Abstract
Background
Three drought yield QTLs, qDTY2.2, qDTY3.1, and qDTY12.1 with consistent effect on grain yield under reproductive stage drought stress were pyramided through marker assisted breeding with the objective of improving the grain yield of the elite Malaysian rice cultivar MR219 under reproductive stage drought stress. Foreground selection using QTL specific markers, recombinant selection using flanking markers, and background selection were performed. BC1F3-derived lines with different combinations of qDTY2.2, qDTY3.1, and qDTY12.1 were evaluated under both reproductive stage drought stress and non-stress during the dry seasons of 2013 and 2014 at IRRI.
Results
The grain yield reductions in the stress trials compared to non-stress trials ranged from 79 to 93 %. In the stress trials, delay in days to flowering and reduction in plant height were observed. In both seasons, MR219 did not produce any yield under stress, however it produced a yield of 5917 kg ha−1 during the 2013 dry season and 8319 kg ha−1 during the 2014 dry season under non-stress. Selected introgressed lines gave a yield advantage of 903 to 2500 kg ha−1 over MR219 under reproductive stage drought stress and a yield of more than 6900 kg ha−1 under non-stress during the 2014 dry season. Among lines with single qDTY, lines carrying qDTY2.2 provided a higher yield advantage under reproductive stage drought stress in the MR219 background. Two-qDTY combinations (qDTY3.1+qDTY2.2 and qDTY3.1+qDTY12.1) performed better than lines with three qDTY combinations, indicating the absence of positive interactions between the three qDTYs.
Conclusion
We successfully developed drought-tolerant MR219 pyramided lines with a yield advantage of more than 1500 kg ha−1. Differential yield advantages of different combinations of the qDTYs indicate a differential synergistic relationship among qDTYs. This is the first report on the successful effect of qDTYs in increasing the yield under drought in genetic backgrounds other than those in which the qDTYs were earlier identified.
Keywords: Drought tolerance, Marker assisted breeding, qDTYs, QTLs pyramiding
Background
Drought is the major constraint to rice production and yield stability in rainfed rice-growing areas in many Asian countries. The severity of drought varies with rainfall pattern, irrigation source, soil type, water availability within and between seasons, and stage of crop growth [1], causing the varied responses of rice cultivars in different years and environments. In South and Southeast Asia as well as in Africa, severe drought is observed almost every year, which drastically affects rice production [2]. In Asia alone, about 45 % of the total rice-growing areas have no assured irrigation access and are subjected to frequent drought [3]. Drought adversely affects the rice crop at all stages of growth and early reproductive stage drought stress (RS), especially during anthesis, has been found to result in significant yield reduction as also observed in wheat and barley [4, 5]. The reduction in rice yield is frequently associated with the increased percentage of spikelet sterility [6–8] and spikelet number per panicle [9]. The degree of seed yield reduction due to water deficit is highly dependent on the timing and duration of stress [10]. Also, water deficit at the meiotic stage has been reported to reduce the seed set in some cultivated rice varieties [11]. The ability of the rice crop to withstand dry conditions and to reproduce in limited water conditions is essential for rice production to still prosper despite drought [12, 13]. It is, therefore, vital to focus on the development of high yielding drought-tolerant rice cultivars which have a targeted yield advantage of at least 1000 kg ha−1 over popular and widely adapted varieties under drought. However, breeding efforts for drought-tolerant rice varieties are limited due to factors such as the difficulty of defining a representative RS condition as well as the low heritability (H) of yield component traits such as spikelet sterility, relative water content, root pulling force, root dry weight, and harvest index under RS as these are highly influenced by multiple genes, the environment, and the interrelation between genotype and environment as well as interaction with other abiotic and biotic stresses [14].
Marker assisted breeding (MAB) has provided new opportunities to introgress regions governing tolerance to RS in drought-tolerant donors through careful QTL identification and fine mapping studies. At the International Rice Research Institute (IRRI), traditional and improved donors were used in developing mapping populations for the identification of major qDTYs [15]. As a result, several qDTYs with large and consistent effects such as qDTY1.1 [16, 17], qDTY2.1 [18], qDTY2.2 [19, 20], qDTY3.1 [18], qDTY4.1 [20], qDTY6.1 [21], qDTY9.1 [20], qDTY10.1 [20], and qDTY12.1 [22] were identified. Generally, these major effect qDTYs have a genetic gain of 10 to 30 %, with a yield advantage of 150 to 500 kg ha−1 under RS. However, to provide more significant economic benefits to farmers, a yield advantage of at least 1000 kg ha−1 is required [1]. In the past few years, consistent efforts have been made to introgress the identified qDTYs into drought-susceptible mega-varieties through the MAB strategy.
Rice breeding programs in Malaysia have focused on developing high productivity rice varieties and have come up with many high-yielding cultivars such as MR84, MR219, and MR220. Most of these cultivars are susceptible to drought. Several studies on the genetic diversity and the morphological, biochemical, and physiological responses of Malaysian rice germplasms under controlled drought environments have been conducted. However, not many studies were undertaken to improve the yield of current popular varieties under drought or to develop new drought-tolerant rice genotypes. Swamy et al. [20] reported that introgression lines with two and three qDTYs in an IR64 background gave a yield advantage of 1200 to 2000 kg ha−1 under RS as well as yields that were similar to that of IR64 under non-stress (NS) conditions, yet the effect of the identified qDTYs in diverse genetic backgrounds remains unknown. Thus, in this study, three drought-tolerant improved lines developed at IRRI and which have performed well under NS conditions were used as qDTY donors. IR 84984-83-15-18-B is the donor of qDTY12.1, the only qDTY for upland environment used in this study. This line was derived from a cross between Way Rarem, a high-yielding drought-sensitive Indonesian upland rice cultivar, and Vandana, a high-yielding and drought-tolerant Indian upland rice cultivar. Way Rarem belongs to the indica group while Vandana has 50 % japonica and 50 % aus background. The qDTY12.1 QTL was flanked between RM28048 and RM511 on chromosome 12. This QTL explained about 51 % of the total genetic variance with an estimated additive effect of 172 kg ha−1 for yield observed under severe upland RS over two years of field evaluation at IRRI [23]. IR 77298-14-1-2-10 and IR 81896-B-B-195 are the donors of qDTY2.2 and qDTY3.1, respectively, which were both identified under severe lowland RS condition. IR 77298-14-1-2-10 was derived from the cross between two indica varieties: AdaySel, a drought-tolerant Indian rice cultivar, and IR64, a modern cultivar grown in South Asia which is highly susceptible to RS. qDTY2.2 was flanked between RM109 and RM279 on chromosome 2 and explained 33 % of the genetic variance under severe lowland RS [18]. IR 81896-B-B-195 was derived from a cross between Apo, an improved indica upland variety with high yield potential under aerobic condition, and Swarna, a widely grown indica rainfed lowland Indian rice cultivar [18, 24]. qDTY3.1 was flanked between RM520 and RM16030 on chromosome 3, explaining about 31 % of the genetic variance for the trait [18]. In the present study, the three qDTYs were pyramided through stepwise marker assisted QTL pyramiding into the high-yielding Malaysian rice cultivar MR219 with the objectives of (i) improving its yield under drought, (ii) understanding the effect of different QTLs in enhancing yield in the background of MR219 individually and in combinations, and (iii) gaining a better understanding of QTL interactions to obtain higher yield advantage under drought.
