Abstract
Phenylketonuria (PKU) is an inborn error of metabolism of the amino acid phenylalanine. It is an autosomal recessive disorder with a rate of incidence of 1 in 10,000 in Caucasian populations. Mutations in the phenylalanine hydroxylase (PAH) gene are the major cause of PKU, due to the loss of the catalytic activity of the enzyme product PAH. Newborn screening for PKU allows early intervention, avoiding irreparable neurological damage and intellectual disability that would arise from untreated PKU. The current primary treatment of PKU is the limitation of dietary protein intake, which in the long term may be associated with poor compliance in some cases and other health problems due to malnutrition. The only alternative therapy currently approved is the supplementation of BH4, the requisite co-factor of PAH, in the orally-available form of sapropterin dihydrochloride. This treatment is not universally available, and is only effective for a proportion (estimated 30%) of PKU patients. Research into novel therapies for PKU has taken many different approaches to address the lack of PAH activity at the core of this disorder: enzyme replacement via virus-mediated gene transfer, transplantation of donor liver and recombinant PAH protein, enzyme substitution using phenylalanine ammonia lyase (PAL) to provide an alternative pathway for the metabolism of phenylalanine, and restoration of native PAH activity using chemical chaperones and nonsense read-through agents. It is hoped that continuing efforts into these studies will translate into a significant improvement in the physical outcome, as well as quality of life, for patients with PKU.
Keywords: Phenylalanine hydroxylase (PAH), phenylketonuria (PKU), mutation, phenotype-genotype correlation, therapy, diet
Phenylketonuria (PKU) and hyperphenylalaninaemia (HPA)
Phenylketonuria (PKU, OMIM 261600) is an inborn error of metabolism, predominantly caused by mutations in the phenylalanine hydroxylase (PAH) gene. The mode of inheritance is autosomal recessive. Mutations in PAH lead to impaired function of the hepatic enzyme PAH (EC 1.14.16.1), which catalyses the conversion of the essential amino acid l-phenylalanine (l-Phe) to l-tyrosine (l-Tyr), a precursor of the neurotransmitters dopamine, noradrenaline and adrenaline. The resulting elevated levels of l-Phe, a condition known as hyperphenylalaninaemia (HPA), which is the primary biochemical marker of PKU and the more benign form mild hyperphenylalaninaemia (MHP).
PKU was described by A. Følling in 1934 (1). The accumulation of l-Phe drives an alternate metabolic pathway, resulting in the detection of phenylketones (phenylpyruvate and phenylacetate) in the urine of PKU patients (2). These ketones are also excreted in sweat, creating a ‘mousy’ odour characteristic of the disease. The unravelling of the metabolic pathway from l-Phe to l-Tyr to subsequent products, and the subsequent development of a newborn screening strategy (3) were significant in the establishment of a treatment and in contributing to the understanding of metabolic diseases at that time. Prior to this, children with PKU failed to attain developmental milestones, ultimately exhibiting profound intellectual impairment. Hyperactivity and seizures also featured later in life, and many untreated individuals have a fairer complexion and hair colour than other family members (4). Most cases of PKU are caused by mutations in the PAH gene (1). The remaining cases are caused by mutations affecting either the synthesis or the regeneration of tetrahydrobiopterin (BH4), the co-factor of PAH (5). The genes involved in the BH4-deficiency type HPA include guanosine triphosphate cyclohydrolase I (GTPCH) (6), 6-pyruvoyl-tetrahydropeterin synthase (PTPS) (7), pterin-4a-carbinolamine dehydratase (PCD) (8), and quinoid dihydropteridine reductase (DHPR) (9). Sepiapterin reductase (SR) is also a genetic factor in BH4-deficiency disorders, but mutations in SR do not lead to HPA (10). The remainder of this review focuses on issues more relevant to HPA caused by a primary deficiency of PAH.
PKU occurs in approximately 1 in 10,000 Caucasian births (11), equivalent to a carrier rate of 1 in 50. However, the incidence rate may vary widely amongst different countries or regions (Table 1). Finland and Japan have the lowest reported incidence of PKU, at 5 and 8 cases per million births respectively (21,23). Incidence rates are highest in Sicily with 1 in 2,700 births diagnosed as PKU, and 1 in 4,200 in Turkey (13,23). There is also a high level of clinical heterogeneity in PKU. Disease severity can be classified by blood Phe levels. In patients with classic PKU, the most severe form, blood Phe levels can rise to over 1,200 µmol/L, compared to 120 µmol/L in a normal healthy person. In moderate and mild PKU, the values range from 900 to 1,200 µmol/L and 600 to 900 µmol/L respectively, and in non-PKU MHP, between 120 and 600 µmol/L (4).
Table 1. Incidence rates of PKU in different populations/countries.
| Ethnic group/population | Incidence of PKU | Reference |
|---|---|---|
| Sicily | 1:2,700 | Guldberg et al. [1993] (12) |
| Turkey | 1:4,200 | Ozalp et al. [2001] (13) |
| Ireland | 1:4,500 | Zschocke et al. [1997] (14) |
| Catalonia | 1:6,600 | Mallolas et al. [1999] (15) |
| Israel | 1:8,200 non-Jews; 1:12,500 Jews |
Bercovich et al. [2008] (16) |
| Caucasian | 1:10,000 | Zschocke [2003] (11) |
| Northern China | 1:11,000 | Song et al. [2005] (17) |
| Cuba | 1:20,000 | Desviat et al. [2001] (18) |
| Korea | 1:41,000 | Lee et al. [2004] (19) |
| Taiwan | 1:55,000 | Chien et al. [2004] (20) |
| Japan | 1:80,500 | Kimura et al. [2001] (21) |
| Finland | 1:100,000 | Guldberg et al. [1995] (22) |
PKU, phenylketonuria.
An additional complication is the effect of maternal PKU on foetal development. The features in the affected newborn (who in most cases does not have PKU) include microcephaly, congenital heart defects, dysmorphic facial features, intrauterine growth retardation, with often severe cognitive impairment becoming apparent in childhood (24,25). Treatment of women with PKU during pregnancy is vital for reducing the likelihood of the so-called maternal PKU syndrome in the offspring, and can be achieved by maintaining a Phe level of 120-360 µmol/L from the first trimester, and ideally even before conception (24).
Molecular bases of PKU
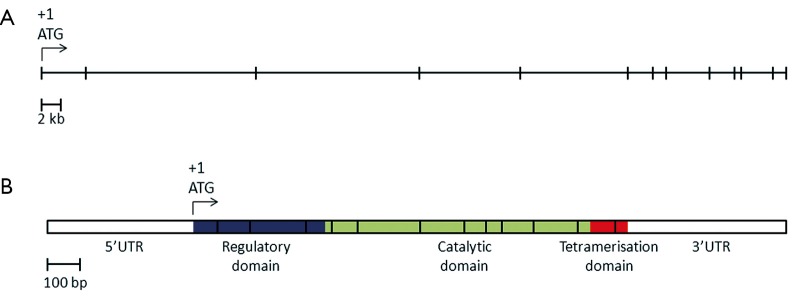
Mutations in the PAH gene accounts for 98% of cases of PKU (25). The human PAH gene maps to chromosome region 12q23.2, on the minus strand, spanning close to 80 kb (NCBI reference sequence NM_000277.1, Figure 1) (26,27). The gene consists of thirteen exons, forming a transcript with an open reading frame of 1,359 bases and encoding a polypeptide of 452 amino acids (NCBI reference sequence NP_000268.1).
Figure 1.
(A) Structure of the human PAH gene. The horizontal line represents the full length of the PAH gene, spanning 79.3 kb. Each vertical bar represents an exon. The location of the start codon ATG in exon 1 is indicated (+1); (B) Schematic representation of PAH mRNA. The vertical lines mark the boundaries between exons. The three functional domain of PAH are coloured purple for the regulatory domain, light green for the catalytic domain and orange for the tetramerisation domain. UTR, untranslated regions; PAH, phenylalanine hydroxylase.
