Abstract
Public interest in the use of “medical marijuana” for the treatment of childhood epilepsy has burgeoned in the last few years. This has occurred in parallel with a growing interest in “medical marijuana” in general. Physicians and pediatricians must balance their patients’ desire for immediate access to these products with the tenets of evidence-based medicine. This review discusses the biochemistry of cannabis products (the phytocannabinoids) setting this in the context of the endogenous endocannabinoid system. The differing and potentially modulating effects of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are reviewed. The evidence-base supporting or not the use of cannabis products for the treatment of neurological disease and specifically epilepsy is explored. The potential for adverse effects and particularly of neurotoxicity is addressed. Finally, public health and sociocultural implications are touched upon. Specific recommendations for interested physicians are provided including advocacy for patients and for a change in the “scheduling” of cannabis in order to better foster much-needed high-quality scientific research in this important area.
Keywords: Cannabidiol (CBD), cannabis, epilepsy, medical marijuana, tetrahydrocannabinol (THC)
Introduction
In just the past 5 years, the remarkable immediacy and reach of modern social media has resulted in a nearly unparalleled expansion of interest in the use of “medical marijuana” products for the treatment of pediatric epilepsy. Until recently this therapeutic option had been largely the fancy of a few cannabis enthusiasts and some dedicated scientists. However, the experiences of two young children with Dravet syndrome who reportedly ceased having seizures and experienced “neurological awakening” after taking cannabidiol (CBD)-rich medical marijuana preparations, led to a public interest in these products that has spread like wildfire (1-3). This phenomenon has rapidly resulted in high-visibility media productions (1), remarkable shifts in public policy (4), legislation of specific medical marijuana laws in multiple states (5), and a high level of interest in these products among physicians, medicinal chemists, and pharmaceutical companies.
These remarkable anecdotal experiences, fueled by an impassioned furor among patient families and advocates, have led to a very high level of expectation regarding the therapeutic potential of cannabinoids for the treatment of epilepsy (3). Families have petitioned their legislators for access to artisanal (vernacular) marijuana products (5), and some have even uprooted their entire family, moving to states with more liberal marijuana policies (6), in order to gain access to these products for their affected children. Meanwhile, several companies are actively developing pharmaceutical products based on medicinal cannabinoids. Physicians are often caught in a quandary complicated by insufficient scientific data. Whereas their patients and families are often demanding access to these products, neurologists and pediatric neurologists may not have the knowledge base or resources to properly address the patient’s concerns nor to advise them in an informed manner (7-9).
This review will seek to provide neurologists and pediatric neurologists a basis to better address these rapidly evolving questions. More than likely, cannabinoids and/or their synthetic derivatives will become a persistent component of our therapeutic arsenal. Hopefully, a solid understanding of cannabinoid chemistry, the endocannabinoid system and the medical evidence surrounding use of cannabis products for the treatment of epilepsy, ultimately can help guide physicians in caring for their patients.
Cannabinoids and chemistry
The plant, Cannabis sativa, often referred to as hemp or marijuana, has been used for its medicinal properties for millennia (10,11). Besides it psychogenic properties, it has been purported to be beneficial for the treatment of a broad range of medical ailments (10-12). The plant contains more than 60 distinct biochemicals which share a common structure most of which presumably have particular bioactive properties (10,11,13,14). Collectively, these are known as the phytocannabinoids. These are terpeno-phenolic compounds, based on their chemical structure (11,13) (Figure 1). The two major phytocannabinoids (Figure 1) are delta-9-tetrahydrocannabinol (THC), the main psychoactive constituent of the marijuana plant, and CBD, a phytocannabinoid that is believed to have no psychoactive properties and is of increasing interest with respect to its therapeutic potential (11-14). The numerous other phytocannabinoids are lesser constituents and are less well studied. However, some may also have medicinal attributes of interest (such as cannabidivarin, which may have antiepileptic properties of its own (15,16).
Figure 1.
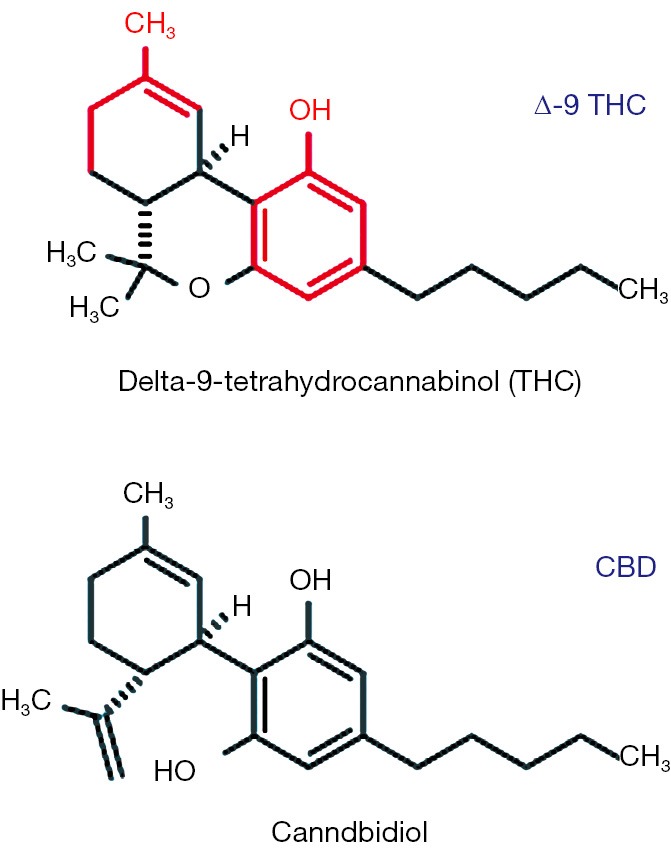
Terpene phenolic heterocyclic structures of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Red portions identify basic terpene (left) and phenol (right) backbones.
