Abstract
Objectives:
To measure the effect of the right and left nostril yoga breathing on frontal hemodynamic responses in 32 right handed healthy male subjects within the age range of 18–35 years (23.75 ± 4.14 years).
Materials and Methods:
Each subject practiced right nostril yoga breathing (RNYB), left nostril yoga breathing (LNYB) or breath awareness (BA) (as control) for 10 min at the same time of the day for three consecutive days, respectively. The sequence of intervention was assigned randomly. The frontal hemodynamic response in terms of changes in the oxygenated hemoglobin (oxyHb), deoxygenated hemoglobin (deoxyHb), and total hemoglobin (totalHb or blood volume) concentration was tapped for 5 min before (pre) and 10 min during the breathing practices using a 16 channel functional near-infrared system (FNIR100-ACK-W, BIOPAC Systems, Inc., U.S.A.). Average of the eight channels on each side (right and left frontals) was obtained for the two sessions (pre and during). Data was analyzed using SPSS version 10.0 through paired and independent samples t-test.
Results:
Within group comparison showed that during RNYB, oxyHb levels increased significantly in the left prefrontal cortex (PFC) as compared to the baseline (P = 0.026). LNYB showed a trend towards significance for reduction in oxyHb in the right hemisphere (P = 0.057). Whereas BA caused significant reduction in deoxyHb (P = 0.023) in the left hemisphere. Between groups comparison revealed that oxyHb and blood volume in the left PFC increased significantly during RNYB as compared to BA (oxyHb: P =0.012; TotalHb: P =0.017) and LNYB (oxyHb: P =0.024; totalHb: P =0.034).
Conclusion:
RNYB increased oxygenation and blood volume in the left PFC as compared to BA and LNYB. This supports the relationship between nasal cycle and ultradian rhythm of cerebral dominance and suggests a possible application of uninostril yoga breathing in the management of psychopathological states which show lateralized cerebral dysfunctions.
Keywords: Blood flow, functional near-infrared spectroscopy, nasal cycle, unilateral nostril breathing, yoga breathing
INTRODUCTION
The alternate congestion-decongestion response of the erectile tissue of nasal turbinate and septum of two nostrils leads to altered unilateral nasal resistance. Thus, the air flow through one nostril is greater than next at any given point of time which later switches to another. This is called nasal cycle.[1] The nasal cycle which lasts for 25 min to 2–3 h is closely related to the ultradian rhythm of cerebral dominance that lasts for 1.5–3 h.[1,2] The dominance of nasal cycle is inversely correlated to the alternating dominance in the cerebral hemispheres; this is regulated by a common mechanism mediated through the autonomic nervous system.[3] Electroencephalogram (EEG) studies have shown that integrated EEG amplitudes are greater over the cerebral hemisphere contralateral to the dominant nostril.[4,5] This rhythm of cerebral dominance plays an important role in cognitive performance, memory processes, mood, and behavior.[2,6]
Pranayama refers to voluntarily regulated rhythmic yoga breathing technique. Slow yogic breathings through a particular nostril have been shown to be effective in improving cardio-respiratory functions, autonomic imbalances, and psychological stress.[7,8,9,10] Studies have observed that left nostril yoga breathing (LNYB) enhanced performance in the right hemisphere dominant tasks such as spatial memory scores[7,8] and right nostril yoga breathing (RNYB) improved left hemisphere dominant tasks such as letter-cancellation and verbal memory scores.[9] An ancient yogic treatise in Samskrit called Shivaswarodaya describes that breathing through a particular nostril affects cognitive activities and emotions of an individual.[11] This has recently been verified by scientific research as well.[12]
Study by Telles et al.[13] showed that right nostril breathing facilitates the left hemisphere activity through a significant reduction in P300 evoked potential latency. The neuroelectric events that underlie P300 generation arise from the interaction between frontal lobe, hippocampal, and temporoparietal function.[13] Thus, researches on uninostril yoga breathing increasingly point towards its activating effect on contralateral brain hemispheres (especially the frontals) in terms of: (a) enhancement of cognitive task performances and (b) greater integrated EEG amplitudes.
