/psychological disorders, epilepsy, and to treat retarded intellect.[11] Further, there are proprietary drugs containing SJ used for various mental disorders [Table 1]. The LD50 of SJ is as high as 1000 mg/kg in rodents with no mortality or adverse effects indicating its safety.[12] In another study, SJ up to 5000 mg/kg for 91 days repeated administration showed long term safety in rats.[13]
Table 1.
Proprietary drugs containing SJ
In the present study, SJ was evaluated for antiepileptic and antipsychotic activities in experimental animals.
MATERIALS AND METHODS
Collection of SJ, drugs and chemicals
SJ powder sample (NRSE50) with batch number SJ/11002 was sourced from Natural Remedies Pvt. Ltd., Bangalore (Karnataka), India. The sample was a dark brown powder and standardized to contain 52.2% w/w fulvic acid and 10% w/w humic acid. SJ quality was analysed by different tests such as microbiology, pesticide residue and heavy metal analysis using induction coupled plasma-mass spectroscopy. The sample met the desired specifications set by different regulatory bodies [Supplementary File [SUPPORTING:1]]. Pentylenetetrazole (PTZ) and apomorphine HCl (Sigma-Aldrich Corporation, St. Louis, USA), isonicotinyl hydrazine (INH: Himedia, Mumbai, India), diazepam injection (Calmpose®, Ranbaxy Ltd., New Delhi, India), phenytoin injection (Eptoin®, Abbot, New Delhi, India), and haloperidol injection (Mindol®, Micro labs, Bangalore, India) were purchased. All other chemicals used were of analytical grade.
Experimental animals and research protocol approval
Young albino Wistar male rats (150-200 g) and Swiss albino mice of either sex (25-30 g) were obtained from the animal house of our institute. The animals were maintained under controlled conditions of temperature (25 ± 2°C) and humidity (45-65%) on a 12 h light: dark cycle and had free access to food and water ad libitum. All the animals were acclimatized for seven days before the study. A total of 60 rats and 90 mice were used in five different animal models to ascertain the antiepileptic and antipsychotic activities of SJ. The animals were randomized into experimental and control groups and housed individually in sanitized polypropylene cages containing sterile paddy husk as bedding. Food, but not water, was withdrawn 3 h before assessing the seizure activity and psychotic behaviours. The experimental protocol was approved by the Institutional Animal Ethics Committee (SSCPT/IAEC.CLEAR/104/2011-12) and conducted according to Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines, Government of India. The different types and stages of seizures, climbing and stereotyped behaviours were evaluated by an independent observer who was blinded to the treatment.
Preparation of solutions and suspensions
PTZ and INH were dissolved in normal saline and distilled water, respectively. Apomorphine HCl and SJ were dissolved in distilled water and used for animal studies. Distilled water was served as a vehicle and administered by the oral route to vehicle control animals. Study duration and doses of SJ (25 and 50 mg/kg, dry weight) were selected based on earlier research on CNS related (anxiety, brain oxidative stress, memory and cognition) disorders, which had shown dose dependent activity.[8,12]
Assessment of antiepileptic activity
Maximal Electro Shock (MES)-induced seizures
Albino Wistar male rats were divided into five groups of six each and treated as Groups 1 and 2 were vehicle and MES control groups, both received distilled water (1 ml/100 g); Group 3: the rats in this group were treated with standard drug phenytoin (90 mg/kg, i.p.); Groups 4 and 5 received SJ (25 and 50 mg/kg, p.o., respectively). All the treatments were given for duration of 15 days. On the concluding day of pretreatment, all groups received electric shock (150 mA, 50 Hz for 0.2 s) on the ear using an electroconvulsiometer (INCO, Ambala Pvt. Ltd., India) except the vehicle control. Total duration of the hind limb tonic extension (HLTE), onset of stupor and time taken for recovery were recorded.[14]
INH-induced seizures