There is considerable confusion concerning the Cannabis plant and its phytochemicals. According to most authorities, “hemp” and “marijuana” refer to different varieties of the same species (C. sativa) (10). Horticultural practices and breeding strategies have led growers to develop varieties that differ tremendously in the relative and absolute content of various cannabinoids and specifically of THC and CBD. In general, plants grown with less light in more tightly grouped plantations are taller, have a higher fiber content and are bred to have a low (less than 0.3%) THC content (10,11,13,14). These are referred to as “hemp.” Other varietals particularly those grown with greater space between plantings and with greater light exposure can result in plants that produce much higher THC concentrations (colloquially referred to as “marijuana”) (10,11,13,14). The portions of the female flowering plants known as trichomes provide the highest yield of phytochemicals (13). These along with leaves, flower and oil are the primary source for “recreational” and medicinal preparations. Consequently, as will be further elaborated upon below, “medical marijuana” is not “one thing.” Rather, there is enormous variability among various preparations, and even among the different pharmaceutical products derived from natural or synthetic cannabinoids. Hence, the ease with which some individuals and organizations tout the benefits and safety of “medical marijuana” either naively ignores or knowingly disregards the actual complexity of the field (17). In this paper, cannabis products that are derived from marijuana or hemp plants and are not subjected to rigorous pharmaceutical grade purification and quality control will be collectively referred to as “artisanal” or “vernacular” preparations.
Because of the high use and abuse of cannabis products, a great deal is already known about the pharmacological properties of these substances (11,13,14). The cannabinoids all share the heterocyclic terpeno-phenolic structure. As large heterocyclic structures they are very lipophilic. Thus they cross the blood brain barrier readily and distribute easily to lipid laden tissues including brain parenchyma and neuronal cell membranes specifically. They may remain in such lipid laden tissues (presumably including the brain) for weeks and from these are released only gradually into the blood stream. CBD and THC, when taken orally, undergo first pass metabolism, thereby affecting bioavailability and dosage (11). Due to this phenomenon, different routes of administration (oral vs. mucosal vs. inhalational) may result in substantially different biological levels of these agents. Furthermore, in the cannabis plant these substances naturally occur in the relatively inactive carboxylated state. Smoking and heating decarboxylates the molecules thus conferring greater bioactivity (11).
Finally, the cannabinoids and CBD in particular are primarily metabolized by the hepatic cytochrome P450 enzyme system, and in turn CBD appears to be an inhibitor of several of the microsomal hepatic metabolic enzymes and in particular of CYP2C19 (11,18). Thus, it is possible that CBD in significant concentrations may increase levels of concomitantly administered drugs metabolized by CYP2C19. This applies to the benzodiazepines and in particular to clobazam whose major active metabolite N-desmethylclobazam is primarily metabolized by CYP2C19 (19,20). Thus seizure control and/or toxicity may result from pharmacokinetic interactions as well as potentially from the direct CNS effect as such of the cannabinoid. Whether or not this degree of enzyme inhibition will result in clinically significant drug interactions is nevertheless still uncertain.
The endocannabinoid system
The very existence of natural substances (the cannabinoids) that have such remarkable and broad effects on human behavior and function presupposes a set of target receptors or endogenous physiological processes upon which these chemicals act. Indeed, the search for the biological targets of THC ultimately lead to the discovery of specific cannabinoid receptors and then to the identification of endogenous ligands for those receptors. Further study of these has greatly enhanced our understanding of the extraordinarily complex and elaborate endogenous system whereby cannabinoid receptors and other targets respond to the endogenous “endocannabinoids” and to the exogenous substances elaborated by the cannabis plant. This complex physiological system is referred to as the endocannabinoid system (21,22).
The two most prevalent cannabinoid receptors are both G-protein-coupled receptors that exert their physiological effects through the adenylate cyclase second messenger system. The CB1 receptor is widely distributed in brain and largely modulates endocannabinoid effects in the CNS (21-23). These receptors are primarily situated pre-synaptically on axon terminals, with their highest density being in the perisynaptic region (Figure 2) (21,22). Activation of the CB1 receptor results primarily in the inhibition of neurotransmitter release (21,22). While CB1 receptors are known to regulate both GABA and glutamate release, a greater density of these receptors exists on inhibitory versus excitatory synapses in most brain regions (21,22).
Figure 2.
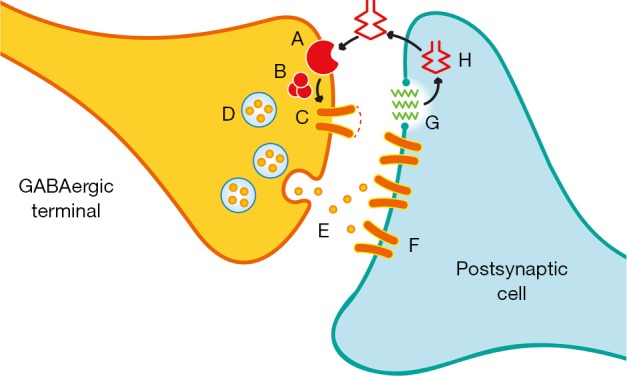
Schematic of a GABAergic synapse modulated by CB1 receptors. The perisynaptic location of CB1 receptors (A) is depicted. GABA (E) stimulates post-synatpic GABA receptors (F). Post synaptic changes induce metabolism of membrane derived phospholipids (G) leading to formation of endocannabinoid (H). The latter diffuses back stimulating the perisynaptic CB1 receptor (A). This in turn modulates neurotransmitter release from presynaptic vesicles (D) via G-protein coupled (B) influence on Ca++ channels (C).
CB2 receptors on the other hand are particularly prevalent on lymphocytes, neoplastic cells and other systemic target tissues (12,21,22,24). Presumably, many of the systemic effects of cannabinoids result from binding to CB2 receptors (22,24). The physiological role of CB2 receptor activation in the periphery is suspected to be similar to the neural modulatory role described above for CB1 receptors though details are not as clearly worked out. In addition to these two best characterized cannabinoid receptors, it is believed that the endocannabinoids and phytocannabinoids may act at a number of other receptor or target sites including GPR55 receptors and TRPV type 1 channels (11,12,25). THC appears to act at CB1 and CB2 receptors as a partial agonist (21-23), whereas CBD appears to have a very low affinity for both of the major cannabinoid receptors (23; see below).