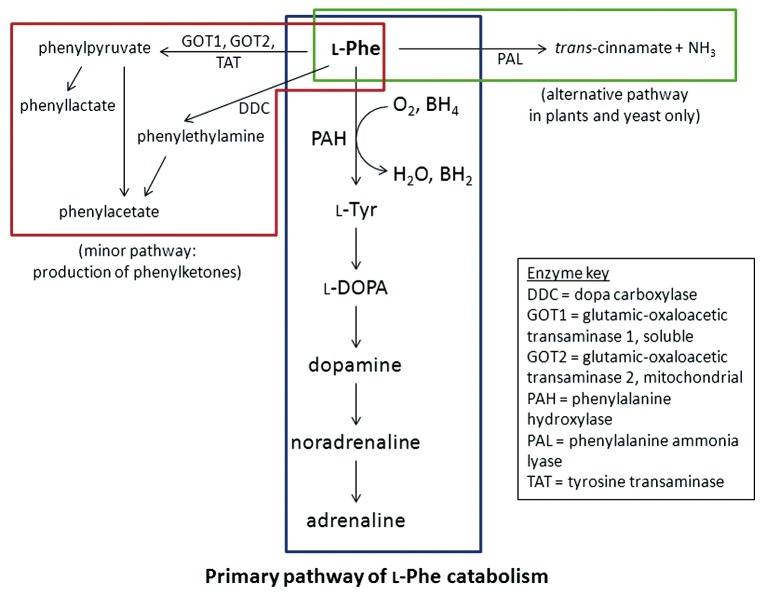
The PAH polypeptide is highly homologous in mammals and retains a high level of homology even in more distant eukaryotes. The 52 kDa polypeptide is divided into three functional domains (28). The N-terminus is the regulatory domain (amino acid residues 1-142), followed by the catalytic domain (residues 143-410) and the C-terminal tetramerisation domain (residues 411-452). The hepatic PAH enzyme catalyses the conversion of l-Phe to l-Tyr, in the presence of the co-factor BH4 [(6R)-l-erythro-5,6,7,8-tetrahydriobiopterin] and O2 (29). The l-Tyr is then further converted to neurotransmitters dopamine, noradrenaline and adrenaline. In the absence of the PAH, an alternative metabolic pathway breaks down l-Phe to phenylketones (Figure 2). In plants and fungi, another enzyme, PAL is involved in the catabolism of l-Phe, and enzyme substitution therapy using PAL is currently being investigated (30).
Figure 2.
Metabolic pathway of l-Phe. The primary pathway (blue box) is the catalytic conversion of l-phe to l-Tyr by phenylalanine hydroxylase (PAH). In phenylketonuria (PKU), the deficiency of PAH enzyme leads to the production of phenylketones by an alternative pathway (red box). A third pathway (green box) can be found in plants and yeast involving the enzyme phenylalanine ammonia lyase (PAL).
There are currently over 560 PAH mutations reported in the PAHdb (http://www.pahdb.mcgill.ca) (31). Mutations causing PKU have been identified in all three domains, but a mutation hotspot in exon 7 of PAH corresponds to the catalytic active site of the protein. The majority of pathogenic mutations in PAH are missense mutations (65.4% of mutations reported in PAHdb), followed by small insertions and/or deletions (both in-frame and frame-shift, 16.4%), mutations affecting splicing (12.2%), then nonsense mutations (5.2%). Large deletions and duplications, involving an entire exon or exons, have also been reported, but they are thought to account for less than 1% of disease alleles within a population (32-34).
As more PAH mutation data were made available, it became clear that there was a relationship between the mutations and the severity of the disorder, allowing classification of some PAH mutations as being likely to cause a particular phenotype (17,35-38). In silico prediction of phenotype (the severity of disease) from genotype may be useful in refining diagnosis and providing a baseline for treatment (36). However, the establishment of a system by which phenotype may be predicted from genotype is hampered by the large number of PAH mutations resulting in a larger number of possible genotype combinations.
The classification of a mutation as ‘severe’, ‘moderate’, ‘mild’ or ‘MHP’ is based upon individuals who are functionally hemizygous, whereby one of the alleles is shown or predicted to be a null allele (39). Null alleles (which are classed as ‘severe’ mutations) include nonsense mutations, frameshift mutations, splicing mutations affecting the canonical AG-GT dinucleotides at the exon-intron boundaries, and missense mutations that result in little residual enzymatic activity, such as p.Arg252Trp, p.Arg408Trp, p.Pro281Leu (36). Although most analyses have shown that this model is successful in correlating phenotype to genotype to a large degree, a significant number of discrepant findings have also been reported. This is most striking in patients who are homozygous for one mutation. For example, of the six patients homozygous for p.Leu48Ser reported in Bercovich et al., (16) two were classified as having classic PKU, 2 with moderate PKU and the other 2 with mild PKU. Similarly, in another study, three patients who are homozygous for p.Ile65Thr had the three classifications PKU, variant and non-PKU MHP (35).
Phenotype classification may vary between different centres and misclassification may contribute to some of the incongruities (36). The method of phenotype classification (by plasma Phe levels or dietary Phe tolerance) is also a possible source of misclassification, with the former more closely aligned with genotype predictions than the latter (36). Different studies or centres may also use different cut-off for classifying the severity of phenotypes. These arbitrary cut-off points may not necessarily reflect the continuum in both the clinical phenotype and in the residual activity in the various mutations. In addition, it is highly probable that other genetic factors play a role in determining PKU severity. Modifier genes, such as the loci involved in the regeneration of BH4, are postulated to have an effect on the phenotypic outcome (40). Similarly, the enzymes involved in the alternate catabolic pathway of l-Phe can modulate dietary Phe tolerance (41). Differences in the transport of l-Phe across the blood-brain barrier have also been suggested as a possible source of phenotypic variance (42).
In vitro studies have been valuable in demonstrating the nature of pathogenicity in many missense mutations and in elucidating the specific role of individual amino acids in the PAH peptide (43-45). In particular, analyses of the enzymatic activity of missense mutations have shown strong correlation to disease severity (46-48), and for rare missense mutations these studies are useful in predicting the metabolic phenotype. Missense PAH mutations may be grouped under broad headings: (I) null mutants with no detectable enzymatic activity; (II) Vmax mutants with reduced maximum activity; (III) kinetic mutants with altered KM for either substrate or co-factor and (IV) unstable mutants with decreased levels of PAH polypeptide (25).
Understanding the molecular defect of the missense mutations may also have therapeutic implications. For example, the aggregation of PAH protein carrying single amino acid changes such as p.Gly46Ser is thought to be caused by misfolding (49). Co-expression of bacterial chaperonins in Escherichia coli and other novel molecular chaperones increase both PAH protein levels and residual activity (50), giving rise to a new class of potential chemical compounds that may be of benefit for the treatment of PKU.
Treatment of PKU
The primary treatment of PKU is the restriction of dietary protein (and thus l-Phe). Due to early detection through newborn screening, treatment can be started in the first weeks of life. Early intervention and good dietary compliance are essential for cognitive outcome (measured as IQ) and for reducing the risk of neurological complications and behavioural problems in PKU patients (51). Whilst foods high in protein, including meat, dairy, nuts and legumes, should be eliminated or highly restricted, the intake of foods high in starch, e.g., potatoes, pasta, bread, needs to be monitored as well. Patients with the severe classic form of PKU must adhere most strictly to the dietary limitations and patients with non-PKU MHP may not require any dietary restrictions at all. Supplementary diet formulae exist for a variety of age ranges, and are critical to ensure the correct balance of the other essential amino acids, vitamins, minerals and trace nutrients are provided to reach recommended daily intake targets (4).
The target level of Phe concentration varies according to the age of the patient (52). In children under two years of age, the target concentration is generally below 360 µmol/L, and increasing to up to 1,200 µmol/L in adults in countries such as Germany, Austria and France, whilst at our clinic the target for adolescents and young adults is 750 µmol/L. The stringent restrictions at an early age are due to the particular sensitivity of the developing brain in young children to the neurotoxic effects of the elevated Phe levels (52). Diet termination at eight years of age resulted in a decrease of intellectual function in children (53), although the effects may be subtle (54). Similar findings have been shown in adult patients, as well as higher risks of eczema, phobias, depression and neurological problems (55). Therefore, maintaining treatment for the duration of their lifespan may be beneficial for patients with PKU. At the same time, it is important to remember that early intervention was only made possible by the introduction of newborn screening for PKU in the 1960s (3), and that the long term effects of treatment in PKU patients are still to be fully determined (56).
Concerns over dietary treatment of PKU
There are three main issues relating to the current treatment regimen for PKU: (I) the level of adherence to Phe-restricted diets, especially in older children and adults; (II) the nutritional adequacy of the Phe-restricted diet; and (III) the effects of the diet and management on physical and psychosocial patient wellbeing, including their quality of life.