Swiss albino female mice were divided into five groups (n = 6). The following treatment was given once daily for 15 days. Groups 1, 4, and 5 received the treatments as described under MES model. Group 2 served as the INH control and received distilled water (1 ml/100g); Group 3 received standard drug diazepam (5 mg/kg, i.p.). On the final day of pretreatment, mice of all groups, except vehicle control, were injected with INH (300 mg/kg, i.p.). INH was given an hour after oral feeding (vehicle and SJ) and 30 min after diazepam. The mice were then placed in an isolated Perspex chamber for 120 min observation and the occurrence of clonic, tonic seizures and death were recorded. The mortality protection was expressed as a percentage.[15]
PTZ-induced seizures
Swiss albino female mice were divided into five groups of six mice each and treated once daily for 15 days. Groups 1, 3, 4, and 5 received the treatments as described in above models. Group 2 severed as the PTZ control and received distilled water (1 ml/100 g). On seizure assessment day, PTZ (80 mg/kg, i.p.) was administered to groups 2, 4, and 5 after one hour of oral treatments and 30 min after i.p. treatment to group 3. All the mice were then placed in an isolated Perspex chamber for observation (duration: 60 min). The occurrence of clonic, tonic seizures and death were recorded during the observation. The protection against mortality was expressed in percentage.[14]
Estimation of brain GABA neurotransmitter
Brain GABA content was estimated in all seizure models according to a published procedure.[16] On the 15th day, after observing the seizures, each animal was sacrificed by decapitation and the brain was dissected out rapidly. It was blotted, weighed and placed in 5 ml of ice-cold trichloroacetic acid (10% w/v), homogenized and centrifuged at 10,000 rpm for 10 min at 0°C. A sample (0.1 ml) of tissue extract was added to 0.2 ml of 0.14 M ninhydrin solution in 0.5 M carbonate-bicarbonate buffer (pH 9.9), kept in a water bath at 60°C for 30 min, cooled and treated with 5 ml of copper tartrate reagent (0.16% w/v disodium carbonate, 0.03% w/v copper sulphate and 0.0329% w/v tartaric acid). After 10 min, fluorescence was recorded at 377/455 nm using spectofluorimeter (Shimadzu, Japan). Brain GABA content was calculated using a standard calibration curve (Y = MX + C) and expressed in ng/mg of wet brain tissue.[17]
Assessment of antipsychotic activity
Apomorphine-induced climbing behaviour in mice
Swiss albino male mice were divided into five groups (n = 6) and treated for 15 days. Groups 1 and 2 were vehicle and apomorphine control, respectively and were treated with distilled water (1 ml/100 g); Groups 3 and 4 were orally treated with SJ at the doses of 25 and 50 mg/kg, respectively. Group 5 was the standard control which received haloperidol (0.1 mg/kg, i.p.). On behavioural assessment day, except group 1, mice of all groups were given apomorphine (3 mg/kg, s.c.) and placed individually in vertical wire mesh stick cages (diameter 12 cm, height 14 cm) for observation of climbing behaviour at every 10 min for 30 min. Before administration of apomorphine, mice were acclimatized to the new environment for a period of 30 min. The method of scoring was: 0 - four paws on the floor, 1 - forefeet holding the vertical bars and 2 - four feet holding the bars.[18]
Apomorphine-induced stereotyped behaviour in rats
Albino Wistar male rats were divided into five groups of six rats each. Groups 1, 2, 3, and 4 received the treatments as described in the previous model. Group 5 served as standard control and received haloperidol (1 mg/kg, i.p.) for 15 days. On the final day of the experiment, all groups, except vehicle control, were given apomorphine (1.5 mg/kg) injection. Immediately after the apomorphine challenge, the rats were placed in individual cages and observed for every 10 min for a total period of 90 min. The intervals permit one to observe the whole course of behavioural effects. The intensity of stereotyped activity was assessed according to a scoring system with 0 - asleep or still, 1 - active, 2 - predominantly active but with bursts of stereotyped sniffing and rearing, 3 - constant stereotyped activity such as sniffing, rearing, or head bobbing, but with locomotor activity still present, 4 - constant stereotyped activity maintained at one location, 5 - constant stereotyped activity but with bursts of licking or gnawing and biting, 6 - continual licking of cage grids and, 7 - continual biting of cage grids.[19]
Statistical evaluation
The data were expressed as mean ± S.E.M. Statistical comparisons were performed using one-way ANOVA followed by Tukey's post-test using Graph Pad Prism version 5.0, USA. A P < 0.05 was considered significant.
