Abstract
Many patients with hepatorenal syndrome (HRS) end up receiving a combined liver and kidney transplant (CKLT) with preservation of native kidneys, specially type 1 HRS since is characterizes by a very rapid deterioration of renal function. Eventually, most of the patients regain renal function, but it is unknown if this is due to the transplanted kidney, the recovery of native renal function, or both. The aim of this study is to evaluate if there is recovery of native renal function in patients with HRS following CKLT. 22 patients (16 men; 6 women) with history of HRS and status post CKLT were studied. Mercapto-acetyltriglycine-3 renograms in the anterior and posterior views with the three kidneys in the field of view were simultaneously acquired. The renograms were analyzed by creating regions of interest around the transplanted and native kidneys. Relative contribution to the renal function, clearance, and effective renal plasma flow for the transplanted and native kidneys were obtained. 1/22 (4.5%) patients presented with a very poor functioning transplanted kidney, in 15/22 (68%) cases the combined native renal function was markedly poorer than the transplanted renal function and in 6/22 (27%) native kidneys showed a contribution to the renal function similar to the transplanted kidney. In conclusion, our series show that around 32% of the HRS patients recovered their native renal function after CKLT. Identification of common factors that affect recovery of native renal function may help to avoid unnecessary renal transplants, significantly reducing morbidity and cost, while facilitating a reallocation of scarce donor resources.
Keywords: Combined liver and kidney transplant, Economic and Social Research Institute, kidney transplant, renal insufficiency, renogram
Introduction
Hepatorenal syndrome (HRS) is the development of acute renal failure, despite histologically normal kidneys, in the setting of chronic hepatic failure.[1,2] Although the incidence of this syndrome is low, morbidity, and mortality for those affected is high. Type 1 HRS is characterized by rapid deterioration of renal function, and mortality tends to occur within the following 21 days following the diagnoses.[3] Although there are various pharmacologic treatment options as well as renal replacement therapy with dialysis, these are to be used as a bridge to transplant.[4,5] At that stage,[6] one of the most accepted transplantation treatments is the combined kidney and liver transplantation (CKLT) leaving the native kidneys in place.[4,7,8]
These patients tend to regain renal function post CKLT, but it is unknown whether this is secondary to the transplanted kidney, to the recovery of the native renal function after improvement in liver function, or both. There is a paucity of publications on the posttransplant renal function in these patients, maybe partially explained because most HRS patients in the past did not survive long enough to have liver transplantation.[3]
As we presented back in 2007,[9,10] there may be a recovery of native renal function in patients with HRS following CKLT, and the aim of this study has been to evaluate, confirm and develop a method to analyze it by means of using mercaptoacetyltriglycine (MAG-3) renogram. If determined, it would then be very important to investigate the factors that potentially affect recovery of native renal function. Furthermore, this could have the potential to enable avoidance of unnecessary renal transplantation.
Materials and Methods
Patient recruitment
Patients were recruited following an institutional review board approved protocol. Inclusion criteria: All patients with type 1 HRS who received a CKLT at the University of California, San Francisco from 1991 to 2007 with a baseline creatinine < 3 mg/dl prior to the onset of HRS and baseline epidermal growth factor receptor ≥ 30 ml/min/1.73 m2.
Of 44 eligible patients, 22 consented to receive a renogram study. Therefore, 22 patients (16 men; 6 women) with a history of type 1 HRS and status post CKLT with recovered renal function were referred by the Department of Nephrology to Nuclear Medicine for postoperative renograms. Mean age ± standard deviation (SD) was 54.9 ± 7.8 years old. The mean time ± SD from CLKT to MAG-3 renogram was 1178 ± 1239 days. There was a median duration of HRS of 29 days, and a need for renal replacement therapy of approximately 3 weeks.
Procedure
We developed the following imaging protocol, after patients were hydrated with 500 cc of water and immediately following the intravenous administration of 3 mCi (111 MBq) of MAG-3 (technetium-99m labeled MAG-3), dynamic imaging was acquired (at 1 min/frame) simultaneously in both the anterior and posterior views using a ADAC gamma camera (Philips (ADAC) Vertex Plus (V60) gamma camera and GE Xeleris PET workstation) with the patient in supine position. Images acquired in a 64 × 64 evaluate with parallel hole collimators. The field of view included all three kidneys on each projection. The dynamic data were acquired for 30 min.