The level of diet adherence in patients with PKU is similar to that reported by the World Health Organization for patients with chronic disease complying with treatment recommendations (57). A study of children in Italy under the age of 18 with PKU found 56% of them to be adherent to the dietary prescriptions, based on food diaries (58). Across ten PKU management centres in Europe, blood Phe concentrations revealed a marked decrease in diet compliance with increasing age, with 88% of children under one year old meeting their target Phe range, compared to only 65% in adults (59). The main challenges to diet adherence are inconvenience of meal preparations, limitations on food choices, palatability of the diet, cost and availability of treatment. Additional barriers include poor social functioning, social and family support and relationships, illiteracy or language difficulties in the patient or carers, social stigma, knowledge and ability of the carer (59). Cotugno et al. also found that the education level of the mother to be a major determinant of diet adherence in their study (58). An overly restrictive PKU diet can lead to growth impairment in early childhood (60), likely due to malnutrition. These diets are also low in long-chain polyunsaturated fatty acids (LCPUFA) and docosahexaenoic acid (DHA), both of which are important in neurological development (61,62). Supplementation of pre-formed LCPUFA have been shown to improve neurological function in children with PKU (63,64). Micronutrient deficiency is far more common and poses a greater danger (60). In patients not taking dietary supplements, deficiencies of vitamins A, C and E, coenzyme Q10, iron, zinc, calcium, manganese and selenium have been reported (52,65). Vitamin B12 deficiency, which can lead to neurological impairment, is also very common in older patients, due to its main source being meat and seafood (66-68). The problem is further compounded by the level of serum vitamin B12 not correlating to the level of functional vitamin B12, masking its deficiency in PKU patients (69). Children on amino acid-restrictive diets also have decreased bone mineral density, placing them at high risk of fractures and osteoporosis, despite receiving adequate levels of calcium, phosphorus and magnesium, and independent on the length of time on the diet (70,71). In addition, recent report has also suggested that patients on Phe-free formulae are at higher risk of developing chronic kidney disease (72).
Additional issues in the dietary treatment that require further addressing are related to the neurological or psychosocial outcomes and quality of life in patients with PKU (73). In spite of early intervention, children with PKU have lower intellectual functioning than their siblings and the general population (74,75), and poorer measures in other neurocognitive outcomes (76). Social and emotional difficulties have also been documented (77,78). Studies have also been carried out to determine how quality of life (QoL) in PKU patients is affected with a general perceived decreased in many aspects of QoL in both children and adults with PKU (79-83).
The discovery that a Phe-restricted diet was beneficial to PKU patients remains a prime example of nurture triumphing over nature (84). However, mounting evidence relating to diet adherence, nutritional deficiency and suboptimal neurological outcomes (and the interplay between the three factors) supports the need to find alternate treatment strategies for PKU.
Tetrahydrobiopterin (Sapropterin) treatment for PKU
Tetrahydrobiopterin (BH4) is the natural co-factor of PAH, and is a requisite for its catalytic activity. In 1999, Kure et al. first demonstrated that pharmacological doses of BH4 may lead to decreases in plasma Phe concentrations in patients with HPA caused by mutations in PAH (85). BH4 supplementation has also been shown to lead to a decrease in blood Phe level, improved Phe tolerance, i.e., the amount of Phe allowed in diet, resulting in relaxation of the Phe-restricted diets in many patients (86-88). Measures of Phe:Tyr and stability of Phe levels, which are hypothesised to be determinants of long-term neurological outcomes, have also been found to improve upon BH4 treatment (89,90). Other benefits reported include improvement of depression and panic attacks in an adult female patient (91).
BH4 supplementation is beneficial only in a proportion of PKU patients (so-called BH4-responsive). BH4-responsiveness can be assessed by a BH4 loading test, in which patients are given a single oral dose of 20 mg/kg. A reduction of Phe levels after 24 h of greater than 30% is defined as responsive (92). However, other variations in loading levels, time points at which measurements are taken, and Phe starting levels, have also been reported (93-95), and these may yield different response classifications, as some patients may show an initial Phe decrease (at 8 h) but return to baseline Phe levels at 24 h (96,97). On the other hand, some patients may return a positive response only at 48 h (94). Studies involving larger cohorts of patients revealed that BH4 supplementation is more effective in decreasing Phe levels in patients with milder forms of PKU, compared to those with the severe classical form (Table 2) (86,93). Overall, the percentage of PKU patients for whom BH4 doses may be of benefit is estimated to be 30% to 50% (106).
Table 2. Proportion of patients with MHP, mild or classical PKU who are BH4-responsive after a BH4-loading test.
| Reference | Phenotypea |
||
|---|---|---|---|
| MHP [%] | Mild/moderate [%] | Classical [%] | |
| Muntau et al., [2002] (86) | 10 of 10 [100] | 17 of 21 [81] | 0 of 7 [0] |
| Desviat et al., [2004] (98) | 6 of 6 [100] | Mild 6 of 12 [50]; moderate 1 of 5 [20] |
1 of 8 [13] |
| Pérez-Dueñas et al., [2004] (99) | 10 of 10 [100] | 9 of 12 [75] | 9 of 42 [21] |
| Hennermann et al., [2005] (100) | 11 of 12 [92] | 4 of 5 [80] | 3 of 23 [13] |
| Fiori et al., [2005] (101) | 87 of 90 [97] | 3 of 7 [43] | 1 of 10 [10] |
| Matalon et al., [2005] (102) | 2 of 3 [67] | 8 of 11 [73] | 12 of 24 [50] |
| Mitchell et al., [2005] (97) | 5 of 6 [83] | Mild 8 of 9 [89]; moderate 3 of 7 [43] |
1 of 15 [7] |
| Leuzzi et al., [2006] (103) | 5 of 5 [100] | 8 of 15 [53] | 4 of 30 [13] |
| Bóveda et al., [2007] (104) | 4 of 4 [100] | 4 of 7 [57] | 2 of 25 [8] |
| Burton et al., [2007]b (95) | 31 of 57 [54] | Mild 38 of 157 [24]; moderate 14 of 135 [10] |
13 of 136 [10] |
| Fiege and Blau, [2007]c (96) | 54 of 64 [84] | Mild 46 of 64 [72]; moderate 24 of 55 [44] |
11 of 110 [10] |
| Burlina and Blau, [2009] (105) | 11 of 11d [100] | 12 of 19d [63] | – |
| Anjema et al., [2011] (94) | 34 of 37 [92] | 33 of 47 [70] | 18 of 84 [21] |
a, unless otherwise specified, phenotypes classed by the following baseline Phe levels: MHP, 300-600 µM; mild, 600-900 µM; moderate, 900-1,200 µM; classical, >1,200 µM. b, Responsiveness was measured at day 8 with daily BH4 dose (10 mg/kg), compared to 24 h and 20 mg/kg BH4 in other studies; c, Data taken for responsiveness (>30% reduction) at 24 h; MHP, <500 µM; mild, 500-900 µM; moderate, 900-1,300 µM; classical, >1,300 µM; d, MHP, <450 µM, mild/moderate, 450-900 µM. PKU, phenylketonuria; MHP, mild hyperphenylalaninaemia.
Certain PAH mutations are frequently identified in BH4-responsive patients, suggesting that genotype may be an important factor in BH4-responsiveness (85). An allele is usually only considered to be BH4-responsive if it is identified in a homozygous patient or a patient compound heterozygous with a second null allele (107). However, there is also evidence that genotype is not the sole determinant of BH4-response. Whilst some mutations (e.g., p.Leu48Ser, p.Ile65Thr, p.Arg261Gln) can be found in BH4-responsive patients at a high frequency, some of these mutations may also be found in patients who are non-BH4-responsive (101,108,109). These discrepancies may be incidental, relating to the methods of ascertaining and interpreting BH4-responsiveness, but there are also possible differences specific to each individual patient, such as BH4-absorption, protein catabolic rate, and Phe intake during the test (94,96,97). The improvement of enzymatic activity in certain mutant PAH proteins expressed in vitro in BH4 supplemented media strongly supports the notion that genotype may play a role in determining BH4-response (110). However, the BH4-loading test is still the best determinant for BH4-responsiveness in PKU patients.
Although the treatment with BH4 offers a safe and effective alternative for many patients with PKU, its appropriateness is limited to primarily patients with a milder biochemical phenotype. Most classical PKU patients, in whom the disease is more difficult to manage and the consequences of dietary non-compliance are greater, are unlikely to benefit from this treatment, and further research into other therapies is required to provide alternative solutions for this group of patients.
Alternate treatments for PKU
New dietary approaches
Research on new dietary supplements for the treatment of PKU is ongoing, including more palatable medical formulae, medical foods using glycomacropeptide (GMP), a naturally low-Phe protein, and supplementation of large neutral amino acids (LNAA). Supplementation of LNAA has been shown to stabilise the concentration of cerebral Phe, despite an increase observed in the plasma level, suggesting that the influx of LNAA may block the transport of Phe across the blood brain barrier (111). Other clinical trials have also found decreases in blood Phe concentrations with the added LNAA intake, although brain Phe levels were not measured in some of these studies, and it has been suggested that these LNAAs may also exert their effect by competing with Phe for active transport across the intestinal mucosa (112-115). This treatment is currently only recommended for adult patients not complying with a low-Phe diet, as those on diets would generally be supplemented with a medical formulae encompassing these amino acids (116).
