Abstract
West syndrome, or infantile spasms syndrome is a frequently catastrophic infantile epileptic encephalopathy with a variety of etiologies. Despite the heterogeneous nature of causes of infantile spasms, a careful diagnostic evaluation can lead to diagnosis in many patients and may guide treatment choices. Magnetic resonance imaging (MRI) brain remains the highest yield initial study in determining the etiology in infantile spasms. Treatment of infantile spasms has little class I data, but adrenocorticotropic hormone (ACTH), prednisolone and vigabatrin have the best evidence as first-line medications. Other therapies including the ketogenic diet and other anti-epileptics medications may also prove useful in the treatment of infantile spasms. In general, more studies are needed to determine the best treatment regimen for this condition. Prognosis is generally poor, with the majority of patients having some or profound neurocognitive delays. Patients without delays at diagnosis and without an identifiable etiology, if treated appropriately, have the greatest likelihood of a normal outcome.
Keywords: Infantile spasms, West syndrome, adrenocorticotropic hormone (ACTH), corticosteroids, hypsarrhythmia
Introduction
In 1841, Dr. William James West wrote a letter to Lancet describing new infantile convulsions in his 4-month-old son (1,2). His apt description of the events, starting with a subtle head drop and progressing to clusters of myoclonic spasms occurring many times daily, accompanied by the regression of development of his previously normal child, remains the hallmark of this condition today. His letter also embodies the distress of a father and medical provider caused by this rare, difficult-to-treat, and frequently, neurologically devastating condition. While infantile spasms were recognized in the medical community prior to this letter, it was a rare disorder with no clear treatment. Over 170 years later, this most common form of infantile epilepsy (3) has more treatment options and identified etiologies, but can still be just as distressing and difficult to treat as in the case of Dr. West’s son.
Infantile spasms syndrome, frequently referred to as West syndrome, is a triad of (I) clinical flexor or extensor spasms, often involving the extremities and head/neck; (II) hypsarrhythmia on electroencephalogram (EEG); and (III) subsequent or concurrent intellectual disability (3,4). In the Delphi consensus, delay is not required (5). It is important to note that although West syndrome and infantile spasms are frequently discussed interchangeably, infantile spasms exist without delay in development and sometimes even without hypsarrhythmia. Patients with infantile spasms are often categorized as symptomatic, cryptogenic or idiopathic (4). Symptomatic infantile spasms have a known structural, metabolic or genetic cause and occur in children with delays present prior to the onset of spasms. Cryptogenic infantile spasms lack a known etiology, but share the prior developmental delays. Finally, idiopathic infantile spasms occur in patients with normal development prior to the onset of spasms and without an identifiable cause. This group continues to decrease in size as more testing reveals previously unknown causes of infantile spasms. The US consensus report on infantile spasms (3) simplifies this categorization to a dichotomous one and defines symptomatic infantile spasms as those patients with either abnormal development and/or a clear etiology for the infantile spasms and cryptogenic spasms as those occurring in the context of normal development without a clear etiology. For simplicity, this review will use the dichotomous definition. Management of infantile spasms should include efforts to determine the etiology if possible, as this can help determine the likelihood of response to treatment, may guide therapeutic decisions, and can help to provide families with a more definitive prognosis for their child (4,6,7).
Infantile spasms occur in roughly 2-3 per 10,000 live births, with peak incidence at 6 months of age and less than 10% of cases presenting after 12 months of age (4,8). Unfortunately, due to the subtle and brief nature of spasms in some patients, there can be delays in diagnosis. There is evidence to suggest that earlier treatment (within one month of onset) is more effective in controlling spasms (9-12), and may improve outcomes. Despite insufficient data to determine if this is true for all etiologies of infantile spasms, treatment should be initiated as soon as possible and providers should be aware of the often subtle presentation to allow for more rapid diagnosis and treatment. The goals of therapy should include a complete cessation of the clinical events and resolution of hypsarrhythmia or modified hypsarrhythmia on video EEG (5). Neurological and/or developmental outcomes in patients with infantile spasms are usually poor (13). Children with symptomatic spasms more frequently exhibit neurological deficits and cognitive and developmental delays, while a higher percentage of patients with idiopathic/cryptogenic infantile spasms may have a normal or near-normal outcome if appropriate treatment is initiated in a timely fashion (9-13). This review will discuss the diagnostic evaluation and treatment options for children diagnosed with infantile spasms, followed by a brief discussion of prognosis. Despite multiple practice parameters and Cochrane reviews in the last 15 years, there remains considerable diversity among providers in their approach to the treatment of infantile spasms (14). This represents the paucity of data to clearly demonstrate a gold standard and limited studies comparing treatment modalities. This is a natural consequence of the varied etiologies and rare occurrence of this condition, and continued efforts are being made worldwide to provide better information to guide decision-making in this severe form of infantile epilepsy.
Diagnostic evaluation
An evaluation including a thorough history and physical examination and EEG, is important in the diagnosis of infantile spasms. Once the diagnosis is established, efforts should be made to establish the underlying etiology, as this significantly affects treatment decisions and prognosis (15). For example, patients with tuberous sclerosis are more likely to respond to treatment with vigabatrin, whereas patients without an identified etiology may respond better to hormonal therapy (16,17). The differential diagnosis in infantile spasms is broad, and it is therefore important that the evaluation follow a step-wise process to limit the number of low-yield or unnecessary tests that are performed.