Result
Development of BC1F3 pyramided lines using marker assisted breeding
The number of selected individuals in every generation of BC1F3 development is shown in Fig. 1. In the 1st season, 96 % of the total F1:1A individuals from Cross 1, 94 % of the total F1:1B individuals from Cross 2, and 96 % of the total F1:1C individuals from Cross 3 amplified the alleles of both parents (heterozygous). This indicated their true hybrid nature as confirmed using peak simple sequence repeat (SSR) markers at each qDTY locus (RM236 for qDTY2.2, RM520 for qDTY3.1,, and RM511 for qDTY12.1). In the 2nd season, Cross 4 was made to develop the F1(2) population by crossing five confirmed F1:1A individuals with 20 confirmed F1:1B individuals. From 587 F1(2) individuals genotyped, only 14 individuals showed donor alleles at both the qDTY2.2 and qDTY3.1 loci when ran with the peak and foreground SSR markers of these loci (OSR17, RM236, RM12460, RM279, RM12569, RM12949, RM12992, RM520, RM416, and DTY3-14). At the same time, 141 BC1F1:1C individuals from Cross 5 were also genotyped for the presence of the qDTY12.1 locus using six peak and foreground SSR markers (RM28076, RM28099, RM28130, RM511, RM1261, and RM28166). However, only 24 BC1F1:1C individuals were amplified qDTY12.1 alleles.
Fig. 1.
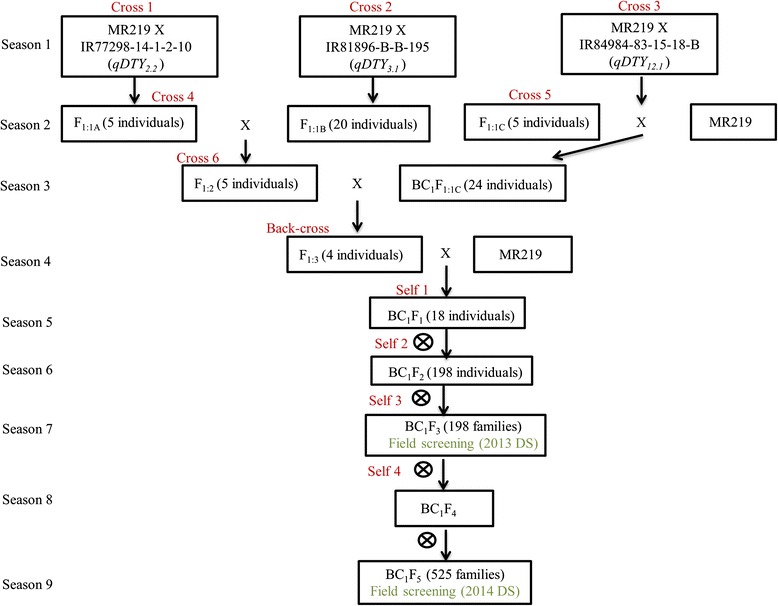
Crossing scheme for the development of BC1F5 MR219 pyramided lines and the number of plants selected at every generation
In the 3rd season, Cross 6 was made to produce the F1(3) population by crossing 24 BC1F1:1C selected individuals with the five F1(2) individuals (IR 97992, IR 97995, IR 97998, IR 98001, and IR 98003). These five F1(2) individuals were selected from the 14 F1(2) individuals with two qDTYs (qDTY2.2 + qDTY3.1) as they had morphological characteristics similar to that of MR219. In the 4th season, a total of 472 F1(3) individuals were genotyped for the presence of all the three qDTY loci and 24 F1(3) individuals were amplified specific alleles of all the three qDTYs. However, only four F1(3) individuals which had similar morphological characteristics as that of the recipient parent and with the higher recipient parent genomes (83 % in IR 98001-7, 89 % in IR 98003-130 and IR 98003-58, and 94 % in IR 98003-257) were further backcrossed with MR219 to produce the BC1F1 population.
In the 5th season, from the total of 1263 BC1F1 individuals genotyped, 104 individuals were amplified specific alleles of all the three qDTYs. However, only 18 BC1F1 individuals which had similar morphological characteristics to that of MR219 were selected. These 18 selected BC1F1 individuals were then genotyped with 48 background SSR markers, results of which showed that the genome recovery varied from 83 % in IR99778-60 to 99 % in IR99784-4. Thus, these 18 selected BC1F1 individuals were selfed to generate a large number of BC1F2 seeds. In the 6th season, a total of 5677 BC1F2 individuals obtained from the 18 individuals from the BC1F1 populations were genotyped for the presence of specific alleles of all the three qDTY loci, and results showed that 437 BC1F2 individuals were homozygotes at the different qDTY loci and their combinations. Furthermore, only 33 BC1F2 individuals carried all the three qDTYs. From a total of 437 BC1F2 individuals that were homozygotes at different qDTY and their combinations, only 198 BC1F2 individuals were finally selected based on their morphological similarity to MR219 and were further advanced in the 7th season to develop 198 BC1F3 families of pyramided lines (PLs).
Imposition of drought stress
Water table depth in the experimental plots of the RS trials during the dry season (DS) of 2013 and 2014 are shown in Fig. 2. Daily rainfall data at the IRRI experimental field during the months of January to April in 2013 DS and 2014 DS were also taken (Fig. 3). In the 2013 DS, the total rainfall was 257.7 mm and the RS treatment was initiated in the 2nd week of February while the stress trial was not irrigated until February 12. However, the stress trials received 137.6 and 37.8 mm rainfall in the 3rd week of February and 1st week of March, respectively, and only 0 to 15.5 mm rainfall from then on up to the last week of April. In the 2014 DS, the RS treatment was initiated also in the 2nd week of February and the stress trial received only 43 mm total rainfall from the day the stress was imposed until harvest. Ground water table continued to decrease to up to 100 cm within a month until harvest. Furthermore, the average water table depth during the critical flowering stage was 100 cm in both seasons, indicating that the crop faced severe RS in both seasons. Figure 4 shows the rice crop in its various growth stages in the RS trials after stress imposition.
Fig. 2.
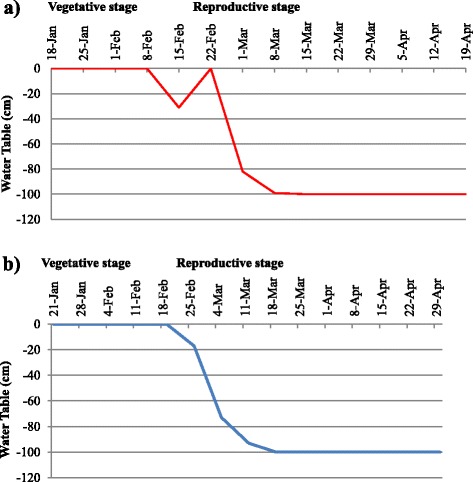
Parching water table in stress trials; a during 2013 dry season; b during 2014 dry season
Fig. 3.
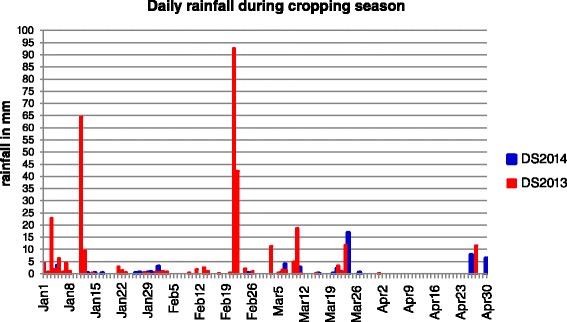
Daily rainfall during the dry season experiment period from January to April in 2013 and 2014
Fig. 4.
Crop at various stages after stress imposition a after 3 weeks of transplanting; b at reproductive stage; c at severe stress showing leaf rolling
Validation of marker assisted breeding for drought tolerance by phenotyping
Line means and heritability
The overall performance of the MR219 PLs and their recipient parent is shown in Table 2. Mean days to 50 % flowering (DTF) in the MR219 PLs varied from 81 to 99 days in the NS trials and 87 to 92 days in RS. Flowering was delayed by 6 to 9 days in the RS trials as compared to the NS trials. Plant height (PH) ranged from 60 to 71 cm in the RS trials and from 87 to 95 cm in the NS trials. RS reduced PH by 24 to 30 cm. The mean grain yield (GY) of PLs ranged from 397 to 920 kg ha−1 in the RS trials and from 6040 to 7120 kg ha−1 in the NS trials. The 86 to 93 % reduction in yield showed that the MR219 PLs were subjected to severe RS in both 2013 DS and 2014 DS. The H of DTF was high in both NS and RS trials (Table 2). For PH, the H value was low to moderate in the RS trials but moderate to high in the NS trials. The H of GY was medium to high in the RS trials and medium in the NS trials.