GMP is a by-product of cheese production, and is a 64 amino peptide with no Phe residues, which makes food made from GMP a good alternative source of protein for patients with PKU (117). The use of these products in the murine model of PKU has shown promising results regarding growth rates and bone mineral density (118,119), and a number of these products are now commercially available (http://www.cambrookefoods.com).
Gene therapy
Research into novel treatments for PKU involves different methods for overcoming the deficiency of the host PAH by gene therapy or enzyme substitution/replacement.
Recombinant adeno-associated virus (AAV) vectors have been used to deliver PAH gene to the liver in a murine PKU model, allowing correction of HPA of up to one year (120-122). The loss of PAH activity over time is due to the continual regeneration of hepatocytes and the loss of the AAV vector. Antibody-mediated immune responses also reduced the efficacy of any reinjections of the same vector. There was also an apparent gender-bias in the efficacy of transduction, with female mice showing a poorer improvement in blood Phe levels (123). Using an AAV8-pseudotype vector with a self-complementary AAV genome, Yagi et al. (124) were able to achieve high levels of liver transduction and expression of PAH with complete phenotypic correction and normal blood Phe for over one year, with equivalent levels of improvement in male and female mice.
Skeletal muscle has been considered a more promising target for gene therapy, as it is easily accessible compared to the liver and the cells are longer-lived (125). On the other hand, the enzymes required for the biosynthesis of BH4, the co-factor of PAH, are not expressed in this tissue. Co-expression of PAH with two of these enzymes, delivered via AAV2 pseudotype 1, led to long-term and stable reduction of blood Phe in the Pahenu2 mouse model (126). Development in viral vectors is likely to continue to drive improvement in both liver- and muscle-directed gene therapies.
Enzyme replacement and substitution therapies
Enzyme replacement via introduction of wild-type, functional PAH protein has been hampered by the instability of the protein produced in vitro (127), rendering large-scale production and purification of the protein costly and inefficient. Although storage of the protein in high glycerol concentrations or mannitol provides some protection against protein denaturation and aggregation, the loss in enzymatic activity after a period of storage is significant (128). Therapeutic liver repopulation, whereby wild-type hepatocytes would be transplanted onto the livers of patients with PKU, has also been suggested as a potential therapy (129). This concept has been proven in the PKU mouse model, in which the introduction of hepatocytes from wild-type and heterozygous (but phenotypically normal) mice to the livers of homozygous affected mice resulted in normal blood Phe levels if there is a high enough level (greater than 10%) of repopulation of wild-type (or heterozygous) cells (130).
An alternative to enzyme replacement is substitution with phenylalanine ammonia lyase (PAL, EC 4.3.1.5). PAL, an enzyme normally found in plants and fungi, catalyses the deamination of phenylalanine to ammonia and trans-cinnamic acid, the latter of which is then quickly converted into hippurate and excreted in urine (131). The use of recombinant PAL (with polyethylene glycol polymers covalently linked to lysine residues, so-called PEGylation) avoids the immune-mediated degradation of the enzyme (132). Weekly subcutaneous injections of PEGylated-PAL enzyme in a murine PKU model can sustain correction of blood Phe for up to one year (133). A phase II clinical trial using this formulation has since commenced. Given the constant requirement of therapy, a less invasive method of delivery would ensure greater compliance to treatment in patients, especially as they enter adulthood. Although initial work on orally-administered PAL enzyme in mice was unsuccessful due to proteolytic degradation in the gut (134,135), there has also been renewed interest in this form of enzyme administration (136). Again, PEGylation of the PAL enzyme is useful in protecting the enzyme from protease degradation in the gut, and oral-administration to mice with HPA significantly reduced (but not completely corrected) blood Phe levels (137).
Chaperone therapy
Another group of novel treatments examine how native PAH in patients can be restored to sufficient catalytic activity such that normal range blood Phe levels can be achieved. Indeed, BH4 (sapropterin) treatment is likely to act via this method, although the exact mechanism is uncertain. During in vitro expression of mutant PAH protein, the presence of BH4 leads to increased protein levels and thus catalytic activity, supporting a chaperone-like role of the BH4, increasing the half-life of the mutant PAH protein and protecting it from targeted degradation in the ubiquitin-dependent proteolytic pathway (138-141). Similar screening of chemical libraries found other candidate chaperones for the p.Val106Ala variant, which is the mutation carried in one of the PKU mouse models, Pahenu1. These compounds not only normalised blood Phe concentrations in vivo, but also showed higher efficacy than BH4 (142). It remains to be seen how these compounds might perform with a wider range of mutations and whether a larger proportion of PKU patients would benefit from treatment with these compounds.
As indicated above, most mutations in the PAH gene cause single amino acid (missense) substitutions. A subset of these mutants are known to cause aggregation of the PAH protein, hypothesised to be a result of misfolding of the mutant polypeptide (49). In other genetic diseases, it has been shown that such misfolding and aggregation would lead to a greater rate of targeted degradation (143,144). By expressing the mutant PAH in an in vitro expression system in the presence of a chemical chaperone, both PAH protein levels and residual activity can be restored, suggesting that such molecular chaperones are good candidates as therapeutic agents for PKU (145). Due to its readiness to form high molecular weight aggregates, the missense mutation p.Gly46Ser (p.G46S) has been used for testing the ability of a range of chaperones to restore proper folding and enzymatic activity (50). Several compounds have shown to inhibit formation of p.Gly46Ser aggregates, including glycerol and Compound III (146), identified as a potential chaperone by Pey et al. (145) for other missense PAH mutations. Other anti-aggregation agents (such as trehalose and sodium 4-phenylbutyrate) may also be good candidate drugs to test in vitro for similar missense mutations causing protein aggregation (147-149).
Nonsense read-through therapy
Another novel type of therapy of genetic disorders is the use of the nonsense read-through agents, such as the aminoglycoside antibiotic gentamicin, for treating individuals with nonsense mutations, e.g., in cystic fibrosis, Duchenne muscular dystrophy (150,151). The development of other novel chemical compounds to overcome toxicity problems associated with traditional aminoglycosides has made nonsense read-through compounds a potentially viable long-term treatment option (152-154). In vitro testing of two aminoglycosides against four PAH nonsense mutations have demonstrated their ability to restore PAH enzyme activity (155). In some populations, the proportion of patients with a nonsense mutation can be as high as 22% (19). This mode of therapy therefore merits further study, in particular in a clinical setting, as to allow evaluation of the extent of the restoration of enzyme activity on regulation of Phe levels and of the short- and long-term effects on the patients.