RESULTS
Effect of SJ on seizure models
Effect of SJ on MES-induced seizures
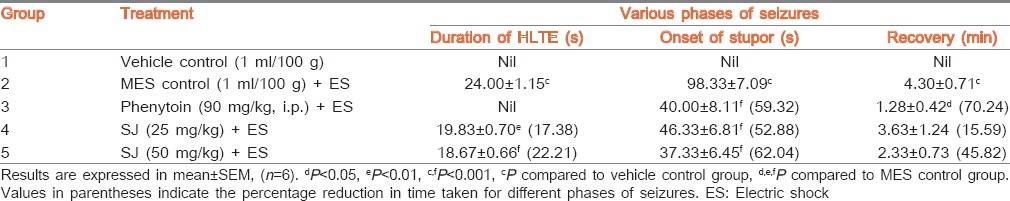
The vehicle control group did not exhibit any phase of seizures. Electric shock produced HLTE and stupor in MES control rats. Pretreatment of rats with SJ (25 and 50 mg/kg) showed significant (P < 0.01 and P < 0.001, respectively) reduction (17.38% and 22.21%, respectively) in the duration of HLTE when compared to MES control rats. SJ at both the doses also significantly (P < 0.001) reduced (52.88% and 62.04%, respectively) the onset of stupor. Phenytoin exhibited complete blockade of hind limb extensor phase and produced significant (P < 0.001) reduction (59.32%) in the stupor phase of seizures [Table 2]. The time taken for recovery in MES control rats was 4.30 ± 0.71 min. Pretreatment of phenytoin and higher dose of SJ, respectively, showed 70.24% and 45.82% reduction in recovery time.
Table 2.
Effect of SJ on MES-induced seizures in rats
Effect of SJ on INH-induced seizures
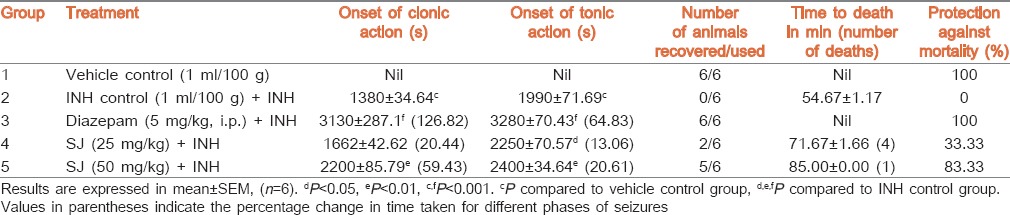
Clonic and tonic seizures were not seen in the vehicle control group. INH (300 mg/kg) injection produced clonic and tonic seizures in INH control group and resulted in 100% death within 54.67 ± 1.17 min. SJ (25 mg/kg) pretreatment significantly (P < 0.05) delayed the onset of tonic seizure (13.06%) and time to death when compared to INH control group. Higher dose of SJ and diazepam also showed significant (P < 0.01 and P < 0.001, respectively) delay in both the onset of clonic and tonic seizures (59.43% and 20.61%, respectively). Pretreatment of mice with SJ (25 and 50 mg/kg) and diazepam, respectively exhibited 33.33%, 83.33% and 100% protection against death [Table 3].
Table 3.
Effect of SJ on INH-induced seizures in mice
Effect of SJ on PTZ-induced seizures
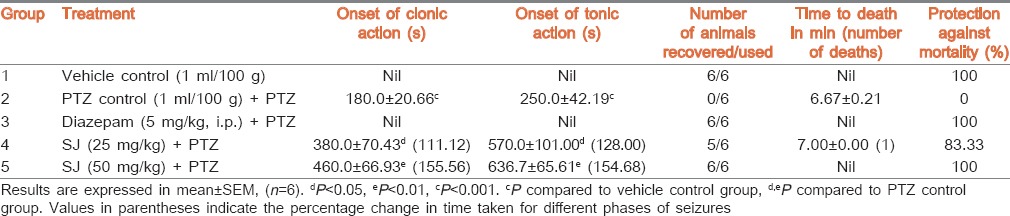
Absence of seizures was observed in mice pretreated with vehicle alone and diazepam. Whereas, administration of PTZ alone produced clonic and tonic seizures in PTZ control group and exhibited 100% death within 6.67 ± 0.21 min. Mice pretreated with SJ (25 and 50 mg/kg) exhibited significant (P < 0.05 and P < 0.01, respectively) and dose dependent delay in the onset of clonic (111.12% and 155.56%, respectively) and tonic seizures (128.00% and 154.68%, respectively) when compared to PTZ control group. SJ at 25 mg/kg showed 83.33% protection against death. However, no deaths were observed in mice pretreated with higher dose of SJ and diazepam indicating 100% protection [Table 4].