This receptor distribution is ideally situated to serve in the capacity of neuromodulation (21,22,26). Indeed a number of complex neuromodulatory processes have been linked to endocannabinoid processing including presynaptic modulation, retrograde neuromodulation and multiple physiologically defined forms of synaptic plasticity including depolarization-induced suppression of inhibition (DISI), depolarization-induced suppression of excitation (DSE) and long-term depression (LTD) (21,22,26). The idea is that synaptic depolarization or hyperpolarization of a post-synaptic membrane results in the induction of post-synaptic metabolic processes which increase the formation and/or release of endocannabinoids. These in turn diffuse back to the presynaptic terminal activating perisynaptic presynaptic CB1 receptors, the activation of which in turn inhibits release of additional neurotransmitter on variable time scales (thereby constituting a form of feedback inhibition) (26). This feedback process may be important during periods of intense synaptic activity.
The two major endocannabinoids are metabolic products of membrane phospholipid metabolism (i.e., of arachidonic acid pathways) (11,13,21-25). These are N-arachidonoyl ethanolamide (AEA; also known as Anandamide, reportedly meaning “inner bliss” in Sanskrit) and 2-arachydonoylglycerol, also referred to as 2AG (Figure 3). 2AG is a full agonist at both CB1 and CB2 receptors and is found at substantially higher levels than AEA, which is a partial agonist at both CB receptor types and at TPVR1 receptors (11,13,21-25). CBD may act by increasing levels of one or more of the endocannabinoids (presumably by interfering with their metabolism or by inhibiting re-uptake; 23). In any case, one can conceptualize these two endogenous cannabinoids as another set of second messenger systems derived from phospholipid metabolism all of which play key roles in neuromodulation.
Figure 3.
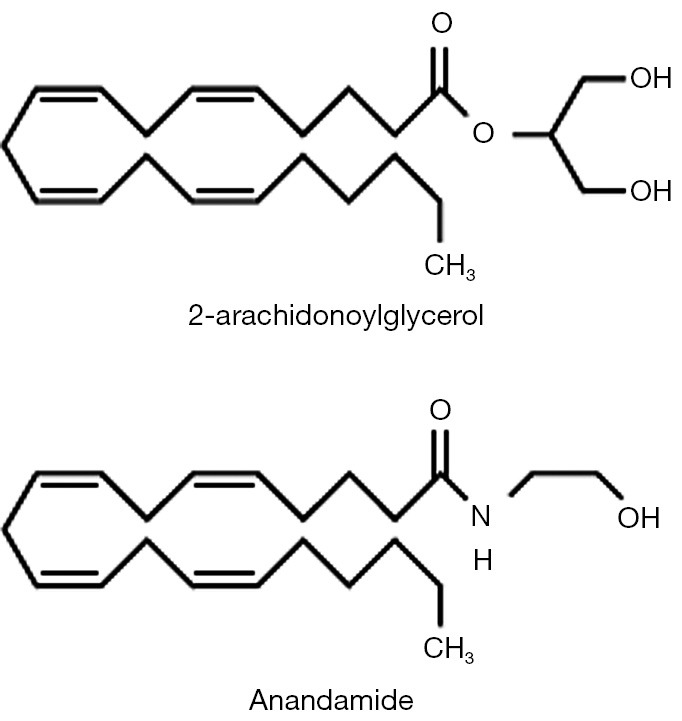
Chemical structures of the two major endocannabinoids: N-arachidonyl ethanolamide (AEA; “anandamide”) and 2 arachidonoylglycerol (2AG). Similarity to arachidonic acid and arachidonic acid metabolites is apparent.
Therefore, overall the evidence suggests that endocannabinoid signaling serves to decrease synaptic transmission during periods of intense cellular activity. The key features of this system include: (I) neuromodulation; (II) widespread CNS effects via CB1 receptor stimulation; (III) widespread systemic effects including anti-inflammatory and immune mediated effects modulated via CB2 receptors; (IV) neuromodulation via inhibitory presynaptic effects with greater influence over inhibitory than excitatory neurotransmission; (V) a pivotal role in retrograde neurotransmission and resultant presynaptic neuromodulation; and finally, (VI) a biphasic effect of the endocannabinoids in numerous physiological systems (21,22,26).
Cannabinoids for neurological disease: what is the medical evidence?
Given the widespread distribution of cannabinoid receptors in brain and body and the key role these receptors play in the modulation of physiological functions, one might anticipate a very broad range of physiological effects resulting from endocannabinoid signaling (12,27). Indeed these range from roles in pain and sensory modulation to vegetative functions, endocrine regulation, and neurophysiological and psychological functions ranging from motor control to mood and behavioral regulation (Table 1). Likewise, it is easy to see why the exogenously administered cannabinoids might have far ranging effects and hence potential broadly distributed therapeutic potential (Table 2). Consequently, the putative therapeutic benefits of the cannabinoids range from treatment of nausea and vomiting, cancer therapeutics to the modulation of neurological and psychiatric disease (12) (Table 2). It is important to recognize, however, that at this stage these extensive putative therapeutic benefits touted by the proponents of medical marijuana are largely unproven (8,9). At present, high quality medical evidence supporting the use of these agents in most of these conditions remains modest at best.
