Abstract
The adequacy of setup margins for various sites in patients treated with helical tomotherapy was investigated. A total of 102 patients were investigated. The breakdown of the patients were as follows: Twenty-five patients each in brain, head and neck (H and N), and pelvis, while 12 patients in lung and 15 in craniospinal irradiation (CSI). Patients were immobilized on the institutional protocol. Altogether 2686 megavoltage computed tomography images were analyzed with 672, 747, 622, 333, and 312 fractions, respectively, from brain, H and N, pelvis, lung, and CSI. Overall systematic and random errors were calculated in three translational and three rotational directions. Setup margins were evaluated using van Herk formula. The calculated margins were compared with the margins in the clinical use for various directions and sites. We found that the clinical isotropic margin of 3 mm was adequate for brain patients. However, in the longitudinal direction it was found to be out of margin by 0.7 mm. In H and N, the calculated margins were well within the isotropic margin of 5 mm which is in clinical use. In pelvis, the calculated margin was within the limits, 8.3 mm versus 10 mm only in longitudinal direction, however, in vertical and lateral directions the calculated margins were out of clinical margins 11 mm versus 10 mm, and 8.7 mm versus 7.0, mm respectively. In lung, all the calculated margins were well within the margins used clinically. In CSI, the variation was found in the middle spine in the longitudinal direction. The clinical margins used in our hospital are adequate enough for sites H and N, lung, and brain, however, for CSI and pelvis the margins were found to be out of clinical margins.
Keywords: Image guidance, margins, setup error
Introduction
The widespread use of intensity-modulated radiation therapy (IMRT) has demonstrated the importance of image guidance. While delivering IMRT plans, often characterized by steep dose gradients, the accuracy of treatment delivery is essential.[1] The sharp dose fall-off near the planning target volume (PTV) and critical structures represents a dosimetric risk of under-dosing the target. Because IMRT plans are more sensitive to uncertainties due to positioning, immobilization is mandated to maintain patient's position during treatments.[2] Immobilization devices should take into consideration various factors such as patient acceptability, radiotherapist's convenience, field placement accuracy, and simulation and treatment delivery times. It should ensure accurate patient positioning with reproducibility throughout the entire course of radiotherapy.[3] Improper immobilization can also be a significant indirect cause of tumor recurrence. IMRT of brain, head and neck (H and N), pelvis, lung, and the special technique of craniospinal irradiation (CSI) requires a good immobilization and stringent image guidance.
Helical tomotherapy (HT) was designed to provide both IMRT and volumetric image-guided radiation therapy (IGRT). Volumetric image guidance was a revolution in radiotherapy. Earlier, at the time of portal imaging, where the matching was done using bony anatomy,[4,5] information regarding the position of the PTV with respect to the bones were unavailable. Volumetric imaging-based guidance has resolved this issue, by acquiring volumetric images for both target and organs-at risks (OARs). By far the most important advantage of volumetric IGRT solutions is the ability to visualize soft tissue prior to treatment and defining the spatial relationship between target and OARs.[6,7,8]
Image matching using computed tomography (CT) data can be done offline or online, but the greatest gain will probably follow from online IGRT. The tomotherapy Hi-Art system incorporates a rapid auto-matching system, so that daily positional correction before treatment delivery is possible. This allows the correction of both random and systematic components of setup errors, thus increasing the accuracy of treatment delivery. The systematic error which is specific for a single patient throughout the course of radiotherapy causes a shift in the entire planned dose distribution relative to the error. These usually arise due to the sudden reduction of postoperative edema, tumor regression, or weight-loss. Random errors cause a blurring of dose distribution over the entire fraction of treatment. It arises due to day-to-day variation in organ motions.[9] In practice, the full characterization of all geometrical uncertainties should lead to objective choice for treatment margins. van Herk et al. statistically analyzed and calculated the systematic and random errors separately and derived appropriate treatment margins.[10]
The primary objective of this study was to evaluate the adequacy of setup margins for various sites including the brain, H and N, lung, pelvis, and CSI for patients treated with HT. The calculated margins were compared with the margins that were in clinical use.