Table 4.
Effect of SJ on PTZ-induced seizures in mice
Estimation of brain GABA content
Except MES model, challenge of chemical inducers (INH and PTZ) resulted in significant (P < 0.05 and P < 0.01, respectively) reduction in brain GABA content when compared to vehicle control. In MES model, SJ (25 and 50 mg/kg) pretreatment (3.85 ± 0.34 and 4.38 ± 0.53 ng/mg, respectively) and also phenytoin (4.57 ± 0.34 ng/mg) to rats failed to show significant increase in brain GABA content in comparison to MES control (3.18 ± 0.38 ng/mg) group. However, INH and PTZ controls produced significant (12.98% and 16.10%, respectively) reduction in brain GABA content when compared to their respective vehicle controls. SJ at lower (15.60% and 15.36%) and higher (approximately 16% and 15.86%) dose as well as diazepam (20.43% and 20.81%) exhibited significant increase in brain GABA content respectively, in INH and PTZ models [Figures 1 and 2].
Figure 1.
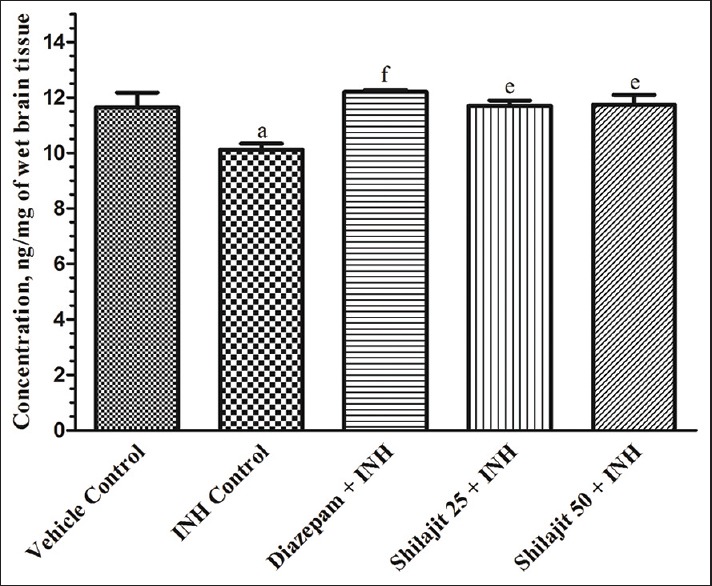
Effect of SJ on brain GABA content in INH-induced seizures in mice. Results are expressed in mean ± S.E.M., (n = 6), aP < 0.05; eP < 0.01; fP < 0.001. aP compared to vehicle control group and e,fP compared to INH control group
Figure 2.
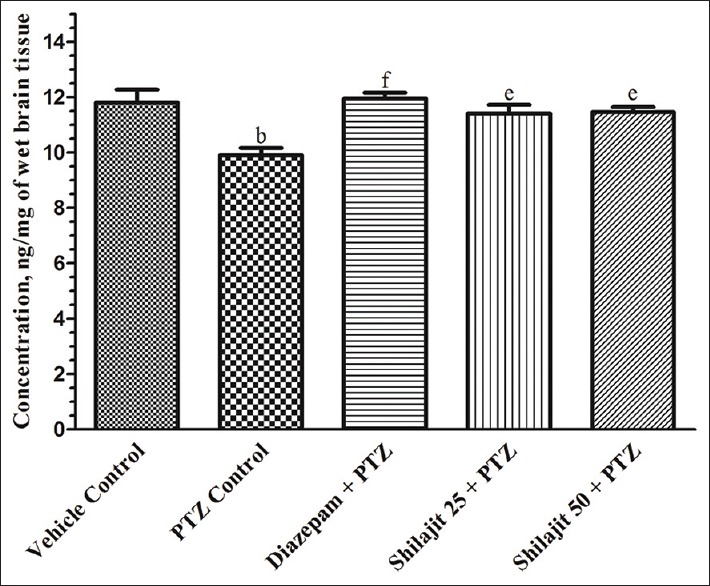
Effect of SJ on brain GABA content in PTZ-induced seizures in mice. Results are expressed in mean ± S.E.M., (n = 6), b,eP < 0.01; fP < 0.001. bP compared to vehicle control group and e,fP compared to PTZ control group
Effect of SJ on psychotic models
Effect of SJ on apomorphine-induced climbing behaviour in mice