History and physical examination
As with most conditions in neurology, the history and physical examination are the first and most important step in evaluating a patient with the concern of infantile spasms. Due to significant advances in personal technology devices, a growing number of parents present with video of the events and have access to internet video of other infants with infantile spasms (18). These clinical events may vary from subtle, single, spasms involving a flexion of the neck or bowing of the head, to full body flexor, extensor or mixed myoclonic spasms occurring in clusters many times throughout the day. Spasms tend to occur more often on awakening or when falling asleep (19). The brief nature of these events, as well as the similarity to other infantile movements (benign sleep myoclonus, Moro reflex, etc.), can lead to a delay in diagnosis (20). When possible, it is helpful for providers to witness the events or capture them with video EEG. If this is not possible, parental reports consistent with infantile spasms should prompt immediate investigation. History should focus on the semiology of the events, frequency, clusters versus single spasms, changes in development or delays prior to the onset of spasms, family history of similar events in infants, and a thorough birth and pregnancy history (3,15,19). Perinatal events are responsible for up to 30% of cases of infantile spasms with a known etiology, and include neonatal strokes, infections and hypoxic-ischemic encephalopathy. It is not clear why some patients with these conditions develop infantile spasms and others do not, but there may be predisposing genetic factors. Prenatal causes include cortical and structural malformations and other genetic causes and are responsible for up to half of symptomatic etiologies (21,22).
Physical examination should focus on neurocutaneous stigmata of disease, which can identify patients with tuberous sclerosis, neurofibromatosis or other phakomatoses. Wood’s lamp evaluation can help identify more subtle cases of tuberous sclerosis. While the majority of patients with infantile spasms do not have tuberous sclerosis, they may make up 25% of symptomatic infantile spasms cases and up to 50% of patients with tuberous sclerosis will develop infantile spasms (17,23). Parents of children with tuberous sclerosis should be counseled regarding infantile spasms early on. Other syndromic features noted on examination can also help to direct the evaluation toward genetic causes of infantile spasms. Trisomy 21 alone accounts for 3-6% of patients with infantile spasms and is nearly always diagnosed prior to the onset of infantile spasms (6,24). Familial infantile spasms are rare, but may have specific genetic mutations (4).
Electroencephalogram (EEG)
EEG should be performed as soon as possible on any infant with concerns for infantile spasms. When possible, 24-hour video EEG is preferred (5). However, a routine video EEG may be adequate when making the diagnosis, especially when the features of hypsarrhythmia (25) are present interictally. Since sleep is an important part of the EEG evaluation for infantile spasms, every effort should be made to include non-REM sleep as part of the EEG evaluation. This may be decreased in infants with infantile spasms, but hypsarrhythmia may be present in this stage of sleep even if absent while awake (26). Up to 1/3 of patients with infantile spasms will not have hypsarrhythmia or may have other EEG abnormalities (19,27). In these cases, if the clinical spasms are consistent with infantile spasms, evaluation and treatment should still proceed accordingly. EEG is used for diagnosis as well as response to treatment (5). There is likely little utility in repeat EEG in patients who are not responding to treatment unless the diagnosis is in question or if the EEG was normal previously.
Brain imaging
Imaging is not required to make the diagnosis of infantile spasms, but is the single-most important method to identify etiology and/or direct further testing in infantile spasms. Fifty to 73 percent of patients have an identifiable etiology on magnetic resonance imaging (MRI) of the brain (6,15,28-30). However, 5-20% of patients may have non-diagnostic abnormalities (3,6). Since tuberous sclerosis warrants a different treatment consideration (13,16), it is recommended that MRI brain be performed prior to treatment initiation when possible. Repeat imaging may be useful if the initial MRI is normal and other testing is not informative (3). Unless there are other factors that suggest that repeat imaging should be done more urgently, it is recommended that repeat MRI be deferred until after 24-36 months in order to increase yield. Focal EEG findings in a refractory patient may prompt repeat imaging sooner, as these patients may benefit from surgical resection.
Computed tomography (CT) is not considered adequate to evaluate for most causes of infantile spasms (4,31), but it is recognized that in some areas of the world, this is the only diagnostic imaging available. CT may help identify evidence of prior brain injury, infection or tumors that can be the cause of infantile spasms (6).
Further evaluation
After history, physical examination, EEG and MRI brain are performed, about 70% of patients have a diagnosis (3). There are limited studies on the recommended evaluation of infantile spasms beyond imaging and wide practice variation exists among pediatric neurology providers, at least in the USA (14). The highest yield studies are genetic studies (6), but there are a variety of available panels and candidate genes in infantile spasms. Single-nucleotide polymorphism (SNP) and comparative genomic hybridization (CGH) microarray are attractive options, but may miss some pathogenic mutations and may also identify variants of unknown significance (VUS). Pathogenic mutations may be identified in about 10-23.5% of patients without a known etiology, with VUS present in 14.8% (6,32). Delayed patients have an even higher yield. Copy number variants (CNV) have also been detected in >10% of patients with infantile spasms and normal imaging as part of the Epilepsy Phenome/Genome Project (EPGP) (32). Candidate and associated genes for infantile spasms include: SCN1A, SCN2A, MAGI2, YWHAD, HIP1, 9p deletion, 15q11 duplications, GABRB3, an unbalanced translocation t(15;16), ARX, CDKL5, TSC1 and 2, trisomy 21 (24), 1p36 deletion, and possible PNPO mutations in cases of pyridoxine-dependent epilepsies with infantile spasms, as well as others (4,21,23,32-35). Karyotype is often performed, and may detect translocations and other rearrangements missed on microarray, but reported yield has been low except in syndromes notable prior to onset of spasms such as trisomy 21 (6). Gene panels may identify genetic causes of IS not evident on other genetic testing and are typically higher yield and less expensive than step-wise individual gene testing. Based on the limited evidence, microarray ± karyotype and followed by an appropriate epilepsy gene panel are likely to have the highest yield (6).