Materials and Methods
A total of 102 patients were investigated. The breakdown of the patients were as follows: Twenty-five patients each in brain, H and N, and pelvis, while 12 patients in lung and 15 in CSI. All the patients were treated with HT with IGRT on a daily basis. Setup correction data for individual patients were acquired. These included the translational displacements, that is, lateral, longitudinal, and vertical directions; and rotational displacements such as pitch, roll, and yaw. Out of which only translational and roll displacements were applied, however, pitch and yaw could not be corrected. Patients were grouped site-wise for the analysis of systematic and random errors. The margins were calculated and compared with treatment margins in clinical use.
Immobilization devices
Patients with brain tumors were immobilized with thermoplastic masks (Orfit) clamped on a base plate by L-shaped rigid polymer that fitted into the cut-out on base plates and locked using three fixation blocks, and head rest on wedges. Masks held the patients tightly preventing major motions and the use of wedges for brain patients helps in flexing the chin, thus reducing the dose to the eyes.
For H and N patients, a similar four clamps thermoplastic mask, head rest, and traction (made of rubber), was used. Rubber traction pulled the shoulder down to avoid the shoulder in the treatment beam.
Patients with lung cancer were immobilized using four clamp thermoplastic body mask with head rest and hands were positioned overhead.
Patients undergoing radiotherapy to pelvis were positioned on a custom-made knee rest made of thermocol and plaster of paris, pillow, and abdominal masks. Abdominal masks were only used in clinically significant cases as rigid immobilization for pelvis can cause greater displacement along lateral directions, especially for obese patients.[11,12]
For CSI, patients were immobilized in the supine position with the same four clamp thermoplastic mask as those used for H and N patients, a head and knee rest, with tattoos matching with the room lasers. Setup was done based on the cranial fiducial markers and midline body tattoos aligned with a room laser system. The alignment of the patient was done rectilinearly on the couch.[13] Figure 1 shows the immobilization devices used at our institution.
Figure 1.
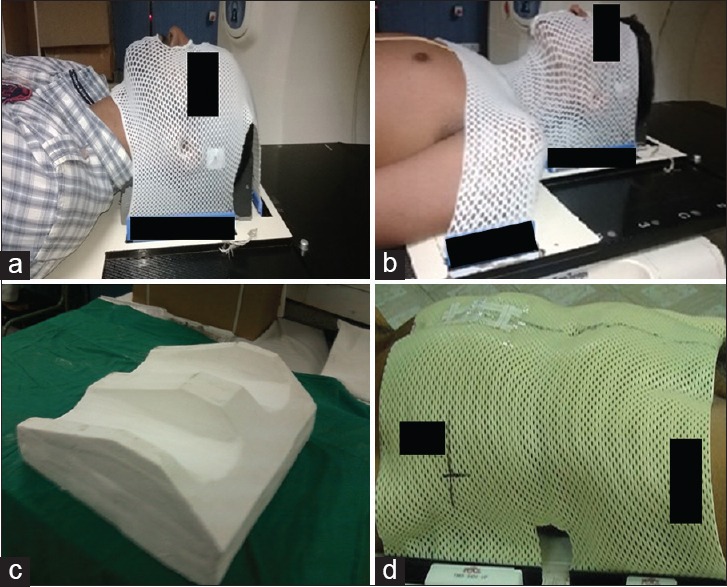
Immobilization devices used in our hospital. (a) Three clamp thermoplastic mask. (b) Four clamp thermoplastic mask. (c) Knee rest. (d) Abdominal thermoplastic mask
Helical tomotherapy based image-guided radiation therapy
It is equipped with a 6 MV linear accelerator capable of delivering helical IMRT by the translation of couch with its 32 binary MLC pairs. It has a degraded energy of 3.17 MV (maximum energy) for CT for IGRT purposes. The transmitted photons are detected by an array of Xenon detectors. More details about the megavoltage CT (MVCT) imaging technique and mechanisms can be found elsewhere.[14] Available options for MVCT image slice width are coarse (6 mm), normal (4 mm), and fine (2 mm) mode. In our institution, the images were acquired in normal mode. The imaging time can depend on the slice thickness and the length of the area to be scanned.