Table 1. Physiological actions/roles of the endocannabinoids.
| Pain/sensory modulation |
| Cognitive/memory processing |
| Mood and behavior |
| Motor control/coordination |
| Endocrine functions |
| Vegetative functions |
| (I) Appetite |
| (II) Temperature control |
| (III) Heart rate regulation |
| (IV) Nausea/vomiting |
| Intraocular pressure |
| Inflammation |
| Immune regulation/recognition |
Table 2. Therapeutic potential of the cannabinoids.
| Nausea/vomiting |
| Cancer chemotherapeutic agents |
| Anorexia |
| Pain/inflammation |
| Inflammatory bowel disease, rheumatic diseases, etc. |
| Neoplastic disease |
| Chemotherapeutic agents?, symptomatic (pain, nausea) |
| Antioxidant |
| Glaucoma |
| Psychiatric/neurological disease |
| MS—spasticity (indication in many countries, not US) |
| Pain, addiction Rx, anxiety, depression, etc. |
Likewise, the widespread physiological effects of the cannabinoids also presuppose the potential for a broad range of toxicities. Thus enthusiasm for therapeutic benefits needs to be tempered by the realistic appreciation of potential adverse effects (Table 3) (4,28,29). Toxicities can be related to direct pharmacologic toxicity of the cannabinoids, including systemic as well as central nervous system toxicity, as well as to coincident harm resulting from associated products inadvertently co-administered with various preparations of “medical marijuana” (8,28-30) (Table 3). The latter could include inhalational injury from smoking, inhalation of associated microorganisms or fungi, inadvertent intake of co-administered pesticides or other byproducts, and so forth.
Table 3. Potential toxicities of cannabinoid preparations.
| General toxicities |
| Tachycardia |
| Pulmonary toxicity (inhaled formulation) |
| Pulmonary infection |
| Inhalation of associated microorganisms/fungi |
| Immune suppression |
| Suppresses macrophages, T-lymphocyte |
| Increased interleukin I release |
| Associated intake of pesticides/byproducts |
| Neurologic and neuropsychiatric toxicities |
| Memory impairment |
| Short and long term |
| Impaired executive functions |
| ↓ concentration, judgment, attention span, motivation, problem solving, reaction time |
| Impaired motor function |
| ↓ coordination |
| Impairment of neural plasticity |
| Neuropsychiatric |
| Anxiety, panic attacks |
| Psychosis |
| ? With or without predisposition |
| Mania, manic episodes |
| ↑ cycling in bipolar |
| Depression |
| “Addiction”/dependence |
| “gateway drug” |
It is important to recall, again, that the cannabis plant produces a remarkably broad array of phytochemicals. It would be anticipated that these could have varying degrees of efficacy as well as toxicity. Specifically, there is considerable evidence that suggests that of the phytocannabinoids, delta-9-THC is likely to be the substance largely responsible for most of the systemic and neurotoxic effects of cannabis preparations (13,14,27). Meanwhile, there is growing evidence that CBD may actually inhibit, reduce or moderate some of these adverse effects (27,31-33). The principal that one endocannabinoid may modulate or act synergistically with another is referred to as the “entourage effect” (13,27). This is the principal that leads some medical marijuana advocates to insist that the “natural product” is preferable to any purified cannabinoid based on the strongly held belief that the synergistic effects of the constituents will be favorable in comparison to the effects of any isolated phytochemical. Obviously, from a scientific standpoint, it becomes virtually impossible to properly conduct carefully controlled studies with vernacular cannabis preparations given that these products likely contain widely disparate relative quantities of the various constituent cannabinoids.
Finally, among the potential toxicities of the cannabinoids, those of greatest concern to neurologists in particular are neurological and neuropsychiatric toxicities (Table 3). Unfortunately, there is considerable high-quality data coming from various sources that indicates that long-term exposure to THC can have serious deleterious effects on neurological functioning (34-37). In particular, there is strong evidence that progressive memory impairment as well as impaired executive functions results (34,37,38). There is additional concern about a deleterious effect on neural plasticity, particularly with regard to the developing brain (39-42). There is strong evidence that cannabis use results in an increased risk of psychosis in predisposed individuals (43,44). Acute and chronic cannabis use have also been linked to aggravation of anxiety, mania and depression. Consequently any future studies of cannabinoid therapy in epilepsy, and in particular in childhood epilepsy, must very carefully assess acute and long-term neurotoxicity.
Cannabinoids in epilepsy: a rapidly evolving field
Despite the extraordinary current enthusiasm for the use of “medical marijuana” in the treatment of epilepsy and specifically for access to CBD among parents of children with intractable epilepsy (2-4,6), the medical evidence supporting the use of “medical marijuana” for the treatment of neurological disease is weak at best (45). A very thorough review of the use of “medical marijuana” in neurological disease was recently published (45). Authors focused on the following neurological conditions: spasticity in patients with MS, central pain and painful spasms in MS, bladder dysfunction in MS, involuntary movements in MS, movement disorders, epilepsy. The overarching conclusion of this systematic review was that there is minimal high quality data to support the use of “medical marijuana” in any neurological condition other than for the treatment of spasticity in multiple sclerosis (45). It is enlightening to briefly review the data that supports this therapy and compare it with what exists with respect to the treatment of epilepsy with cannabis products.
Controlled trials of various pharmaceutical cannabis products for the treatment of multiple sclerosis (specifically the painful spasms of MS) began in the early 2000s (45). Initial studies did use a variety of preparations (Table 4). This culminated in a series of high quality placebo-controlled trials with Sativex (a 50:50 mixture of THC and CBD) from 2006 to 2011 (46,47). These trials demonstrated a statistically significant benefit of Sativex in the management of painful MS spasms, resulting in the marketing of this agent in England and many other countries (not including the United States). However, quick perusal of Table 4 demonstrates that well over 2000 patients were studied in this fashion. In addition, published post-marketing data regarding well over 10,000 patient-years of experience with Sativex demonstrates relatively low toxicity and statistical absence of serious adverse events (46-49). Since Sativex is a 50-50 mixture of THC and CBD, these observations do provide some reassurance that CBD itself may have minimal toxicity and a low propensity to result in dangerous or serious adverse effects.