Inborn errors of metabolism are detected in up to 5% of patients with infantile spasms (6). Imaging and, where available, newborn screening may suggest a possible inborn error of metabolism. Patients with early onset spasms and/or refractory to treatment warrant further evaluation with metabolic studies, recognizing the low-yield, but possible treatment changes to improve outcomes (6,15). Metabolic testing may include acylcarnitine profile, total and free carnitine, serum and CSF lactate and glucose, serum pyruvate, urine organic acids, serum and CSF amino acids, and CSF neurotransmitter metabolites, including pyridoxal-5’-phosphate.
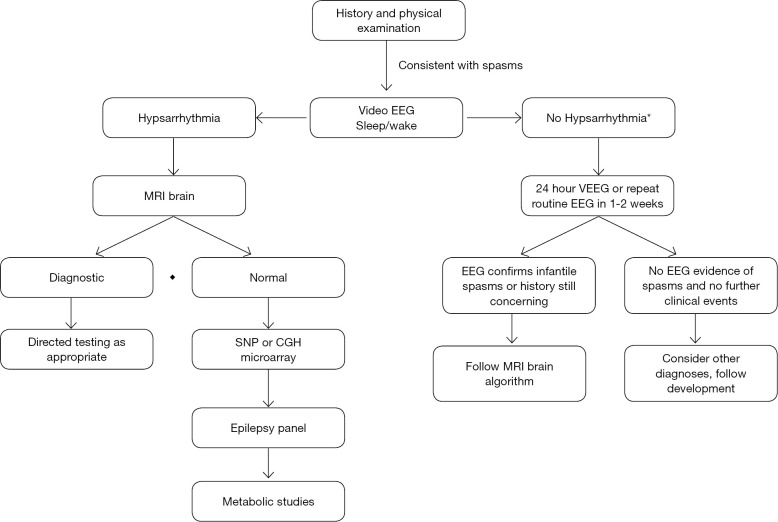
Based on probability of diagnostic yield, a sequential diagnostic algorithm is recommended (Figure 1). These diagnostic methods yield an etiology in 70-85% of patients (4,6,15).
Figure 1.
Diagnostic algorithm for infantile spasms. As noted, imaging has the highest yield, followed by genetic studies. *, Patients with a convincing history of infantile spasms and abnormalities other than hypsarrhythmia on EEG, may proceed to imaging without repeat EEG. If the concern for infantile spasms is significant and development is affected, it is not recommended to wait for EEG abnormalities, as up to 1/3 of patients may not have hypsarrhythmia. Treatment should begin once imaging is complete unless there is concern for infection and other work-up is needed. EEG, electroencephalogram; MRI, magnetic resonance imaging; ACTH, adrenocorticotrophic hormone; SNP, single nucleotide polymorphism; CGH, comparative genomic hybridization.
Treatment
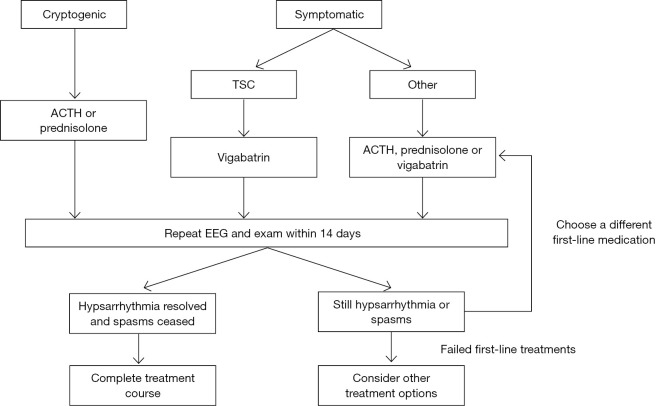
There are limited data to guide the treatment of infantile spasms (13,16,26). Most studies are retrospective or small prospective studies. EEG data is not available in all studies as some rely on parental report. Some studies use improvement in spasm frequency while others use complete resolution of spasms. We will only discuss results in terms of complete resolutions of infantile spasms as this is the typical goal of treatment and partial resolution is more subjective (5,13,16,26). However, even when complete cessation of spasms is required, there are some variations in the timeframe/method that this is reported. In addition, many studies report a rate of relapse as high as nearly 50% after initial response (13,36-38), further complicating interpretation of the results. Cost and availability of medications vary substantially throughout the world and may limit choices in some regions and patient populations. Hormonal therapies, including adrenocorticotropic hormone (ACTH) and corticosteroids, and vigabatrin have the most evidence to support their use in infantile spasms (13,16). It is important to note, however, that robust data is lacking in all treatment options (26), and randomized, controlled, multicenter comparative trials are needed. Many other treatment options continue to be explored, including the ketogenic diet, traditional anti-epileptic medications, and resective surgery in select cases (15). A treatment algorithm is suggested (Figure 2), with the recognition that some treatment choices will still be guided by provider preference and experience due to limited data. Doses and treatment lag for each medication vary and are described in Table 1.
Figure 2.