Functional near-infrared spectroscopy (fNIRS) is a noninvasive optical method that can measure the real time change in oxygenated hemoglobin (oxyHb), deoxygenated hemoglobin (deoxyHb), and their sum that is, total hemoglobin (totalHb) or blood volume in different brain regions including bilateral prefrontal cortices (PFCs). Basics of the NIRS device are described elsewhere.[14] Though the spatial resolution of the fNIRS device is coarse, its temporal resolution is excellent and fNIRS results are physiologically comparable to fMRI results.[15] A recent study used fNIRS to study the effect of yoga breathing technique called Kapalabhati (KB) on blood flow changes in PFCs of 18 healthy individuals and 18 schizophrenia patients. There was a significant increase in bilateral prefrontal oxyHb (in µMol/L) in healthy subjects during the practice of KB.[16] This suggests probable effect of yoga breathing on brain hemodynamics and necessitates deeper exploration.
Thus, in order to understand the mechanism through which the nasal cycle relates to ultradian rhythm of cerebral dominance, present study was planned to understand the effect of yoga breathing through a particular nostril on oxygenation and blood flow changes in bilateral prefrontal cortices in healthy individuals using fNIRS.
MATERIALS AND METHODS
Subjects
Thirty-two male subjects with ages ranging between 18 and 35 years (group mean ± standard deviation [SD], 23.75 ± 4.14 years) and average education of 15.78 ± 2.88 (mean ± SD) years were included in the study. Demographic details of the subjects are provided in Table 1. Sex differences have been documented in structure, function, and chemistry of the brain, and different phases of menstrual cycle have been shown to influence the cerebral blood flow,[17,18] hence the study included only male participants. The subjects were students of graduation and postgraduation studies from a Yoga University. They had an experience of practicing the three yoga breathing techniques that are, RNYB, LNYB and breathe awareness (BA) ranging between 3 and 36 months (group mean ± SD, 12.5 ± 8.6 months). All of them had completed a residential training course in yoga which was for 1-month. In addition to this, all the subjects included in the study were given week long training in the breathing practices assessed in the present study for 30 min each day for a week before starting the study. This 1-week of supervised practice was to ensure the uniformity among all the subjects. All subjects were checked for their health using general health questionnaire (GHQ) and those with GHQ score ≥7 were excluded. None of them had a history of smoking or respiratory ailments including nasopharyngeal abnormalities. They were all right handed dominant based on their response to the Edinburgh handedness inventory.[19] Also, none of them was taking medication and they did not use any other wellness strategy. The variables to be recorded and the study design were described to the subjects after which their signed consent to participate in the study was obtained. None of them was aware of the hypothesis of the study. The project had the approval of the Institutional Review Board.
Table 1.
Demographic data of the subjects

Assessment
Each subject performed single intervention on each day at the same time, empty stomach, but the intervention was randomized using slips numbered from 1 to 3.
Recorded audio-tape of instructions was played during the time of the experiment for RNYB, LNYB, and BA, respectively. Assessment schedule is provided in Table 2. The inhalation and exhalation ratio was 2:3, the duration of inhalation was 6 s and exhalation was 9 s. Therefore, the breathing time was 15 s for a single breath, approximately 4 breaths in a minute and 80 breaths in the total duration of 10 min. Each session lasted for 15 min where the first 5 min was for baseline recording (pre) than any one of yogic breathing was performed for 10 min. The intervention of RNYB, LNYB, and BA was practiced for 10 min continuously without any interval. The oxy-hemoglobin (oxyHb) and deoxy-hemoglobin (deoxyHb) concentration were assessed over the left and right hemisphere. Recordings were taken only when the subject performed the practice correctly and comfortably. A chest pressure transducer was used to monitor the technique of breathing objectively.
Table 2.
Assessment schedule of the study

General health questionnaire
GHQ was used to establish healthy status. It has 28 questions with four different sub scales to assess the physical fitness, anxiety and insomnia, social dysfunction, and depression. It gives the information about the recent mental status and general health. The questioner has acceptable psychometrics and has intimae consistency and reliability with Cronbach's alpha of 0.85 and validity of 0.76.[20]
Functional near-infrared spectroscopy device
The system (FNIR100-ACK-W, BIOPAC Systems, Inc., U.S.A.) is a continuous wave device which measures changes in attenuation at 2 wavelengths (730 and 850 nm, ±15 nm), sampling at 25 kHz and allows for the differentiation of two dynamic absorbers (oxyHb and deoxyHb). It has 4 light emitting and 10 detector probes with 16 channels that can be measured quasi simultaneously. Concentration changes in oxyHb and deoxyHb were calculated based on a modified Beer–Lambert approach.[14] The optodes were affixed to a probe set with an inter-optode distance of 2.5 cm covering an area of ∼6 cm × 18 cm. The probe set was fastened to the participant's head by elastic straps. The head band was placed on the forehead and covered with a black cloth. The recording was made in a dark sound attenuated cabin. For horizontal fixation, the lower edge of the probe set was fixed 1 cm above the nasion.