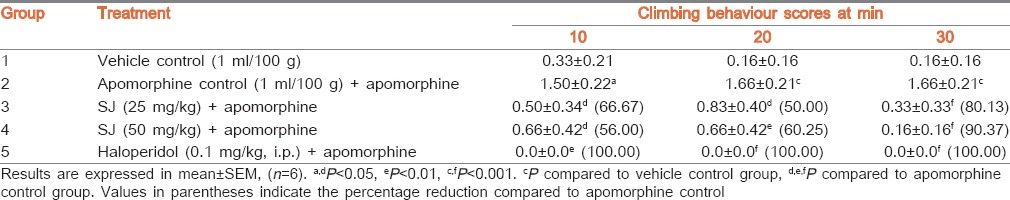
Vehicle control mice displayed least climbing behaviour. Haloperidol pretreated group did not exhibit any climbing behaviour indicating 100% protection and higher activity, whereas administration of apomorphine alone to group 2 significantly (P < 0.001) increased the climbing behaviour when compared to vehicle control. Pretreatment of mice with SJ (25 mg/kg), time dependently and significantly reduced the intensity of climbing behaviour at all the observed intervals with higher activity at 30 min (P < 0.001) and the percentage reductions were, respectively 66.67%, 50.00%, and 80.13%. Further, SJ at 50 mg/kg produced gradual significant (P < 0.001) reduction in climbing behaviour with 56.00% to 90.37% reductions [Table 5].
Table 5.
Effect of SJ on apomorphine-induced climbing behaviour in mice
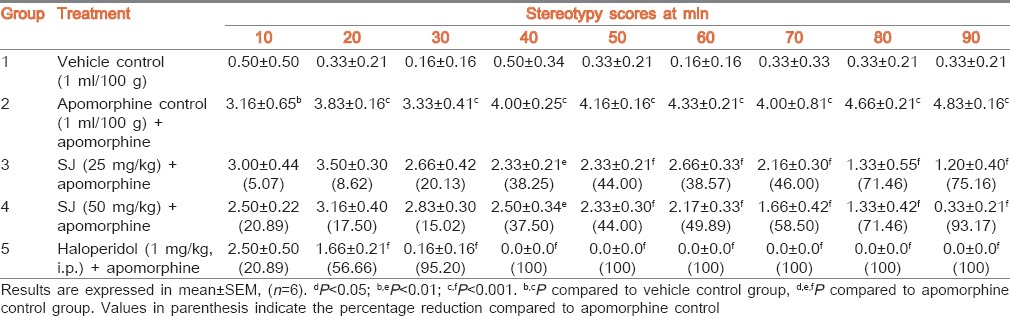
Effect of SJ on apomorphine-induced stereotyped behaviour in rats
Injection of apomorphine alone produced significant stereotyped behaviour in apomorphine control rats. Pretreatment of rats with SJ (25 and 50 mg/kg) produced significant reduction in stereotyped score at 40 (P < 0.01) to 90 (P < 0.001) min intervals and the percentage reductions gradually increased from 38.25% to 75.16% and 37.50% to 93.17%, respectively. Haloperidol pretreated group also revealed a statistically significant (P < 0.001) reduction in stereotyped score at 20-90 min time intervals with percentage reductions from 56.66% to 100%. The vehicle control group exhibited minimal stereotyped behaviour [Table 6].
Table 6.
