Abstract
The main purpose of this study is to know the effect of three different photon energies viz., 6, 10, and 15 mega voltage (MV) on RapidArc (RA) planning for deep-seated cervix tumor and to develop clinically acceptable RA plans with suitable photon energy. RA plans were generated for 6, 10, and 15 MV photon energies for twenty patients reported with cervix carcinoma. RA plans were evaluated in terms of planning target volume (PTV) coverage, dose to organs at risk (OARs), conformity index (CI), homogeneity index (HI), gradient measure, external volume index of dose distribution produced, total number of monitor units (MUs), nontumor integral dose (ID), and low dose volume of normal tissue. A two-sample paired t-test was performed to compare the dosimetric parameters of RA plans. Irrespective of photon energy used for RA planning, plans were dosimetrically similar in terms of PTV coverage, OARs sparing, CI and HI. The numbers of MUs were 13.4 ± 1.4% and 18.2 ± 1.5% higher and IDs were 2.7 ± 0.8% and 3.7 ± 0.9% higher in 6 MV plans in comparison to that in the 10 and 15 MV plans, respectively. V1Gy, V2Gy, V3Gy, and V4Gy were higher in 6 MV plans in comparison to that in 10 and 15 MV plans. Based on this study, 6 MV photon beam is a good choice for RA planning in case of cervix carcinoma, as it does not deliver additional exposure to patients caused by photoneutrons produced in high energy beams.
Keywords: Dose distribution, neutron dose, photon energy, RapidArc
Introduction
Cervical cancer remains the most common gynecological cancer worldwide. Radiotherapy is commonly used to treat cervix cancer.[1,2] RapidArc (RA) is one of the advanced technologies available for cancer treatment in radiotherapy. It is the extension of the principle of intensity modulated arc therapy proposed by Yu in 1995,[3] which involves simultaneous rotational movement of the linear accelerator's gantry along with movement of the multi-leaf collimator (MLC) leaves to produce fluence modulation while beam is on.[4] The ability of the RA technique to synchronize dose rate, gantry speed, and MLC motion during radiation beam-on makes RA superior than intensity modulation radiotherapy (IMRT).[5]
Many authors have conducted a study on IMRT but Otto et al.[6] and Palma et al.[7] reported that RA technique generates superior target coverage and provides superior organs at risk (OARs) sparing in comparison to IMRT. RA is more efficient in treatment delivery as it reduces number of monitor units (MUs) and requires less beam-on time.
Rao et al.[8] compared volumetric modulated arc therapy (VMAT) with fixed field IMRT and helical tomotherapy. They reported that VMAT time varied from 2.1 to 4.6 min, IMRT treatment varied from 7.9 to 11.1 min, and tomotherapy time varied from 4 to 7 min.
Verbakel et al.[9] and Otto et al.[6] found that VMAT is more MU efficient in comparison to IMRT. As VMAT requires less beam-on time, it will result in reduced leakage and integral dose (ID) to OARs.
The above facts state that RA technique is superior in comparison to IMRT, hence, in this study, we have tried to investigate the effect of different photon energies on cervix RA planning and to evaluate their dosimetric parameters in terms of planning target volume (PTV) coverage, OARs sparing, different physical indices, MUs, and ID to normal tissues with 6, 10, and 15 mega voltage (MV) photon beam energies.
Materials and Methods
Immobilization devices
All the patients were immobilized in supine position with the help of All-in One board (AIO, Orfit Industry NV, Belgium), thermoplastic mold cast (Orfit Industry NV, Belgium), and knee rest.
Simulation
Siemens SOMATOM Sensation Open CT Scanner (Siemens Medical Systems, Germany) was utilized for computed tomography (CT) of all the patients. CT images of 3.0 mm slice thickness were acquired extending from L2 to proximal third femoral diaphysis. All patients were scanned with full bladder as per institutional protocol.
Target and organs at risks delineation
Target volumes were delineated on CT images for clinical target volume (CTV) and PTV as per Radiation Therapy Oncology Group guidelines.[10] PTV was defined by adding a 5 mm margin to CTV that includes the cervix, uterus, parametrial tissues, and pelvic nodes including presacral. OARs such as bladder, rectum, femoral heads, and bowel were also delineated.