Treatment algorithm. Suggested sequence of treatment, with etiology incorporated. Data is inadequate to recommend a consistent first-line therapy, except in the case of tuberous sclerosis. It is recommended to try two first-line therapies before moving on to other options, as there is less data to support the second-line treatments. Studies have reported patients that respond to ACTH after failing corticosteroids and vice-versa. TSC, tuberous sclerosis complex; ACTH, adrenocorticotropic hormone; EEG, electroencephalogram.
Table 1. Treatment options in infantile spasms.
| Medication | Dose | Route | Time to effect | Duration of therapy | Side effects |
|---|---|---|---|---|---|
| First-line | |||||
| ACTH | 150 units/m2/day | IM | 14 days | 14 days, then taper | Hypertension, infection, weight gain, irritability |
| Prednisolone | 8 mg/kg/day or 40-60 mg/day | PO | 14 days | 14 days, then taper | |
| Vigabatrin | 50-150 mg/kg/day | PO | 14 days | 6-9 months, then taper | Visual field loss, MRI changes, irritability |
| Second-line | |||||
| Ketogenic diet | 3:1-4:1 ratio | PO | 1-3 months | At least 6 months | Constipation, nephrolithiasis, acidosis |
| Topiramate | 4-20 mg/kg/day | PO | 1+ months | Unclear | Anorexia, hypohidrosis, nephrolithiasis |
| Zonisamide | 4-20 mg/kg/day | PO | Up to 3 weeks | Unclear | |
| Pyridoxine | 100 mg | IV | Immediate | Once only | Cardiorespiratory depression |
| Daily: 100-400 mg/day | PO | 1-2 weeks | Lifelong | Neuropathy at high doses | |
First-line medications have the most data to support their use. Order is not in order of recommendation of use for either set of medications. Pyridoxine is given in a one-time dose by IV with EEG and cardiorespiratory monitoring and repeated orally if effective. Common side effects listed, but not an inclusive list of all side effects. ACTH, adrenocorticotropic hormone; EEG, electroencephalogram; MRI, magnetic resonance imaging; IM, intramuscular; PO, by mouth; IV, intravenous.
ACTH
ACTH is probably the most universally accepted first-line treatment of infantile spasms, stemming from the class 1 randomized controlled trials performed from 1983-1999 that show its efficacy (36-39). In 2004, the American Academy of Neurology and Child Neurology Society concluded that ACTH is “probably effective” in the treatment of infantile spasms (13). Five studies were considered class 1 evidence, and the rates of cessation of spasms were better than any previously reported, ranging from 42-87% (13,36-39). Response is typically seen within 14 days or sooner (13,36-40). Despite the promising data, ACTH has its own drawbacks and limitations.
First, the relapse rate in these studies is high in ACTH (13,36-38). There are variations in dosing and duration that make comparison and recommended treatment regimens difficult to formulate (26). Side effects have been severe and frequent. In addition, the cost of ACTH in the United States and elsewhere continues to climb (27,38). However, in a recent survey, about 2/3 of providers were using ACTH as first-line treatment of infantile spasms, with varying doses and durations of treatment (14). The relative strength of the data in favor of ACTH, as well as the difficulty in recruiting enough subjects in infantile spasms studies, has resulted in a paucity of studies evaluating ACTH further. Only one other prospective study (UKISS) has looked at ACTH since 2000, and it did not use EEG to track response (10,40,41). However, the rate of response was similar to that previously reported (13). In addition, although this study suggests equal efficacy between synthetic ACTH and prednisolone, it was not adequately powered to determine superiority of either hormonal treatment. It was primarily designed to compare hormonal treatments to vigabatrin.
Subsequently, the 2012 revisions to the AAN/CNS Evidence-based recommendations in the treatment of infantile spasms had no new evidence to evaluate the efficacy of ACTH. Review of previous studies, suggests that low-dose ACTH may be equivalent to high-dose (150 units/m2), but it is not entirely clear which low dose is to be used, with doses varying from 0.2 units/kg to 20 units/m2 (16). Hrachovy et al. showed equivalency between low-dose (20 units/day) and high-dose (150 units/m2/day) ACTH with response rates of 50% and 58%, respectively (42). It may be appropriate to start with either 20 units/day or 150 units/m2 as the treatment dose for ACTH. However, if a patient does not respond to the lower dose, it is recommended to try the higher dose (13,16).
Corticosteroids
There is an ongoing and increasing interest in the use of corticosteroids in the treatment of infantile spasms. Prednisolone is appealing due to its low cost, ready availability in many countries, ease of administration, and growing evidence that it may be similar in efficacy to ACTH and vigabatrin.
Initially, studies suggested that corticosteroids were inferior to ACTH. Prednisone/prednisolone were used in lower doses, ranging from 2-3 mg/kg/day (36,37,39,43), with response rates from 25-59%. In most cases, these were significantly lower than the response to ACTH.
Multiple studies have subsequently used higher doses of prednisolone, primarily either 40-60 mg/day (38,40,41,44) or weight-based dosing of 8 mg/kg/day with a maximum dose of 60 mg/day (27). These have shown rates of efficacy similar to ACTH, ranging from 67-80%. Relapse rate was also similar to ACTH or lower. However, other than the UK study, these are all retrospective studies. As noted above, although UKISS was not powered to compared ACTH with prednisolone, they had similar efficacy (40,41).
While the data are still limited, there is promise for using prednisolone in the treatment of infantile spasms. Indeed, more providers are using prednisolone as first-line treatment (14,38) and the most recent AAN parameter acknowledges the growing evidence that suggests prednisolone may be a reasonable initial treatment option (16). Prednisolone could possibly be equivalent to low-dose ACTH (36) or even high-dose ACTH, when used at higher doses of either 8 mg/kg/day or 40-60 mg/day (27,38,40,41). The side effect profile of prednisolone has been tolerable (44) and may be better than that of ACTH (45). Like ACTH, response is typically within 14 days, and if there is not complete spasm cessation, ACTH, vigabatrin or other treatments should be considered.