Table 2.
Means for days to flowering (DTF), plant height (PH) and grain yield (GY) of MR219 PLs as compared to MR219 under lowland reproductive stage drought stress and non-stress conditions
| Season/Year | Stress | Duration | No. of MR219 PLs | DTF | PH (cm) | GY (kg ha-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean MR219 PLs | Mean MR219 | Trial H | Mean MR219 PLs | Mean MR219 | Trial H | Mean MR219 PLs | RYR (%) | Mean MR219 | Trial H | ||||
| DS2013 | Non-stress | Short | 108 | 81 | 89 | 0.82 | 90 | 104 | 0.54 | 6040 | 93 | 6639 | 0.53 |
| DS2013 | Drought | Short | 108 | 87 | - | 0.81 | 60 | - | 0.27 | 397 | - | 0 | 0.76 |
| DS2013 | Non-stress | Medium | 90 | 85 | 91 | 0.75 | 95 | 91 | 0.75 | 6774 | 86 | 5917 | 0.54 |
| DS2013 | Drought | Medium | 90 | 92 | - | 0.88 | 71 | - | 0.36 | 920 | - | 0 | 0.87 |
| DS2014 | Non-stress | Medium | 525 | 99 | 100 | 0.86 | 87 | 97 | 0.7 | 7120 | 91 | 8319 | 0.68 |
| DS2014 | Drought | Medium | 525 | 90 | - | 0.78 | 60 | - | 0.52 | 672 | - | 0 | 0.58 |
MR219 did not flower under drought stress
Dry season (DS), Days to 50 % flowering (DTF), and plant height (PH, in cm), broad-sense heritability (H), grain yield (GY, in kg ha−1) and relative yield reduction in RS compared to NS (RYR, in percentage)
Performance of promising drought tolerant pyramided lines
The yield performance of 16 most promising drought-tolerant BC1F5 MR219 PLs is presented in Table 3. In the 2013 DS and 2014 DS, the recipient parent, MR219, did not flower in the RS trials. The differences in the DTF of the MR219 PLs under NS and RS conditions were considerable (86 to 94 days under NS and 92 to 105 days under RS). In the NS trials, the DTF for the chosen MR219 PLs was relatively lower than that of MR219 (data not shown). Differences in PH for the chosen MR219 PLs under RS and NS were also notable (82 to 101 under NS and 55 to 69 cm under RS). Mean PH for the selected MR219 PLs in both RS and NS trials was lower than that of MR219. The GY of selected MR219 PLs ranged from 6947 to 11,672 kg ha−1 in the NS trials and from 903 to 2523 kg ha−1 in the RS trials. MR219 produced very little or no GY under RS. However, under NS, the mean GY of MR219 ranged from 5917 to 8319 kg ha−1. Thus, the yield advantage of the PLs over MR219 under RS ranged from 756 to 2521 kg ha−1 in the 2013 DS and from 923 to 2523 kg ha−1 in the 2014 DS. In NS, most of these lines yielded similar to MR219 while some lines recorded higher yields than MR219 (Table 3).
Table 3.
QTL presence, and grain yield (GY) of 16 chosen best performers - across two seasons of lowland drought stress and non-stress conditions
| Line | Duration in 2013DS | qDTY 12.1 | qDTY 3.1 | qDTY 2.2 | GY (kg ha−1) | |||
|---|---|---|---|---|---|---|---|---|
| DS2013 | DS2014 | |||||||
| RS | NS | RS | NS | |||||
| IR 99784-156-137-1-1 | Medium | - | √ | - | 1362 | 6200 | 2523 | 10713 |
| IR 99784-255-7-2-5 | Medium | √ | √ | - | 1346 | 7783 | 1591 | 8383 |
| IR 99784-188-202-1-2 | Medium | - | √ | √ | 939 | 9431 | 1562 | 8369 |
| IR 99784-188-202-1-1 | Medium | - | √ | √ | 939 | 9431 | 1478 | 7937 |
| IR 99784-255-68-1-5 | Medium | √ | √ | √ | 1611 | 6905 | 1183 | 7782 |
| IR 99784-188-202-1-3 | Medium | - | √ | √ | 939 | 9431 | 1058 | 6947 |
| IR 99784-255-55-2-5 | Medium | √ | - | √ | 2073 | 8525 | 1049 | 7858 |
| IR 99784-188-201-B-1 | Medium | √ | - | √ | 977 | 8610 | 1042 | 9975 |
| IR 99784-255-7-2-2 | Medium | √ | √ | - | 1346 | 7783 | 983 | 9438 |
| IR 99784-40-1-B-6 | Medium | - | - | √ | 1164 | 8168 | 942 | 8254 |
| IR 99784-11-35-2-2 | Short | - | √ | √ | 1300 | 8633 | - | - |
| IR 99784-255-9-1-3 | Medium | √ | √ | - | 888 | 8174 | 936 | 9014 |
| IR 99784-156-87-1-9 | Medium | √ | √ | √ | 923 | 5808 | 930 | 11672 |
| IR 99784-188-179-1-2 | Medium | √ | √ | - | 1459 | 9107 | 927 | 7419 |
| IR 99784-11-8-1-5 | Short | - | - | √ | 756 | 9573 | - | - |
| IR 99784-255-49-1-1 | Medium | √ | √ | - | 2521 | 5832 | 903 | 8152 |
| MR 219 | - | - | - | 13 | 5917 | 0 | 8322 | |
MR219 did not flower under drought stress
Performance of different combinations of qDTY
The mean GY of the MR219 PLs with single and different combinations of qDTYs (QTL class – A, B, C, D, E, F, and G) alongside the recipient parent (no QTL class) is presented in Table 4. Under RS, the mean GY for the PLs was significantly higher than that of MR219. Generally, among MR219 PLs with a single qDTY, the mean GY of Class G (with qDTY2.2) was highest, followed by Class F (with qDTY3.1) and Class E (with qDTY12.1). On the other hand, among the PLs with two qDTYs, Class E (qDTY2.2 + qDTY3.1) followed by Class C (qDTY12.1 + qDTY3.1) and Class B (qDTY12.1 + qDTY2.2) provided a significant yield advantage over MR219 under RS. However, under NS, the yield levels of the recipient parent were higher compared to other QTL classes. These results indicate that PLs with qDTY/s were quite effective in enhancing GY under severe RS conditions (Fig. 5).
Table 4.
QTL class mean comparisons for grain yield in kg ha−1 under reproductive stage drought stress (stress) and irrigated control (non-stress) in MR219 as the recipient parent in trials conducted during dry season 2013 and 2014
| QTL class label | QTL | 2013 - short duration | 2013 - medium duration | 2014 - medium duration | |||
|---|---|---|---|---|---|---|---|
| NS | RS | NS | RS | NS | RS | ||
| A | qDTY 2.2 + qDTY 3.1 + qDTY 12.1 | 4600 ba | 475.55 c | 6799 ab | 642 b | 7706 d | 442 b |
| B | qDTY 12.1 + qDTY 3.1 | 6577 d | 179.57 ab | 6652 ac | 1072 d | 7532 cd | 794 e |
| C | qDTY 12.1 + qDTY 2.2 | 4518 ba | 376.55 c | 7633 b | 761 bc | 7364 ac | 698 d |
| D | qDTY 2.2 + qDTY 3.1 | 6867 d | 444.98 c | 7158 bc | 1112 d | 6843 b | 571 c |
| E | qDTY 12.1 | 5935 c | 373.46 cb | 6229 a | 654 b | 6967 b | 301 a |
| F | qDTY 3.1 | 6366 dc | 582.25 c | 6488 a | 890 c | 7374 ac | 568 c |
| G | qDTY 2.2 | 4148 a | 514.48 c | 6760 ab | 1104 d | 7079 ba | 669 dc |
| X (MR 219) | NO QTL | 6713 bdc | 0 a | 5917 ab | 13 a | 8321 bcd | 0 ab |
| F- value | 7.48 | 3.27 | 2 | 11.76 | 9.45 | 19.39 | |
| p-value | <0.0001 | 0.0043 | 0.07 | <0.0001 | <0.0001 | <0.0001 | |
Means followed by the same letter are not significantly different
Fig. 5.
