Abstract
Background
The neuropeptides orexin A and B play a role in reward and feeding and are critical for arousal. However, it was not initially appreciated that most prepro-orexin synthesizing neurons are almost exclusively concentrated in the perifornical hypothalamus, which when stimulated elicits panic-associated behavior and cardiovascular responses in rodents and self-reported “panic attacks” and “fear of dying” in humans. More recent studies support a role for the orexin system in coordinating an integrative stress response. For instance, orexin neurons are highly reactive to anxiogenic stimuli, are hyperactive in anxiety pathology, and have strong projections to anxiety and panic-associated circuitry. Although the two cognate orexin receptors are colocalized in many brain regions, the orexin 2 receptor (OX2R) most robustly maps to the histaminergic wake-promoting region, while the orexin 1 receptor (OX1R) distribution is more exclusive and dense in anxiety and panic circuitry regions, such as the locus ceruleus. Overall, this suggests that OX1Rs play a critical role in mobilizing anxiety and panic responses.
Methods
Here, we used a CO2-panic provocation model to screen a dual OX1/2R antagonist (DORA-12) to globally inhibit orexin activity, then a highly selective OX1R antagonist (SORA1, Compound 56) or OX2R antagonist (SORA2, JnJ10397049) to assess OX1R and OX2R involvement.
Results
All compounds except the SORA2 attenuated CO2-induced anxiety-like behaviors, and all but the SORA2 and DORA attenuated CO2-induced cardiovascular responses.
Conclusions
SORA1s may represent a novel method of treating anxiety disorders, with no apparent sedative effects that were present with a benzodiazepine.
Keywords: hypercapnia, panic, anxiety, orexin, hypocretin, hypothalamus, chemoreception, COPD, bronchitis, asthma
INTRODUCTION
Panic, fear, and anxiety states are adaptive responses to cope with an immediate imminent threat, potential imminent threat, or a nonimminent threat (e.g., distal or temporal), respectively. Responses consist of an integrated pattern of behaviors such as fleeing or fighting during panic, vigilance or freezing during fear, and avoidance during anxiety states. Panic- and fear-associated behaviors are also strongly associated with robust cardiovascular and respiratory responses. In the 1940s, Hess and Brugger found a panic-generating site in the perifornical hypothalamic region (PeF) of cats where electrical stimulation produced strong behavioral and autonomic responses resembling panic/defense reactions (e.g., increased blood pressure, piloerection, hissing, and arching of back).[1] Such responses are mirrored in rodents.[2–6] Furthermore, stimulating the PeF region in humans produces self-reports of panic and fear of dying that are accompanied by panic- and fear-associated autonomic responses (e.g., tachycardia, increased blood pressure, hyperventilation, thermal sensations, and paresthesias);[7–9] all symptoms associated with panic attacks (PAs) in patients with panic disorder (PD).[10] Chronic disinhibition of the PeF also produces rats that are vulnerable to displaying panic-like responses to interoceptive stimuli, such as hypercapnia (elevated blood pCO2), that signals suffocation (see review[11]) and provokes PAs in subjects with PD.[12–14] This region has only recently gained further attention in the context of arousal, anxiety, fear, and panic with the discovery of orexin (OX) neurons in 1998.[15,16] These neurons are almost exclusively localized within the PeF and adjacent lateral hypothalamus of rodents[17] and humans,[18] and are critical for arousal.[19]
Orexin’s role in more complex emotional responses is evolving, and recent studies have demonstrated that orexin neurons are highly responsive to anxiogenic stimuli; strongly innervate anxiety and panic-associated neural systems; and are involved in mobilizing an adaptive, integrated stress response [see reviews[20,21]]. Additionally, we recently determined that this system plays a critical role in coordinating panic-associated anxiety and cardiovascular responses in a panic vulnerability model, and neuropsychiatric patients with increased anxiety symptoms had elevated cerebrospinal fluid orexin levels.[22] Subsequent studies have determined that optogenetically stimulating orexin neurons in rats increase anxiety states and activity in anxiety-related neural circuits,[23] and the orexin system is also implicated in fear-associated learning.[24–26]
Orexins (OXA and OXB) are derived from a common precursor, prepro-orexin, and have high sequency homology; human and rat forms of OXA are 100% conserved.[16] There are two cognate G-protein coupled receptors for orexins that are colocalized in many brain regions, but the orexin 2 receptor (OX2R) is most dominantly located in wake-promoting systems (e.g., histaminergic system). In contrast, the orexin 1 receptor (OX1R) is almost exclusively expressed in vigilance and anxiety/fear/panic regions, such as the locus ceruleus (LC). Moreover, the OX1 to OX2 expression ratio is also higher in the panic, fear, and anxiety-associated circuitry, such as the bed nucleus of the stria terminalis BNST, amygdala, and anterior cingulate.[27,28] Important with respect to translational studies, conservation between rat and human OX1R and OX2R is very high—94 and 95%, respectively.[16] Overall, these studies suggest that selective OX1R antagonists (SORA1) may represent a novel method of treating anxiety.