Institutional image-guided radiation therapy protocol
The patients were positioned on Hi-Art tomotherapy couch with appropriate immobilization devices. The tattoos marked on the patient body were then aligned with respect to red lasers and were allowed for automatic positioning in the scan plane. Entire PTV was selected as the region of interest.
For brain patients with small tumors, scanning was done in a fine mode with a slice thickness of 2 mm. The scans acquired were registered to the treatment planning CT by using rigid registration algorithm. The registration was carried out by the treating physician on the day-1, followed by the radiation therapy technologists for subsequent fractions. Matching was carried out over appropriate landmarks for each disease site and positions of PTV.
The registration protocol of brain patients was carried out with respect to the supra-orbital ridge which remains intact within the immobilization mask, in addition to the chin and other stable anatomical structures. For, H and N and pelvis patients, bony structures surrounding the PTV were considered as the landmarks. The shifts observed along lateral, longitudinal, and vertical directions; and the rotational shifts such as pitch, roll, and yaw were noted. The translational shifts, in lateral, longitudinal, and vertical directions and rotational errors in roll directions were applied before treatment delivery, however, rotational errors in pitch and yaw directions were not applied for treatment delivery. Since, the treatment length of CSI is generally longer, the scan was carried out in three parts of the region of interest, viz upper spine - at the junction of brain and spine, middle spine, and lower spine. The details of the technique, setup errors were described in our previous publications.[13]
For pelvis patients, PTV plus bladder was selected as the region to be scanned. For a reproducible bladder filling, patients with ca cervix was under a bladder protocol, where the patient was asked to drink 500 ml of water and wait for 30 min, followed by treatment. For H and N, pelvis, and lung with large tumors the scan was performed in a coarse mode where the slice thickness was 6 mm.
The imaging of patients analyzed was carried out daily and no corrections were applied if the displacements were of the order of 1 mm and 3 mm for brain and H and N, and for lung and pelvis, respectively. For CSI, the imaging was carried out daily, and the correction was applied, based on the hospital protocol.[13]
Data collection and analysis
Setup correction data for individual patients for each MVCT session were acquired. These included the translational displacements that is, lateral, longitudinal, and vertical directions; and rotational displacements like pitch, roll, and yaw. Patients were grouped site-wise for the analysis of systematic and random errors. Systematic errors were computed by taking the standard deviation of the mean of displacements of the individual patients over a population in each disease site. The random error was defined as the root mean square of the random error distribution, which was computed from the standard deviations of individual patients over a population in each site. These were calculated for all directions and sites and compared with treatment margins in clinical use. van Herk's recipe was used to generate the margins. van Herk et al. had analytically devised a margin recipe between clinical target volume (CTV) and PTV, which ensures a minimum dose to CTV of 95% for 90% of patients. The recipe in millimeters is simplified as:
2.5Σ+0.7∂
Σ- Systematic error.
∂ - Random error.
Systematic errors are stochastic in nature for a group of patients. It is specific for each patient, for one patient it may be due to postoperative edema and for another it may be due to the wrong positioning of the patient at the time of simulation. Hence, these errors are to be analyzed statistically, with site specification. The errors observed can be more easily correlated with sites. The average of standard deviations of the mean of shifts per patient along particular directions can easily quantify systematic errors along that direction. Random deviation occurs due to day-to-day variations in patient setup. These include internal organ motion and can be quantified as the root mean square of all patients’ random deviations. The systematic error requires margins 3–4 times as large as comparable to random errors.[15,16,17]
Results
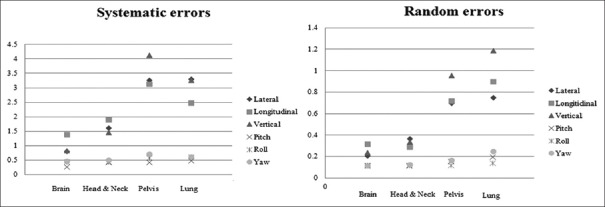
The systematic error along the lateral, longitudinal, and vertical directions for all sites ranges from 0.8 mm to 1.4 mm, 0.8 to mm 3.3 mm, and 3.1 mm to 4.1 mm, respectively and the results obtained are plotted in Figure 2. All the random errors were within 1 mm except for lung with an error of 1.5 mm along the vertical direction. The systematic error was found to be 4.1 mm and 3.6 mm along the vertical direction for pelvis and lung patients respectively, which was the highest. Similarly, among the random error, it was observed that the highest error of 1.0 mm and 1.5 mm was found for lung and pelvis in the vertical direction. The systematic rotational errors ranged from 0.3° to 0.5° along vertical (pitch) axis, while it was 0.5° to 0.6° along lateral (roll) and 0.5° to 0.7° along vertical (yaw) axes for all disease sites.