ADAC and GE Xeleris software packages were available for processing these studies. These software packages are standardized for subjects with a single kidney as well as two kidneys, but not for three kidneys. The analytic approach consisted in manually drawing a region of interest (ROI) with background correction including both native kidneys (trying to avoid as much as possible inclusion of background or aortic activity) (native counts) and a single ROI around the transplanted kidney with background correction (trying to avoid nearby urinary activity) (transplanted counts). This was performed in the anterior and the posterior projections.
An initial attempt to analyze the renal function in the kidneys was by calculating a geometric mean of the total counts obtained in the native and transplanted ROIs according to the formula.
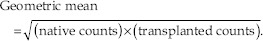
For instance
However, the geometric mean indicates the central tendency or typical value of a set of numbers and clearly could underestimate/overestimate renal function, especially by the kidney/s with the highest/lowest contribution.
Therefore, a new approach was pursued. Without attenuation correction, the closest approach to analyze real total counts within the ROIs would be by using the counts from the native kidneys in the posterior view and the counts from the transplanted kidney in the anterior view. Therefore, and following this argument, total counts = native counts (posterior) + transplanted counts (anterior).
For instance

Both approaches were used for the analysis of the first 10 patients. The values obtained with these two methods were correlated with the patient's clearance and effective renal plasma flow (eRPF). Correlation coefficients were obtained. Then, a decision was made to choose the most accurate method for the analysis and presentation of the rest of the data.
Time activity curves were generated, as well as relative contributions to the overall renal function of each kidney to global renal function, using the 1st 2 min. Camera-based MAG-3 clearance without blood or urine sampling as previously described by Taylor et al.[11] (normal for adult men 238 ml/min/1.73 m2; 226 ml/min/1.73 m2 for women) was calculated.[12]
eRPF = RPF × extraction ratio
(where extraction ratio is the ratio of compound entering the kidney that was excreted into the final urine) from the transplanted and native kidneys were also calculated using clinical package software in a dedicated workstation using the Schlegel's method.[12,13] Since eRPF does not show significant differences between genders, but it is known to decrease with age, based on Lin et al. calculations (group 51–60 years normal eRPF value of 559.5 ± 102 ml/min) and considering that our cohort has a mean age of 54.9 ± 7.8 years old[14] an eRPF value of 559.5 ± 102 ml/min was considered normal. No depth correction was calculated, but parameters were corrected for body surface area.
Each case was analyzed a minimum of 3 times by the same American Board of Neurophysiologic Monitoring certified MD. A second nuclear physician was trained to perform the same analysis.
Results
The method of analysis showed no significant minimal inter or intraobserver variability.
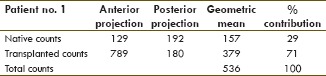
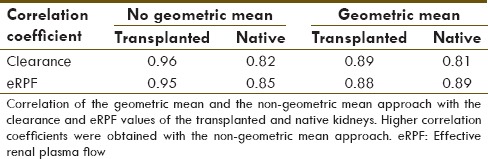
The comparison of the two approaches to calculate % contribution to the renal function in the first 10 patients showed, as hypothesized, that the geometric mean underestimates the renal function of the kidney/s with the highest contribution and overestimating the ones with the lowest contribution [Table 1]. In addition, it correlates better with the eRPF and clearance of the patients [Table 2]. Therefore, the nongeometric mean approach was used for the presentation of the following data.
Table 1.
Geometric mean vs Non geometric mean
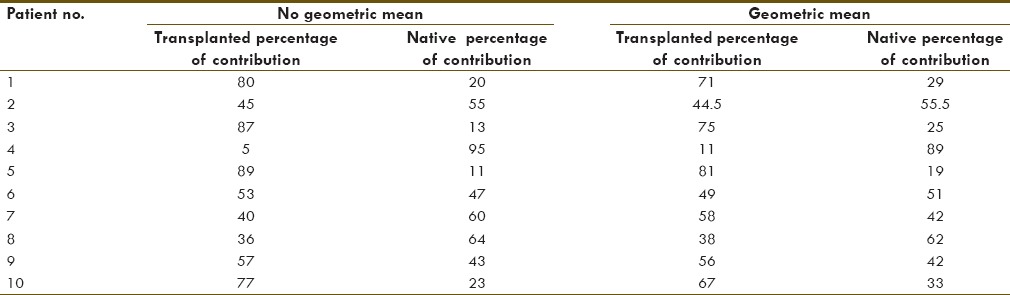
Table 2.