Consistent with this hypothesis is that systemically administering the centrally active SORA1 SB334867[29] attenuates panic-associated responses following panicogenic challenges (e.g., “suffocation”-associated stimuli (CO2),[30] sodium lactate,[22] and anxiogenic drugs[31]). However, there are concerns that SB334867 is hydrolytically unstable[32] and has off target effects at higher concentrations (unpublished results in review[33]).
Therefore, in the present series of studies, we used a 20% CO2-panic provocation model to screen highly selective and well-characterized orexin receptor antagonists (i.e., a dual OX1/2r antagonist [DORA-12] to globally inhibit orexin activity; then a highly selective SORA1 (Compound 56) and SORA2 (JnJ10397049) to assess OX1r and OX2r involvement) alongside a benzodiazepine positive control for panicolytic properties. In rats, the 20% CO2-induced panic provocation produces anxiety and fear-associated behaviors, and marked cardiovascular and thermoregulatory responses associated with panic states,[30,34,35] replicating a similar 20% CO2-provocation model used in healthy humans that evokes symptoms consistent with criteria for PAs.[36]
MATERIALS AND METHODS
ANIMALS AND HOUSING CONDITIONS
Adult male Sprague-Dawley rats (300–350 g, Harlan Laboratories, Indianapolis, IN) were housed individually under standard environmental conditions (22°C; 12-h light:12-hdark cycle; lights on at 7:00 a.m.) for 5–7 days prior to the surgical manipulations. Food and water were provided ad libitum. Animal care procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals, 8th Edition and the guidelines of the IUPUI Institutional Animal Care and Use Committee.
Telemetry Probe Implantation
Prior to and during surgery, rats were anesthetized with isoflurane (MGX Research Machine; Vetamic, Rossville, IN), then had a radio-telemetric probe (Model. HD-S11, Data Sciences International, St. Paul, MN) implanted into the peritoneal cavity and a pressure transducer implanted into the femoral artery as described previously,[30] which can simultaneously measure mean arterial blood pressure (MAP), heart rate (HR), core body temperature (CBT), and general locomotor activity in freely moving rats.
Description of Hypercarbic or Atmospheric Gas Infusions and Drug Treatment Protocol
We previously used CO2 (ProCO2) and O2 (ProO2) sensors in our enclosed flow cages (12″ width × 12″ height × 24″ length) and verified that O2 and CO2 concentrations remain normal with atmospheric air infusion, and only the CO2 concentrations rapidly increase from <1 to 20% at the 5-min time point for CO2 challenge.[37] In a counter-balanced design (i.e., all rats receive each drug treatment with at least 48 hr between treatments), rats were systemically treated with a control vehicle or different doses of compounds, then placed into the chamber where atmospheric air was being infused. All rats had infusions of the following: (1) 5 min infusion of atmospheric gas (<1% CO2, 21% O2, 79% N2: Praxair, Inc., Indianapolis, IN) for baseline measurements (approximately 45–60 min posttreatment), then (2) either the control gas or experimental normoxic, hypercarbic gas (20% CO2, 21% O2, 59% N2: Praxair) for 5 min (note: for control rats the atmospheric gas was turned off and back on again at the beginning and end of this infusion to be identical to the manipulations for the hypercarbic gas challenge), and finally, (3) 5 min infusion of atmospheric gas. Following exposure to hypercarbic and atmospheric air gases, rats were immediately placed in the open field box for 5 min, then assessed in a social interaction (SI) test for 5 min. The data reported are changes in activity, expressed in 1 min bins, relative to the average of the baseline measurement (t − 5 min to t − 1 min) from each rat.
SI and Open Field Behavioral Testing
The open field arena is 90 cm × 90 cm, with 40 cm walls, and divided into a 6 × 6 grid of equally sized squares. Four squares form the center, and constitute the starting position of the test.