Conclusions
Different modes of therapy for PKU are now under development. These therapies illustrates the range of solutions that can be deployed to address the root problem of PKU, the loss of catalytic activity of the PAH enzyme. Although many of these have been high efficacious in the murine model of PKU, they all require greater research efforts and clinical testing to ensure the safety and longevity of treatment, with the goal of eliminating the rigorous dietary restrictions in many, if not all, patients with PKU. These treatment solutions may also be highly applicable to other genetic disorders of metabolism, and research into PKU therapies will have far-reaching consequences in the field of genetic medicine in the next few decades.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Scriver CR, Eisensmith RC, Woo SL, et al. The hyperphenylalaninemias of man and mouse. Annu Rev Genet 1994;28:141-65. [DOI] [PubMed] [Google Scholar]
- 2.Penrose LS. Inheritance of phenylpyruvic amentia (Phenylketonuria). Lancet 1935;2:192-4. [Google Scholar]
- 3.Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963;32:338-43. [PubMed] [Google Scholar]
- 4.Blau N, Van Spronsen FJ, Levy HL. Phenylketonuria. Lancet 2010;376:1417-27. [DOI] [PubMed] [Google Scholar]
- 5.Thöny B, Blau N. Mutations in the BH4-metabolizing genes GTP cyclohydrolase I, 6-pyruvoyl-tetrahydropterin synthase, sepiapterin reductase, carbinolamine-4a-dehydratase, and dihydropteridine reductase. Hum Mutat 2006;27:870-8. [DOI] [PubMed] [Google Scholar]
- 6.Blau N, Ichinose H, Nagatsu T, et al. A missense mutation in a patient with guanosine triphosphate cyclohydrolase I deficiency missed in the newborn screening program. J Pediatr 1995;126:401-5. [DOI] [PubMed] [Google Scholar]
- 7.Thöny B, Leimbacher W, Blau N, et al. Hyperphenylalaninemia due to defects in tetrahydrobiopterin metabolism: molecular characterization of mutations in 6-pyruvoyl-tetrahydropterin synthase. Am J Hum Genet 1994;54:782-92. [PMC free article] [PubMed] [Google Scholar]
- 8.Citron BA, Kaufman S, Milstien S, et al. Mutation in the 4a-carbinolamine dehydratase gene leads to mild hyperphenylalaninemia with defective cofactor metabolism. Am J Hum Genet 1993;53:768-74. [PMC free article] [PubMed] [Google Scholar]
- 9.Howells DW, Forrest SM, Dahl HH, et al. Insertion of an extra codon for threonine is a cause of dihydropteridine reductase deficiency. Am J Hum Genet 1990;47:279-85. [PMC free article] [PubMed] [Google Scholar]
- 10.Bonafé L, Thöny B, Penzien JM, et al. Mutations in the sepiapterin reductase gene cause a novel tetrahydrobiopterin-dependent monoamine-neurotransmitter deficiency without hyperphenylalaninemia. Am J Hum Genet 2001;69:269-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zschocke J. Phenylketonuria mutations in Europe. Hum Mutat 2003;21:345-56. [DOI] [PubMed] [Google Scholar]
- 12.Guldberg P, Romano V, Ceratto N, et al. Mutational spectrum of phenylalanine hydroxylase deficiency in Sicily: implications for diagnosis of hyperphenylalaninaemia in southern Europe. Hum Mol Genet 1993;2:1703-7. [DOI] [PubMed] [Google Scholar]
- 13.Ozalp I, Coşkun T, Tokatli A, et al. Newborn PKU screening in Turkey: at present and organization for future. Turk J Pediatr 2001;43:97-101. [PubMed] [Google Scholar]
- 14.Zschocke J, Mallory JP, Eiken HG, et al. Phenylketonuria and the peoples of Northern Ireland. Hum Genet 1997;100:189-94. [DOI] [PubMed] [Google Scholar]
- 15.Mallolas J, Vilaseca MA, Campistol J, et al. Mutational spectrum of phenylalanine hydroxylase deficiency in the population resident in Catalonia: genotype-phenotype correlation. Hum Genet 1999;105:468-73. [DOI] [PubMed] [Google Scholar]
- 16.Bercovich D, Elimelech A, Zlotogora J, et al. Genotype-phenotype correlations analysis of mutations in the phenylalanine hydroxylase (PAH) gene. J Hum Genet 2008;53:407-18. [DOI] [PubMed] [Google Scholar]
- 17.Song F, Qu YJ, Zhang T, et al. Phenylketonuria mutations in Northern China. Mol Genet Metab 2005;86 Suppl 1:S107-18. [DOI] [PubMed] [Google Scholar]
- 18.Desviat LR, Pérez B, Gutiérrez A, et al. Molecular basis of phenylketonuria in Cuba. Hum Mutat 2001;18:252. [DOI] [PubMed] [Google Scholar]
- 19.Lee DH, Koo SK, Lee KS, et al. The molecular basis of phenylketonuria in Koreans. J Hum Genet 2004;49:617-21. [DOI] [PubMed] [Google Scholar]
- 20.Chien YH, Chiang SC, Huang A, et al. Mutation spectrum in Taiwanese patients with phenylalanine hydroxylase deficiency and a founder effect for the R241C mutation. Hum Mutat 2004;23:206. [DOI] [PubMed] [Google Scholar]
- 21.Kimura T, Ikeda H, Akaba K, et al. Mutation analysis of phenylketonuria in Yamagata prefecture, Japan. Pediatr Int 2001;43:1-3. [DOI] [PubMed] [Google Scholar]
- 22.Guldberg P, Henriksen KF, Sipilä I, et al. Phenylketonuria in a low incidence population: molecular characterisation of mutations in Finland. J Med Genet 1995;32:976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guldberg P, Henriksen KF, Sipilä I, et al. Phenylketonuria in a low incidence population: molecular characterisation of mutations in Finland. J Med Genet 1995;32:976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch R, Hanley WB, Levy HL, et al. The maternal phenylketonuria international study: 1984-2002. Pediatrics 2003;112:1523-9. [PubMed] [Google Scholar]
- 25.Scriver CR, Kaufman S. Hyperphenylalaniaemia: phenylalanine hydroxylase deficiency. In: Scriver CR, Beaudet AL, Sly WS, et al. eds. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill, New York, 2001:1667-724. [Google Scholar]
- 26.Woo SL, Lidsky AS, Güttler F, et al. Cloned human phenylalanine hydroxylase gene allows prenatal diagnosis and carrier detection of classical phenylketonuria. Nature 1983;306:151-5. [DOI] [PubMed] [Google Scholar]
- 27.Kwok SC, Ledley FD, DiLella AG, et al. Nucleotide sequence of a full-length complementary DNA clone and amino acid sequence of human phenylalanine hydroxylase. Biochemistry 1985;24:556-61. [DOI] [PubMed] [Google Scholar]
- 28.Erlandsen H, Stevens RC. The structural basis of phenylketonuria. Mol Genet Metab 1999;68:103-25. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman S. A new cofactor required for the enzymatic conversion of phenylalanine to tyrosine. J Biol Chem 1958;230:931-9. [PubMed] [Google Scholar]
- 30.Sarkissian CN, Gámez A. Phenylalanine ammonia lyase, enzyme substitution therapy for phenylketonuria, where are we now? Mol Genet Metab 2005;86 Suppl 1:S22-6. [DOI] [PubMed] [Google Scholar]
- 31.Scriver CR, Hurtubise M, Konecki D, et al. PAHdb 2003: what a locus-specific knowledgebase can do. Hum Mutat 2003;21:333-44. [DOI] [PubMed] [Google Scholar]
- 32.Gable M, Williams M, Stephenson A, et al. Comparative multiplex dosage analysis detects whole exon deletions at the phenylalanine hydroxylase locus. Hum Mutat 2003;21:379-86. [DOI] [PubMed] [Google Scholar]
- 33.Birk Møller L, Nygren AO, Scott P, et al. Low proportion of whole exon deletions causing phenylketonuria in Denmark and Germany. Hum Mutat 2007;28:207. [DOI] [PubMed] [Google Scholar]
- 34.Desviat LR, Pérez B, Ugarte M. Identification of exonic deletions in the PAH gene causing phenylketonuria by MLPA analysis. Clin Chim Acta 2006;373:164-7. [DOI] [PubMed] [Google Scholar]
- 35.Kayaalp E, Treacy E, Waters PJ, et al. Human phenylalanine hydroxylase mutations and hyperphenylalaninemia phenotypes: a metanalysis of genotype-phenotype correlations. Am J Hum Genet 1997;61:1309-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guldberg P, Rey F, Zschocke J, et al. A European multicenter study of phenylalanine hydroxylase deficiency: classification of 105 mutations and a general system for genotype-based prediction of metabolic phenotype. Am J Hum Genet 1998;63:71-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasnauskienė J, Cimbalistienė L, Kučinskas V. Validation of PAH genotype-based predictions of metabolic phenylalanine hydroxylase deficiency phenotype: investigation of PKU/MHP patients from Lithuania. Med Sci Monit 2003;9:CR142-6. [PubMed] [Google Scholar]
- 38.Güttler F, Azen C, Guldberg P, et al. Relationship among genotype, biochemical phenotype, and cognitive performance in females with phenylalanine hydroxylase deficiency: report from the maternal phenylketonuria collaborative study. Pediatrics 1999;104:258-62. [DOI] [PubMed] [Google Scholar]