Vigabatrin
Vigabatrin was approved in the UK in 1989 and the United States in 2009 for the treatment of infantile spasms and intractable complex partial/focal seizures (46). The largest randomized study of vigabatrin in infantile spasms was an open-label, randomized, single-masked, multi-center 3-year study comparing high and low-dose vigabatrin (47). This 2001 study included both patients with tuberous sclerosis and other causes and had a response rate of 36% overall and 52% among patients with TSC. High-dose vigabatrin (150 mg/m2/day) was also found to be more effective than low-dose. Other studies have shown response rates of 35-82% among cryptogenic cases and response rates of 27-59% in symptomatic cases, with overall rates of response ranging from 26-76%, most of which are greater than 50% (48-51). Response occurred within 2 weeks of therapy in most patients and not after 12 weeks (49). It is recommended to stop therapy after 14 days if no response is noted (3).
Vigabatrin is the preferred first-line therapy for patients with infantile spasms and tuberous sclerosis (16), with some studies showing efficacy of greater than 90% in patients with tuberous sclerosis (17). In general, vigabatrin has been thought to be less effective than ACTH in other patient populations (40,41,52), although long-term outcomes may be similar (41). Relapse rates range from 16-21% (47). Reversible MRI changes have been observed in as many as 30% of patients (53,54). Peripheral visual field constriction (46,49), has been noted as a serious side effect of vigabatrin in 15-30% of patients, although it is thought that this rate may be lower among patients with infantile spasms. In responders, it is recommended to discontinue therapy after 6 months to limit the chances of peripheral visual field constriction (55). Less severe side effects include drowsiness, hypotonia and irritability in 13-25% of patients (47,48,50,52,56).
Ketogenic diet
The ketogenic diet is used often in intractable or profound epilepsies, including infantile spasms, with or without the concurrent use of medications (35,57-59). Spasm freedom has been reported in 14-65% of patients within 1-3 months (57,60-63). Diet ratio ranged from 3:1 to 4:1. Use as first-line therapy is limited as most studies focused on patients with intractable seizures and already on 1-3 anti-epileptic medications. Efficacy was higher in infantile spasms patients treated prior to 1 year of age (60) and among patients with cryptogenic infantile spasms (63). Some patients had improved seizure control (57,60), were able to reduce medications (60), and had cognitive improvements (61), even without cessation of spasms. Ketogenic formula and young age make the diet an attractive option, but there are inadequate data to recommend the diet as a sole first-line therapy (64).
Other treatments
There are limited data to support the use of other anti-epileptics in the treatment of infantile spasms. Some have advocated the use of pyridoxine (7,65) as a brief and safe trial at the onset of spasms as it can be quickly and safely trialed, using 100 mg IV once with continuous EEG and cardiorespiratory monitoring during administration (15). In Japan, pyridoxine has been used as first-line therapy for infantile spasms with 15% efficacy (65), which is less than the rate of spontaneous remission of spasms (4,19). Pyridoxine-dependent epilepsy can present as infantile spasms and should be considered as a possible etiology, especially in patients with younger onset.
Topiramate has some efficacy in the resolution of spasms, with response rate ranging from 10-48% (65-67), with doses of up to 30 mg/kg being used (18) and mean dose ranging from 10-16 mg/kg/day (68). Most studies are retrospective and are often confounded by concomitant use of other anti-epileptic medications and small numbers of patients.
Zonisamide has been used in doses ranging from 4-20 mg/kg/day with effective doses ranging from 5-12.5 mg/kg/day. Rates of response range from 26-41%. Time to response was up to 19 days (69-70).
Levetiracetam has very limited data regarding its use in infantile spasms and will not be discussed in detail. There is inadequate evidence to recommend its use as a second-line therapy.
Benzodiazepines have been shown to have little efficacy and possibly increased risk of morbidity and mortality in the treatment of infantile spasms (15).
Prognosis
There is no evidence that treatment alters long-term outcome in infantile spasms (19). In general, outcome is poor and the underlying genetic and structural brain issues that accompany some infantile spasms likely predispose to poor development, regardless of treatment. Epilepsy is present in up to 50% of patients (12,18,19). Autism is present in 15-33% of patients with infantile spasms and as high as 70% patients with tuberous sclerosis and infantile spasms (9). Even within patients with tuberous sclerosis, the presence of infantile spasms increases the risk of poor neurodevelopmental outcome (71). However, this is not the case in trisomy 21 (24).
Normal or near normal development is present in only 15-25% (12,18-19). In 20-35 years of follow up, nearly all patients with a normal or near normal outcome held jobs (12). Seventeen percent had an IQ >85.
Etiology is the most important predictor of outcome (10,33,72). Better developmental outcomes are noted in patients without an identified etiology (cryptogenic/idiopathic) and even better in those treated with hormonal therapy rather than vigabatrin (72). Short treatment lag (<1 month) is also associated with improved outcome (10-12,72), especially among cryptogenic patients (73). Cryptogenic patients may have a normal or near-normal outcome up to 54.3% of the time (74), while only 12.5% of symptomatic patients have a good outcome (11), Mortality may be as high as 10% at 3 years of age and 19% at age 10 years. ACTH therapy had an associated 12% mortality (12). Nearly all patients stop having spasms by 3 years of age.