Figure 2.
The distribution of systematic and random errors along translational and rotational directions for brain, H and N, pelvis and lung patients. Errors are in millimeters along translational direction and in degrees along rotational directions
In CSI, the highest systematic errors were observed along the longitudinal direction, 3.9 mm, 4.6 mm, and 4.2 mm for upper, middle, and lower spine, respectively. Table 1 gives the systematic and random errors obtained for patients undergoing CSI. The largest random error of 0.9 mm was observed along the lateral direction for lower spine. In comparison with the spine, the systematic and random errors along all the three directions were lesser for MVCT scans obtained at the junction of brain and spine.
Table 1.
Systematic and random errors for CSI patients along lateral, longitudinal, and vertical directions
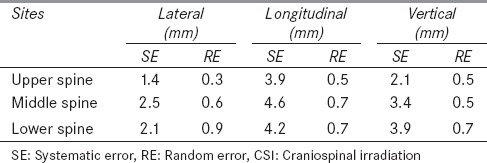
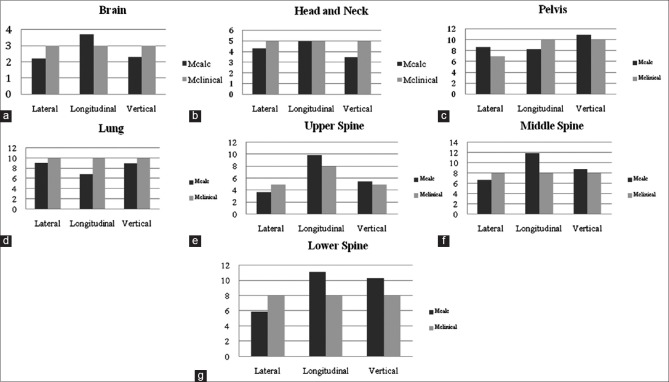
Figure 3 gives the derived CTV to PTV margins along lateral, longitudinal, and vertical directions and its comparisons with the margins used clinically. It was found that the clinical isotropic margin of 3 mm is adequate for brain patients. However, in the longitudinal direction it was found to be out of margin by 0.7 mm. In H and N, the calculated margins were well within the isotropic margin of 5 mm which is in clinical use. In pelvis, only in longitudinal direction, the calculated margin was within the limits, 8.3 mm versus 10 mm, however, in vertical and in lateral direction the calculated margins were out of clinical margins 11 mm versus 10 mm, and 8.7 mm versus 7.0 mm, respectively. In the lung, all the calculated margins were well within the margins used clinically. In CSI, the variation was found in the middle spine in the longitudinal direction.
Figure 3.
Comparison of calculated clinical target volume to planning target volume margins to the margins (in millimeters) in clinical use for sites (a) brain, (b) head and neck, (c) pelvis, (d) lung, (e) upper spine, (f) middle spine, and (g) lower spine. Mcalc: Calculated margins, Mclinical: Margins in clinical use
Discussion
IMRT provides highly conformal dose distributions providing a steep dose fall-off to the normal tissues, thus the accuracy of daily setup of patients is essential. Treatment margins have to be appropriate to prevent the geometric miss of PTV and irradiation of OARs. In our institution, the margins for brain tumors is 3 mm, H and N 5 mm, and lung 7-10 mm. Pelvis patients, largely comprises ca cervix, with a margin of 10 mm applied in both vertical and longitudinal direction, where as a margin of 7 mm along the lateral direction. The results of the present study, in general, indicate that the margins used clinically for the sites investigated were adequate for sites brain, H and N, and lung and thus, improves the confidence. However, for CSI and pelvis the margins were the out of clinical margins. Further, it acts as guidance when MVCT imaging is not possible during the treatment for various reasons. It also opens up the possibility of reducing the frequency of MVCT imaging to improve the throughput of the machine in a busy center such as ours, or to increase the frequency of imaging whenever appropriate. In addition, this study also may throw some insight in margin reduction in some critical patients such as re-irradiation and dose escalation for appropriate sites.