Table 4. Clinical studies of cannabinoids in multiple sclerosis.
| Study | Product | Design | No. of Pts | “Result” | Other comment |
|---|---|---|---|---|---|
| Killestein, 2002 | THC vs. C. sativa extract | 20 wks, R, DB, Placebo | 16 | No change AS | Worsening in MSFC |
| CAMS, Zajicek, 2003 | Marinol, Cannador | 15 wks, R, Placebo | 667 | No signif change in AS | ↓ 10 m walking time; subj imp. Spasticity, pain |
| Vaney, 2004 | THC 2.5 mg CBD 0.9 mg | Pro, R, DB, placebo, X-over | 57 | No signif diff | trend in favor of ↓ spasm freq; imp. sleep, mobility |
| CAMS-ext Zajicek, 2005 | Marinol, Cannador | Up to 12 mos | 502 (80% of CAMS) | Small imp AS, Marinol + Cannador | |
| Wade, 2006 | Sativex | Open label, ~434 days, sub 6 wks placebo controlled | 137 | ↓ in VAS score; pain, tremor, bladder—neg | 42.3% withdrew from lack of efficacy |
| Collin, 2007 | Sativex | 6 wks, DB, Placebo cont | 189 | ↓ spasticity by NRS score | No other sign effect |
| Novotna, 2011 | Sativex | Unusual design: initial 4 wks single blind; Phase B: R, DB, Placebo with “early responders” | 572 phase A 241 phase B | Highly sign improvement spasticity (NRS) in phase B | Also, ↓ freq of spasms, sleep disturbances |
| Notcutt, 2012 | Sativex | Blinded withdrawal in long-term treated pts | 36 (18 per group) | Time to treatment failure | Global imp of change scales (pt and caregiver) |
CBD, cannabidiol; AS, Ashworth scale (“objective” spasticity scale); MSFC, MS Functional Composite; VAS, visual analogue scale rating spasticity subjectively; NRS, subjective numerical rating scale for spasticity; wks, weeks; mos, months; pts, patients; signif, significant; diff, difference; freq, frequency; subj, subject; imp, important; neg, negative. Marinol, synthetic THC; Cannador, oral C. Sativa extract.
However contrast this with the published data regarding the use of medical marijuana for the treatment of epilepsy (Table 5) (50-53). Until the last few years, the published data was minimal (Table 5) and included less than 70 subjects. Very few of these were children. Furthermore, none of these studies would meet criteria as Class I-III clinical trials (50-53). However this state of affairs is rapidly changing given the current climate. In 2013, Porter and Jacobson (54) published the self-reported experience of 19 patients whose families had given their children some form of high-CBD medical marijuana product for severe intractable epilepsy. In this group, the reported “doses” of CBD administered ranged from <0.5 mg/kg/day to ~29 mg/kg/day. The majority of families reported improvement: Ten reported > 80% improvement, while two patients reported complete cessation of seizures. Others reported an ability to discontinue previously administered medications (54). Though clearly this data would not be considered high quality medical evidence, the information at the time was tantalizing.
Table 5. Clinical studies of cannabinoids in epilepsy- pre-2013.
| Study | No. of patients | Dose/duration | Results |
|---|---|---|---|
| Mechoulam, Carlini 1978 | 9 (R, 4 CBD) epilepsy | 200 mg/day 3 months | 2/4 CBD: seizure free; 0/5 P |
| Cunha et al., 1980 | Phase 1: 16 healthy; R, 8 CBD | 200-300 mg/day | Phase 1: 2/8 CBD-somnolence |
| Phase 2: R, 15; 2e Gen Epi with TL Focus; 8 CBD | Phase 2: 4/8 CBD-almost sz free | ||
| 3/8 partial improvement | |||
| 1/7 P seizure free | |||
| Ames, Cridland, 1986 | 12; MR; Intr Epi institutionalized | 200 mg/day (open label) | somnolence |
| Trembly 1990 (abstract only) | 12 pts; placebo phase, then R x-over | Placebo 6 mos 300 mg/day | “some benefit” |
CBD, cannabidiol; pts, patients.
In addition, considerable preclinical evidence regarding the potential efficacy of cannabinoids for the treatment of epilepsy does exist. Some of these studies began as early as the 1970s. Phytocannabinoids (particularly CBD) have been studied in a wide array of animal models of epilepsy (55-59). For the most part, these have demonstrated substantial efficacy. There is some evidence that THC itself can be pro- convulsant in some animal models (57). More recently, efficacy in animal models of temporal lobe epilepsy and partial seizures has been demonstrated (59). In addition there is some evidence that tolerance to the anticonvulsant effects of CBD is not a prominent feature in animal models of epilepsy (55). Thus based on these preclinical studies, one would be excited about the potential therapeutic potential of the cannabinoids. However, it is undeniable that the complex regulation that surrounds these schedule I substances has impeded scientific investigation of their therapeutic potential.
Spurred by the widespread interest in the therapeutic potential of CBD for the treatment of intractable childhood epilepsies, GW pharmaceuticals (the makers of Sativex) developed a pure CBD product known as Epidiolex. Epidiolex has been granted orphan drug status through the FDA for Dravet syndrome and Lennox-Gastaut syndrome. More than 20 expanded access IND’s were granted for the use of Epidiolex in up to 25 or more children per site for the treatment of severe intractable epilepsy. Initial reports of the experience with these children (again uncontrolled) were recently presented (60). 137 patients had received epidiolex for >3 months. Overall seizure frequency was reduced by 54% in all patients and by 63% in Dravet syndrome patients. At 3 months 9% of patients and 16% of Dravet patients were seizure free. Adverse effects were modest (somnolence, diarrhea, fatigue and decreased appetite). Though 22 severe adverse effects were deemed “possibly related” to study medication, only 14 of a total of 213 initially treated patients withdrew due to lack of efficacy or side effects (60). While the results remain promising, outcome data nevertheless is based on self-reported seizure frequencies, is uncontrolled and may suffer from the same methodological problems to some degree as did the Porter and Jacobson report. However, placebo controlled trials of Epidiolex for Dravet and Lennox-Gastaut syndrome are now in process. Consequently higher quality medical evidence surrounding the use of CBD for the treatment of pediatric epilepsy syndromes will be forthcoming. Meanwhile Insys Therapeutics, Inc. has developed a synthetic form of CBD and clinical trials have been initiated (61).