Patient characteristics
Twenty patients reported with cervix carcinoma (stages II–IIIB) who were treated by IMRT and RA techniques (8 patients treated with IMRT and 12 with RA using 6 MV) were selected retrospectively for this study. The patient anterior-posterior mean separation was 22.0 ± 2.3 cm ranging from 18.4 cm to 26.0 cm and right-lateral mean separation was 35.9 ± 4.8 cm ranging from 29.2 cm to 48 cm. Mean PTV volume was 1318.9 ± 189.0 cc ranging from 1112.0 cc to 1710.6 cc. Bladder and rectum mean volume was 379.6 ± 189.0 cc ranging from 169.7 cc to 748.0 cc and 64.6 ± 22.0 cc ranging from 45.9 cc to 101.4 cc, respectively.
Bladder and rectum volumes overlapped with PTV were also calculated by Boolean operation. Non-overlapping volumes of bladder (bladder minus PTV) and rectum (rectum minus PTV) were also calculated using Boolean operation. Bladder minus PTV mean volume was 38.5 ± 6.9% ranging from 29.7cc to 48.7cc. Rectum minus PTV mean volume was 40.7 ± 9.8% ranging from 24.9cc to 51.3cc.
Planning objective and prescription
RA plans were generated with 6, 10, and 15 MV photon beams for the prescribed dose (PD) of 50.4 Gray (Gy) in 28 fractions to the PTV at the rate of 1.8 Gray per fraction. Planning objective was to deliver 100% PD to 95% of PTV with no more than 2% of PTV volume receiving 107% of PD as recommended in International Commission on Radiation Units and Measurements report number 50 (ICRU 50)[11] and ICRU 62.[12] Dose to bladder and rectum was restricted in such a way that V50Gy (volume receiving 50 Gy) should be <50% of OARs volume and mean dose of both femoral heads should remain within 20 Gy as per institutional protocol.
Planning technique
RA plans were generated for delivery on linac True Beam STx (Varian Medical Systems, Palo Alto, CA, USA) which is capable of delivering IMRT and RA. This linac is equipped with high definition-MLC of 60 pairs, inner 32 leaf pairs of 0.25 cm, and outer 28 leaf pairs of 0.50 cm projection width at isocenter. Machine was calibrated at 1 cGy/MU as per Technical Reports Series No. 398 of International Atomic Energy Agency[13] for all the energies.
Treatment planning system (TPS) Eclipse version 10.0 (Varian Medical Systems, Palo Alto, CA, USA) was used for RA planning. Double arcs were used for all the RA plans. The first arc was clockwise with gantry angle 179–181° and collimator angle 10–30° and the second arc was counter clockwise with gantry angle 181–179° and collimator angle 10–30°. Collimator rotation was used to cover entire target volume and reduce tongue and groove effect during gantry rotation, which subsequently minimizes inter-leaf leakage.[14,15] The progressive resolution optimizer algorithm was used for optimization and anisotropic analytical algorithm with 0.25 cm grid size was used for photon dose calculation for all plans. All the plans were generated with all the three energies viz., 6, 10, and 15 MV with dose rate of 600 MU/min.
Dosimetric comparison and plan evaluation
Cumulative dose volume histogram generated by TPS was used to evaluate dosimetric parameters. PTV coverage was evaluated by calculating mean dose, V95%(PTV volume receiving 95% of PD), V98%, V100%, and V107%. Bladder and rectum were evaluated for mean dose, V30Gy(volume receiving 30 Gy), V40Gy, and V50Gy. Femoral heads were also evaluated for mean dose, maximum dose, V10Gy(volume receiving 10 Gy), V20Gy, V30Gy, and V40Gy.
The homogeneity index (HI) and conformity index (CI) were calculated using following formulae
HI = D5%/D95%[16]
Where D5% and D95% are the doses to 5% and 95% PTV volumes, respectively.
CI (for 98% of PD) = Volume receiving 98% of PD/PTV[17]
Gradient measure (GM) was calculated as radius difference between the equivalent spheres of prescription and half prescription isodose volumes, which indicates dose falloff around PTV. Small value of GM indicates higher dose gradient around PTV.[18]
External volume index (EVI) was calculated as follows;
EVI = Volume of normal tissue receiving reference dose (NTVDRef)/PTV.[19]
Integral dose
ID is the dose deposited to the normal tissues outside the PTV in a patient. It is also the area under the curve of a differential absolute-dose, absolute volume histogram. It was calculated to assess the plan quality based on the following formula considering uniform tissue density:
Nontumor integral dose (NTID) = mean dose × volume of normal tissue outside PTV.[20]
For low dose volume evaluation of normal tissues, D1%(dose to 1% volume of normal tissues), D2%, D5%, V1Gy(volume receiving 1 Gy), V2Gy, V3Gy, V4Gy, and V5Gy were calculated.