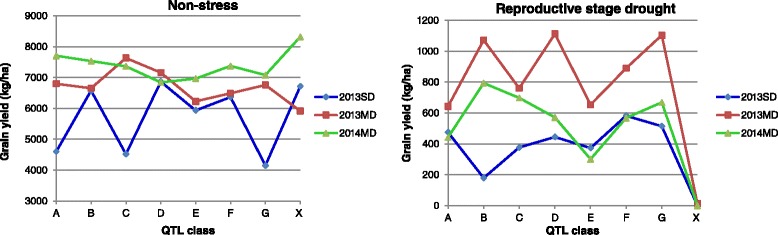
Graph showing QTL classes (X axis) and mean grain yield (Y axis) of short (SD) and medium duration (MD) lines with MR219 as the recipient parent. Trials were conducted during dry seasons of 2013 and 2014
Discussion
Selection of parents
Developing and improving rice varieties with high GY under RS through different breeding strategies is necessary for obtaining sustainable rice yields even as the frequency and severity of drought are predicted to increase. However, like in all breeding programmes to develop superior lines, the selection of parents is a critical step. Until now, only one study has reported on improving drought tolerance in the Malaysian mega-variety MR219 which is known to be highly sensitive to drought [25]. However, the drought stress level used in the study was mild as the yield reduction of improved lines in the RS trials was less than 20 % compared to the control. According to [26], severe levels of RS with more than 65 % yield reduction in RS trials compared to control are necessary to identify true drought-tolerant lines.
Despite having a high adaptability to drought, several Malaysian traditional rice varieties and landraces usually carry undesirable traits such as low yield potential, poor response to high-input management, and taller plant type. One or more of these traits located close to qDTY regions, thus becoming a potential linkage drag in breeding for drought tolerance using conventional approaches. Moreover, in quantitative traits such as GY and PH, variability might be controlled by many minor QTLs. Thus, introgression of several minor genes to improve one quantitative trait is difficult to achieve due to two reasons: (i) the effect of minor genes is regularly inconsistent and (ii) there will be too many markers to handle in the breeding program. Different QTLs segregating in different mapping populations, QTL x genetic background interaction, and QTL x environment interaction could be the reasons for the inconsistency in the estimated QTL effects [27]. Thus, the selection of major effect QTLs to be used in breeding programs is essential. qDTY12.1 has shown the largest effect on GY under upland RS conditions while qDTY2.2 and qDTY3.1 have shown the larger effect on GY under lowland RS conditions [18, 22, 28]. It is interesting to note that these three qDTYs were identified in severe levels of RS under field conditions. Furthermore, qDTY2.2, qDTY3.1, and qDTY12.1 also exhibited a consistent effect in different mapping populations. The use of pre-breeding lines or near isogenic lines with major effect QTL is the key to be successful QTL pyramiding [1, 29].
Pre-breeding lines that possess drought yield QTLs coupled with appropriate plant type and high yield have been generated at IRRI. These lines were produced by crossing low-yielding but drought-tolerant donors with high-yielding but drought-susceptible recipients [1]. In this study, MR219 was used as recipient parent as it carries desirable traits such as high yield potential, appropriate PH, and moderate to high tolerance to multiple pests and diseases. Pre-breeding lines in the background of high-yielding mega-varieties with a major and consistent effect qDTY, namely, IR 77298-14-1-2-10, IR 81896-B-B-195, and IR 84984-83-15-18-B were used as donor parents.
Development of drought tolerant MR219 PLs using stepwise MAB technique
MAB has been an effective and efficient strategy in crop improvement as it speeds up and simplifies the selection process especially for complex traits [30–32]. Several researchers successfully pyramided QTLs/genes for multiple disease resistance to provide a broader spectrum of resistance than those conferred by a single QTL/gene [33–36].
Swamy and Kumar [1] reported that Vandana-introgressed lines with qDTY12.1 showed a yield advantage of only 500 kg ha−1 over the drought-tolerant cultivar Vandana. Thus, introgression of only a single qDTY into drought-susceptible mega-varieties may not produce adequate yield increments under RS conditions. In this study, several MR219 PLs gave 1000 kg ha−1 or more yield in the RS trials. This result indicates that marker-assisted QTL pyramiding of major-effect qDTYs through backcross breeding is an appropriate strategy to achieve an increase of at least 1000 kg ha−1 of GY under RS conditions.
Marker assisted pyramiding can also enable breeders to introgress two or more QTLs controlling various traits associated with biotic and abiotic stresses in plants. Frisch and Melchinger [37] concluded that the effectiveness of marker assisted breeding/pyramiding depends on the availability of closely linked markers and/or flanking markers for the target locus, the size of the population as well as the number of backcrosses, and the position and number of background markers. However, another study [38] indicated that the selection of the recipient and donor parents was more crucial. MAB with a stepwise screening technique was applied to select genotypes with desirable genes/QTLs by reducing a number of selected individuals in each step [35, 39].
In the present study, peak markers tightly-linked to the QTL region were selected. The linkage drag was eliminated using flanking markers while background markers were used for the fast recovery of the recipient parent’s genetic background [40]. Donor fragments of approximately 15 Mbp of the three drought yield QTLs, qDTY2.2 (10 Mbp), qDTY3.1 (1.7 Mbp), and qDTY12.1 (3.5 Mbp) were introgressed into MR219 crosses. The PLs in which all the qDTYs were successfully introgressed represented about 3.8 % of the genome. With this size of the introgressed fragments, linkage drag might have occurred and affected the phenotype of the plants with qDTYs. This is partly evident from the variability in the DTF of the MR219 PLs as compared to the recipient parent. Dixit et al. [41] reported that the qDTY2.2 and qDTY12.1 regions were narrowed down, thus, introgression of these refined QTL regions could minimize the undesirable linkage of the qDTY donors.
The probability of obtaining at least one ideotype carrying homozygous loci in the F2 population is 4n, where ‘n’ is the number of target loci [42]. In the current study though, only 14 (F1:2) individuals with two qDTYs (qDTY2.2 + qDTY3.1) were identified from the total of 587 individuals produced in Cross 6. This indicates that the ideotype plant was absent probably because the crossing program involved four different genetic backgrounds. Therefore, a greater number of the F1:2 population is required to get one ideotype. Similarly in BC1F2 populations, only 33 individuals from the total of 5677 individuals with all three qDTYs in homozygous loci were identified, which confirmed that at least a doubled population size is required to get one desired ideotype. The same observation was reported by [35]. As the QTL regions were large, more than one marker for each QTL was used in step-wise system for the foreground selection. The larger size of the individual QTL regions as well as the necessity to pyramid two or more QTLs to obtain a sufficient number of positive plants with combinations of qDTYs requires maintaining a larger population than pyramiding genes/QTLs having tightly linked markers.
Introgression of QTLs involved in stress tolerance often induces undesirable traits from the donor parents. This might be due to the lack of a precise knowledge of the key genes underlying the QTLs controlling the target traits [38]. Servin and Hospital [43] reported that two to four markers on a chromosome of about 100 cM distance could provide adequate coverage of the genome on backcross programs through MAB simulation study. In this study, at least four markers per chromosome with an average distance of 35 cM between markers were used for background selection in the BC1F1 populations as recommended by [39, 40]. The selected BC1F1 MR219 PLs had 83 to 99 % of the recipient genome.