- 39.Guldberg P, Mikkelsen I, Henriksen KF, et al. In vivo assessment of mutations in the phenylalanine hydroxylase gene by phenylalanine loading: characterization of seven common mutations. Eur J Pediatr 1995;154:551-6. [DOI] [PubMed] [Google Scholar]
- 40.Blau N, Thöny B, Cotton RG, et al. Disorders of tetrahydrobiopterin and related biogenic amines. In: Scriver CR, Beaudet AL, Sly WS, et al. eds. The metabolic & molecular bases of inherited disease. McGraw-Hill, New York, 2001. [Google Scholar]
- 41.Treacy E, Pitt JJ, Seller K, et al. In vivo disposal of phenylalanine in phenylketonuria: a study of two siblings. J Inherit Metab Dis 1996;19:595-602. [DOI] [PubMed] [Google Scholar]
- 42.Gizewska M, Cabalska B, Cyrytowski L, et al. Different presentations of late-detected phenylketonuria in two brothers with the same R408W/R111X genotype in the PAH gene. J Intellect Disabil Res 2003;47:146-52. [DOI] [PubMed] [Google Scholar]
- 43.Waters PJ, Parniak MA, Nowacki P, et al. In vitro expression analysis of mutations in phenylalanine hydroxylase: linking genotype to phenotype and structure to function. Hum Mutat 1998;11:4-17. [DOI] [PubMed] [Google Scholar]
- 44.Daniele A, Cardillo G, Pennino C, et al. Five human phenylalanine hydroxylase proteins identified in mild hyperphenylalaninemia patients are disease-causing variants. Biochim Biophys Acta 2008;1782:378-84. [DOI] [PubMed]
- 45.Gjetting T, Romstad A, Haavik J, et al. A phenylalanine hydroxylase amino acid polymorphism with implications for molecular diagnostics. Mol Genet Metab 2001;73:280-4. [DOI] [PubMed] [Google Scholar]
- 46.Mirisola MG, Calì F, Gloria A, et al. PAH gene mutations in the Sicilian population: association with minihaplotypes and expression analysis. Mol Genet Metab 2001;74:353-61. [DOI] [PubMed] [Google Scholar]
- 47.Pey AL, Desviat LR, Gámez A, et al. Phenylketonuria: genotype-phenotype correlations based on expression analysis of structural and functional mutations in PAH. Hum Mutat 2003;21:370-8. [DOI] [PubMed] [Google Scholar]
- 48.Gjetting T, Petersen M, Guldberg P, et al. In vitro expression of 34 naturally occurring mutant variants of phenylalanine: correlation with metabolic phenotypes and susceptibility toward protein aggregation. Mol Genet Metab 2001;72:132-43. [DOI] [PubMed] [Google Scholar]
- 49.Waters PJ. How PAH gene mutations cause hyper-phenylalaninemia and why mechanism matters: insights from in vitro expression. Hum Mutat 2003;21:357-69. [DOI] [PubMed] [Google Scholar]
- 50.Leandro J, Saraste J, Leandro P, et al. The G46S-hPAH mutant protein: a model to study the rescue of aggregation-prone PKU mutations by chaperones. Mol Genet Metab 2011;104 Suppl:S40-4. [DOI] [PubMed] [Google Scholar]
- 51.González MJ, Gutiérrez AP, Gassió R, et al. Neurological complications and behavioral problems in patients with phenylketonuria in a follow-up unit. Mol Genet Metab 2011;104 Suppl:S73-9. [DOI] [PubMed] [Google Scholar]
- 52.Demirkol M, Giżewska M, Giovannini M, et al. Follow up of phenylketonuria patients. Mol Genet Metab 2011;104 Suppl:S31-9. [DOI] [PubMed] [Google Scholar]
- 53.Seashore MR, Friedman E, Novelly RA, et al. Loss of intellectual function in children with phenylketonuria after relaxation of dietary phenylalanine restriction. Pediatrics 1985;75:226-32. [PubMed] [Google Scholar]
- 54.Channon S, Goodman G, Zlotowitz S, et al. Effects of dietary management of phenylketonuria on long-term cognitive outcome. Arch Dis Child 2007;92:213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koch R, Burton BK, Hoganson G, et al. Phenylketonuria in adulthood: a collaborative study. J Inherit Metab Dis 2002;25:333-46. [DOI] [PubMed] [Google Scholar]
- 56.Trefz F, Maillot F, Motzfeldt K, et al. Adult phenylketonuria outcome and management. Mol Genet Metab 2011;104:S26-30. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organisation, Adherence to long-term therapies. Evidence for action. WHO, Geneva, 2003. [Google Scholar]
- 58.Cotugno G, Nicolò R, Cappelletti S, et al. Adherence to diet and quality of life in patients with phenylketonuria. Acta Paediatr 2011;100:1144-9. [DOI] [PubMed] [Google Scholar]
- 59.MacDonald A, Gokmen-Ozel H, van Rijn M, et al. The reality of dietary compliance in the management of phenylketonuria. J Inherit Metab Dis 2010;33:665-70. [DOI] [PubMed] [Google Scholar]
- 60.MacDonald A, Rocha JC, van Rijn M, et al. Nutrition in phenylketonuria. Mol Genet Metab 2011;104 Suppl:S10-8. [DOI] [PubMed] [Google Scholar]
- 61.Horrocks LA, Yeo YK. Health benefits of docosahexaenoic acid (DHA). Pharmacol Res 1999;40:211-25. [DOI] [PubMed] [Google Scholar]
- 62.Koletzko B, Lien E, Agostini C, et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med 2008;36:5-14. [DOI] [PubMed] [Google Scholar]
- 63.Koletzko B, Beblo S, Demmelmair H, et al. Does dietary DHA improve neural function in children? Observations in phenylketonuria. Prostaglandins Leukot Essent Fatty Acids 2009;81:159-64. [DOI] [PubMed] [Google Scholar]
- 64.Koletzko B, Sauerwald T, Demmelmair H, et al. Dietary long-chain polyunsaturated fatty acid supplementation in infants with phenylketonuria: a randomized controlled trial. J Inherit Metab Dis 2007;30:326-32. [DOI] [PubMed] [Google Scholar]
- 65.MacDonald A, Lee P, Davies P, et al. Long-term compliance with a novel vitamin and mineral supplement in older people with PKU. J Inherit Metab Dis 2008;31:718-23. [DOI] [PubMed] [Google Scholar]
- 66.Robinson M, White FJ, Cleary MA, et al. Increased risk of vitamin B12 deficiency in patients with phenylketonuria on an unrestricted or relaxed diet. J Pediatr 2000;136:545-7. [DOI] [PubMed] [Google Scholar]
- 67.Hanley WB, Feigenbaum AS, Clarke JT, et al. Vitamin B12 deficiency in adolescents and young adults with phenylketonuria. Eur J Pediatr 1996;155 Suppl 1:S145-7. [DOI] [PubMed] [Google Scholar]
- 68.Aung TT, Klied A, McGinn J, et al. Vitamin B12 deficiency in an adult phenylketonuria patient. J Inherit Metab Dis 1997;20:603-4. [DOI] [PubMed] [Google Scholar]
- 69.Vugteveen I, Hoeksma M, Monsen AL, et al. Serum vitamin B12 concentrations within reference values do not exclude functional vitamin B12 deficiency in PKU patients of various ages. Mol Genet Metab 2011;102:13-7. [DOI] [PubMed] [Google Scholar]
- 70.Allen JR, Humphries IR, Waters DL, et al. Decreased bone mineral density in children with phenylketonuria. Am J Clin Nutr 1994;59:419-22. [DOI] [PubMed] [Google Scholar]
- 71.Koura HM, Abdallah Ismail N, Kamel AF, et al. A long-term study of bone mineral density in patients with phenylketonuria under diet therapy. Arch Med Sci 2011;7:493-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hennermann JB, Roloff S, Gellermann J, et al. Chronic kidney disease in adolescent and adult patients with phenylketonuria. J Inherit Metab Dis 2013;36:747-56. [DOI] [PubMed] [Google Scholar]
- 73.Enns GM, Koch R, Brumm V, Blakely E, et al. Suboptimal outcomes in patients with PKU treated early with diet alone: revisiting the evidence. Mol Genet Metab 2010;101:99-109. [DOI] [PubMed] [Google Scholar]
- 74.Gassió R, Artuch R, Vilaseca MA, et al. Cognitive functions in classic phenylketonuria and mild hyperphenylalaninaemia: experience in a paediatric population. Dev Med Child Neurol 2005;47:443-8. [DOI] [PubMed] [Google Scholar]
- 75.Moyle JJ, Fox AM, Arthur M, et al. Meta-analysis of neuropsychological symptoms of adolescents and adults with PKU. Neuropsychol Rev 2007;17:91-101. [DOI] [PubMed] [Google Scholar]
- 76.Gassió R, Fusté ME, Lopez-Sala A, et al. School performance in early and continuously treated phenylketonuria. Pediatr Neurol 2005;33:267-71. [DOI] [PubMed] [Google Scholar]
- 77.Smith I, Knowles J. Behaviour in early treated phenylketonuria: a systematic review. Eur J Pediatr 2000;159:S89-93. [DOI] [PubMed] [Google Scholar]
- 78.Stemerdink BA, Kalverboer AF, van der Meere JJ, et al. Behaviour and school achievement in patients with early and continuously treated phenylketonuria. J Inherit Metab Dis 2000;23:548-62. [DOI] [PubMed] [Google Scholar]