Data analysis
Sample size was calculated using two-tailed G power (calculated sample size = 32; effect size 1.05, alpha = 0.05, power = 0.80). The calculated sample size came out to be 32. The waveforms of oxyHb and deoxyHb changes in bilateral PFC were acquired from all the subjects in all 16 channels, and the data was averaged according to the task condition (pre, during and post). The average of the oxyHb, deoxyHb, and totalHb levels on both right (channels 1–8) and left side (channels 9–16) of the brain[16] was taken. Thereby, one mean value of each condition (pre and during) for each side of the brain (right and left) was obtained for each participant. The data were analyzed by the statistician using Statistical Package for Social Sciences version 10.0 (IBM India Private Limited). Shapiro–Wilk's test was used to check the normality of the data. As the data was found to be normally distributed, paired samples t-test was used to measure the changes in oxyHb, deoxyHb, and totalHb levels, respectively, during RNYB, LNYB, and BA practices from the baseline (pre) levels in all the subjects and independent samples t-test was used for between group comparisons. Alpha (P value) <0.05 was considered to be statistically significant.
RESULTS
Oxygenated hemoglobin changes
Within group comparisons [Table 3 and Figure 1] revealed that during RNYB oxyHb levels increased significantly in the left PFC as compared to the baseline (P = 0.026), whereas no significant change was observed in the right hemisphere (P = 0.654). During LNYB, we observed a trend towards significance for a reduction in oxyHb in the right hemisphere (P = 0.057). In the left PFC, there was no significant change in oxyHb levels as compared to the baseline (P = 0.854). The control intervention BA did not show any significant changes in the levels of oxyHb in bilateral PFC (left PFC: [P = 0.145]; right PFC [P = 0.061]).
Table 3.
Comparison within the group before and during RNYB, LNYB and BA groups for changes in oxy-Hb, deoxy-Hb, total-Hb
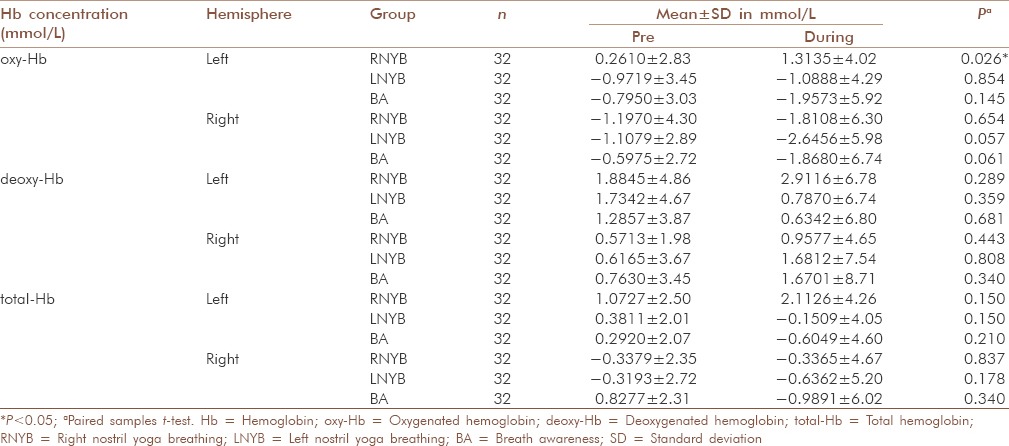
Figure 1.