Conclusions
Infantile spasms remain a difficult-to-treat and frequently devastating infantile epileptic encephalopathy. Etiology can be identified in 70% or more of patients and it is expected that this will continue to improve with more widely-available genetic testing. However, MRI brain remains the study with the highest yield in identifying etiology. Etiology guides treatment decisions and affects prognosis. Although treatment may not affect the outcomes for all patients, it is likely that for some cryptogenic patients it may be critical. First-line therapies include hormonal treatments and vigabatrin and efficacy can usually be determined within about 2 weeks. Use of a standardized approach to treatment is associated with a higher rate of use of first-line therapies and better rates of spasm cessation at 3-month follow-up (75). Further studies are needed to better compare first-line treatments and to determine efficacy of second-line treatments. The goal of treatment of infantile spasms remains complete cessation of spasms. Patients diagnosed and treated in a timely and appropriate fashion, especially those with cryptogenic/idiopathic infantile spasms, have the highest likelihood of a normal or near-normal outcome.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.West WJ. On a peculiar form of infantile convulsions. Lancet 1841;1:724-5. [Google Scholar]
- 2.Lux AL. West & son: the origins of West syndrome. Brain Dev 2001;23:443-6. [DOI] [PubMed] [Google Scholar]
- 3.Pellock JM, Hrachovy R, Shinnar S, et al. Infantile spasms: a U.S. consensus report. Epilepsia 2010;51:2175-89. [DOI] [PubMed] [Google Scholar]
- 4.Nordphysicianguides.org/Infantile-Spasms. Available online: http://nordphysicianguides.org/Infantile-Spasms/
- 5.Lux AL, Osborne JP. A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: consensus statement of the West Delphi group. Epilepsia 2004;45:1416-28. [DOI] [PubMed] [Google Scholar]
- 6.Wirrell EC, Shellhaas RA, Joshi C, et al. How should children with West syndrome be efficiently and accurately investigated? Results from the National Infantile Spasms Consortium. Epilepsia 2015;56:617-25. [DOI] [PubMed] [Google Scholar]
- 7.Shields WD. West's syndrome. J Child Neurol 2002;17 Suppl 1:S76-9. [DOI] [PubMed] [Google Scholar]
- 8.Wang CJ, Jonas R, Fu CM, et al. Quality-of-care indicators for infantile spasms. J Child Neurol 2013;28:13-20. [DOI] [PubMed] [Google Scholar]
- 9.Askalan R, Mackay M, Brian J, et al. Prospective preliminary analysis of the development of autism and epilepsy in children with infantile spasms. J Child Neurol 2003;18:165-70. [DOI] [PubMed] [Google Scholar]
- 10.O'Callaghan FJ, Lux AL, Darke K, et al. The effect of lead time to treatment and of age of onset on developmental outcome at 4 years in infantile spasms: evidence from the United Kingdom Infantile Spasms Study. Epilepsia 2011;52:1359-64. [DOI] [PubMed] [Google Scholar]
- 11.Widjaja E, Go C, McCoy B, et al. Neurodevelopmental outcome of infantile spasms: A systematic review and meta-analysis. Epilepsy Res 2015;109:155-62. [DOI] [PubMed] [Google Scholar]
- 12.Riikonen R. Long-term outcome of patients with West syndrome. Brain Dev 2001;23:683-7. [DOI] [PubMed] [Google Scholar]
- 13.Mackay MT, Weiss SK, Adams-Webber T, et al. Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology 2004;62:1668-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mytinger JR, Joshi S, Pediatric Epilepsy Research Consortium, et al . The current evaluation and treatment of infantile spasms among members of the Child Neurology Society. J Child Neurol 2012;27:1289-94. [DOI] [PubMed] [Google Scholar]
- 15.Shields WD. Infantile spasms: little seizures, BIG consequences. Epilepsy Curr 2006;6:63-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Go CY, Mackay MT, Weiss SK, et al. Evidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2012;78:1974-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiele EA. Managing epilepsy in tuberous sclerosis complex. J Child Neurol 2004;19:680-6. [DOI] [PubMed] [Google Scholar]
- 18.Kossoff EH. Infantile spasms. Neurologist 2010;16:69-75. [DOI] [PubMed] [Google Scholar]
- 19.Hrachovy RA, Frost JD, Jr. Infantile epileptic encephalopathy with hypsarrhythmia (infantile spasms/West syndrome). J Clin Neurophysiol 2003;20:408-25. [DOI] [PubMed] [Google Scholar]
- 20.Appleton RE. West syndrome: long-term prognosis and social aspects. Brain Dev 2001;23:688-91. [DOI] [PubMed] [Google Scholar]
- 21.Osborne JP, Lux AL, Edwards SW, et al. The underlying etiology of infantile spasms (West syndrome): information from the United Kingdom Infantile Spasms Study (UKISS) on contemporary causes and their classification. Epilepsia 2010;51:2168-74. [DOI] [PubMed] [Google Scholar]