- 79.Bik-Multanowski M, Didycz B, Mozrzymas R, et al. Quality of life in noncompliant adults with phenylketonuria after resumption of the diet. J Inherit Metab Dis 2008;31 Suppl 2:S415-8. [DOI] [PubMed] [Google Scholar]
- 80.van Spronsen FJ, Huijbregts SC, Bosch AM, et al. Cognitive, neurophysiological, neurological and psychosocial outcomes in early-treated PKU-patients: a start toward standardized outcome measurement across development. Mol Genet Metab 2011;104:S45-51. [DOI] [PubMed] [Google Scholar]
- 81.Landolt MA, Nuoffer JM, Steinmann B, et al. Quality of life and psychologic adjustment in children and adolescents with early treated phenylketonuria can be normal. J Pediatr 2002;140:516-21. [DOI] [PubMed] [Google Scholar]
- 82.Simon E, Schwarz M, Roos J, et al. Evaluation of quality of life and description of the sociodemographic state in adolescent and young adult patients with phenylketonuria (PKU). Health Qual Life Outcomes 2008;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gassió R, Campistol J, Vilaseca MA, et al. Do adult patients with phenylketonuria improve their quality of life after introduction/resumption of a phenylalanine-restricted diet? Acta Paediatr 2003;92:1474-8. [PubMed] [Google Scholar]
- 84.Bickel H, Gerrard J, Hickmans EM. Influence of phenylalanine intake on phenylketonuria. Lancet 1953;265:812-3. [DOI] [PubMed] [Google Scholar]
- 85.Kure S, Hou DC, Ohura T, et al. Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. J Pediatr 1999;135:375-8. [DOI] [PubMed] [Google Scholar]
- 86.Muntau AC, Röschinger W, Habich M, et al. Tetrahydrobiopterin as an alternative treatment for mild phenylketonuria. N Engl J Med 2002;347:2122-32. [DOI] [PubMed] [Google Scholar]
- 87.Leuret O, Barth M, Kuster A, et al. Efficacy and safety of BH4 before the age of 4 years in patients with mild phenylketonuria. J Inherit Metab Dis 2012;35:975-81. [DOI] [PubMed] [Google Scholar]
- 88.Singh RH, Quirk ME, Douglas TD, et al. BH4 therapy impacts the nutrition status and intake in children with phenylketonuria: 2-year follow-up. J Inherit Metab Dis 2010;33:689-95. [DOI] [PubMed] [Google Scholar]
- 89.Burton BK, Bausell H, Katz R, et al. Sapropterin therapy increase stability of blood phenylalanine levels in patients with BH4-responsive phenylketonuria (PKU). Mol Genet Metab 2010;101:110-4. [DOI] [PubMed] [Google Scholar]
- 90.Humphrey M, Nation J, Francis I, et al. Effect of tetrahydrobiopterin on Phe/Tyr ratios and variation in Phe levels in tetrahydrobiopterin responsive PKU patients. Mol Genet Metab 2011;104:89-92. [DOI] [PubMed] [Google Scholar]
- 91.Koch R, Güttler F, Blau N. Mental illness in mild PKU responds to biopterin. Mol Genet Metab 2002;75:284-6. [DOI] [PubMed] [Google Scholar]
- 92.Blau N, Bélanger-Quintana A, Demirkol M, et al. Optimizing the use of sapropterin (BH(4)) in the management of phenylketonuria. Mol Genet Metab 2009;96:158-63. [DOI] [PubMed] [Google Scholar]
- 93.Bernegger C, Blau N. High frequency of tetrahydrobiopterin-responsiveness among hyperphenylalaninemias: a study of 1919 patients observed from 1988 to 2002. Mol Genet Metab 2002;77:304-13. [DOI] [PubMed] [Google Scholar]
- 94.Anjema K, Venema G, Hofstede FC, et al. The 48-hour tetrahydrobiopterin loading test in patients with phenylketonuria: evaluation of protocol and influence of baseline phenylalanine concentration. Mol Genet Metab 2011;104 Suppl:S60-3. [DOI] [PubMed] [Google Scholar]
- 95.Burton BK, Grange DK, Milanowski A, et al. The response of patients with phenylketonuria and elevated serum phenylalanine to treatment with oral sapropterin dihydrochloride (6R-tetrahydriobiopterin): a phase II, multicentre, open-label, screening study. J Inherit Metab Dis 2007;30:700-7. [DOI] [PubMed] [Google Scholar]
- 96.Fiege B, Blau N. Assessment of tetrahydrobiopterin responsiveness in phenylketonuria. J Pediatr 2007;150:627-30. [DOI] [PubMed] [Google Scholar]
- 97.Mitchell JJ, Wilcken B, Alexander I, et al. Tetrahydrobiopterin-responsive phenylketonuria: the New South Wales experience. Mol Genet Metab 2005;86 Suppl 1:S81-5. [DOI] [PubMed] [Google Scholar]
- 98.Desviat LR, Pérez B, Bélanger-Quintana A, et al. Tetrahydrobiopterin responsiveness: results of the BH4 loading test in 31 Spanish PKU patients and correlation with their genotype. Mol Genet Metab 2004;83:157-62. [DOI] [PubMed] [Google Scholar]
- 99.Pérez-Dueñas B, Vilaseca MA, Mas A, et al. Tetrahydrobiopterin responsiveness in patients with phenylketonuria. Clin Biochem 2004;37:1083-90. [DOI] [PubMed] [Google Scholar]
- 100.Hennermann JB, Bührer C, Blau N, et al. Long-term treatment with tetrahydrobiopterin increases phenylalanine tolerance in children with severe phenotype of phenylketonuria. Mol Genet Metab 2005;86:S86-90. [DOI] [PubMed] [Google Scholar]
- 101.Fiori L, Fiege B, Riva E, et al. Incidence of BH4-responsiveness in phenylalanine-hydroxylase-deficient Italian patients. Mol Genet Metab 2005;86:S67-74. [DOI] [PubMed] [Google Scholar]
- 102.Matalon R, Michals-Matalon K, Koch R, et al. Response of patients with phenylketonuria in the US to tetrahydrobiopterin. Mol Genet Metab 2005;86 Suppl 1:S17-21. [DOI] [PubMed] [Google Scholar]
- 103.Leuzzi V, Carducci C, Carducci C, et al. The spectrum of phenylalanine variations under tetrahydrobiopterin load in subjects affected by phenylalanine hydroxylase deficiency. J Inherit Metab Dis 2006;29:38-46. [DOI] [PubMed] [Google Scholar]
- 104.Bóveda MD, Couce ML, Castiñeiras DE, et al. The tetrahydrobiopterin loading test in 36 patients with hyperphenylalaninaemia: evaluation of response and subsequent treatment. J Inherit Metab Dis 2007;30:812. [DOI] [PubMed] [Google Scholar]
- 105.Burlina A, Blau N. Effect of BH4 supplementation on phenylalanine tolerance. J Inherit Metab Dis 2009;32:40-5. [DOI] [PubMed] [Google Scholar]
- 106.Burnett JR. Sapropterin dihydrochloride (Kuvan/phenoptin), an orally active synthetic form of BH4 for the treatment of phenylketonuria. IDrugs 2007;10:805-13. [PubMed] [Google Scholar]
- 107.Zurflüh MR, Zschocke J, Lindner M, et al. Molecular genetics of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Hum Mutat 2008;29:167-75. [DOI] [PubMed] [Google Scholar]
- 108.Pérez-Dueñas B, Vilaseca MA, Mas A, et al. Tetrahydrobiopterin responsiveness in patients with phenylketonuria. Clin Biochem 2004;37:1083-90. [DOI] [PubMed] [Google Scholar]
- 109.Karacić I, Meili D, Sarnavka V, et al. Genotype-predicted tetrahydrobiopterin (BH4)-responsiveness and molecular genetics in Croatian patients with phenylalanine hydroxylase (PAH) deficiency. Mol Genet Metab 2009;97:165-71. [DOI] [PubMed] [Google Scholar]
- 110.Kim SW, Jung J, Oh HJ, et al. Structural and functional analyses of mutations of the human phenylalanine hydroxylase gene. Clin Chim Acta 2006;365:279-87. [DOI] [PubMed] [Google Scholar]
- 111.Pietz J, Kreis R, Rupp A, et al. Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J Clin Invest 1999;103:1169-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koch R, Moseley KD, Yano S, et al. Large neutral amino acid therapy and phenylketonuria: a promising approach to treatment. Mol Genet Metab 2003;79:110-3. [DOI] [PubMed] [Google Scholar]
- 113.Matalon R, Michals-Matalon K, Bhatia G, et al. Large neutral amino acids in the treatment of phenylketonuria (PKU). J Inherit Metab Dis 2006;29:732-8. [DOI] [PubMed] [Google Scholar]
- 114.Matalon R, Michals-Matalon K, Bhatia G, et al. Double blind placebo control trial of large neutral amino acids in treatment of PKU: effect on blood phenylalanine. J Inherit Metab Dis 2007;30:153-8. [DOI] [PubMed] [Google Scholar]
- 115.Schindeler S, Ghosh-Jerath S, Thompson S, et al. The effects of large neutral amino acid supplements in PKU: an MRS and neuropsychological study. Mol Genet Metab 2007;91:48-54. [DOI] [PubMed] [Google Scholar]
- 116.Rocha JC, Martel F. Large neutral amino acids supplementation in phenylketonuric patients. J Inherit Metab Dis 2009;32:472-80. [DOI] [PubMed] [Google Scholar]