The results of the present study agree with Schubert et al., which resulted in a smaller frequency of three-dimensional vector displacements for brain and H and N cases in comparison to pelvis and lung. However, as compared to Schubert et al., our rotational displacements for brain and H and N had a smaller frequency than those of pelvis and lung. It was observed that the displacement along the longitudinal direction for brain and H and N cases was more than the displacements in lateral and vertical directions. The systematic errors were 0.8 mm along lateral and vertical directions while it was 1.4 mm along longitudinal directions for brain. Four out of 25 brain patients had an average displacement >3 mm longitudinally in 113 fractions which led to a margin above 3 mm along the longitudinal direction. For H and N, it was 1.6 mm along lateral and 1.5 mm along vertical directions, while 2.0 mm along the longitudinal direction. It is to be noted that for HT treatments the patients are positioned outside the bore, which does not take into account the absolute tomotherapy couch sag. This systematic error due to the sag in the couch was nullified by acquiring the corrected vertical values of the couch on the 1st day of treatment. A large systematic error would otherwise been observed along vertical direction because of the increased effects of couch sag which is inherent to HT.[18] A minor shift existing along the superior-inferior direction due to the same sag could not be corrected, which resulted in systematic displacements for all brain and H and N patients longitudinally. This error in the entire patient population resulted in an increased systematic error along longitudinal directions.
Moreover, deviation from the IGRT protocol due to individual patient variations, poor image quality, may cause larger displacements from the population mean. For example, in brain patients, instead of matching the supra-orbital ridge sometimes, it is preferred by the radiotherapists to match the rigid structures near the chin in MVCT scans, which can cause displacements in both systematic and random errors. This may happen, when the image quality is poor and the structures cannot be visualized in individual patients. For pelvis patients, the highest systematic error of 0.7° in rotational displacements was observed along yaw, which may be attributed to the poor immobilization, bladder filling, and abdominal fat due to obesity among patients. This also resulted in an increase of random errors which was 1.0 mm along vertical direction. We agree with the findings of Nutting et al. that the use of immobilization devices helped in reducing the random errors in comparison with no immobilization.[19] It is also to be noted that large systematic error at the time of CT simulation leads to its nonreproducibility at the time of daily setup. Hence, it is very important that during CT simulation the patient need to be simulated in a treatment position which can be reproduced in all subsequent fractions.
For lung, greater displacements are expected along inferior–superior and anterio–posterior direction[20,21,22] due to breathing motion. No significant correlations were observed in the expected and observed displacements over the entire population of patients. This analysis lacked the grouping of tumors with respect to various quarters within the lung. Tumors attached to the ribs behave differently from those near the heart, hence to define margins for tumors within lung with proper classification is necessary and the analysis is beyond the scope of this study.
The margins used for the brain was found to be adequate along a lateral and vertical direction, however, along the longitudinal direction, the margins calculated were found to be out of clinically practiced margins. The results of the present study may also indicate the need for larger margins for upper and lower spine along longitudinal and vertical directions in CSI patients. This is in agreement with the findings of Gupta et al. of greater isotropic margins of 8.5 mm for upper spine and 11.5 mm for lower spine.
It is being recognized that the modern radiation therapy delivery devices, such as HT with IGRT facility, has improved the therapeutic ratio, by minimizing the toxicity, and maximizing the tumor dose.[23] However, we should recognize that these clinical gains with new technologies may be limited by uncertainties in the various stages of the treatment process. Every step in the process of radiotherapy has an uncertainty component, which needs to be identified so that, appropriate steps can be taken to keep them under control. In the present study, only interfraction setup errors that may have certain impact on PTV margins have been investigated, however, it should be recognized that in the end-to-end process, the uncertainties may arise from various sub-processes such as immobilization, imaging, definition of target volume/OARs, dosimetry, plan optimization, dose calculation, delivery, and verification.[24] The uncertainty arises from each of the above steps has to be evaluated for complete uncertainty analysis, which is beyond the scope of the study.