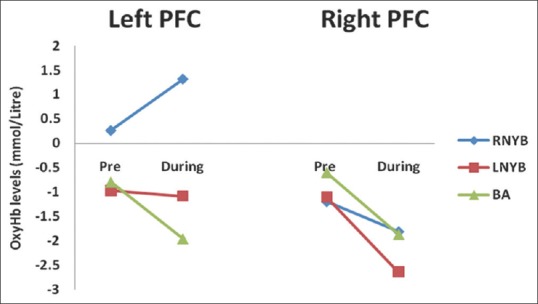
Changes in oxygenated hemoglobin levels before and during right nostril yoga breathing, left nostril yoga breathing and breath awareness in left and right hemispheres. OxyHb = Oxygenated hemoglobin; RNYB = Right nostril yoga breathing; LNYB = Left nostril yoga breathing; BA = Breath awareness; PFC = Prefrontal cortex
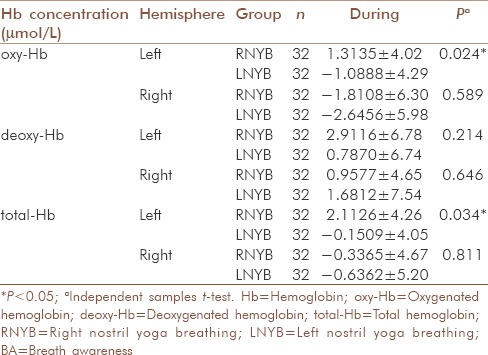
For oxyHb levels, comparison between RNYB and BA showed that oxyHb increased significantly in the left PFC during RNYB as compared to BA [P = 0.012, Table 4]; with no significant difference between them in right PFC [P = 0.972, Table 4]. Similarly, the comparison between the oxyHb levels during RNYB and LNYB revealed a significant increase in oxyHb in left PFC during RNYB [P = 0.024, Table 5] as compared to LNYB, with no such difference in right PFC [P = 0.589, Table 5]. No significant differences were observed between oxyHb levels during LNYB and BA in both the PFC [Table 5].
Table 4.
Comparison between RNYB and BA groups for changes in oxy-Hb, deoxy-Hb, total-Hb during the intervention
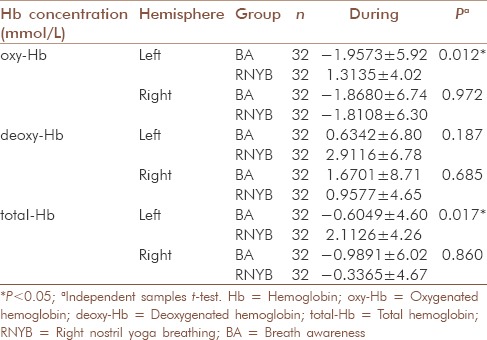
Table 5.
Comparison between RNYB and LNYB groups for changes in oxy-Hb, deoxy-Hb, total-Hb during the intervention
Deoxygenated hemoglobin changes
During RNYB, we did not observe any significant change in deoxyHb levels in the left as well as right PFC, respectively, as compared to the baseline [left PFC (P = 0.289); right PFC (P = 0.443); Table 3 and Figure 2]. Similar results were seen during LNYB as well (left PFC: [P = 0.359; Table 3]; right PFC: [P = 0.808; Table 3]). Practice of BA also revealed no significant changes during the practice in both the hemispheres but after the practice of BA, there was a significant reduction in deoxyHb in the left PFC [P = 0.023, Table 3].
Figure 2.
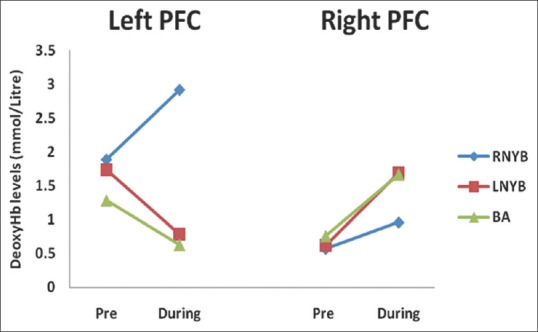
Changes in deoxygenated hemoglobin levels before and during right nostril yoga breathing, left nostril yoga breathing and breath awareness in left and right hemispheres. OxyHb = Oxygenated hemoglobin; RNYB = Right nostril yoga breathing; LNYB = Left nostril yoga breathing; BA = Breath awareness; PFC = Prefrontal cortex
Comparison between deoxyHb levels during RNYB and BA, RNYB and LNYB, or LNYB and BA did not show any significant differences in both the hemispheres, respectively [Table 4].