In parallel, families throughout the United States are gaining access to various vernacular “hemp oil” preparations with high CBD, low THC content. Here again the analysis of outcomes is complicated by the extraordinary variability between these products, relative lack of consistency in dosing, variable quality control, and uncertainty with respect to the presence or absence of other potentially bioactive constituents within these products (i.e., could there be an “entourage effect” in some instances?). A recent report from Colorado is intriguing in this respect (6). The authors indicate that while 57% of families reported positive results, there was no evidence of improvement in electroencephalogram patterns in 8 of the responders for whom data was available. In addition, there were significant adverse effects including increased seizures in 13%, status epilepticus and even death. Finally, it is intriguing to consider that the highest reported rate of benefit came from families who had specifically moved to Colorado in order to gain access to “hemp oil” products (47%) versus those who already lived in the state (22%) (6). This could strongly suggest a significant placebo effect in the self-reported seizure outcomes.
Clearly, this conundrum with respect to the outcomes of treatment of epileptic children with “medical marijuana” illustrates how challenging this field is given the “dizzying array” of preparations (6,45). These range from natural products to synthetic agents, substances delivered by inhalation versus those that are swallowed or even delivered by an oral-mucosal spray. Again, this makes comparison of outcomes among various studies nearly impossible. In general, as shown in Table 6, there are clearly distinct advantages to the use of a highly purified, pharmaceutical product. However, advocates of “medical marijuana” might argue that this approach ignores the potentially beneficial impact of the “entourage effect” (4,13).
Table 6. AAP Policy Statement concerning Marijuana, April 2015 (29).
| Opposed to Mj use in children and adolescents |
| Opposes MM use (outside of FDA etc…) |
| Recognizes option in desperate situations |
| Opposes legalization due to perceived risks |
| In states with legalized Mj, favors strict regulation of access |
| Supports R+D of Cannabinoid products |
| Recommends change to schedule II |
| In states with legalized Mj, we should advocate for controls similar to alcohol and tobacco |
| Revenue from regulation should go to research |
| Childproof packaging and related precautions |
| Supports decriminalization of possession |
| Strongly opposes smoked Mj |
| Discourages use of Mj by adults in presence of minors |
Mj, Marijuana; MM, medical marijuana; R-D, research and development.
Sociocultural considerations
Clearly, it is very difficult for the pediatric neurologist to navigate the complex legal, medical, psychological and sociocultural complexities surrounding the use of “medical marijuana” in children with epilepsy. Challenges exist in general when it comes to balancing the increasing interest espoused by families in complementary or alternative therapeutic strategies with the trend in allopathic medicine toward increasing reliance on “evidence-based medicine.” This becomes even more complicated when the substance in question is controlled by rigid and intimidating federal regulation, while simultaneously being subject to highly variable, contradictory and rapidly changing state regulations (4,5,8). Specifically according to the Controlled Substance Act of 1970, the federal government has placed marijuana and THC under Schedule I of controlled substances. This designation indicates that marijuana and THC are considered to have: (I) high potential for abuse; (II) no currently accepted medical use in the United States; and (III) lack of accepted safety. In addition, this federal regulation specifies that all species of plants from which controlled substances in Schedule I derive are similarly controlled and subject to the same penalties. By virtue of this extended concept of what constitutes a marijuana product, the DEA and FDA have made it clear that CBD falls under this same rubric (Schedule I). This is despite the fact that most experts and considerable evidence now suggest that this particular phytochemical in fact does not have abuse potential and is clearly of substantial medical interest at this time (13,25,27).
In the meanwhile, state regulation of marijuana ranges from complete proscription to full legalization. Some states have enacted CBD-specific laws (Utah, Georgia, others) which allow families to administer CBD-rich, THC-poor marijuana products to their children with intractable epilepsy under very specific circumstances in part regulated by the respective state health departments (5). However, it is left up to families themselves, with or without the tacit assistance of physicians, to determine how to obtain these substances, judge the quality of the available products, and determine what amount to administer (2,6,54). The role of the pediatric neurologist in this setting is complicated by the fact that it is technically illegal for a physician to “prescribe” these substances under federal law. Furthermore, institutions such as children’s hospitals may decide that administration of these substances within the confines of the hospital may put their staff or even their credentialing at risk. Consequently, there is the uncomfortable potential that a child benefiting from a vernacular “hemp-oil” product would not be permitted to receive it in the hospital, conceivably putting the child at risk in the event the vernacular substance is in fact functioning as an effective anticonvulsant.
There is also considerable concern that the “legalization” of marijuana or the liberalization of medical marijuana laws within states could have a variety of deleterious public health effects (4,8,62-65). Concerns have been raised with respect to increase in violent crime or delinquent behavior (66), potential for increase in driving related accidents or deaths (8), potential for neurotoxicity due to increased use of recreational marijuana products (62), unintended neurotoxicity in children (65), and the theoretical (largely unproven) proposition that increased access to cannabis serves as a gateway to more serious drug abuse behaviors (28,29). With respect to these various public health concerns, actual data is in fact conflicting (28,66) and to some degree data available so far is less alarming than might be imagined by those who strongly oppose the liberalization or legalization of medicinal marijuana products. On the other hand, the American Academy of pediatrics has recently published a position paper/consensus statement with respect to this (28,29) (Figure 4) which clearly states the Academy’s position against the legalization of marijuana but in favor of changing the schedule status of marijuana from Schedule I to Schedule II to facilitate quality scientific research in this area.
Figure 4.
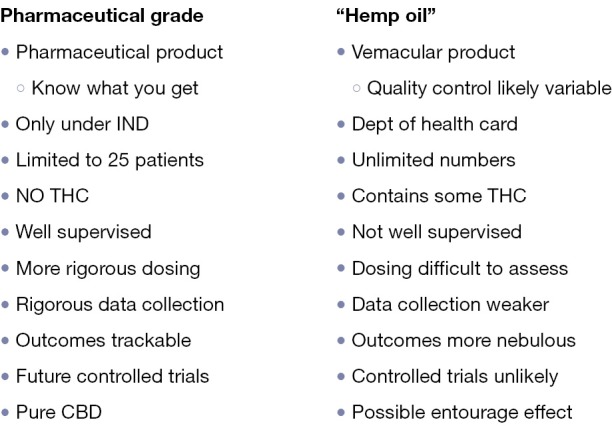
Comparison of Pharmaceutical Grade Cannabidiol (CBD) vs. Vernacular Preparations (“hemp-oil”). THC, tetrahydrocannabinol.