Conclusion
The PTV margins were analyzed for various disease sites for patients treated with HT based IGRT technique. The present study gives us the confidence on the margins that were being used clinically in our institution. The results of the present study indicate that the clinical margins used in our hospital are adequate enough for sites, brain (other than longitudinally by 0.7 mm), H and N, and lung, however, for CSI and pelvis, the margins calculated were found to be out of clinical margins. Since daily image guidance is used, there could be a potential to reduce the margin if a situation arises for appropriate sites. On the other hand, if daily image guidance is not possible, the clinical margins used in this protocol along with the immobilization devices will keep the setup errors within the acceptable tolerance. The calculated margins obtained in the present study may serve as a reference for margins in non IGRT setup; however, the conditions such as immobilization and contouring protocols need to be taken into account.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Duma MN, Kampfer S, Wilkens JJ, Schuster T, Molls M, Geinitz H. Comparative analysis of an image-guided versus a non-image-guided setup approach in terms of delivered dose to the parotid glands in head-and-neck cancer IMRT. Int J Radiat Oncol Biol Phys. 2010;77:1266–73. doi: 10.1016/j.ijrobp.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 2.Velec M, Waldron JN, O’Sullivan B, Bayley A, Cummings B, Kim JJ, et al. Cone-beam CT assessment of interfraction and intrafraction setup error of two head-and-neck cancer thermoplastic masks. Int J Radiat Oncol Biol Phys. 2010;76:949–55. doi: 10.1016/j.ijrobp.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Sharp L, Lewin F, Johansson H, Payne D, Gerhardsson A, Rutqvist LE. Randomized trial on two types of thermoplastic masks for patient immobilization during radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61:250–6. doi: 10.1016/j.ijrobp.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 4.Persoon L, Murrer L, Postma E. 286 registration of megavoltage and kilovoltage images for automated setup verification on electronic portal imaging device (EPID) images. Radiother Oncol. 2005;76(Supplement 2):S132–3. [Google Scholar]
- 5.Van de Steene J, Van den Heuvel F, Bel A, Verellen D, De Mey J, Noppen M, et al. Electronic portal imaging with on-line correction of setup error in thoracic irradiation: Clinical evaluation. Int J Radiat Oncol Biol Phys. 1998;40:967–76. doi: 10.1016/s0360-3016(97)00925-5. [DOI] [PubMed] [Google Scholar]
- 6.Verellen D, De Ridder M, Tournel K, Duchateau M, Reynders T, Gevaert T, et al. An overview of volumetric imaging technologies and their quality assurance for IGRT. Acta Oncol. 2008;47:1271–8. doi: 10.1080/02841860802244182. [DOI] [PubMed] [Google Scholar]
- 7.McBain CA, Henry AM, Sykes J, Amer A, Marchant T, Moore CM, et al. X-ray volumetric imaging in image-guided radiotherapy: The new standard in on-treatment imaging. Int J Radiat Oncol Biol Phys. 2006;64:625–34. doi: 10.1016/j.ijrobp.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Santanam L, Esthappan J, Mutic S, Klein EE, Goddu SM, Chaudhari S, et al. Estimation of setup uncertainty using planar and MVCT imaging for gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2008;71:1511–7. doi: 10.1016/j.ijrobp.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 9.Vaandering A, Lee JA, Renard L, Grégoire V. Evaluation of MVCT protocols for brain and head and neck tumor patients treated with helical tomotherapy. Radiother Oncol. 2009;93:50–6. doi: 10.1016/j.radonc.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 10.van Herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: Dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–35. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 11.Song PY, Washington M, Vaida F, Hamilton R, Spelbring D, Wyman B, et al. A comparison of four patient immobilization devices in the treatment of prostate cancer patients with three dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34:213–9. doi: 10.1016/0360-3016(95)02094-2. [DOI] [PubMed] [Google Scholar]