Statistical analysis
The comparison between dosimetric parameters of 6, 10, and 15 MV RA plans was performed using two-sample paired t-test. The analyses were performed with International Business Machines Corporation (IBM), IBM SPSS Statistics for Windows, (Version 20.0. Armonk, NY: IBM Corp). A P < 0.05 was considered as statistically significant.
Results
Table 1 presents the different dosimetric parameters of RapidArc plans for 6, 10, and 15 MV photon energies. There was no statistically significant (P > 0.05) difference found in terms of PTV coverage for 6, 10, and 15 MV energies. There was a slight increase in PTV volume receiving dose 107% of the PD with increase in energy, but results were not statistically significant (P > 0.05).
Table 1.
DVH dosimetric data of PTV for RA using 6, 10, and 15 MV photon energies
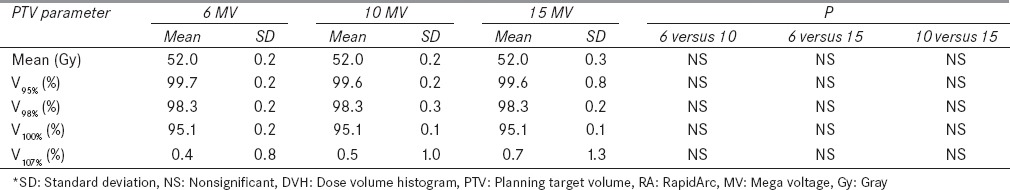
Figure 1 represents the isodose distribution resulting from RA planning with 6, 10, and 15 MV photon energies for a representative patient along axial, coronal and sagittal views, in which one can easily distinguish the difference in 50% isodose line in RA plans using 6, 10, and 15 MV energies.
Figure 1.
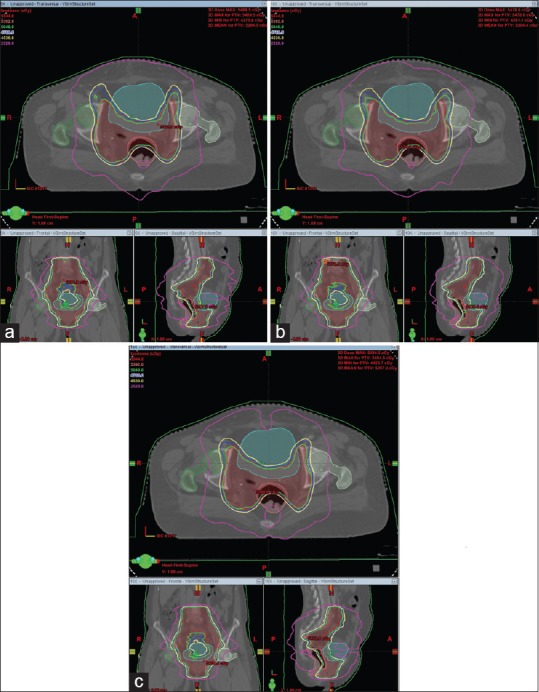
The isodose distribution generated from RapidArc planning in case of Ca-Cervix for same patient in axial, coronal and sagittal planes with (a) 6 MV, (b) 10 MV, and (c) 15 MV photon beam energies
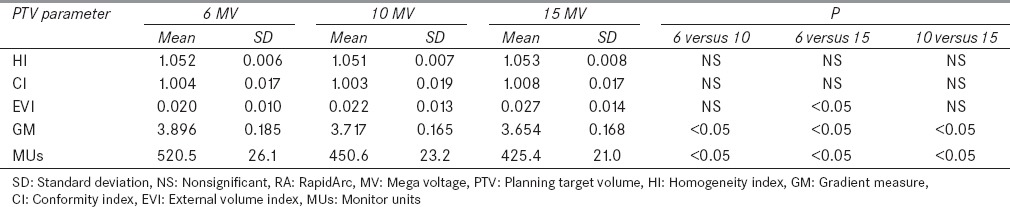
The P value for HI and CI of all the plans with all the three different energies viz., 6, 10, and 15 MV was found to be >0.05, thus there is no statistical significant difference with respect to change in energy. However, Table 2 shows that 10 MV plans have slightly better HI and CI as compared to 6 and 15 MV plans. The P value for GM was found to be <0.05 with respect to change in energy. There was a decrease in GM value with increase in photon energy. The 15 MV plans have 6.6% and 1.7% improved GM as compared to that of 6 and 10 MV plans respectively. EVI value in 6 MV plans was smaller in comparison to that in 10 and 15 MV. The P value for the EVI of 6 MV versus 15 MV plans was found to be <0.05 which shows the significant difference.