Correlation coefficient
One patient 1/22 (4.5%) presented with a very poor functioning transplanted kidney (contribution to renal function: 4.7%, clearance: 42 ml/min, and eRPF: 34 ml/min). Contribution to renal function by the native kidneys was 95% [Table 3 and Figure 1].
Table 3.
Patient summary table
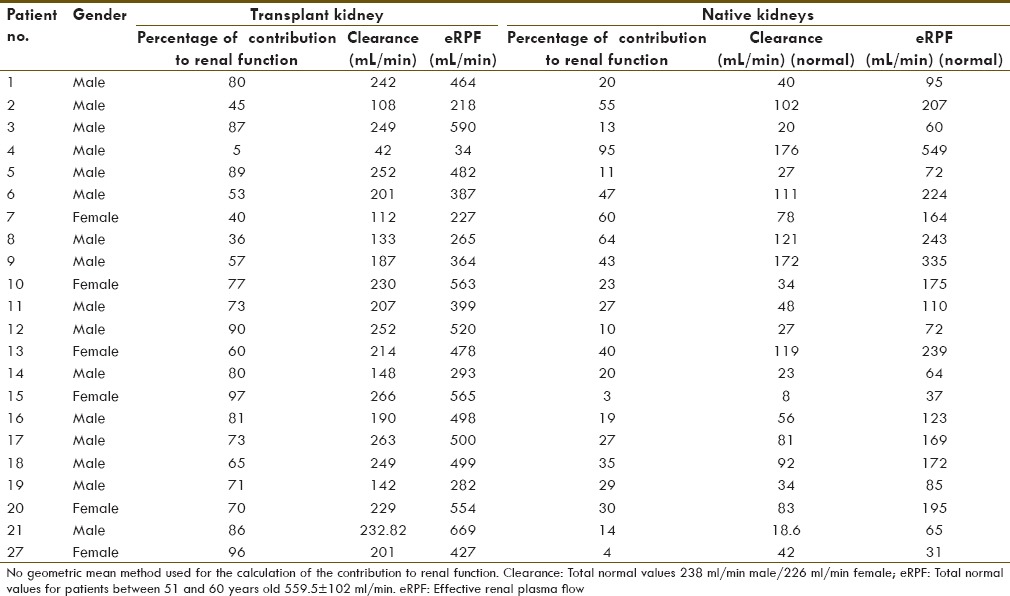
Figure 1.