- 12.Bieri S, Miralbell R, Nouet P, Delorme H, Rouzaud M. Reproducibility of conformal radiation therapy in localized carcinoma of the prostate without rigid immobilization. Radiother Oncol. 1996;38:223–30. doi: 10.1016/0167-8140(95)01699-6. [DOI] [PubMed] [Google Scholar]
- 13.Gupta T, Upasani M, Master Z, Patil A, Phurailatpam R, Nojin S, et al. Assessment of three-dimensional set-up errors using megavoltage computed tomography (MVCT) during image-guided intensity-modulated radiation therapy (IMRT) for craniospinal irradiation (CSI) on helical tomotherapy (HT) Technol Cancer Res Treat. 2015;14:29–36. doi: 10.7785/tcrt.2012.500391. [DOI] [PubMed] [Google Scholar]
- 14.Burnet NG, Adams EJ, Fairfoul J, Tudor GS, Hoole AC, Routsis DS, et al. Practical aspects of implementation of helical tomotherapy for intensity-modulated and image-guided radiotherapy. Clin Oncol (R Coll Radiol) 2010;22:294–312. doi: 10.1016/j.clon.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Broggi S, Cozzarini C, Fiorino C, Maggiulli E, Alongi F, Cattaneo GM, et al. Modeling set-up error by daily MVCT for prostate adjuvant treatment delivered in 20 fractions: Implications for the assessment of the optimal correction strategies. Radiother Oncol. 2009;93:246–52. doi: 10.1016/j.radonc.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Stroom JC, de Boer HC, Huizenga H, Visser AG. Inclusion of geometrical uncertainties in radiotherapy treatment planning by means of coverage probability. Int J Radiat Oncol Biol Phys. 1999;43:905–19. doi: 10.1016/s0360-3016(98)00468-4. [DOI] [PubMed] [Google Scholar]
- 17.Stroom JC, Heijmen BJ. Geometrical uncertainties, radiotherapy planning margins, and the ICRU-62 report. Radiother Oncol. 2002;64:75–83. doi: 10.1016/s0167-8140(02)00140-8. [DOI] [PubMed] [Google Scholar]
- 18.Schubert LK, Westerly DC, Tomé WA, Mehta MP, Soisson ET, Mackie TR, et al. A comprehensive assessment by tumor site of patient setup using daily MVCT imaging from more than 3,800 helical tomotherapy treatments. Int J Radiat Oncol Biol Phys. 2009;73:1260–9. doi: 10.1016/j.ijrobp.2008.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nutting CM, Khoo VS, Walker V, McNair H, Beardmore C, Norman A, et al. A randomized study of the use of a customized immobilization system in the treatment of prostate cancer with conformal radiotherapy. Radiother Oncol. 2000;54:1–9. doi: 10.1016/s0167-8140(99)00181-4. [DOI] [PubMed] [Google Scholar]
- 20.Seppenwoolde Y, Shirato H, Kitamura K, Shimizu S, van Herk M, Lebesque JV, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53:822–34. doi: 10.1016/s0360-3016(02)02803-1. [DOI] [PubMed] [Google Scholar]
- 21.Mechalakos J, Yorke E, Mageras GS, Hertanto A, Jackson A, Obcemea C, et al. Dosimetric effect of respiratory motion in external beam radiotherapy of the lung. Radiother Oncol. 2004;71:191–200. doi: 10.1016/j.radonc.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Panakis N, McNair HA, Christian JA, Mendes R, Symonds-Tayler JR, Knowles C, et al. Defining the margins in the radical radiotherapy of non-small cell lung cancer (NSCLC) with active breathing control (ABC) and the effect on physical lung parameters. Radiother Oncol. 2008;87:65–73. doi: 10.1016/j.radonc.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Gupta T, Wadasadawala T, Master Z, Phurailatpam R, Pai-Shetty R, Jalali R. Encouraging early clinical outcomes with helical tomotherapy-based image-guided intensity-modulated radiation therapy for residual, recurrent, and/or progressive benign/low-grade intracranial tumors: A comprehensive evaluation. Int J Radiat Oncol Biol Phys. 2012;82:756–64. doi: 10.1016/j.ijrobp.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 24.Vienna: International Atomic Energy Agency; 2004. International Atomic Energy Agency. Technical Report Series 430. Commissioning and Quality Assurance of Computerized Planning Systems for Radiation Treatment of Cancer. [Google Scholar]