Effect of SJ on apomorphine-induced stereotypy in rats
DISCUSSION
The present study is a preliminary attempt to evaluate the antiepileptic and antipsychotic effects of SJ. The natural seizure pattern was mimicked in rodent models using MES-induced seizures in rats; and INH and PTZ-induced seizures in mice. The classical rodent seizure models were selected on the basis of the underlying mechanisms involved in the antiepileptic drugs (AEDs). The seizure pattern in MES for all laboratory animals and man are similar except for the time scale.[20] MES-induced HLTE seizures are blocked by AEDs such as phenytoin, which are known to inhibit voltage sensitive Na+ channels.[21] The epileptic action of INH (a GABA-synthesis inhibitor) involves disruption of GABAergic neurotransmission in the CNS.[22] PTZ acts by selective blocking of Cl− channel coupled to the GABAA receptor complex. INH and PTZ-induced clonic-tonic seizures are blocked by AEDs such as diazepam by acting on the GABAA receptor mediated Cl− conductance.[23] It has been scientifically affirmed that intra-peritoneal INH and PTZ-induced seizure identifies compounds that are effective against petit-mal and myoclonic types of seizures, whereas MES test identifies agents with activity against grand-mal epilepsy.[18]
SJ produced a significant protective effect against seizures induced by electric shock. Seizures produced by MES can be explained by three phases such as, tonic extensor, stupor and recovery or death[13] which was also seen in the present study. A fast recovery in the extension phase (HLTE) and onset of stupor were observed in rats pretreated with SJ, thus justifying its antiepileptic activity. In addition, SJ pretreated animals also showed fast recovery from seizures which further supports its protective activity against seizures. Phenytoin, a diphenylhydantoin, also significantly suppressed HLTE, stupor and fast recovery from seizures.
The antiepileptic activity of SJ is further confirmed by using INH and PTZ-induced seizures in mice. SJ pretreated mice in INH model exhibited significant delay in the onset of tonic seizures at both the doses. However, only higher dose of SJ showed significant postponement in the onset of clonic seizure and maximum mortality protection as well as delayed death. Further, in PTZ-induced seizure model, SJ pretreated mice produced a statistically significant delay in the onset of clonic and tonic seizures at both the doses with 100% mortality protection at higher dose.
Mice pretreated with SJ showed significant increase in brain GABA content when compared to INH and PTZ control mice, suggesting the antiepileptic effect of SJ probably through elevation of brain GABA content. However, SJ pretreatment failed to exhibit a significant increase in brain GABA content in MES model. This may be due to the fact that GABAergic system not being disturbed in MES model unlike other tested models.[24] Electric shock mainly triggers the opening of Na+ channels and higher influx of Na+ ions, resulting in depolarization of nerve cell membranes, causing increased action potential and excitation of neuronal cells resulting in epileptic seizures.[1] It is well known that anxiolytic drugs inhibit clonic and tonic seizures in mice.[18] SJ is reported to possess anxiolytic activity[8] and in some recent studies, it has also been reported with neuroprotective, glycine and GABA-mimetic actions.[9,10] It is as well reported that picrotoxin, a GABAA receptor antagonist suppresses SJ-induced responses and completely blocked by strychnine, a glycine receptor antagonist.[10] All of these studies justify the observed antiepileptic effect of SJ through GABA-mimetic actions. Further, fulvic and humic acids, major derivatives of SJ, exhibited several neurobehavioural protective characteristics, including hypnosedative, anxiolytic, neuroprotective and antioxidative effects.[6,7] Hence, it is also reasonable to assume that the antiepileptic action of SJ may also be because of the abovementioned neurodefensive effects.
The antipsychotic activity of SJ was evaluated by apomorphine-induced climbing and stereotyped behaviours in rodents. Apomorphine, an aporphine derivative, is well known direct dopamine D2 agonist and in higher dose it causes stimulant effects such as hyperactivity and stereotypy via stimulation of the central mesolimbic and striatal dopaminergic, serotonergic, noradrenergic neurotransmitters (via α1A,2A and β pathways) as well as inhibition of GABA system.[25,26,27] Moreover, mesolimbic dopaminergic mechanisms have been associated with a critical role in the demonstration of stereotypy after acute psychostimulant administration; some of the specific behaviours produced in these models closely resemble that of humans and are considered to be particularly relevant to psychiatric disorders.[18]
The peripheral administration of apomorphine to mice and rats typically results in an increased locomotion and stereotyped behaviour (rearing, sniffing, licking, biting and gnawing), respectively,[18] which was also observed in the present study. SJ pretreatment successfully decreased locomotory and stereotypy behaviours in both mice and rats in a dose dependent manner. The effect of higher dose of SJ on the neuro-behavioural activity was more potent than the lower dose. The drugs which antagonize climbing and stereotyped behaviours in rodents have been correlated with neuroleptic activity. Further, dopamine D2, serotonin and noradrenergic receptor blockade and GABA-mimetic actions are suggested in the management of psychosis.[18]
Some noradrenergic neuron blocking agents such as reserpine blocks the granular reuptake of noradrenaline and 5-HT by the vesicular amine transporters and inhibits stereotyped behaviour.[28] Furthermore, serotonin (5-HT2A) receptor antagonists such as clozapine, olanzapine and amperozide are used as antipsychotics. It is also reported that the GABAergic and dopaminergic systems influence one another to enhance their antagonistic activity in apomorphine-induced climbing and stereotyped behaviours.[29] If GABA agonists purely act by inhibiting dopamine neurons, then their mode of action in apomorphine-induced climbing and stereotyped behaviours would be similar to the action of neuroleptics, namely, by reducing dopaminergic transmission in mesolimbic and striatal areas.[25] The above hypothesis further supports the antipsychotic and GABA-mimetic actions of SJ.