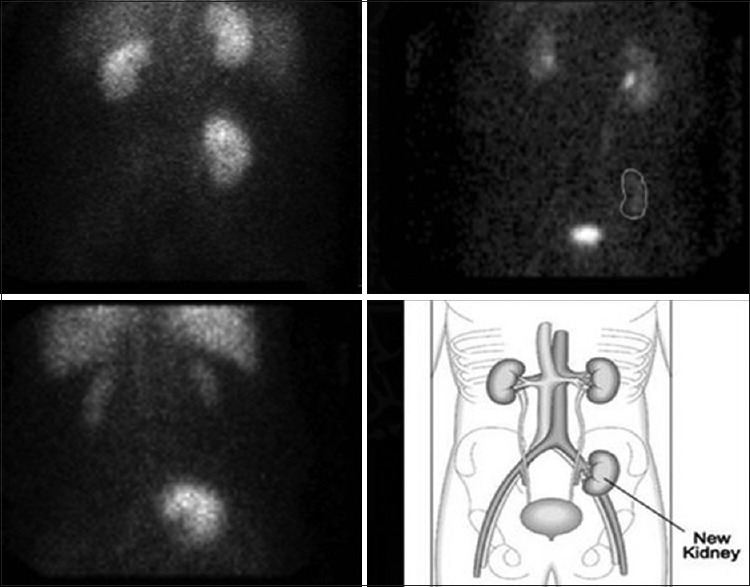
Three different examples of the type of renograms that were seen in the study. The different panels show a sum image of the 30 min dynamic acquisition in an anterior view with the three kidneys in the field of view. Panel A shows a sum image of the mercapto-acetyltriglycine (MAG-3) renogram of patient no. 7. The study shows similar contribution to the renal function by the three kidneys. Panel B shows a sum image of the MAG-3 renogram of patient no. 5. The study shows decreased contribution to the renal function by the native kidneys. Panel C shows a sum image of the MAG-3 renogram of patient no. 4. The study shows decreased contribution to the renal function by the transplanted kidney. Panel D is a sketch to facilitate identification of the three kidneys in the anterior view
In 15/22 (68%) cases, the combined native renal function was poorer than the transplanted renal function, with a contribution to the renal function ≤ 40% (contribution to renal function: 21 ± 6 native vs. 81 ± 6% transplanted, clearance: 34 ± 21 native vs. 223 ± 39 ml/min transplanted, and eRPF: 97 ± 46 native vs. 479 ± 89 ml/min transplanted). In 7/22 (32%) cases, the combined native renal function was severely poorer than the transplanted renal function, with a contribution to the renal function ≤ 20% [Table 3].
In 6/22 (27%) native kidneys showed a contribution to the renal function similar to the transplanted kidney, between 40% and 64% (contribution to real function: 48 ± 9 native vs. 52 ± 9% transplanted, clearance: 117 ± 31 native vs. 159 ± 47 ml/min transplanted, eRPF: 235 ± 57 native vs. 323 ± 103 ml/min transplanted) [Table 3].
Of note, some patients (patients no. 7, 14 and 19) showed moderately decreased MAG-3 camera-based clearance and eRPF.
In all cases, each native kidney contributed similarly to the native renal function.
Discussion
Combined liver and kidney transplant is a common and very aggressive treatment of type 1 HRS that involves significant morbidity, very high cost and uses significant donor resources. Eventually, most of these patients regain renal function post CKLT; however, since these native kidneys characteristically show a normal histology, it is unclear whether these are able to become functional again after normalization of the liver function or stay indefinitely damaged.
Our group has been interested since 2007[9,10] in knowing if there is a recovery of native renal function in patients with HRS and also in knowing how to calculate and quantify it by using MAG-3 renograms.
There are Food and Drug Administration approved software packages available for processing of MAG-3 renograms. These packages are standardized for subjects with a single kidney and two kidneys, but not for three kidneys (our case). Therefore, we developed an imaging protocol that included the three kidneys within the field of view and dynamic images were acquired simultaneously in anterior and posterior. Then, we developed an analytic approach that consisted in manually drawing a ROI with background correction including both native kidneys (native counts) and another single ROI around the transplanted kidney (transplanted counts). This was performed by in the anterior and the posterior projections.
An initial attempt to analyze the renal function in the kidneys was by calculating a geometric mean of the total counts obtained in the native and transplanted ROIs. Other groups have used this method.[15] However, the geometric mean indicates the central tendency or typical value of a set of numbers and expected underestimate and overestimate renal function in the kidney/s with the highest and lowest contribution, respectively.
Therefore, a new approach was pursued. Without attenuation correction, the closest approach to analyze real total counts within the ROIs would be by using the counts from the native kidneys in the posterior view and the counts from the transplanted kidney in the anterior view. Therefore, and following this argument, total counts = native counts (posterior) + transplanted counts (anterior). By using this approach, the contribution to the renal function correlated better with the clearance and eRPF of the native and transplanted kidneys, than the geometric mean.
Although correction for attenuation definitely makes the calculation of real function more accurate, as already published in the literature,[16] the corrections are not performed without complex calculations. Besides, although calculation of the distance skin-renal hilum is a great way of calculating the depth of the kidneys, it is still not ideal since not all the events arising from a bean shape organ with a volume of ~145 cm3 are at the same distance from the skin as the hilum.