Severity of reproductive stage drought stress trials
The level of drought in the 2013 DS and 2014 DS was considered as severe based on the mean yield reduction of more than 65 % as compared to NS (Table 2). The soil showed a deep crack due to insufficient water moisture (Figs. 2 and 4). In a different study, [44] reported yield reductions of up to 70 % upon imposing drought for 15 days at panicle initiation stage and 88 and 52 % reduction when stress was introduced at flowering and grain filling stage, respectively. Although [45] proposed that 50 % reduction in yield is required to identify true drought-tolerant lines, [46] did not, however, observe any response to selection in screens that had a yield reduction of up to 56 %. They recommended that a screening protocol that could reduce the mean yield by at least 65 % under RS as compared to irrigated control is required in order to identify true drought-tolerant lines.
Agronomic performance of MR219 PLs under RS and NS conditions
Delay in flowering that was observed in the RS trials in this study confirmed that water stress affected flowering time. Similar results were reported by [16, 17, 19, 47–53].
MR219 was extremely sensitive to severe RS conditions as it did not flower under RS. However, MR219 PLs were less affected by RS probably due to the QTL alleles that exhibited a tendency to reduce delay in flowering under stress conditions. Similar results were observed in NS trials where MR219 PLs flowered earlier than their recipient parent. Bernier et al. [54] and Venuprasad et al. [19] showed that qDTY12.1 and qDTY3.1 affected both GY and DTF under RS conditions, suggesting that genes within these QTLs are probably associated with early DTF. In this study, stress was imposed 30 days after transplanting to ensure that even lines with earliest flowering did not escape drought and that the selected lines are truly drought tolerant.
Performance of identified improved drought tolerance PLs
Sixteen BC1F5 MR219 PLs with yield advantages of 903 to 2523 kg ha−1 under RS during the 2014 DS were identified. The selected MR219 PLs also performed well in the NS trials. Two PLs, IR 99784-156-137-1-1 and IR 99784-188-201-B-1, gave yield advantages of 1042 kg ha−1 and 2523 kg ha−1, respectively, under severe RS and 1653 kg ha−1 and 2391 kg ha−1 yield advantages over MR219 under NS conditions. Comparison among the chosen PLs showed that 75 % and 63 % of them carried qDTY3.1 and qDTY2.2 either as a single qDTY or a combination with other qDTYs. PLs with qDTY12.1 either singly or in combination with other qDTYs was the lowest (56 %). However, no definite pattern could be assessed as to the performance of selected lines under RS due to the interaction between QTLs and background genotyping. This implies that QTL pyramiding using MAB technology is an effective method to improve current mega-varieties and to develop new rice cultivars that are tolerant to drought. PLs with good yield potential and an appreciable yield under RS can be effectively disseminated for cultivation by rice farmers in drought-prone environments of Malaysia.
Performance of different combinations of qDTY
The higher mean GY of MR219 PLs compared to the recipient parent MR219 under RS is an indication of the positive effect of introgressed qDTYs under these conditions. Mean comparisons of qDTY classes show that introgressed lines with a single qDTY provided a significant yield increase over MR219 under RS without any yield reduction in NS, indicating the positive effect of qDTY2.1, qDTY3.1, and qDTY12.1 in the MR219 background to increase yield under RS. These QTLs have been found to show some effect in the background of the high-yielding varieties IR 64 [20], Swarna, and TDK 1 [19, 55]. This is the first report of introgression and yield increase produced by qDTY2.1, qDTY3.1, and qDTY12.1 in genetic backgrounds other than varieties against which the individual qDTYs were identified.
Mean comparisons of qDTY classes also show that introgressed lines with two qDTYs (qDTY2.2 + qDTY3.1, qDTY2.2 + qDTY12.1, and qDTY3.1 + qDTY12.1) provided a significant yield advantage over MR219 under RS. However, the yield advantage provided by combining three qDTYs (qDTY2.2 + qDTY3.1 + qDTY12.1) was lower than the two-qDTY combinations. This demonstrates the absence of a positive interaction between three qDTYs even though each shows a positive interaction with each other. Mean comparisons of qDTY classes further reveal the role of interaction between qDTYs and between qDTYs and recipient genetic background. The results indicate: (i) a non-linear interaction between multiple qDTYs and (ii) the presence of a differential synergistic relationship between qDTY combinations [20, 56]. The qDTY combinations that provide higher yield advantage under RS may vary from genotype to genotype due to qDTY x qDTY and qDTY x genetic background interactions. The results imply the necessity to identify qDTY combinations with positive interaction against different genetic backgrounds for their precise use in MAB.
Conclusion
Drought is a major challenge in achieving sustainable world rice production in the rainfed ecosystems of Asia. Breeding drought-tolerant rice cultivars can increase rice production yields especially in rainfed ecosystems under drought stress. The identification and introgression of QTL regions with a large and consistent effect on GY under RS presents an opportunity to improve high-yielding drought-susceptible mega-varieties through MAB. The selected MR219 PLs developed in this study conferred a yield advantage of 903 to 2523 kg ha−1 over their recipient parent under RS conditions and maintained high yield potential similar to or higher than MR219 under NS, indicating that drought tolerance can be successfully combined with high yield potential in the background of semi-dwarf varieties.
Methods
Plant materials and breeding scheme for development of BC1F5 population
All the plant materials including the mapping populations and PLs used in the study were developed at IRRI, Philippines. A total of 198 BC1F3 and 525 BC1F5 PLs were used in this study and were derived from the crossing of the drought-susceptible Malaysian rice cultivar MR219 (recurrent parent) with three drought-tolerant donor parents, namely IR 77298-14-1-2-10, IR 81896-B-B-195, and IR 84984-83-15-18-B. MR219 is a high-yielding indica rice cultivar with desirable traits such as short maturity, appropriate plant height with strong culms, and resistance to blast and bacterial leaf blight, while the grain can be marketed as a long-grain variety [57]. However, it is highly susceptible to drought. The donor parents, meanwhile, are the pre-breeding lines developed from mapping populations generated for QTL identification study by crossing drought-susceptible mega-varieties with drought-tolerant donors. Donor parents (Table 1) also carried desirable traits such as high yield, appropriate plant height, and medium to high resistance to pests and diseases as they were in the background of mega-varieties.
Table 1.
Details on drought yield QTLs (qDTYs) used in the study
| Recipient | Donor | Donor line used | Ecosystem | qDTY name | Chromosome | Interval | Peak marker | a | R2 |
|---|---|---|---|---|---|---|---|---|---|
| IR64 | Adaysel | IR77298-14-1-2-10 | Lowland | qDTY 2.2 | 2 | RM236-RM279 | RM236 | 14 | 6 |
| Swarna | Apo | IR81896-B-B-195 | Lowland | qDTY 3.1 | 3 | RM520-RM16030 | RM520 | 30 | 27 |
| Vandana | Way Rarem | IR84984-83-15-18-B | Upland | qDTY 12.1 | 12 | RM28048-RM511 | RM511 | 47 | 33 |
Additive effect compared to trial mean (a, in percent), phenotypic variance (R2, in percent)
The three qDTYs were introgressed into MR219 using the QTL pyramiding technique as suggested by [39] and [35] which involved six crosses, followed by a backcross and four times of selfing thereafter (Fig. 1).
Genotyping and marker assisted breeding
The DNA marking work was conducted at the Molecular Marker Applications Laboratory (MMAL) of the Plant Breeding, Genetics, and Biotechnology Division of IRRI. Fresh leaves from all lines were collected and freeze-dried. DNA extraction of leaf samples was carried out using the modified CTAB protocol [58]. A total of 125 SSR markers linked to the three qDTY regions (foreground selection) and an additional 711 SSR markers distributed in the whole rice genome which are unlinked to the qDTY regions (background selection) were tested in a polymorphism survey. However, only three peak markers and additional 13 flanking markers were found to be polymorphic in the three qDTY regions and were used in foreground selection in every generation. The peak markers linked to the three qDTY regions on chromosomes 2, 3, and 12 were RM236, RM520, and RM511, respectively (Table 1). All SSR markers were assayed on the MR219 rice population as described by [59]. The polymerase chain reaction products were separated in 6 % or 8 % non-denaturing polyacrylamide gel electrophoresis. DNA fragments were then stained with SYBR Safe and visualized with UV trans-illuminator. DNA profiles from such markers were scored in comparison with their parents. Plant selection in each generation was dependent on a number of plants that carried the target regions. Step-wise marker assisted selection and phenotyping technique was applied to select, to advance the chosen plants, and to decrease the number of samples in every generation.