Total hemoglobin (blood volume) changes
Though within group comparison of totalHb levels did not show significant change from the baseline during all the three interventions in both the hemispheres, respectively [Table 3 and Figure 3], between group comparisons revealed a significant increase in blood volume in the left PFC during RNYB as compared to BA [P = 0.017, Table 4]. A significant increase in blood volume was seen during RNYB as compared to LNYB as well [P = 0.034, Table 5]. Other between the group comparisons for change in blood volume did not reveal any significant change on both the PFC [Table 5].
Figure 3.
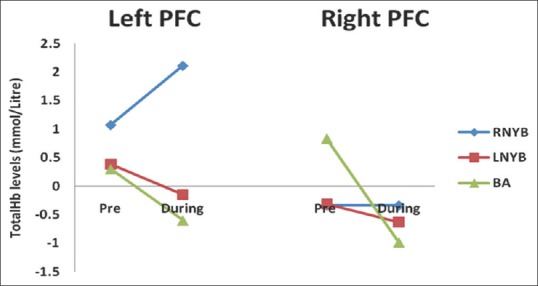
Changes in total hemoglobin levels before and during right nostril yoga breathing and left nostril yoga breathing and breath awareness in left and right hemispheres. OxyHb = Oxygenated hemoglobin; RNYB = Right nostril yoga breathing; LNYB = Left nostril yoga breathing; BA = Breath awareness; PFC = Prefrontal cortex
DISCUSSION
The present study was planned to see the effects of yoga breathing through a particular nostril on the hemodynamic changes in the bilateral PFC on 32 healthy male volunteers. We found that RNYB lead to a significant increase in the level of oxyHb and totalHb in the left PFC in comparison to BA or LNYB, also, there was a trend toward significant reduction in oxyHb in right PFC during LNYB and BA, respectively.
Increase in totalHb and oxyHb and a reciprocal decrease in deoxyHb are expected to be observed in activated areas of the brain in NIRS measurements which indicates an increase in the blood flow,[14] though researchers have found activation of a brain region when there is: (1) No change in totalHb alongside an increase in oxyHb and a reciprocal decrease in deoxyHb and (2) an increase or no change in deoxyHb accompanying increases in totalHb and oxyHb.[14,21] OxyHb is considered as the most sensitive indicator of changes in regional cerebral blood flow in NIRS measurements.[21] We found an increase in both oxyHb and totalHb with no change in deoxyHb in the left PFC during RNYB. Thus, the present study suggests activation or increase in the blood flow in the left PFC during RNYB and trend towards deactivation of right PFC during LNYB and BA, respectively.
These results support the relationship between nasal cycle and ultradian rhythm of cerebral dominance and point toward change in the blood flow and oxygenation in contralateral brain regions as one of the mechanisms underlying this relationship. Present study also explains: (1) Better performance in the left hemisphere dominant tasks such as verbal memory scores and letter-cancellation task and (2) increased integrated EEG amplitudes and reduced P300 latency in left hemisphere observed immediately after RNYB respectively.[6,13]
We also observed a trend toward the significant decrease in oxyHb [P = 0.053; Table 3 and Figure 1] along with no change in deoxyHb or totalHb in right PFC during LNYB. This suggests a probable deactivating or relaxing effect of LNYB on right PFC. But studies have observed the better performances in right hemisphere dominant tasks and higher integrated EEG amplitudes in the right hemisphere after LNYB. Thus, why we did not get activating effects on right hemisphere following LNYB or in contrast why our results show a deactivating or relaxing trend is difficult to explain and more detailed studies to understand the underlying mechanisms are needed in future. Here one important aspect is the traditional ancient yogic view on uninostril yogic breathing where LNYB also known as Chandra anulomaviloma is believed to produce the calming and relaxing effect on the body in contrast to RNYB or Surya anulomaviloma which is more activating and energizing.[11] Results in the present study support this view. Previously, similar diverging effects of RNYB and LNYB have been observed on autonomic nervous system, where RNYB lead to higher sympathetic activity but LNYB, on the other hand, brought parasympathetic dominance.[22]