Table 2.
Plan comparison parameters for RA plans using 6, 10, and 15 MV energies
Dose to bladder
Table 3 shows that 15 MV offers statistically significant (P< 0.05) improvement in mean dose and V30Gy of the bladder in comparison to 6 MV. There were gradual improvements in mean dose and V30Gy of bladder with increase in energy. There was no statistically significant (P > 0.05) difference found in V40Gy and V50Gy of the bladder, but there was improvement in mean bladder dose for 15 MV in comparison to 6 and 10 MV.
Table 3.
Dose-volume parameter for different OARs for RA plans using 6, 10, and 15 MV energies
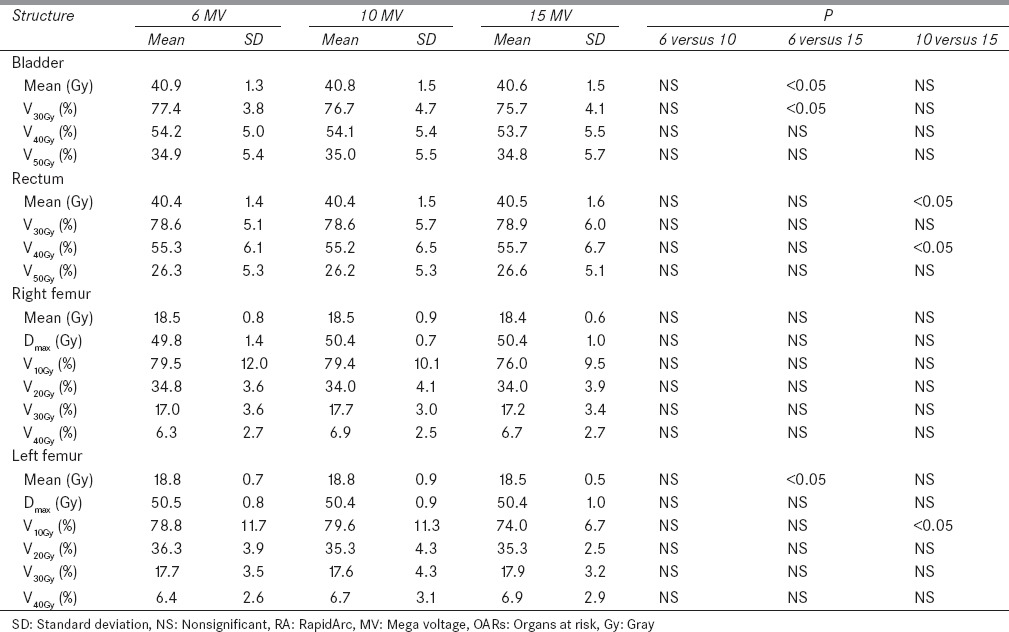
Dose to rectum
Table 3 represents that 10 MV offers statistically significant improvement (P < 0.05) in mean dose and V40Gy of rectum in comparison to 15 MV. There was a slight improvement in rectum dose for 10 MV in comparison to 6 and 15 MV. Results show that 15 MV delivers higher mean dose to rectum. V30Gy and V50Gy of rectum were also evaluated, but results were not statistically significant (P > 0.05).
Dose to femoral heads
Femoral heads were evaluated for mean dose, Dmax V10Gy, V20Gy, V30Gy, and V40Gy, but results were not found statistically significant (P > 0.05) as shown in Table 3.
Integral dose to normal tissues (nontumor integral dose)
There was a statistically significant (P < 0.05) improvement in NTID with increase in photon energy. There was a reduction of 2.7 ± 0.8% and 3.7 ± 0.9% in NTID in 10 and 15 MV plans respectively in comparison to that in 6 MV plans. NTID in 15 MV plans was 1.0 ± 0.5% less in comparison to that in 10 MV plans.