Selection process
In this study, the selection process involved four steps. First is the foreground selection for the MR219 population where the qDTY2.2, qDTY3.1 and qDTY12.1 loci were monitored by RM236, RM520, and RM511 markers, respectively, which are tightly linked with those QTLs [18, 22]. Once the individuals with donor alleles at the peak of the qDTY region/s were identified, these individuals were genotyped with an additional three to six markers flanking both sides of the qDTY region/s. This second step is also known as recombinant selection and its main purpose is to increase the efficiency of selection by reducing linkage drag [40, 60]. Moreover, the use of flanking markers for recombinant selection also assisted in recovering the important traits of the recipient parent and in minimizing the effects of linkage drag from the qDTY donors. In the third step, the individuals which passed the foreground and recombinant selections will undergo phenotypic screening. Here, only individuals with desired plant traits such as appropriate plant height (90 to 120 cm), long grains, and free of diseases were short listed. The first three steps of selection were performed in every generation of development of the PLs. However, only in the BC1F1 generation, selected individuals were also genotyped with background markers in order to determine the percentage of the recipient parent genome. In the fourth step, plants showing positive interactions between QTLs/QTL combinations and background as evidenced by higher yield under drought were selected.
Performance of pyramided lines
After genotyping, 198 BC1F3 (separated between short and medium duration PLs) and 525 BC1F5 PLs with different qDTYs and their combinations were selected based on similarity in morphological characteristics with MR219. These 198 BC1F3 and 525 BC1F5 PLs were evaluated together with their recipient and donor parents in the field under lowland RS and NS conditions during the 2013 DS and 2014 DS. Field-based phenotyping trials were conducted at the IRRI farms in lowland transplanted conditions (IRRI, Los Banos, Philippines, 140 N 1210E, 21 m above sea level). Lowland refers to field trials conducted under flooded, puddled, and transplanted conditions. In total, six lowland trials (three under RS and three under NS) were conducted using this population. MR219 PLs and MR219 were evaluated in an alpha lattice design with two replications [61] in plot sizes of two rows of 5 m length at 25 cm × 25 cm spacing. Missing hills were replanted with stock seedlings within 10 days of transplanting. A total of 50 plants were maintained in each plot. Inorganic fertilizers (N:P:K) were applied at the rate of 90:30:30 kg ha−1. The post emergence herbicide Sofit (pretilachlor 0.3 kg a.i. ha−1) was applied four days after transplanting and hand weeding was done for weed control. For the control of stem borers and other insects, Furadan (carbofuran 1 kg a.i. ha−1) at five days after transplanting and Cymbush (cypermethrin 1 kg a.i. ha−1) at 16 days after transplanting were applied. To control snails, a molluscicide, Bayluscide (niclosamide 0.25 kg a.i. ha−1) was also applied to the fields.
For the RS trials, the fields were irrigated to maintain soil moisture at field capacity or above for four weeks after transplanting. RS was imposed four weeks after transplanting by draining water from the field. Perforated PVC pipes were placed at a 100-cm soil depth in four different points in the field. Daily water table depth was measured in the RS trials after stress initiation. Data on daily rainfall, daily maximum and minimum temperature, and relative humidity for the trial period were recorded. The fields were allowed to dry until the soil cracked and the surface was completely dry. When the target level of soil dried and the check varieties as well as 70 % of the entries showed severe leaf rolling, and the water table reached below 100 cm and remained the same for about three weeks, irrigation was introduced through flash flooding. The fields were drained again after 24 h [19] to impose the second cycle of stress. Parching water table was measured from all pipes every day after draining the field until the crop reached 50 % maturity.
In the NS trials, 5 cm water level was maintained in the fields throughout the crop season until draining before harvesting. The NS trials were conducted to obtain the data on the performance of PLs under control condition to select lines combining high yield under NS and considerably good yield under RS conditions.
Data collection
Data for DTF, PH, and GY were recorded from all trials. DTF was recorded as the number of days from sowing till the day when 50 % of the plants had flowering tillers. PH (in cm) of three plants from each plot was measured at maturity from ground level to the tip of the tallest tiller and averaged for analysis. GY from each plot was harvested at physiological maturity, dried to 14 % moisture content, and weighed. The measured GY was then converted to kg ha−1.
Statistical analysis
Recorded data from the RS and NS trials were compiled separately. Data from each trial were analyzed using CROP STAT v7.2 and PB Tools v1.1.0 softwares (http://bbi.irri.org/products) based on a mixed model that considers replications and blocks within replications as random effect and the genotypes as fixed effect. Trial-wise broad-sense heritability (H) for each trait was calculated as:
where σ2g is genotypic variance, σ2e is error variance, and r is the number of replications.
Selection of drought-tolerant pyramided lines
BC1F5 PLs with different qDTY combinations that produced more than 900 kg ha−1 under RS but yielding similar or more than MR219 under NS were identified during the 2014 DS. These identified PLs were classified as drought tolerant. A total of 16 BC1F5 PLs were classified as the most promising as they produced more stable and consistent GY across the two dry seasons. The MR219 PLs with single and different combinations of qDTYs were categorized into eight classes depending on whether they possessed one (class E, F, and G), two (class B, C, and D) or three (class A) qDTYs or none (class X).
QTL combinations class analysis
The performance of the genotype nested within the QTL class in the block within the replicate is modelled as follows:
where μ is the population mean, rk is the effect of the kth replicate, b(r)kl + qi is the effect of the lth block within the kth replicate, qi is the effect of the ith QTL, g(q)ij is the effect of the jth genotype nested within the ith QTL and eijkl is the error [62]. The effects of QTL and genotypes within QTL are considered fixed while the replicate and blocks within replicate effects are considered random.
Availability of data and material
All the data from which conclusions of this research are drawn are present in Tables 1, 2, 3 and 4 and Figs. 1, 2, 3, 4 and 5.
Acknowledgments
The authors would like to thank the Global Partnership Initiative for Plant Breeding Capacity (GIPB) for their research fund support, Faculty of Science and Technology (FST) and International Rice Research Institute (IRRI) for the laboratory, field and glasshouse facilities support.
Abbreviations
- DTF
days to flowering
- qDTYs
drought yield QTLs
- DS
dry season
- GY
grain yield
- H
heritability
- IRRI
International Rice Research Institute
- KDML 105
Khao Dawk Mali 105
- MAB
marker assisted breeding
- MMAL
molecular marker application
- NS
non-stress
- PBGB
plant breeding, genetics, and biotechnology
- PH
plant height
- PLs
pyramided lines
- QTLs
quantitative trait loci
- RS
reproductive stage drought stress
- SSR
simple sequence repeat
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NAAS carried out the genotyping and phenotyping studies, contributed to statistical analysis, and manuscript draft preparation, MS was associated with genotyping of the population, WR contributed to the selection of recipient variety from Malaysia for QTL introgression and manuscript preparation, TSC contributed to phenotyping of the PLs across seasons, AR contributed to statistical analysis and manuscript draft preparation and AK conceptualized the study, supervised the genotyping, phenotyping and contributed to manuscript preparation. All authors read and approved the final manuscript.
Contributor Information
Noraziyah Abd Aziz Shamsudin, Email: nora_aziz@ukm.edu.my.
B. P. Mallikarjuna Swamy, Email: m.swamy@irri.org.
Wickneswari Ratnam, Email: wicki@ukm.edu.my.
Ma. Teressa Sta. Cruz, Email: m.stacruz@irri.org.
Anitha Raman, Email: a.raman@irri.org.
Arvind Kumar, Email: a.kumar@irri.org.