CONCLUSION
Present study provides the scientific basis for the ethno medicinal usage of SJ in treating epileptic and psychotic disorders. The useful effect of SJ observed in this study may be due to the presence of several active constituents such as benzocoumarins, triterpenes, phenolic compounds, fulvic and humic acids either in combination or alone, which needs to be investigated. Further neurochemical studies in the brain are warranted to confirm the specific mode of said hypotheses.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are thankful to Natural Remedies Pvt. Ltd., Bangalore, India, for providing test drug SJ. Sharanbasappa Durg thanks M.S. University of Baroda, Gujarat and AICTE, New Delhi, for the award of GPAT (GATE Pharmaceutical Sciences; AIR-345) fellowship.
REFERENCES
- 1.Bromfield EB, Cavazos JE, Sirven JI, editors. West Hartford (CT): American Epilepsy Society; 2006. An Introduction to Epilepsy. [PubMed] [Google Scholar]
- 2.World Health Organization. Epilepsy Fact Sheet N999. [Last accessed on 2014 Jun 12]. Available from: http://www.who.int/mediacentre/factsheets/fs999/en/
- 3.Van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: Evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–95. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 4.Poole K, Moran N, Bell G, Solomon J, Kendall S, McCarthy M, et al. Patients’ perspectives on services for epilepsy: A survey of patient satisfaction, preferences and information provision in 2394 people with epilepsy. Seizure. 2000;9:551–8. doi: 10.1053/seiz.2000.0450. [DOI] [PubMed] [Google Scholar]
- 5.Beaubrun G, Gray GE. A review of herbal medicines for psychiatric disorders. Psychiatr Serv. 2000;51:1130–4. doi: 10.1176/appi.ps.51.9.1130. [DOI] [PubMed] [Google Scholar]
- 6.Ali M, Sahrawat I, Singh O. Phytochemical investigation of shilajit. Indian J Chem. 2004;43B:2217–22. [Google Scholar]
- 7.Wilson E, Rajamanickam GV, Dubey GP, Klose P, Musial F, Saha FJ, et al. Review on shilajit used in traditional Indian medicine. J Ethnopharmacol. 2011;136:1–9. doi: 10.1016/j.jep.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Jaiswal AK, Bhattacharya SK. Effects of shilajit on memory, anxiety and brain monoamines in rats. Indian J Pharmacol. 1992;24:12–7. [Google Scholar]
- 9.Khaksari M, Mahmmodi R, Shahrokhi N, Shabani M, Joukar S, Aqapour M. The effects of shilajit on brain edema, intracranial pressure and neurologic outcomes following the traumatic brain injury in rat. Iran J Basic Med Sci. 2013;16:858–64. [PMC free article] [PubMed] [Google Scholar]
- 10.Yin H, Yang EJ, Park SJ, Han SK. Glycine- and GABA-mimetic Actions of shilajit on the substantia gelatinosa neurons of the trigeminal subnucleus caudalis in mice. Korean J Physiol Pharmacol. 2011;15:285–9. doi: 10.4196/kjpp.2011.15.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delhi, India: Controller of Publications; 2003. The Ayurvedic Formulary of India, Part I and II. Second Revised English edition. Department of Indian Systems of Medicine and Homeopathy, Ministry of Health and Family Welfare, Government of India. [Google Scholar]
- 12.Mittal P, Kaushik D, Gupta V, Bansal P, Khokra S. Therapeutic potentials of “Shilajit rasayana” – A review. Int J Pharm Clin Res. 2009;1:47–9. [Google Scholar]