- 22.Poulat AL, Lesca G, Sanlaville D, et al. A proposed diagnostic approach for infantile spasms based on a spectrum of variable aetiology. Eur J Paediatr Neurol 2014;18:176-82. [DOI] [PubMed] [Google Scholar]
- 23.Paciorkowski AR, Thio LL, Dobyns WB. Genetic and biologic classification of infantile spasms. Pediatr Neurol 2011;45:355-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanmaneechai O, Sogawa Y, Silver W, et al. Treatment outcomes of West syndrome in infants with Down syndrome. Pediatr Neurol 2013;48:42-7. [DOI] [PubMed] [Google Scholar]
- 25.Gibbs EL, Fleming MM, Gibbs FA. Diagnosis and prognosis of hypsarhythmia and infantile spasms. Pediatrics 1954;13:66-73. [PubMed] [Google Scholar]
- 26.Hancock EC, Osborne JP, Edwards SW. Treatment of infantile spasms. Cochrane Database Syst Rev 2013;6:CD001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain SA, Shinnar S, Kwong G, et al. Treatment of infantile spasms with very high dose prednisolone before high dose adrenocorticotropic hormone. Epilepsia 2014;55:103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer WD, Haller JS, Sullivan LR, et al. The value of neuroradiology in infantile spasms. J Pediatr 1982;100:47-50. [DOI] [PubMed] [Google Scholar]
- 29.Aydinli N, Calişkan M, Ozmen M, et al. Neuroradiologic aspects of West syndrome. Pediatr Neurol 1998;19:211-6. [DOI] [PubMed] [Google Scholar]
- 30.Saltik S, Kocer N, Dervent A. Magnetic resonance imaging findings in infantile spasms: etiologic and pathophysiologic aspects. J Child Neurol 2003;18:241-6. [DOI] [PubMed] [Google Scholar]
- 31.van Bogaert P, Chiron C, Adamsbaum C, et al. Value of magnetic resonance imaging in West syndrome of unknown etiology. Epilepsia 1993;34:701-6. [DOI] [PubMed] [Google Scholar]
- 32.Epilepsy Phenome/Genome Project & Epi4K Consortium. Copy number variant analysis from exome data in 349 patients with epileptic encephalopathy. Annals of Neurology 2015;78:323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavone P, Striano P, Falsaperla R, et al. Infantile spasms syndrome, West syndrome and related phenotypes: what we know in 2013. Brain Dev 2014;36:739-51. [DOI] [PubMed] [Google Scholar]
- 34.Mastrangelo M, Leuzzi V. Genes of early-onset epileptic encephalopathies: from genotype to phenotype. Pediatr Neurol 2012;46:24-31. [DOI] [PubMed] [Google Scholar]
- 35.Lux AL. Latest American and European updates on infantile spasms. Curr Neurol Neurosci Rep 2013;13:334. [DOI] [PubMed] [Google Scholar]
- 36.Hrachovy RA, Frost JD, Jr, Kellaway P, et al. Double-blind study of ACTH vs prednisone therapy in infantile spasms. J Pediatr 1983;103:641-5. [DOI] [PubMed] [Google Scholar]
- 37.Snead OC, 3rd, Benton JW, Myers GJ. ACTH and prednisone in childhood seizure disorders. Neurology 1983;33:966-70. [DOI] [PubMed] [Google Scholar]
- 38.Kossoff EH, Hartman AL, Rubenstein JE, et al. High-dose oral prednisolone for infantile spasms: an effective and less expensive alternative to ACTH. Epilepsy Behav 2009;14:674-6. [DOI] [PubMed] [Google Scholar]
- 39.Baram TZ, Mitchell WG, Tournay A, et al. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics 1996;97:375-9. [PMC free article] [PubMed] [Google Scholar]
- 40.Lux AL, Edwards SW, Hancock E, et al. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet 2004;364:1773-8. [DOI] [PubMed] [Google Scholar]
- 41.Lux AL, Edwards SW, Hancock E, et al. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol 2005;4:712-7. [DOI] [PubMed] [Google Scholar]
- 42.Hrachovy RA, Frost JD, Jr, Glaze DG. High-dose, long-duration versus low-dose, short-duration corticotropin therapy for infantile spasms. J Pediatr 1994;124:803-6. [DOI] [PubMed] [Google Scholar]
- 43.Lombroso CT. A prospective study of infantile spasms: clinical and therapeutic correlations. Epilepsia 1983;24:135-58. [DOI] [PubMed] [Google Scholar]
- 44.Hancock E, Osborne J. Treatment of infantile spasms with high-dose oral prednisolone. Dev Med Child Neurol 1998;40:500. [PubMed] [Google Scholar]
- 45.Snead OC, 3rd. Treatment of infantile spasms. Pediatr Neurol 1990;6:147-50. [DOI] [PubMed] [Google Scholar]
- 46.Shields WD, Pellock JM. Vigabatrin 35 years later - from mechanism of action to benefit-risk considerations. Acta Neurol Scand Suppl 2011;(192):1-4. [DOI] [PubMed] [Google Scholar]
- 47.Elterman RD, Shields WD, Mansfield KA, et al. Randomized trial of vigabatrin in patients with infantile spasms. Neurology 2001;57:1416-21. [DOI] [PubMed] [Google Scholar]
- 48.Fejerman N, Cersósimo R, Caraballo R, et al. Vigabatrin as a first-choice drug in the treatment of West syndrome. J Child Neurol 2000;15:161-5. [DOI] [PubMed] [Google Scholar]
- 49.Willmore LJ, Abelson MB, Ben-Menachem E, et al. Vigabatrin: 2008 update. Epilepsia 2009;50:163-73. [DOI] [PubMed] [Google Scholar]