References
- 1.Swamy BPM, Kumar A. Genomics-based precision breeding approaches to improve drought tolerance in rice. Biotechnol Adv. 2013;31:1308–18. doi: 10.1016/j.biotechadv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Luo LJ, Zhang QF. The status and strategy on drought resistance of rice (Oryza sativa L.) Chin J Rice Sci. 2001;15:209–14. [Google Scholar]
- 3.Crosson P. Fragile lives in fragile ecosystems, Proceedings of the International Rice Research Conference. Los Banos: International Rice Research Institute; 1995. Natural resource and environmental consequences of rice production; pp. 83–100. [Google Scholar]
- 4.Boyer JS, Westgate ME. Grain yields with limited water. J Trialal Bot. 2004;55:2385–94. doi: 10.1093/jxb/erh219. [DOI] [PubMed] [Google Scholar]
- 5.Barnabas B, Jager K, Feher A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008;31:11–38. doi: 10.1111/j.1365-3040.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- 6.Fukai S, Pantuwan G, Jongdee B, Cooper M. Screening for drought resistance in rainfed lowland rice. Field Crops Res. 1999;64:61–74. doi: 10.1016/S0378-4290(99)00051-9. [DOI] [Google Scholar]
- 7.Jongdee B, Fukai S, Cooper M. Leaf water potential and osmotic adjustment as physiological traits to improve drought tolerance in rice. Field Crops Res. 2002;76:153–63. doi: 10.1016/S0378-4290(02)00036-9. [DOI] [Google Scholar]
- 8.Liu JX, Liao DQ, Oane R, Estenor L, Yang XE, Li ZC, et al. Genetic variation in the sensitivity of anther dehiscence to drought stress in rice. Field Crops Res. 2006;97:87–100. doi: 10.1016/j.fcr.2005.08.019. [DOI] [Google Scholar]
- 9.Boonjung H, Fukai F. Effects of soil water deficit at different growth stage on rice growth and yield under upland conditions. 2. phenology, biomass production and yield. Field Crops Res. 1996;48:47–55. doi: 10.1016/0378-4290(96)00039-1. [DOI] [Google Scholar]
- 10.Garrity DP, O’Toole JC. Screening rice for drought resistance at the reproductive phase. Field Crops Res. 1994;39:99–110. doi: 10.1016/0378-4290(94)90012-4. [DOI] [Google Scholar]
- 11.Sheoran IS, Saini HS. Drought- induced sterility in rice: changes in carbohydrate levels and enzymes activities associated with the inhibition of starch accumulation in pollen. Sex Plant Reprod. 1996;9:1661–9. doi: 10.1007/BF02221396. [DOI] [Google Scholar]
- 12.Ashley J. Drought and crop adaptation. In: Rowland JRJ, editor. Dryland farming in Africa. UK: Macmillan Press Limited; 1993. pp. 46–67. [Google Scholar]
- 13.Turner NC. Drought resistance and adaptations to water deficits in crop plants. In: Mussel H, Staples RC, editors. Stress physiology of crop plants. New York: Wiley Interscience; 1979. pp. 343–7. [Google Scholar]
- 14.Kumar R, Venuprasad R, Atlin GN. Genetic analysis of rainfed lowland rice drought tolerance under naturally-occurring stress in Eastern India: heritability and QTL effects. Field Crops Res. 2007;103:42–52. doi: 10.1016/j.fcr.2007.04.013. [DOI] [Google Scholar]
- 15.Swamy BPM, Kumar A. Sustainable rice yield in water-short drought-prone environments. In: Teang SL, editor. Conventional and molecular approaches, irrigation systems and practices in challenging environments. 2012. [Google Scholar]
- 16.Vikram P, Swamy BPM, Dixit S, Ahmad H, Sta Cruz MT, Singh AK, et al. qDTY1.1, a major QTL for rice grain yield under drought with a consistent effect in multiple elite genetic backgrounds. BMC Genet. 2011;12:1–30. doi: 10.1186/1471-2156-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghimire KH, Lenie AQ, Vikram P, Swamy BPM, Dixit S, Ahmed H, et al. Identification and mapping of a QTL (qDTY1.1) with a consistent effect on grain yield under drought. Field Crops Res. 2012;131:88–96. doi: 10.1016/j.fcr.2012.02.028. [DOI] [Google Scholar]
- 18.Venuprasad R, Dalid CO, Del Valle M, Zhao D, Espiritu M, Sta Cruz MT, et al. Identification and characterization of large-effect quantitative trait loci for grain yield under lowland drought stress in rice using bulk-segregant analysis. Theor Appl Genet. 2009;120:177–90. doi: 10.1007/s00122-009-1168-1. [DOI] [PubMed] [Google Scholar]
- 19.Venuprasad R, Zenna N, Choi RI, Amante M, Virk PS, Kumar A. Identification of marker loci associated with tungro and drought tolerance in near-isogenic rice lines derived from IR64/Aday Sel. IRRN 32.1. 2007. pp. 270–9. [Google Scholar]
- 20.Swamy BPM, Ahmed HU, Henry A, Mauleon R, Dixit S, Vikram P, et al. Genetic, physiological, and gene expression analyses reveal multiple QTL enhance the yield of rice mega-variety mega-variety IR64 under drought. PLoS One. 2013;8:e62795. doi: 10.1371/journal.pone.0062795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venuprasad R, Bool ME, Quiatchon L, Atlin GN. A QTL for rice grain yield in aerobic environments with large effects in three genetic backgrounds. Theor Appl Genet. 2012;124:323–32. doi: 10.1007/s00122-011-1707-4. [DOI] [PubMed] [Google Scholar]
- 22.Bernier J, Kumar A, Venuprasad R, Spaner D, Atlin GN. A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice. Crop Sci. 2007;47:507–16. doi: 10.2135/cropsci2006.07.0495. [DOI] [Google Scholar]
- 23.Bernier J, Kumar A, Venuprasad R, Spaner D, Verulkar S, Mandal NP, et al. Characterization of the effect of a QTL for drought resistance in rice, qtl12.1, over a range of environments in the Philippines and Eastern India. Euphytica. 2009;166:207–17. doi: 10.1007/s10681-008-9826-y. [DOI] [Google Scholar]
- 24.George T, Magbanua R, Garrity DP, Tubana BS, Quinton J. Rapid yield loss of rice cropped successively in aerobic soil. Agron J. 2002;94:981–9. doi: 10.2134/agronj2002.0981. [DOI] [Google Scholar]
- 25.Rusli I, Zaiton ARH. Symposium on radiation and nuclear technologies for crop improvement and productivity in sustainable agriculture. Kajang, Malaysia. 2013. A success story of FNCA mutation breeding project in Malaysia; pp. 20–7. [Google Scholar]
- 26.Kumar A. Breeding rice for drought tolerance and adaptation to climate change. 2008. [Google Scholar]
- 27.Bernando R. Molecular markers and selection for complex traits in plants: learning from the last 20 years. Crop Sci. 2008;48:1649–64. doi: 10.2135/cropsci2008.03.0131. [DOI] [Google Scholar]
- 28.Swamy BPM, Vikram P, Dixit S, Ahmed HU, Kumar A. Meta-analysis of grain yield QTL identified during agricultural drought in grasses showed consensus. BMC Genomics. 2011;12:319. doi: 10.1186/1471-2164-12-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye G, Smith KF. Marker-assisted gene pyramiding for cultivar development. In: Janick J, editor. Plant breeding reviews. 33. Hoboken: Wiley; 2010. [Google Scholar]
- 30.Sirithunya P, Sriprakhon S, Vongsaprom C, Sreewongchai T, Vanavichit A, Toojinda T. Discovery of broad spectrum blast resistance in rice. In: Vanavichin A, editor. The 1st International Conference on Rice for the Future 31 Aug - 3 Sep 2004. Bangkok: Kasetsart University; 2004. pp. 160–3. [Google Scholar]
- 31.Collard BCY, Jahufer MZZ, Brouwer JB, Pang ECK. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: the basic concepts. Euphytica. 2005;142:169–96. doi: 10.1007/s10681-005-1681-5. [DOI] [Google Scholar]