Evaluation for low dose volumes of normal tissue
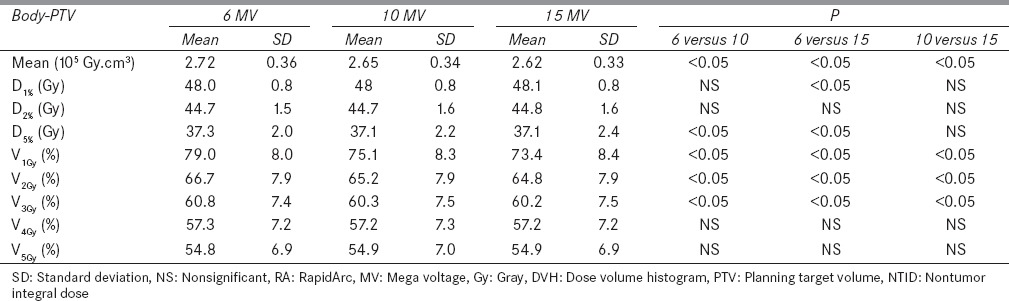
Dose to the volumes of 1%, 2%, and 5% and volumes of 1, 2, 3, 4, and 5 Gy of normal tissues were also calculated as shown in Table 4 and compared for analysis. For the 6, 10 and 15 MV plans, there were no significant (P > 0.05) differences in doses to 1%, 2% and 5% of normal tissues whereas the differences were significant in the cases of percentage volumes receiving 1, 2 and 3 Gy doses with 6, 10 and 15 MV beams. There was a gradual decrease found in 1, 2, and 3 Gy volumes of normal tissues with increase in photon beam energies. V4Gy and V5Gy were not found significantly (P > 0.05) different for 6, 10, and 15 MV plans.
Table 4.
DVH parameters of normal tissues (body-PTV) for NTID and low doses from RA plans using 6, 10, and 15 MV
Monitor units
There was a statistically significant (P < 0.05) reduction in number of MUs with increase in energy. Table 2 shows that number of MUs in 15 MV plans were 18.3 ± 1.6% and 5.6 ± 1.9% less in comparison to that in 6 and 10 MV plans. Also, the number of MUs in 10 MV plans is 13.4 ± 1.4% less in comparison to that in 6 MV plans.
Discussion
This study represents a thorough investigation of dose distribution in RA plans for cervix cancer using 6, 10, and 15 MV photon beam energies. The analysis is to evaluate the dosimetric impact of 6, 10, and 15 MV photon energies on RA plans for cervix cancer. The calculated P value for the PTV coverage was >0.05 in 6, 10, and 15 MV plans, which does not show statistically significant (P > 0.05) difference. The results of this study concurred with the results presented already by few authors. Sternick et al.[21] reported in their study that there was no significant difference in the dose distribution in rotational IMRT plans using energies ranging from 4 to 18 MV in the case of prostate cancer.
Ost et al.[22] also reported no advantage of high energy over low energy for IMRT and VMAT plans for primary prostate radiotherapy with simultaneous integrated boost.
Plans with 15 MV photon beam offer statistically significant difference only for mean bladder dose, V30Gy of the bladder, GM, NTID, and number of MUs in comparison to 6 MV. But there will be neutron production in case of 15 MV, and inclusion of neutron will eventually increase the risk of secondary malignancies.[23]
Thangavelu et al.[24] reported that 15 MV provides slightly better target coverage and better OARs sparing, but it cannot be considered as better choice as there is risk of secondary malignancies due to neutron production.
The 10 MV beam offers statistically significant sparing of rectum mean dose and V40Gy in comparison to 15 MV, and slightly better sparing of bladder and rectum in comparison to 6 MV. It also offers better results for GM (4.8%), MUs (13.4%), lesser NTID (2.7%), less V1Gy, V2Gy, V3Gy, and D5% of normal tissue volume in comparison to 6 MV plans. The results of this study are inconsistent with the results recently reported by Mattes et al.[25] Their study evaluated the dosimetric effect of photon energy on quality of VMAT for large number of prostate cancer patients and found that the 10 MV plan delivered lower NTID (4.1%), GM (4.1%), and 13% lesser number of MUs than the 6 MV plans, although in their study they did not evaluate low dose volume of normal tissue and EVI.
Pasler et al.[26] also assessed treatment plan quality and dosimetric accuracy of VMAT and IMRT plans using 6, 10, and 15 MV photon energies for prostate and found only statistically significant difference in NTID for 10 MV in comparison to 6 MV, they did not evaluate the difference in MUs, GM, EVI, and low dose volume of normal tissues. Onal et al.[27] also compared IMRT and VMAT plans with different energy levels 6, 10, and 15 MV using Monte-Carlo algorithm for prostate cancer. They found the significant difference only in number of MUs for 10 MV and 6 MV plans, as they did not calculate GM, EVI, and low dose volume of normal tissue.