Some authors have recommended single-photon emission computed tomography/computed tomography (SPECT/CT) after MAG-3 dynamic acquisition for a more accurate correction for attenuation.[17] Although, this is probably the most accurate method to correct for attenuation, 16 slice SPECT/CT scanners are not the community standard. SPECT alone is still considered community standard although it is moving toward at least one single slice SPECT/CT scanner. However, the hilum would be difficult to identify in a single slice. In addition, the acquisition of the additional SPECT/CT postrenogram would add significant time and extra radiation to the study, difficult to justify since the dynamic scan, which is the one that provides the diagnostic, has already been performed; unless it shows significant gain like changes in patient's management.
Patients post CKLT have three kidneys, two natives and one transplanted pelvic kidney. If these three kidneys were to be healthy, meaning that there was no intrinsic renal disease in either the native kidneys prior to transplant or the transplanted renal allograft, it would be anticipated that each one of them should contribute 33% to the patient's global renal function. Therefore, a complete recovery of native renal function should have an anticipated contribution to renal function of ~66%. In our series, only 2/22 patients showed a native contribution close to ~66%, patient nos. 7 and 8, with a native contribution of 60% and 64%, respectively. However, a total of 6/22 (27%) patients showed a significant contribution to the renal function similar to that of the transplanted kidney, between 40% and 64%, respectively.
Although our number of patients is small (22 patients), the importance of our findings could be significant considering the rareness of the HRS. This % of contribution certainly opens to discussion the need for renal transplantation in all these patients and prompts the need for studies to investigate the factors that potentially affect/predict recovery of native renal function post CLKT.
It was clear in our study that the transplanted kidneys as part of a CKLT overall were of good quality and were significant contributors to the patient's global renal function. However, in our series, one patient 1/22 (4.5%) presented with a poorly functioning transplanted kidney (contribution to renal function: 4.7%, clearance: 42 ml/min, and eRPF: 34 ml/min), unperceived due to an otherwise unknown complete recovery of the native kidneys. Without a renal allograft biopsy it is difficult to determine why this transplanted kidney had such poor function; but possibilities include severe postoperative acute tubular necrosis that did not recover, rejection, or undiagnosed intrinsic renal disease in the donor. Although a single case in our series, this discovery raises the following questions: Would, and perhaps more importantly, how often do the native kidneys have recovered after liver transplant alone? Does the alteration in renal blood flow that occurs with a third kidney result in delayed native recovery? Should these patients receive a closer evaluation with a noninvasive method that allows characterization and quantification of the native and the transplanted renal function, independently? MAG-3 renograms have all those attributes and provide minimum radiation (0.8 mSv), equivalent to the radiation received for 95 days from natural background radiation normal function.[18]
Conclusion
We presented in 2007 that some patients with HRS status post CKLT are able to have significant recovery of their native kidneys with MAG-3 renograms. Since then, we have been evaluating more cases and perfecting our imaging technique for qualitative and quantitative purposes, as presented here.
In our series, 32% of patients diagnosed with type 1 HRS and recover renal function were able to recover functionality of their native kidneys after CKLT. One of the patients had an unknown nonfunctional transplanted kidney.