The SI test was done in the open field arena and is a fully validated test of experimental anxiety-like behavior in rats.[38,39] The “experimental” rat and an unfamiliar “partner” rat are both placed in the center of the box, and the total duration (second) of nonaggressive physical contact (grooming, sniffing, crawling over and under, etc.) initiated by the “experimental” rat is quantified over 5 min. All behaviors were videotaped, and sessions were scored using ANY-maze (Stoelting, Wood Dale, IL) for open field (Stoelting), or by Stephanie D. Fitz (whom was blind to treatments) for SI.
Experiment 1–2: Effects of High and Low Doses of Lorazepam on CO2 Induced Responses
In the first experiment, rats were injected intraperitoneal (i.p.) with a control vehicle (0.2 ml/100 g volume dimethyl sulfoxide [DMSO]) or one of two higher doses of the benzodiazepine lorazepam (0.5 or 1 mg/kg, laboratory animal resource center LARC). In a second experiment, rats were injected i.p. with a control vehicle (0.2 ml/100 g volume DMSO) or one of two lower doses of the benzodiazepine lorazepam (0.1 or 0.3 mg/kg).
Experiment 3: Effects of DORA-12 on CO2-Induced Responses
Five days prior to experiments, rats were trained with a mock gavage. On the experimental day, rats received an oral gavage of a control vehicle (0.2 ml/100 g volume 20% vitamin E/TPGS) or 30 mg/kg of a dual orexin 1/2 receptor antagonist, DORA-12 (Merck & Co.) with balanced potency for OX1R (Ki = 1.8 nM, FLIPR IC50 = 27 nM) and OX2R (Ki = 0.17 nM, FLIPRIC50 = 27 nM) and good brain exposure (passive permeability of 38 × 10−6 cm/s and Pgp efflux of 0.6 (BA/AB), 47% oral bioavailability, and a favorable brain to plasma ratio of 0.4–0.6.[40,41] This compound is a close structural analogue of suvorexant, and the 30 mg/kg PO dose of DORA-12 used here achieves a plasma Cmax of 2.02 μM with CSF exposure of 66 nM and ex vivo occupancy of 97%, and has been shown to promote sleep in rats.[40,42]
Experiment 4: Effects of SB334867 on CO2-Induced Responses
All rats were injected i.p. with a control vehicle (0.2 ml/100 g volume DMSO) or a 30 mg/kg dose of SB334867 (Tocris Bioscience, in 0.2 ml/100 g volume DMSO, i.p.) which has selectivity of 50× for the OX1 receptor compared to the OX2R[33] and attenuates stress-induced anxiety-like behavior and panic-associated cardioexcitatory responses without inducing somnolence in the CO2 model described here.[30] Subcutaneous administration of this SORA1 crosses the blood–brain barrier, and in an ex vivo receptor-binding assay of a brain region with high expression of OX1Rs, a 10 mg/kg dose occupies approximately 80% of OX1Rs 30–60 min postinjection, and a 30mg/kg dose displays almost 100% occupancy of OX1Rs.[43]
Experiment 5: Effects of Compound 56 on CO2-Induced Responses
All rats were injected subcutaneous (s.c.) with a control vehicle (0.2 ml/100 g volume DMSO) or one of three doses of the highly SORA1 Compound 56 (either 3, 10, or 30 mg/kg (N-({3-[(3-ethoxy-6-methylpyridin-2-yl)carbonyl]-3-azabicyclo[4.1.0]hept-4-ylmethyl)-5-(trifluoromethyl)pyrimidin-2-amine),[44] which has 44× selectivity for the OX1 receptor compared to the OX2 receptor.[43] Subcutaneous administration of this SORA1 crosses the blood–brain barrier, and in an ex vivo receptor binding assay of a brain region with high expression of OX1Rs, a 10 mg/kg dose occupies approximately >90% of OX1Rs 30–60 min postinjection.[43] Unlike SB334867, which has been shown to have off-target affinities for non-OXRs,[33] in a binding assay panel of 50 receptors, ion channels, and transporters, Compound 56 did not exhibit a significant affinity to anything other than the OX1R.[43]
Experiment 6: Effects of JnJ10397049 on CO2-Induced Responses
All rats were injected s.c. with a control vehicle (0.2 ml of 10% pharmasolve, 5% solutol, and 85% dextrose in water) or a dose of JnJ10397049 (10 or 30 mg/kg, Tocris Biosciences, in 0.2 ml) which has 630× selectivity for the OX2R compared to the OX1R.[33] Subcutaneous administration of this SORA2 crosses the blood–brain barrier, and in an ex vivo receptor-binding assay of a brain region with high expression of OX2Rs and OX1Rs, a 30mg/kg dose occupies approximately 75–80% of OX2Rs, but does not bind to OX1Rs, 30—60 min postinjection.[45]
Experiment 7: Effects of Repeated Hypercarbic Gas Exposure on Behavior and Physiology
Since all of the above experiments were done in a crossover design, a group of six rats were implanted with radiotelemetry probes and exposed to 20% CO2, normoxic air, as described previously, on day 1, day 3, and day 5 to confirm that repeated 20% CO2, normoxic gas exposure did not result in exacerbated or diminished anxiety or physiological responses.