This study revealed that variation in NTID was <5% for RA plans using 6, 10, and 15 MV energies. Pirzkall et al.[28] also reported a variation of 5% in NTID among prostate IMRT plans using 6, 10, and 18 MV energies. D’Souza and Rosen[20] reported that higher energy beams reduced the NTID and this effect is approximately independent of the numbers of beams, their beam orientation, and relative weights. Table 4 presents that 6 MV delivers 2.7 ± 0.8% and 3.7 ± 0.9% more NTID in comparison to 10 and 15 MV, respectively, and this is consistent with the results of the studies already published.[20,28,29] Our study also evaluated the D1%, D2%, and D5% and found D5% to be significantly high in 6 MV plans as compared to that in 10 and 15 MV plans. The normal tissue volumes receiving 1, 2, and 3 Gy in 6 MV plans were significantly highest.
Hall et al.[30,31] illustrated in their study that this low dose volume may not cause acute or subacute clinical morbidity but could potentially be carcinogenic. They reported that IMRT is likely to have 1–1.75% higher incidence of secondary malignancies compared to conventional radiotherapy in the patients surviving for 10 years. Followill et al.[32] estimated whole-body dose equivalent resulting from IMRT, they concluded that IMRT may increase the risk of secondary cancers by 0.4–1% as compared to conventional radiotherapy.
Kry et al.[33,34] also calculated the risk of second fatal malignancies. They reported that risk of second fatal malignancies in patients treated with 6 MV can be 38 times higher than that in patients treated with 10 MV. They reported the conservative maximum risk of fatal second malignancy was 2.1% for IMRT using 10 MV and 5.1% for IMRT using 18 MV. Intermediate risk associated with IMRT using 6 MV beam were 2.9% for treatment with Varian linear accelerator and 3.7% for treatment with Siemens linear accelerator, as well as using 15 MV X-rays 3.4% for Varian and 4.0% for Siemens linear accelerators respectively.
Major limitation of this study is that it does not consider the contribution of dose deposited by photoneutrons produced in high energy beam of 10 and 15 MV.[35] Dose from neutrons is more important because of their high relative biological effectiveness (RBE) and also radiation weighting factor of 20, hence higher biological damage compared to photons.[36]
In case of 6 MV RA plans, there is no photoneutron production, thereby reducing the biological damage. Also, there is no statistical significant difference between 6, 10, and 15 MV plans in terms of target coverage, OARs sparing, HI, and CI. Six mega voltage plans delivers significantly higher number of MUs, NTID and expose more normal tissues to low doses. However, this can be accepted against the higher risk of secondary cancer associated with photoneutrons in high energy beam. Many authors have also reported 6 MV as a good choice for treating deep-seated tumors like cervix and prostate.[16,28,37]
Conclusion
This study has been done to compare the dosimetric impact of different photon energy on carcinomas of cervix RA radiotherapy planning. There were no statistical significant differences in the 6, 10, and 15 MV plans in terms of PTV coverage, OARs sparing, HI, and CI. Although the number of MUs exposure of normal tissues to low doses was significantly higher in 6 MV plans compared to that in 10 and 15 MV plans, these drawbacks can be neglected as the probability of risk of secondary malignancies due to photoneutron production in 10 and 15 MV plans is higher. Hence, it can be concluded that RA technique using 6 MV beam is dosimetrically better in comparison to 10 and 15 MV.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, Heintz AP, et al. Carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2003;83(Suppl 1):41–78. doi: 10.1016/s0020-7292(03)90115-9. [DOI] [PubMed] [Google Scholar]
- 2.Ohno T, Kato S, Sato S, Fukuhisa K, Nakano T, Tsujii H, et al. Long-term survival and risk of second cancers after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2007;69:740–5. doi: 10.1016/j.ijrobp.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Yu CX. Intensity-modulated arc therapy with dynamic multileaf collimation: An alternative to tomotherapy. Phys Med Biol. 1995;40:1435–49. doi: 10.1088/0031-9155/40/9/004. [DOI] [PubMed] [Google Scholar]