- 32.Dayteg C, Tuvesson S, Merker A, Jahoor A, Kolodinska-Brantestam A. Automation of DNA marker analysis for molecular breeding in crops: practical experience of a plant breeding company. Plant Breed. 2007;126:410–5. doi: 10.1111/j.1439-0523.2007.01306.x. [DOI] [Google Scholar]
- 33.Castro AJ, Capettini F, Corey AE, Filichkina T, Hayes PM, Kleinhofs A, et al. Mapping and pyramiding of qualitative and quantitative resistance to stripe rust in barley. Theor Appl Genet. 2003;107:922–30. doi: 10.1007/s00122-003-1329-6. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Li X, Jiang G, Xu Y, He Y. Pyramiding of Xa7 and Xa21 for the improvement of disease resistance to bacterial blight in hybrid rice. Plant Breed. 2006;125:600–5. doi: 10.1111/j.1439-0523.2006.01281.x. [DOI] [Google Scholar]
- 35.Sreewongchai T, Toojinda T, Thanintor N, Kosawang C, Vanavichit A, Tharreau D, et al. Development of elite indica rice lines with wide spectrum of resistance to Thai blast isolates by pyramiding multiple resistance QTLs. Plant Breed. 2010;129:176–80. doi: 10.1111/j.1439-0523.2009.01669.x. [DOI] [Google Scholar]
- 36.Pinta W, Toojinda T, Thummabenjapone P, Sanitchon J. Pyramiding of blast and bacterial leaf blight resistance genes into rice cultivar RD6 using marker assisted selection. Acad J. 2013;12:4432–8. [Google Scholar]
- 37.Frisch M, Melchinger AE. Selection theory for marker-assisted backcrossing. Genetics. 2005;170:909–17. doi: 10.1534/genetics.104.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muthurajan R, Balasubramaniam P. Pyramiding gene for enhancing tolerance to abiotic and biotic stresses. In: Mohan Jain S, Brar DS, editors. Molecular techniques in crop improvement. 2. London: Springer Dordrecht Heidelberg; 2012. pp. 163–84. [Google Scholar]
- 39.Servin B, Martin OC, Mezard M, Hospital F. Toward a theory of marker-assisted gene pyramiding. Genetics. 2004;168:513–23. doi: 10.1534/genetics.103.023358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neeraja CN, Maghirang-Rodriguez R, Pamplona A, Heuer S, Collard BCY, Septiningsih EM, et al. A marker-assisted backcross approach for developing submergence-tolerant rice cultivars. Theor Appl Genet. 2007;115:767–76. doi: 10.1007/s00122-007-0607-0. [DOI] [PubMed] [Google Scholar]
- 41.Dixit S, Swamy BPM, Vikram P, Ahmed HU, Sta Cruz MT, Amante M, et al. Fine mapping of QTLs for rice grain yield under drought reveals sub-QTLs conferring a response to variable drought severities. Theor Appl Genet. 2012;125:155–69. doi: 10.1007/s00122-012-1823-9. [DOI] [PubMed] [Google Scholar]
- 42.Witcombe JR, Hash CT. Resistance gene deployment strategies in cereal hybrids using marker-assisted selection: gene pyramiding, three-way hybrids, and synthetic parent populations. Euphytica. 2000;112:175–86. doi: 10.1023/A:1003836132603. [DOI] [Google Scholar]
- 43.Servin B, Hospital F. Optimal positioning of markers to control genetic background in marker-assisted backcrossing. J Hered. 2002;93:214–7. doi: 10.1093/jhered/93.3.214. [DOI] [PubMed] [Google Scholar]
- 44.Yambao EB, Ingram KT. Drought stress index for rice. Philipp J Crop Sci. 1988;13(20):105–11. [Google Scholar]
- 45.Lafitte HR. Management of water controlled drought in breeding plots. In: Fischer KS, Lafitte R, Fukai S, Atlin G, Hardy B, editors. Breeding rice for drought-prone environments. Los Banos: International Rice Research Institute; 2003. pp. 23–6. [Google Scholar]
- 46.Kumar A, Bernier J, Verulkar S, Lafitte HR, Atlin GN. Breeding for drought tolerance: direct selection for yield, response to selection and use of drought-tolerant donors in upland and lowland-adapted populations. Field Crops Res. 2008;107:221–31. doi: 10.1016/j.fcr.2008.02.007. [DOI] [Google Scholar]
- 47.Pantuwan G, Fukai S, Cooper M, Rajatasereekul S, O’Toole JC. Yield response of rice (Oryza sativa L.) to drought under rainfed lowlands: 1. grain yield and yield components. Field Crops Res. 2002;73:153–68. doi: 10.1016/S0378-4290(01)00187-3. [DOI] [Google Scholar]
- 48.Lanceras CL, Pantuwan G, Jongdee B, Toojinda T. Quantitative trait loci associated with drought tolerance at reproductive stage in rice. Plant Physiol. 2004;135:384–99. doi: 10.1104/pp.103.035527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atlin GN, Lafitte HR, Tao D, Laza M, Amante M, Courtois B. Developing rice cultivars for high fertility upland systems in the Asian Tropics. Field Crops Res. 2006;97:43–52. doi: 10.1016/j.fcr.2005.08.014. [DOI] [Google Scholar]
- 50.Jongdee B, Pantuwan G, Fukai S, Fisher K. Improving drought tolerance in rainfed lowland rice: an example from Thailand. Agric Water Manag. 2006;80:225–40. doi: 10.1016/j.agwat.2005.07.015. [DOI] [Google Scholar]
- 51.Ouk M, Basnayake J, Tsubo M, Fukai S, Fischer KS, Cooper M, et al. Use of drought response index for identification of drought tolerant genotypes in rain fed lowland rice. Field Crops Res. 2006;99:48–58. doi: 10.1016/j.fcr.2006.03.003. [DOI] [Google Scholar]
- 52.Zhao X, Qin Y, Sohn JK. Identification of main effects, epistatic effects and their environmental interactions of QTLs for yield traits in rice. Genes Genomics. 2010;32:37–45. doi: 10.1007/s13258-010-0786-y. [DOI] [Google Scholar]
- 53.Blum A. Plant water relations, plant stress and plant production. In: Blum A, editor. Plant breeding for water-limited environments. New York, Dordrecht, Heidelberg, London: Springer; 2011. pp. 11–52. [Google Scholar]
- 54.Bernier J, Atlin G, Serraj R, Kumar A, Spaner D. Review: breeding upland rice for drought resistance. J Sci Food Agric. 2008;88:927–39. doi: 10.1002/jsfa.3153. [DOI] [Google Scholar]
- 55.Dixit S, Singh A, Sta Cruz MT, Maturan PT, Amante M, Kumar A. Multiple major QTLs lead to stable yield performance of rice cultivars across variable drought intensities. BMC Genet. 2014;15:16. doi: 10.1186/1471-2156-15-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dixit S, Swamy BPM, Vikram P, Ahmed HU, Sta Cruz MT. Fine mapping four large effect QTLs for rice grain yield under drought. Theor Appl Genet. 2011;125:155–69. doi: 10.1007/s00122-012-1823-9. [DOI] [PubMed] [Google Scholar]
- 57.MARDI . FFTC research highlight: MR 219, a new high-yielding rice variety with yields of more than 10 mt/ha. 2002. [Google Scholar]
- 58.Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–6. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panaud O, Chen X, McCouch S. Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.) Mol Gen Genet. 1996;252:597–607. doi: 10.1007/BF02172406. [DOI] [PubMed] [Google Scholar]
- 60.Hospital F, Charcosset A. Marker-assisted introgression of quantitative trait loci. Genetics. 1997;147:1469–85. doi: 10.1093/genetics/147.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patterson HD, Williams ER. A new class of resolvable incomplete block designs. Biometrika. 1976;63:83–92. doi: 10.1093/biomet/63.1.83. [DOI] [Google Scholar]
- 62.Knapp SJ. Mapping quantitative trait loci. In: Philipps RL, Vasil IK, editors. DNA based markers in plants. 2001. pp. 58–96. [Google Scholar]