- 50.Aicardi J, Mumford JP, Dumas C, et al. Vigabatrin as initial therapy for infantile spasms: a European retrospective survey. Sabril IS Investigator and Peer Review Groups. Epilepsia 1996;37:638-42. [DOI] [PubMed] [Google Scholar]
- 51.Pesaturo KA, Spooner LM, Belliveau P. Vigabatrin for infantile spasms. Pharmacotherapy 2011;31:298-311. [DOI] [PubMed] [Google Scholar]
- 52.Vigevano F, Cilio MR. Vigabatrin versus ACTH as first-line treatment for infantile spasms: a randomized, prospective study. Epilepsia 1997;38:1270-4. [DOI] [PubMed] [Google Scholar]
- 53.Thelle T, Gammelgaard L, Hansen JK, et al. Reversible magnetic resonance imaging and spectroscopy abnormalities in the course of vigabatrin treatment for West syndrome. Eur J Paediatr Neurol 2011;15:260-4. [DOI] [PubMed] [Google Scholar]
- 54.Dracopoulos A, Widjaja E, Raybaud C, et al. Vigabatrin-associated reversible MRI signal changes in patients with infantile spasms. Epilepsia 2010;51:1297-304. [DOI] [PubMed] [Google Scholar]
- 55.Westall CA, Wright T, Cortese F, et al. Vigabatrin retinal toxicity in children with infantile spasms: An observational cohort study. Neurology 2014;83:2262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Partikian A, Mitchell WG. Major adverse events associated with treatment of infantile spasms. J Child Neurol 2007;22:1360-6. [DOI] [PubMed] [Google Scholar]
- 57.Hong AM, Turner Z, Hamdy RF, et al. Infantile spasms treated with the ketogenic diet: prospective single-center experience in 104 consecutive infants. Epilepsia 2010;51:1403-7. [DOI] [PubMed] [Google Scholar]
- 58.Kossoff EH, Hartman AL. Ketogenic diets: new advances for metabolism-based therapies. Curr Opin Neurol 2012;25:173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kossoff EH, Thiele EA, Pfeifer HH, et al. Tuberous sclerosis complex and the ketogenic diet. Epilepsia 2005;46:1684-6. [DOI] [PubMed] [Google Scholar]
- 60.Kossoff EH, Pyzik PL, McGrogan JR, et al. Efficacy of the ketogenic diet for infantile spasms. Pediatrics 2002;109:780-3. [DOI] [PubMed] [Google Scholar]
- 61.Pires ME, Ilea A, Bourel E, et al. Ketogenic diet for infantile spasms refractory to first-line treatments: an open prospective study. Epilepsy Res 2013;105:189-94. [DOI] [PubMed] [Google Scholar]
- 62.Kossoff EH, Hedderick EF, Turner Z, et al. A case-control evaluation of the ketogenic diet versus ACTH for new-onset infantile spasms. Epilepsia 2008;49:1504-9. [DOI] [PubMed] [Google Scholar]
- 63.Eun SH, Kang HC, Kim DW, et al. Ketogenic diet for treatment of infantile spasms. Brain Dev 2006;28:566-71. [DOI] [PubMed] [Google Scholar]
- 64.Kossoff EH. International consensus statement on clinical implementation of the ketogenic diet: agreement, flexibility, and controversy. Epilepsia 2008;49 Suppl 8:11-3. [DOI] [PubMed] [Google Scholar]
- 65.Overby PJ, Kossoff EH. Treatment of infantile spasms. Curr Treat Options Neurol 2006;8:457-64. [DOI] [PubMed] [Google Scholar]
- 66.Peltzer B, Alonso WD, Porter BE. Topiramate and adrenocorticotropic hormone (ACTH) as initial treatment for infantile spasms. J Child Neurol 2009;24:400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zou LP, Ding CH, Fang F, et al. Prospective study of first-choice topiramate therapy in newly diagnosed infantile spasms. Clin Neuropharmacol 2006;29:343-9. [DOI] [PubMed] [Google Scholar]
- 68.Korinthenberg R, Schreiner A. Topiramate in children with west syndrome: a retrospective multicenter evaluation of 100 patients. J Child Neurol 2007;22:302-6. [DOI] [PubMed] [Google Scholar]
- 69.Yanai S, Hanai T, Narazaki O. Treatment of infantile spasms with zonisamide. Brain Dev 1999;21:157-61. [DOI] [PubMed] [Google Scholar]
- 70.Lotze TE, Wilfong AA. Zonisamide treatment for symptomatic infantile spasms. Neurology 2004;62:296-8. [DOI] [PubMed] [Google Scholar]
- 71.Goh S, Kwiatkowski DJ, Dorer DJ, et al. Infantile spasms and intellectual outcomes in children with tuberous sclerosis complex. Neurology 2005;65:235-8. [DOI] [PubMed] [Google Scholar]
- 72.Darke K, Edwards SW, Hancock E, et al. Developmental and epilepsy outcomes at age 4 years in the UKISS trial comparing hormonal treatments to vigabatrin for infantile spasms: a multi-centre randomised trial. Arch Dis Child 2010;95:382-6. [DOI] [PubMed] [Google Scholar]
- 73.Hamano S, Yoshinari S, Higurashi N, et al. Developmental outcomes of cryptogenic West syndrome. J Pediatr 2007;150:295-9. [DOI] [PubMed] [Google Scholar]
- 74.Karvelas G, Lortie A, Scantlebury MH, et al. A retrospective study on aetiology based outcome of infantile spasms. Seizure. 2009;18:197-201. [DOI] [PubMed] [Google Scholar]
- 75.Fedak EM, Patel AD, Heyer GL, et al. Optimizing Care With a Standardized Management Protocol for Patients With Infantile Spasms. J Child Neurol 2015;30:1340-2. [DOI] [PubMed] [Google Scholar]