- 4.Auj-E-Taqassas . USA: Author House™; 2011. Investigation of VMAT Algorithms and Dosimetry. ISBN: 978-1-4567-7418-9(e) [Google Scholar]
- 5.Cozzi L, Dinshaw KA, Shrivastava SK, Mahantshetty U, Engineer R, Deshpande DD, et al. A treatment planning study comparing volumetric arc modulation with RapidArc and fixed field IMRT for cervix uteri radiotherapy. Radiother Oncol. 2008;89:180–91. doi: 10.1016/j.radonc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Otto K, Milette M, Wu J. Temporal delivery efficiency of a novel single gantry arc optimization technique for treatment of recurrent nasopharynx cancer. Int J Radiat Oncol Biol Phys. 2007;69:S703. [Google Scholar]
- 7.Palma D, Vollans E, James K, Nakano S, Moiseenko V, Shaffer R, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: Comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 8.Rao M, Yang W, Chen F, Sheng K, Ye J, Mehta V, et al. Comparison of Elekta VMAT with helical tomotherapy and fixed field IMRT: Plan quality, delivery efficiency and accuracy. Med Phys. 2010;37:1350–9. doi: 10.1118/1.3326965. [DOI] [PubMed] [Google Scholar]
- 9.Verbakel WF, Cuijpers JP, Hoffmans D, Bieker M, Slotman BJ, Senan S. Volumetric intensity-modulated arc therapy vs. conventional IMRT in head-and-neck cancer: A comparative planning and dosimetric study. Int J Radiat Oncol Biol Phys. 2009;74:252–9. doi: 10.1016/j.ijrobp.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 10.Radiation Therapy Oncology Group (RTOG-0418) Protocol: A Phase II Study of Intensity Modulated Radiation Therapy (IMRT) to the Pelvis +/. Chemotherapy for Post. operative Patients with either Endometrial or Cervical Carcinoma. Available from: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0418 .
- 11.Bethesda: International Commission on Radiation Units and Measurements; 1993. ICRU. ICRU Report 50. Prescribing, Recording, and Reporting Photon Beam Therapy. [Google Scholar]
- 12.Bethesda: International Commission on Radiation Units and Measurements; 1999. ICRU. ICRU Report 62. Prescribing, Recording, and Reporting Photon Beam Therapy. Supplement to ICRU Report 50. [Google Scholar]
- 13.Vienna: IAEA; 2000. IAEA. An International Code of Practice for Dosimetry based on absorbed dose to Water, IAEA Technical Series No.398, absorbed Dose Determination in External Beam Radiotherapy. [Google Scholar]
- 14.Otto K, Clark BG. Enhancement of IMRT delivery through MLC rotation. Phys Med Biol. 2002;47:3997–4017. doi: 10.1088/0031-9155/47/22/307. [DOI] [PubMed] [Google Scholar]
- 15.Deng J, Pawlicki T, Chen Y, Li J, Jiang SB, Ma CM. The MLC tongue-and-groove effect on IMRT dose distributions. Phys Med Biol. 2001;46:1039–60. doi: 10.1088/0031-9155/46/4/310. [DOI] [PubMed] [Google Scholar]
- 16.Zhai DY, Yin Y, Gong GZ, Liu TH, Chen JH, Ma CS, et al. RapidArc radiotherapy for whole pelvic lymph node in cervical cancer with 6 and 15 MV: A treatment planning comparison with fixed field IMRT. J Radiat Res. 2013;54:166–73. doi: 10.1093/jrr/rrs066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bethesda: International Commission on Radiation Units and Measurements; 2010. ICRU. ICRU Report 83. Prescribing, Recording, and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT) [Google Scholar]
- 18.Sung W, Park JM, Choi CH, Ha SW, Ye SJ. The effect of photon energy on intensity-modulated radiation therapy (IMRT) plans for prostate cancer. Radiat Oncol J. 2012;30:27–35. doi: 10.3857/roj.2012.30.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meertens H, Borger J, Steggerda M, Blom A. Evaluation and optimization of interstitial brachytherapy dose distribution. In: Mould RF, Battermann JJ, Martinez AA, Speiser BL, editors. Brachytherapy from Radium to Optimization. Veenendaal, the Netherlands: Nucletron International; 1994. pp. 300–6. [Google Scholar]
- 20.D’Souza WD, Rosen II. Nontumor integral dose variation in conventional radiotherapy treatment planning. Med Phys. 2003;30:2065–71. doi: 10.1118/1.1591991. [DOI] [PubMed] [Google Scholar]