- 117.van Calcar SC, Ney DM. Food products made with glycomacropeptide, a low-phenylalanine whey protein, provide a new alternative to amino Acid-based medical foods for nutrition management of phenylketonuria. J Acad Nutr Diet 2012;112:1201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Solverson P, Murali SG, Brinkman AS, et al. Glycomacropeptide, a low-phenylalanine protein isolated from cheese whey, supports growth and attenuates metabolic stress in the murine model of phenylketonuria. Am J Physiol Endocrinol Metab 2012;302:E885-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Solverson P, Murali SG, Litscher SJ, et al. Low bone strenght is a manifestation of phenylketonuria in mice and is attenuated by a glycomacropeptide diet. PLoS ONE 2012;7:e45165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ledley FD, Grenett HE, diLella AG, et al. Gene transfer and expression of human phenylalanine hydroxylase. Science 1985;228:77-9. [DOI] [PubMed] [Google Scholar]
- 121.Ding Z, Georgiev P, Thöny B. Administration-route and gender-independent long-term therapeutic correction of phenylketonuria (PKU) in a mouse model by recombinant adeno-associated virus 8 pseudotyped vector-mediated gene transfer. Gene Ther 2006;13:587-93. [DOI] [PubMed] [Google Scholar]
- 122.Harding CO, Gillingham MB, Hamman K, et al. Complete correction of hyperphenylalaninemia following liver-directed, recombinant AAV2/8 vector-mediated gene therapy in murine phenylketonuria. Gene Ther 2006;13:457-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rebuffat A, Harding CO, Ding Z, et al. Comparison of adeno-associated virus pseudotype 1, 2, 8 vectors administered by intramuscular injection in the treatment of murine phenylketonuria. Hum Gene Ther 2010;21:463-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yagi H, Ogura T, Mizukami H, et al. Complete restoration of phenylalanine oxidation in phenylketonuria mouse by a self-complementary adeno-associated virus vector. J Gene Med 2011;13:114-22. [DOI] [PubMed] [Google Scholar]
- 125.Thöny B. Long-term correction of murine phenylketonuria by viral gene transfer: liver versus muscle. J Inherit Metab Dis 2010; 33: 677-80. [DOI] [PubMed] [Google Scholar]
- 126.Ding Z, Harding CO, Rebuffat A, et al. Correction of murine PKU following AAV-mediated intramuscular expression of a complete phenylalanine hydroxylating system. Mol Ther 2008;16:673-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Woo SL, Gillam SS, Woolf LI. The isolation and properties of phenylalanine hydroxylase from human liver. Biochem J 1974;139:741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nascimento C, Leandro J, Lino PR, et al. Polyol additives modulate the in vitro stability and activity of recombinant human phenylalanine hydroxylase. Appl Biochem Biotechnol 2010;162:192-207. [DOI] [PubMed] [Google Scholar]
- 129.Harding CO, Gibson KM. Therapeutic liver repopulation for phenylketonuria. J Inherit Metab Dis 2010;33:681-7. [DOI] [PubMed] [Google Scholar]
- 130.Hamman KJ, Winn SR, Harding CO. Hepatocytes from wild-type or heterozygous donors are equally effective in achieving successful therapeutic liver repopulation in murine phenylketonuria (PKU). Mol Genet Metab 2011;104:235-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hoskins JA, Hollidays SB, Greenway AM. The metabolism of cinnamic acid by healthy and phenylketonuric adults; a kinetic study. Biomed Mass Spectrom 1984; 11: 296-300. [DOI] [PubMed] [Google Scholar]
- 132.Gámez A, Wang L, Sarkissian CN, et al. Structure-based epitope and PEGylation sites mapping of phenylalanine ammonia-lyase for enzyme substation treatment of phenylketonuria. Mol Genet Metab 2007;91:325-34. [DOI] [PubMed] [Google Scholar]
- 133.Sarkissian CN, Gámez A, Wang L, et al. Preclinical evaluation of multiple species of PEGylated recombinant phenylalanine ammonia lyase for the treatment of phenylketonuria. Proc Natl Acad Sci USA 2008;105:20894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gilbert HJ, Jack GW. The effect of proteinases on phenylalanine ammonia-lyase from the yeast rhodotorula glutinis. Biochem J 1981;199:715-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Safos S, Chang TM. Enzyme replacement therapy in ENU2 phenylketonuric mice using oral microencapsulated phenylalanine ammonia-lyase: a preliminary report. Artif Cells Blood Substit Immobil Biotechnol 1995;23:681-92. [DOI] [PubMed] [Google Scholar]
- 136.Kang TS, Wang L, Sarkissian CN, et al. Converting an injectable protein therapeutic into an oral form: phenylalanine ammonia lyase for phenylketonuria. Mol Genet Metab 2010;99:4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sarkissian CN, Kang TS, Gámez A, et al. Evaluation of orally administered PEGylated phenylalanine ammonia lyase in mice for the treatment of Phenylketonuria. Mol Genet Metab 2011;104:249-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Erlandsen H, Pey AL, Gámez A, et al. Correction of kinetic and stability defects by tetrahydrobiopterin in phenylketonuria patients with certain phenylalanine hydroxylase mutations. Proc Natl Acad Sci USA 2004;101:16903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thöny B, Ding Z, Martínez A. Tetrahydrobiopterin protects phenylalanine hydroxylase activity in vivo: implications for tetrahydrobiopterin-responsive hyperphenylalaninemia. FEBS Lett 2004;577:507-11. [DOI] [PubMed] [Google Scholar]
- 140.Pey AL, Pérez B, Martínez MA, et al. Mechanisms underlying responsiveness to tetrahydrobiopterin in mild phenylketonuria mutations. Hum Mutat 2004;24:388-99. [DOI] [PubMed] [Google Scholar]
- 141.Aguado C, Pérez B, Ugarte M, et al. Analysis of the effect of tetrahydrobiopterin on PAH gene expression in hepatoma cells. FEBS Lett 2006;580:1697-701. [DOI] [PubMed] [Google Scholar]
- 142.Santos-Sierra S, Kirchmair J, Perna AM, et al. Novel pharmacological chaperones that correct phenylketonuria in mice. Hum Mol Genet 2012;21:1877-87. [DOI] [PubMed] [Google Scholar]
- 143.Ulloa-Aguirre A, Janovick JA, Brothers SP, et al. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic 2004;5:821-37. [DOI] [PubMed] [Google Scholar]
- 144.Gregersen N. Protein misfolding disease: pathogenesis and intervention. J Inherit Metab Dis 2006;29:456-70. [DOI] [PubMed] [Google Scholar]
- 145.Pey AL, Ying M, Cremades N, et al. Identification of pharmacological chaperones as potential therapeutic agents to treat phenylketonuria. J Clin Invest 2008;118:2858-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Leandro J, Simonsen N, Saraste J, et al. Phenylketonuria as a protein misfolding disease: The mutation pG46S in phenylalanine hydroxylase promotes self-association and fibril formation. Biochim Biophys Acta 2011;1812:106-20. [DOI] [PubMed]
- 147.de Almeida SF, Picarote G, Fleming JV, et al. Chemical chaperones reduce endoplasmic reticulum stress and prevent mutant HFE aggregate formation. J Biol Chem 2007;282:27905-12. [DOI] [PubMed] [Google Scholar]
- 148.Kubota K, Niinuma Y, Kaneko M, et al. Suppressive effects of 4-phenylbutyrate on the aggregation of Pael receptors and endoplasmic reticulum stress. J Neurochem 2006;97:1259-68. [DOI] [PubMed] [Google Scholar]
- 149.Davies JE, Sarkar S, Rubinsztein DC. Trehalose reduces aggregate formation and delays pathology in a transgenic mouse model of oculopharyngeal muscular dystrophy. Hum Mol Genet 2006;15:23-31. [DOI] [PubMed] [Google Scholar]
- 150.Howard M, Frizzell RA, Bedwell DM. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat Med 1996;2:467-9. [DOI] [PubMed] [Google Scholar]
- 151.Barton-Davis ER, Cordier L, Shoturma DI, et al. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J Clin Invest 1999;104:375-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Du L, Damoiseaux R, Nahas SA, et al. Nonaminoglycoside compounds induce readthrough of nonsense mutations. J Exp Med 2009;206:2285-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Welch EM, Barton ER, Zhuo J, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature 2007;447:87-91. [DOI] [PubMed] [Google Scholar]
- 154.Nudelman I, Glikin D, Smolkin B, et al. Repairing faulty genes by aminoglycosides: development of new derivatives of geneticin (G418) with enhanced suppression of disease-causing nonsense mutations. Bioorg Med Chem 2010;18:3735-46. [DOI] [PubMed] [Google Scholar]
- 155.Ho G, Reichardt J, Christodoulou J. In vitro read-through of phenylalanine hydroxylase (PAH) nonsense mutations using aminoglycosides: a potential therapy for phenylketonuria. J Inherit Metab Dis 2013;36:955-9. [DOI] [PubMed] [Google Scholar]