STATISTICAL ANALYSES
For experiments 1–7, dependent variables for analyses of open field and SI behaviors, cardiovascular (HR, MAP), locomotor activity, and CBT were analyzed using an ANOVA with drug/gas as main factor and, additionally, time as a repeated measures for physiological parameters. In the presence of significant main effects or main effect × time interactions, Fisher’s least significant difference (LSD), protected by ANOVAs at each time point (when relevant), was used for post hoc analyses. The alpha level was set at 0.05.
RESULTS
EXPERIMENT 1–2: EFFECTS OF HIGH AND LOW DOSES OF LORAZEPAM ON CO2-INDUCED RESPONSES
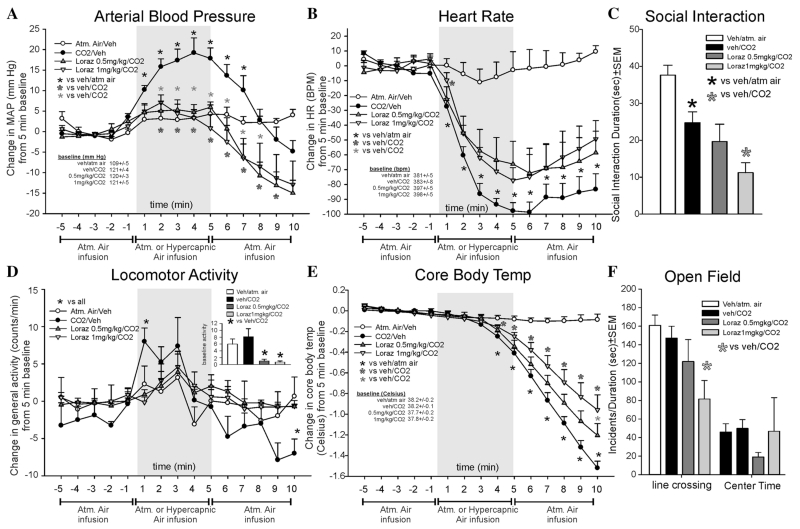
In experiment 1, lorazepam blocked CO2-induced pressor responses (F(42,378) = 4.8, P < .001, n = 8, 7, 8, 8, Fig. 1a). One CO2/Veh rat was removed due=to a series of MAP that were more than two standard deviations from the mean. The high dose attenuated the onset of the CO2-induced bradycardia (F(42,392) = 18.5, P < .001, n = 8/group, Fig. 1b). For locomotor activity, the CO2-induced increase was reduced at both doses (F(42,392) = 2.6., P = 0.017, n = 8/group, Fig. 1d, n = 8/group). However, locomotor activity was reduced prior to gas challenges and potentially indicates a sedative effect (F(3,31) = 6.5, P = 0.002, see bar graph inset to top right). Lorazepam attenuated the CO2-induced decrease in CBT (F(14,80) = 23.7, P < .001, n = 6/group, Fig. 1e). For CBT, two rats were removed due to consistently nonphysiological CBT. There were no differences noted at baseline between treatment groups for MAP, HR, or CBT.
Figure 1. Line graphs indicate the effects of systemic intraperitoneal injections of 0.5 and 1 mg/kg of the benzodiazepine lorazepam on 20% CO2-induced changes (from 5 min baseline) in (a) mean arterial blood pressure (MAP, mm Hg), (b) HR (beats/min, BPM), (d) general locomotor activity (counts/min), and decreased CBT (Celsius) in freely moving rats surgically implanted with radiotelemetry probes. There was no significant differences in baseline MAP, HR, or CBT (see legends on figures with baselines ± SEM). However, these doses of lorazepam did decrease general locomotor activity between groups (see bar graph inset in d). A similar decrease in mean behavioral activity was also observed in the (c) SI test, and (f) line crossings and center time in the open field test that are represented as bar graphs. Gray shaded area represents the 5 min CO2 or atmospheric air challenge, where atmospheric air was also infused prior to and after for 5 min (also indicated at bottom of x-axis). *, Between subjects differences using a Fisher’s LSD post hoc test that was protected with a repeated measures ANOVA and an ANOVA at each time point.