- 13.Velmurugan C, Vivek B, Wilson E, Bharathi T, Sundaram T. Evaluation of safety profile of black shilajit after 91 days repeated administration in rats. Asian Pac J Trop Biomed. 2012;2:210–4. doi: 10.1016/S2221-1691(12)60043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine. 2004;11:56–64. doi: 10.1078/0944-7113-00376. [DOI] [PubMed] [Google Scholar]
- 15.Ramdhave AS, Badole SL, Bodhankar SL. Anticonvulsant activity of stem bark of Pongamia pinnata. Biomed Aging Pathol. 2011;1:147–57. [Google Scholar]
- 16.Lowe IP, Robins E, Eyerman GS. The fluorometric measurement of glutamic decarboxylase and its distribution in brain. J Neurochem. 1958;3:8–18. doi: 10.1111/j.1471-4159.1958.tb12604.x. [DOI] [PubMed] [Google Scholar]
- 17.Sutton I, Simmonds MA. Effects of acute and chronic pentobarbitone on the gamma-aminobutyric acid system in rat brain. Biochem Pharmacol. 1974;23:1801–8. doi: 10.1016/0006-2952(74)90188-9. [DOI] [PubMed] [Google Scholar]
- 18.Voghel HG, Voghel WH. Berlin: Springer-Verlag; 1997. Drug Discovery and Evaluation: Pharmacological Assays. [Google Scholar]
- 19.Gupta G, Kazmi I, Afzal M, Rahman M, Saleem S, Ashraf MS, et al. Sedative, antiepileptic and antipsychotic effects of Viscum album L. (Loranthaceae) in mice and rats. J Ethnopharmacol. 2012;141:810–6. doi: 10.1016/j.jep.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Toman JE, Loewe S, Goodman LS. Physiology and therapy of convulsive disorders: Effect of anticonvulsant drugs on electroshock seizures in man. Arch Neurol Psychiatry. 1947;58:312–24. doi: 10.1001/archneurpsyc.1947.02300320063003. [DOI] [PubMed] [Google Scholar]
- 21.Holmes GL, Zhao Q. Choosing the correct antiepileptic drugs: From animal studies to the clinic. Pediatr Neurol. 2008;38:151–62. doi: 10.1016/j.pediatrneurol.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurnani A, Chawla R, Kundra P, Bhattacharya A. Acute isoniazid poisoning. Anaesthesia. 1992;47:781–3. doi: 10.1111/j.1365-2044.1992.tb03256.x. [DOI] [PubMed] [Google Scholar]
- 23.Riss J, Cloyd J, Gates J, Collins S. Benzodiazepines in epilepsy: Pharmacology and pharmacokinetics. Acta Neurol Scand. 2008;118:69–86. doi: 10.1111/j.1600-0404.2008.01004.x. [DOI] [PubMed] [Google Scholar]
- 24.Castel-Branco MM, Alves GL, Figueiredo IV, Falcão AC, Caramona MM. The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs. Methods Find Exp Clin Pharmacol. 2009;31:101–6. doi: 10.1358/mf.2009.31.2.1338414. [DOI] [PubMed] [Google Scholar]
- 25.Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, et al. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22:2873–84. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee EH, Wang FB, Tang YP, Geyer MA. Gabaergic interneurons in the dorsal raphe mediate the effects of apomorphine on serotonergic system. Brain Res Bull. 1987;18:345–53. doi: 10.1016/0361-9230(87)90012-8. [DOI] [PubMed] [Google Scholar]
- 27.Dunn RB, Kruse H, Geyer HM, Novick WJ, Fielding S. The effects of GABA agonists and antagonists on apomorphine-induced climbing behavior. Brain Res Bull. 1980;5:433–7. [Google Scholar]
- 28.Ayhan IH, Randrup A. Role of brain noradrenaline in morphine-induced stereotyped behaviour. Psychopharmacologia. 1972;27:203–12. doi: 10.1007/BF00422800. [DOI] [PubMed] [Google Scholar]
- 29.Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: Sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol. 1995;355:418–26. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]