Recommendations for the future
It is an exciting time with respect to the study of phytochemicals in their application to the treatment of epilepsy and in particular intractable pediatric epilepsies. It is particularly satisfying to see that the grassroots experiences of families who have children suffering from severe intractable epilepsy have been able to move the field forward so rapidly. The upwelling of interest has already had a remarkable impact and in the space of a few years a large body of medical evidence of increasing quality has been accumulated. Ongoing and anticipated double-blind placebo controlled trials promise the availability of high quality medical evidence in the near future. Hopefully, CBD and possibly other phytocannabinoids or combinations thereof will prove to be beneficial for at least a subset of epileptic children.
In order to foster this progress it is suggested that pediatric neurologists may wish to advocate for the following:
Improved public understanding of the complexity of “medical marijuana” and of the value of high quality medical evidence in guiding therapeutic decisions;
Improved physician understanding of “medical marijuana”, the broad range of preparations subsumed under this misleadingly simple term, and the potential risks and benefits deriving from the disparate chemical substances and products falling under this rubric;
A change in federal regulations that would facilitate carefully conducted, scientifically driven, basic, preclinical and clinical studies of phytocannabinoids in the treatment of various neurological diseases including epilepsy.
Furthermore, pediatric neurologists are encouraged to inform themselves on the specifics of federal and local state regulations so as to be able to best inform and advocate for their patients.
Acknowledgements
The author thanks Claire Filloux, Ph.D., for preparation of Figures 1,3, and Matthew Sweney, MS, MD for careful review of the manuscript.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Gupta S. Why I changed my mind on weed. 2013. Available online: http://www.cnn.com/2013/08/08/health/gupta-changed-mind-marijuana/
- 2.Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia 2014;55:783-6. [DOI] [PubMed] [Google Scholar]
- 3.Welty TE, Luebke A, Gidal BE. Cannabidiol: promise and pitfalls. Epilepsy Curr 2014;14:250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clin Proc 2012;87:172-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom S. CBD Oil Now Legal in 15 States. 2015. Available online: http://www.celebstoner.com/news/marijuana-news/2014/03/13/four-states-on-verge-of-passing-cbd-only-laws/
- 6.Press CA, Knupp KG, Chapman KE. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav 2015;45:49-52. [DOI] [PubMed] [Google Scholar]
- 7.Greydanus DE, Hawver EK, Greydanus MM, et al. Marijuana: current concepts(†). Front Public Health 2013;1:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson ST, D’Souza DC. Problems with the medicalization of marijuana. JAMA 2014;311:2377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubens M. Political and medical views on medical marijuana and its future. Soc Work Public Health 2014;29:121-31. [DOI] [PubMed] [Google Scholar]
- 10.Hill RJ. Marijuana, Cannabis sativa L: Moraceae, Cannaboideae. Regulatory horticulture 1983. Available online: http://www.agriculture.pa.gov/Protect/PlantIndustry/NIPPP/Documents/marijuana%20article.pdf
- 11.Borgelt LM, Franson KL, Nussbaum AM, et al. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 2013;33:195-209. [DOI] [PubMed] [Google Scholar]
- 12.Fine PG, Rosenfeld MJ. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med J 2013;4:e0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011;163:1344-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill AJ, Williams CM, Whalley BJ, et al. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther 2012;133:79-97. [DOI] [PubMed] [Google Scholar]
- 15.Hill TD, Cascio MG, Romano B, et al. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br J Pharmacol 2013;170:679-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci 2014;5:1131-41. [DOI] [PubMed] [Google Scholar]
- 17.Clark PA, Capuzzi K, Fick C. Medical marijuana: medical necessity versus political agenda. Med Sci Monit 2011;17:RA249-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang R, Yamaori S, Okamoto Y, et al. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinet 2013;28:332-8. [DOI] [PubMed] [Google Scholar]
- 19.Walzer M, Bekersky I, Blum RA, et al. Pharmacokinetic drug interactions between clobazam and drugs metabolized by cytochrome P450 isoenzymes. Pharmacotherapy 2012;32:340-53. [DOI] [PubMed] [Google Scholar]
- 20.Geffrey AL, Pollack SF, Bruno PL, et al. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015;56:1246-51. [DOI] [PubMed] [Google Scholar]
- 21.Kano M, Ohno-Shosaku T, Hashimotodani Y, et al. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 2009;89:309-80. [DOI] [PubMed] [Google Scholar]
- 22.Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci 2012;35:529-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 2008;153:199-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rom S, Persidsky Y. Cannabinoid receptor 2: potential role in immunomodulation and neuroinflammation. J Neuroimmune Pharmacol 2013;8:608-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014;55:791-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol 2009;71:283-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izzo AA, Borrelli F, Capasso R, et al. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 2009;30:515-27. [DOI] [PubMed] [Google Scholar]
- 28.Ammerman S, Ryan S, Adelman WP, et al. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics 2015;135:e769-85. [DOI] [PubMed] [Google Scholar]
- 29.Committee on Substance Abuse, Committee on Adolescence ; Committee on Substance Abuse Committee on Adolescence. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics 2015;135:584-7. [DOI] [PubMed] [Google Scholar]