The exact mechanism through which uninostril breathing influences cerebral blood flow is not known. PFC receives serotonergic input from dorsal raphe nucleus of the brainstem, as well as noradrenergic input from another brainstem nucleus, the locus coeruleus (LC). A large number of studies have shown that these two neurotransmitter systems (serotonin and nordrenaline) modulate the functional properties of the PFC in both humans and animal models. Studies have revealed that breathing through a particular nostril can alter metabolism and autonomic activites.[22] The mechanical receptors in the nasal mucosa are activated with airflow into the nostrils, and this signal is unilaterally transmitted to the hypothalamus thereby altering the autonomic functions mediated via hypthalamo-pituitary-adrenal (HPA) axis.[1] Telles et al. found that regular practice of RNYB for a month leads to a significant increase of 37% in baseline oxygen consumption. The authors attributed this increase in metabolism to increased sympathetic discharge mediated by increased output of adrenaline from the adrenal medulla.[22] Interestingly, LC has been identified as an upstream component of circuitry providing for dorsal medial PFC modulation of emotional stress-induced (HPA) activation.[23] Thus, uninostril yoga breathing may influence PFC through HPA-LC mediated noradrenaline release. Another neurotransmitter which may play an important role in mediating PFC activation is serotonin. In order to understand the neuro-physiological mechanisms involved in Zen meditation, another study used 24-channel near-infrared spectroscopy during a 20-min session of abdominal (Tanden) breathing in 15 healthy volunteers. They found a significant increase in the level of oxyHb in anterior PFC during Tanden breathing, accompanied by a reduction in feeling of negative mood compared to the baseline. They also observed changes in EEG such as increased alpha band activity and decreased theta band activity during Tanden breathing and EEG changes were correlated with a significant increase in whole blood serotonin (serotonin) levels. Thus, the author concluded that Tanden breathing lead to the activation of the anterior PFC and serotonin system. This may be responsible for the improvement of negative mood and EEG signal changes observed during Tanden breathing.[24] Another study assessed three primary lines of evidence that comprised of the effects of serotonin and noradrenaline on impulsivity, cognitive flexibility, and working memory and found supporting evidence toward the activating effect of serotonin and deactivating effect of noradrenaline on PFC.[25] Improvement in mood and EEG changes have been observed with RNYB,[13] as these changes are correlated with serotonin level as well and serotonin has an activating effect on PFC, it appears probable that RNYB may be mediating its PFC activating effect via increased serotonin release from the dorsal raphe nucleus in the brainstem. Now the question arises, what makes RNYB specifically engage the dorsal raphe nucleus pathway? It is well known that right and LNYB have diverging effects on the autonomic nervous system, where RNYB causes sympathetic activation and LNYB leads to parasympathetic dominance. These effects are exerted via autonomic neurons in the paraventricular nucleus of the hypothalamus.[22] RNYB stimulates the paraventricular nucleus and may selectively increase corticotrophin releasing hormone (CRH) and cortisol by modulation of HPA axis. CRH is the stress neurotransmitter which plays an important role in the activation of the central sympathetic and serotonergic systems and release of serotonin from dorsal raphe nucleus has been shown to be mediated by the release of CRH.[26] Thus, it is hypothesized that RNYB causes prefrontal activation through HPA-CRH-dorsal raphe nucleus mediated serotonin release. LNYB, on the other hand, leads to parasympathetic dominance by suppressing the activation of the paraventricular nucleus and thereby decreasing CRH and cortisol secretion. This may exert opposing effects. It is known that neural connections exist between the CRH neurons in the paraventricular nucleus of the hypothalamus and noradrenergic neurons in LC.[27] Also, increased parasympathetic response (as observed after LNYB) could result in a decrease in both heart rate and respiration that may lead to the stimulation of LC by the paragigantocellular nucleus.[28] Thus, two breathing techniques may follow different pathways to cause the activation or deactivation of contralateral hemispheres [Figure 4]. A hypothetical mechanism can be postulated to explain the activating and deactivating effects of RNYB and LNYB as observed in our study: This involves HPA-LC mediated noradrenaline release to cause PFC deactivation and HPA-CRH-dorsal raphe nucleus mediated serotonin release to cause PFC activation [Figure 4].
Figure 4.