According to our findings, it appears that these patients could benefit from closer evaluations with a noninvasive method like MAG-3 renogram, since it allows characterization and quantification of the native and the transplanted renal function, independently. It also may be helpful to know the factors that potentially affect recovery of native renal function, in order to anticipate those HRS patients whose native renal function is likely to return following liver transplantation. If determined, this has the potential to enable avoidance of unnecessary renal transplantation, which would significantly reduce the morbidity and cost associated with treating HRS patients, while also facilitating reallocation of scarce donor resources.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164–76. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 2.Carl DE, Sanyal A. The management of hepatorenal syndrome. Minerva Gastroenterol Dietol. 2009;55:207–26. [PubMed] [Google Scholar]
- 3.Munoz SJ. The hepatorenal syndrome. Med Clin North Am. 2008;92:813. doi: 10.1016/j.mcna.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Kiser TH, Maclaren R, Fish DN. Treatment of hepatorenal syndrome. Pharmacotherapy. 2009;29:1196–211. doi: 10.1592/phco.29.10.1196. [DOI] [PubMed] [Google Scholar]
- 5.Slack AJ, Wendon J. The liver and kidney in critically ill patients. Blood Purif. 2009;28:124–34. doi: 10.1159/000227281. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, Ling Q, Zhang M, Gao F, He Z, You J, et al. Outcome of patients with hepatorenal syndrome type 1 after liver transplantation: Hangzhou experience. Transplantation. 2009;87:1514–9. doi: 10.1097/TP.0b013e3181a4430b. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland SM, Alexander SR, Sarwal MM, Berquist WE, Concepcion W. Combined liver-kidney transplantation in children: Indications and outcome. Pediatr Transplant. 2008;12:835–46. doi: 10.1111/j.1399-3046.2008.01041.x. [DOI] [PubMed] [Google Scholar]
- 8.del Pozo AC, Martín JD, Rodriguez-Laiz G, Sturdevant M, Iyer K, Schwartz M, et al. Outcome of combined liver and kidney transplantation in hepatitis C: A single-center long-term follow-up experience. Transplant Proc. 2009;41:1713–6. doi: 10.1016/j.transproceed.2009.02.103. [DOI] [PubMed] [Google Scholar]
- 9.Carlson D, Liou D, Nguyen N, Qian J, Hawkins R, Mari C. Evaluation of recovery of native renal function in patients with hepatorenal syndrome following liver and kidney transplant. Is Kidney Transplantation Necessary? WRMSNM. 2007 [Google Scholar]
- 10.Jesse Q, Mari C, Vincenti F. Recovery of native function from hepatorenal syndrome after combined kidney-liver transplant. Transplant Summit. 2007 [Google Scholar]
- 11.Taylor A, Jr, Corrigan PL, Galt J, Garcia EV, Folks R, Jones M, et al. Measuring technetium-99m-MAG3 clearance with an improved camera-based method. J Nucl Med. 1995;36:1689–95. [PubMed] [Google Scholar]
- 12.Esteves FB, Taylor A, Manatunga A, Folks RD, Krishnan M, Garcia E. 99mTc-MAG3 renography: Normal values for MAG3 clearance and curve parameters, excretory parameters, and residual urine volume. AJR Am J Roentgenol. 2006;187:610–7. doi: 10.2214/AJR.05.1550. [DOI] [PubMed] [Google Scholar]
- 13.Riedinger-Berriolo A, Touzery C, Chevet D, Toubeau M, Riedinger JM, Mezeray C, et al. Normal range of [99mTc] MAG-3 renogram parameters in renal transplant recipients. Transplant Proc. 1994;26:301–2. [PubMed] [Google Scholar]
- 14.Lin WY, Changlai SP, Kao CH. Normal ranges of renal physiological parameters for technetium-99m mercaptoacetyltriglycine and the influence of age and sex using a camera-based method. Urol Int. 1998;60:11–6. doi: 10.1159/000030196. [DOI] [PubMed] [Google Scholar]
- 15.Mansouri AM, Vejdani K, Bastani B, Nguyen NC. Calculation of renal differential function following renal transplant: Retrospective validation of a simplified method. Clin Nucl Med. 2012;37:931–8. doi: 10.1097/RLU.0b013e31826382e9. [DOI] [PubMed] [Google Scholar]
- 16.Francis JM, Palmer MR, Donohoe K, Curry M, Johnson SR, Karp SJ, et al. Evaluation of native kidney recovery after simultaneous liver-kidney transplantation. Transplantation. 2012;93:530–5. doi: 10.1097/TP.0b013e3182449161. [DOI] [PubMed] [Google Scholar]
- 17.Palmer MR, Donohoe KJ, Francis JM, Mandelbrot DA. Evaluation of relative renal function for patients who had undergone simultaneous liver–kidney transplants usingTc-99m-MAG3 scintigraphy with attenuation correction from anatomical images and SPECT/CT. Nucl Med Commun. 2011;32(8):738–44. doi: 10.1097/MNM.0b013e328347e958. [DOI] [PubMed] [Google Scholar]
- 18. [Last accessed on 2007 Feb 17]. Available from: http://www.doseinfo.radar.com .