- 30.Gordon AJ, Conley JW, Gordon JM. Medical consequences of marijuana use: a review of current literature. Curr Psychiatry Rep 2013;15:419. [DOI] [PubMed] [Google Scholar]
- 31.Henquet C, Kuepper R. Does cannabidiol protect against the negative effects of THC? Br J Psychiatry 2010;197:259-60. [DOI] [PubMed] [Google Scholar]
- 32.Schubart CD, Sommer IE, van Gastel WA, et al. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr Res 2011;130:216-21. [DOI] [PubMed] [Google Scholar]
- 33.Morgan CJ, Schafer G, Freeman TP, et al. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study. Br J Psychiatry 2010;197:285-90. [DOI] [PubMed] [Google Scholar]
- 34.Bolla KI, Brown K, Eldreth D, et al. Dose-related neurocognitive effects of marijuana use. Neurology 2002;59:1337-43. [DOI] [PubMed] [Google Scholar]
- 35.Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: an FMRI study. Drug Alcohol Depend 2009;105:139-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gruber SA, Silveri MM, Dahlgren MK, et al. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Exp Clin Psychopharmacol 2011;19:231-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith MJ, Cobia DJ, Wang L, et al. Cannabis-Related Working Memory Deficits and Associated Subcortical Morphological Differences in Healthy Individuals and Schizophrenia Subjects. Schizophr Bull 2014;40:287-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavisian B, MacIntosh BJ, Szilagyi G, et al. Effects of cannabis on cognition in patients with MS: a psychometric and MRI study. Neurology 2014;82:1879-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trezza V, Cuomo V, Vanderschuren LJMJ. Cannabis and the developing brain: Insights from behavior. Eur J Pharmacol 2008;585:441-52. [DOI] [PubMed] [Google Scholar]
- 40.Batalla A, Bhattacharyya S, Yücel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One 2013;8:e55821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zalesky A, Solowij N, Yücel M, et al. Effect of long-term cannabis use on axonal fibre connectivity. Brain 2012;135:2245-55. [DOI] [PubMed] [Google Scholar]
- 42.Jager G, Ramsey NF. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: an overview of animal and human research. Curr Drug Abuse Rev 2008;1:114-23. [DOI] [PubMed] [Google Scholar]
- 43.Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol 2010;92:370-85. [DOI] [PubMed] [Google Scholar]
- 44.Griffith-Lendering MF, Wigman JT, Prince van Leeuwen A, et al. Cannabis use and vulnerability for psychosis in early adolescence--a TRAILS study. Addiction 2013;108:733-40. [DOI] [PubMed] [Google Scholar]
- 45.Koppel BS, Brust JC, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014;82:1556-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Notcutt W, Langford R, Davies P, et al. A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex® (nabiximols). Mult Scler 2012;18:219-28. [DOI] [PubMed] [Google Scholar]
- 47.Flachenecker P. A new multiple sclerosis spasticity treatment option: effect in everyday clinical practice and cost-effectiveness in Germany. Expert Rev Neurother 2013;13:15-19. [DOI] [PubMed] [Google Scholar]
- 48.Tanasescu R, Constantinescu CS. Pharmacokinetic evaluation of nabiximols for the treatment of multiple sclerosis pain. Expert Opin Drug Metab Toxicol 2013;9:1219-28. [DOI] [PubMed] [Google Scholar]
- 49.Leussink VI, Husseini L, Warnke C, et al. Symptomatic therapy in multiple sclerosis: the role of cannabinoids in treating spasticity. Ther Adv Neurol Disord 2012;5:255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev 2012;6:CD009270. [DOI] [PubMed] [Google Scholar]
- 51.Ames FR, Cridland S. Anticonvulsant effect of cannabidiol. S Afr Med J 1986;69:14. [PubMed] [Google Scholar]
- 52.Trembly B. Sherman M. Double-blind clinical study of cannabidiol as a secondary anticonvulsant. Marijuana’90 International Conference on Cannabis and Cannabinoids. Kolympari, Crete 1990;8-11. [Google Scholar]
- 53.Cunha JM, Carlini EA, Pereira AE, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 1980;21:175-85. [DOI] [PubMed] [Google Scholar]
- 54.Porter BE, Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav 2013;29:574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karler R, Turkanis SA. The Cannabinoids as Potential Antiepileptics. J Clin Pharmacol 1981;21:437S-48S. [DOI] [PubMed] [Google Scholar]
- 56.Turkanis SA, Karler R. Electrophysiologic properties of the cannabinoids. J Clin Pharmacol 1981;21:449S-63S. [DOI] [PubMed] [Google Scholar]
- 57.Karler R, Calder LD, Turkanis SA. Prolonged CNS hyperexcitability in mice after a single exposure to delta-9-tetrahydrocannabinol. Neuropharmacology 1986;25:441-6. [DOI] [PubMed] [Google Scholar]
- 58.Jones NA, Hill AJ, Smith I, et al. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther 2010;332:569-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones NA, Glyn SE, Akiyama S, et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure 2012;21:344-52. [DOI] [PubMed] [Google Scholar]
- 60.Devinsky O, Sullivan J, Friedman D, et al. Epidiolex (Cannabidiol) in Treatment Resistant Epilepsy. AAN 67th annual meeting abstract, 2015. [Google Scholar]
- 61.Available online: http://www.insysrx.com/investors/recent-news/
- 62.Jouanjus E, Leymarie F, Tubery M, et al. Cannabis-related hospitalizations: unexpected serious events identified through hospital databases. Br J Clin Pharmacol 2011;71:758-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woods JA, Wright NJ, Gee J, et al. Cannabinoid Hyperemesis Syndrome: An Emerging Drug-Induced Disease. Am J Ther 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh TS, Van Dyke M, Maffey A, et al. Medical marijuana’s public health lessons--implications for retail marijuana in Colorado. N Engl J Med 2015;372:991-3. [DOI] [PubMed] [Google Scholar]
- 65.Wang GS, Roosevelt G, Heard K. Pediatric marijuana exposures in a medical marijuana state. JAMA Pediatr 2013;167:630-3. [DOI] [PubMed] [Google Scholar]
- 66.Morris RG, TenEyck M, Barnes JC, et al. The effect of medical marijuana laws on crime: evidence from state panel data, 1990-2006. PLoS One 2014;9:e92816. [DOI] [PMC free article] [PubMed] [Google Scholar]