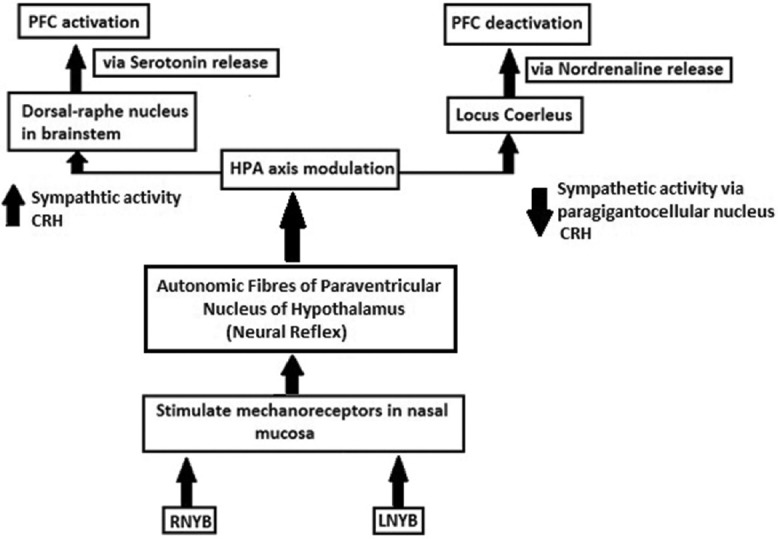
Pathway showing hypothetical mechanism of action of uninostril yoga breathing on brain hemodynamics. CRH = Corticotrophin releasing hormone; PFC = Prefrontal cortex; RNYB = Right nostril yoga breathing; LNYB = Left nostril yoga breathing
Present study has several limitations. A randomized controlled design would have been better, but this could not be achieved in the present study due to limited subject availability. Second, we did not assess the post-breathing baseline. The main objective of present study was to understand the mechanism through which uninostril yoga breathing affects cognition. Thus, this preliminary study was planned to see the effect during the breathing process on PFC activation. In future, we plan to assess the post-breathing effects as well to find out the duration for which the effect lasts after the breathing technique. Future studies should also use other comprehensive imaging techniques such as fMRI to confirm these findings and should observe the effect of uninostril breathings on other brain areas as well. Effect of uninostril yoga breathing on neuro-chemicals should also be assessed in future studies to understand the mechanism behind activation or deactivation of PFCs.[25] Future studies should also apply and observe the effects of uninostril yoga breathing on individuals with various psychopathologies where lateralized cerebral dysfunctions are prominent, viz., attention deficit hyperactivity disorder, alzheimer's disease, depression, obsessive compulsive disorder etc.[29]
CONCLUSION
Yoga breathing through a particular nostril was found to have an effect on contralateral frontal hemodynamics. This may be the probable mechanism behind the cognitive changes induced by uninostril yoga breathing. These findings support the relationship between nasal cycle and ultradian rhythm of cerebral dominance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Keuning J. On the nasal cycle. Int J Rhinol. 1968;6:99–136. [Google Scholar]
- 2.Shannahoff-Khalsa D. The ultradian rhythm of alternating cerebral hemispheric activity. Int J Neurosci. 1993;70:285–98. doi: 10.3109/00207459309000583. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy B, Ziegler MG, Shannahoff-Khalsa DS. Alternating lateralization of plasma catecholamines and nasal patency in humans. Life Sci. 1986;38:1203–14. doi: 10.1016/0024-3205(86)90175-x. [DOI] [PubMed] [Google Scholar]
- 4.Werntz DA, Bickford RG, Shannahoff-Khalsa D. Selective hemispheric stimulation by unilateral forced nostril breathing. Hum Neurobiol. 1987;6:165–71. [PubMed] [Google Scholar]
- 5.Werntz DA, Bickford RG, Bloom FE, Shannahoff-Khalsa DS. Alternating cerebral hemispheric activity and the lateralization of autonomic nervous function. Hum Neurobiol. 1983;2:39–43. [PubMed] [Google Scholar]
- 6.Shannahoff-Khalsa DS, Boyle MR, Buebel ME. The effects of unilateral forced nostril breathing on cognition. Int J Neurosci. 1991;57:239–49. doi: 10.3109/00207459109150697. [DOI] [PubMed] [Google Scholar]
- 7.Pal GK, Velkumary S, Madanmohan Effect of short-term practice of breathing exercises on autonomic functions in normal human volunteers. Indian J Med Res. 2004;120:115–21. [PubMed] [Google Scholar]
- 8.Brown RP, Gerbarg PL. Sudarshan Kriya yogic breathing in the treatment of stress, anxiety, and depression: Part I – Neurophysiologic model. J Altern Complement Med. 2005;11:189–201. doi: 10.1089/acm.2005.11.189. [DOI] [PubMed] [Google Scholar]