- 21.Sternick ES, Bleier AR, Carol MP, Curran BH, Holmes TW, Kania AA, et al. Intensity modulated radiation therapy: What photon energy is best? In: Leavitt DD, editor. Proc. of 12th International Conference on the use of Computers in Radiation Therapy Salt Lake City, Utah. Madison, WI: Medical Physics Publishing; 1997. pp. 418–9. [Google Scholar]
- 22.Ost P, Speleers B, De Meerleer G, De Neve W, Fonteyne V, Villeirs G, et al. Volumetric arc therapy and intensity-modulated radiotherapy for primary prostate radiotherapy with simultaneous integrated boost to intraprostatic lesion with 6 and 18 MV: A planning comparison study. Int J Radiat Oncol Biol Phys. 2011;79:920–6. doi: 10.1016/j.ijrobp.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 23.Kry SF, Salehpour M, Followill DS, Stovall M, Kuban DA, White RA, et al. Out-of-field photon and neutron dose equivalents from step-and-shoot intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62:1204–16. doi: 10.1016/j.ijrobp.2004.12.091. [DOI] [PubMed] [Google Scholar]
- 24.Thangavelu S, Jayakumar S, Govindarajan KN, Supe SS, Nagarajan V, Nagarajan M. Influence of photon energy on the quality of prostate intensity modulated radiation therapy plans based on analysis of physical indices. J Med Phys. 2011;36:29–34. doi: 10.4103/0971-6203.75469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattes MD, Tai C, Lee A, Ashamalla H, Ikoro NC. The dosimetric effects of photon energy on the quality of prostate volumetric modulated arc therapy. Pract Radiat Oncol. 2014;4:e39–44. doi: 10.1016/j.prro.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Pasler M, Georg D, Wirtz H, Lutterbach J. Effect of photon-beam energy on VMAT and IMRT treatment plan quality and dosimetric accuracy for advanced prostate cancer. Strahlenther Onkol. 2011;187:792–8. doi: 10.1007/s00066-011-1150-0. [DOI] [PubMed] [Google Scholar]
- 27.Onal C, Arslan G, Parlak C, Sonmez S. Comparison of IMRT and VMAT plans with different energy levels using Monte-Carlo algorithm for prostate cancer. Jpn J Radiol. 2014;32:224–32. doi: 10.1007/s11604-014-0291-3. [DOI] [PubMed] [Google Scholar]
- 28.Pirzkall A, Carol MP, Pickett B, Xia P, Roach M, 3rd, Verhey LJ. The effect of beam energy and number of fields on photon-based IMRT for deep-seated targets. Int J Radiat Oncol Biol Phys. 2002;53:434–42. doi: 10.1016/s0360-3016(02)02750-5. [DOI] [PubMed] [Google Scholar]
- 29.Aoyama H, Westerly DC, Mackie TR, Olivera GH, Bentzen SM, Patel RR, et al. Integral radiation dose to normal structures with conformal external beam radiation. Int J Radiat Oncol Biol Phys. 2006;64:962–7. doi: 10.1016/j.ijrobp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Hall EJ, Wuu CS. Radiation-induced second cancers: The impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83–8. doi: 10.1016/s0360-3016(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 32.Followill D, Geis P, Boyer A. Estimates of whole-body dose equivalent produced by beam intensity modulated conformal therapy. Int J Radiat Oncol Biol Phys. 1997;38:667–72. doi: 10.1016/s0360-3016(97)00012-6. [DOI] [PubMed] [Google Scholar]
- 33.Kry SF, Salehpour M, Followill DS, Stovall M, Kuban DA, White RA, et al. The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62:1195–203. doi: 10.1016/j.ijrobp.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 34.Kry SF, Followill D, White RA, Stovall M, Kuban DA, Salehpour M. Uncertainty of calculated risk estimates for secondary malignancies after radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1265–71. doi: 10.1016/j.ijrobp.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Gurjar OP, Jha VK, Sharma SD. Radiation dose to radiotherapy technologists due to induced activity in high energy medical electron linear accelerators. Radiat Prot Environ. 2014;37:25–9. [Google Scholar]
- 36.Bethesda, Maryland: NCRP; 1987. NCRP. NCRP Report No. 79: Neutron Contamination for Medical Electron Accelerators. [Google Scholar]
- 37.Tyagi A, Supe SS, Sandeep, Singh MP. A dosimetric analysis of 6 MV versus 15 MV photon energy plans for intensity modulated radiation therapy (IMRT) of carcinoma of cervix. Rep Pract Oncol Radiother. 2010;15:125–31. doi: 10.1016/j.rpor.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