- 9.Ravindra PN, Madanmohan, Pavithran P. Effect of pranayam (yoga breathing) and shavasan (relaxation training) on the frequency of benign ventricular ectopics in two patients with palpitations. Int J Cardiol. 2006;108:124–5. doi: 10.1016/j.ijcard.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Jerath R, Edry JW, Barnes VA, Jerath V. Physiology of long pranayamic breathing: Neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med Hypotheses. 2006;67:566–71. doi: 10.1016/j.mehy.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 11.Muktibodhananda S. Munger, Bihar: Yoga Publications Trust; 2004. Swara Yoga: The Tantric Science of Brain Breathing Including the original Sanskrit text of the Shiva Swarodaya with English translation. [Google Scholar]
- 12.Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol. 1998;106:156–64. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- 13.Telles S, Joshi M, Somvanshi P. Yoga breathing through a particular nostril is associated with contralateral event-related potential changes. Int J Yoga. 2012;5:102–7. doi: 10.4103/0973-6131.98220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jöbsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–7. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 15.Yuan Z, Ye J. Fusion of fNIRS and fMRI data: Identifying when and where hemodynamic signals are changing in human brains. Front Hum Neurosci. 2013;7:676. doi: 10.3389/fnhum.2013.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhargav H, Nagendra HR, Gangadhar BN, Nagarathna R. Frontal hemodynamic responses to high frequency yoga breathing in schizophrenia: A functional near-infrared spectroscopy study. Front Psychiatry. 2014;5:29. doi: 10.3389/fpsyt.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krejza J, Siemkowicz J, Sawicka M, Szylak A, Kochanowicz J, Mariak Z, et al. Oscillations of cerebrovascular resistance throughout the menstrual cycle in healthy women. Ultrasound Obstet Gynecol. 2003;22:627–32. doi: 10.1002/uog.907. [DOI] [PubMed] [Google Scholar]
- 18.Kochanowicz J, Krejza J, Koroza O, Mariak Z. Influence of estrogens on the impedance of cerebral blood vessels. Neurol Neurochir Pol. 2005;39:175–80. [PubMed] [Google Scholar]
- 19.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg D, Williams P. Swindon, Wiltshire, UK: nferNelson; 2000. General Health Questionnaire (GHQ) [Google Scholar]
- 21.Obrig H, Villringer A. Beyond the visible – Imaging the human brain with light. J Cereb Blood Flow Metab. 2003;23:1–18. doi: 10.1097/01.WCB.0000043472.45775.29. [DOI] [PubMed] [Google Scholar]
- 22.Telles S, Nagarathna R, Nagendra HR. Breathing through a particular nostril can alter metabolism and autonomic activities. Indian J Physiol Pharmacol. 1994;38:133–7. [PubMed] [Google Scholar]
- 23.Radley JJ, Williams B, Sawchenko PE. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J Neurosci. 2008;28:5806–16. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arita H. Anterior prefrontal cortex and serotonergic system activation during Zen meditation practice induces negative mood improvement and increased alpha band in EEG. Rinsho Shinkeigaku. 2012;52:1279–80. doi: 10.5692/clinicalneurol.52.1279. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald PJ. A neurochemical yin and yang: Does serotonin activate and norepinephrine deactivate the prefrontal cortex? Psychopharmacology (Berl) 2011;213:171–82. doi: 10.1007/s00213-010-1856-1. [DOI] [PubMed] [Google Scholar]
- 26.Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002;22:1020–6. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chrousos GP. Organization and Integration of the Endocrine System. Sleep Med Clin. 2007;2:125–145. doi: 10.1016/j.jsmc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newberg AB, Iversen J. The neural basis of the complex mental task of meditation: Neurotransmitter and neurochemical considerations. Med Hypotheses. 2003;61:282–91. doi: 10.1016/s0306-9877(03)00175-0. [DOI] [PubMed] [Google Scholar]
- 29.Shannahoff-Khalsa DS. Selective unilateral autonomic activation: Implications for psychiatry. CNS Spectr. 2007;12:625–34. doi: 10.1017/s1092852900021428. [DOI] [PubMed] [Google Scholar]