CO2 reduced SI behaviors (F(3,28) = 10.8, P < .001, n = 8/group, Fig. 1c). However, the highest dose of lorazepam decreased the SI time further. Given that locomotor activity was decreased prior to gas challenges (Fig. 1d inset), these data suggest that lorazepam was sedative. In the open field test, the highest dose of lorazepam decreased line crossing (F(3,28) = 3.9, P = .019, Fig. 1f), which is another indication of a potential sedative effect. There were no differences in center time (F(3,28) = 0.5, P = .655, Fig. 1f).
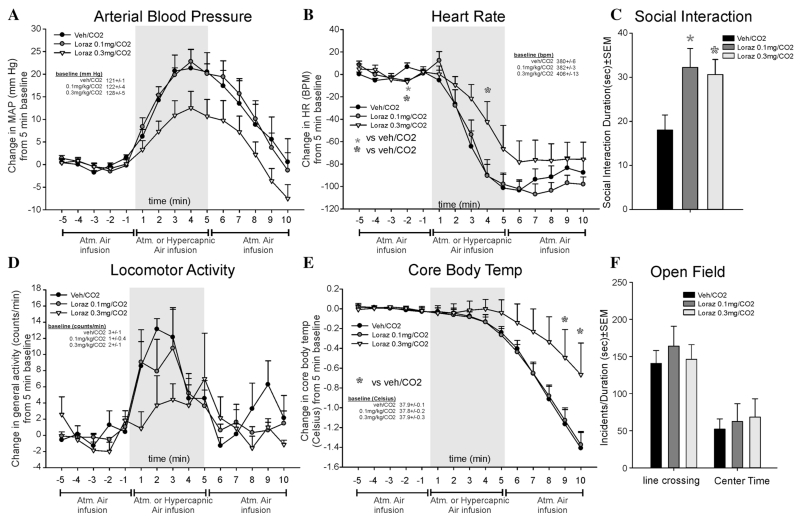
In light of potential sedative effects and no detected anxiolytic effect of lorazepam, experiment 2 was conducted with lower doses. Here, lorazepam did not alter CO2-induced pressor responses (F(28,252) = 1.2, P = .223, n = 7/group, Fig. 2a), and the 0.3 mg/kg dose of lorazepam attenuated the CO2-induced bradycardia at one time point (F(28,252) = 1.5, P = .047, Fig. 2b). Lorazepam did not alter baseline locomotor activity over time (F(2,18) = 1.0, P = .395), and there were no overall treatment effects (F(28,252) = 1.1., P = .323, Fig. 2d). CO2-induced drops in CBT (F(28,252) = 3.0, P <.001) were attenuated with the 0.3 mg/kg dose of lorazepam (Fig. 2e). No significant baseline differences were noted for MAP, HR, or CBT.
Figure 2. Line graphs indicate the effects of systemic intraperitoneal injections of 0.1 and 0.3 mg/kg of the benzodiazepine lorazepam on 20% CO2-induced changes (from 5 min baseline) in (a) MAP (mm Hg), (b) HR (beats/min, BPM), (d) general locomotor activity (counts/min), and decreased CBT (Celsius) in freely moving rats surgically implanted with radiotelemetry probes. There were no significant differences in baseline MAP, HR, locomotor activity, or CBT (see legends on figures with baselines ± SEM). Gray shaded area represents the 5 min CO2 or atmospheric air challenge, where atmospheric air was also infused prior to and after for 5 min (also indicated at bottom of x-axis). Bar graphs indicate effects of systemic injections of 0.1 and 0.3 mg/kg of the benzodiazepine lorazepam on 20% CO2-induced changes in (c) SI in the SI test, and (f) line crossings and center time in the open field test. *, Between subjects differences using a Fisher’s LSD post hoc test that was protected with a repeated measures ANOVA and an ANOVA at each time point.
Both doses of lorazepam increased SI behaviors (F(2,18) = 4.3, P = .030, n = 7/group, Fig. 2c). In the open field test, there was no effect on line crossing (F(2,18) = .3, P = .737, n = 7/group) or center time (F(2,18) = 0.1, P = .865, n = 7/group, Fig. 2f).
EXPERIMENT 3: EFFECTS OF DORA-12 ON CO2-INDUCED RESPONSES
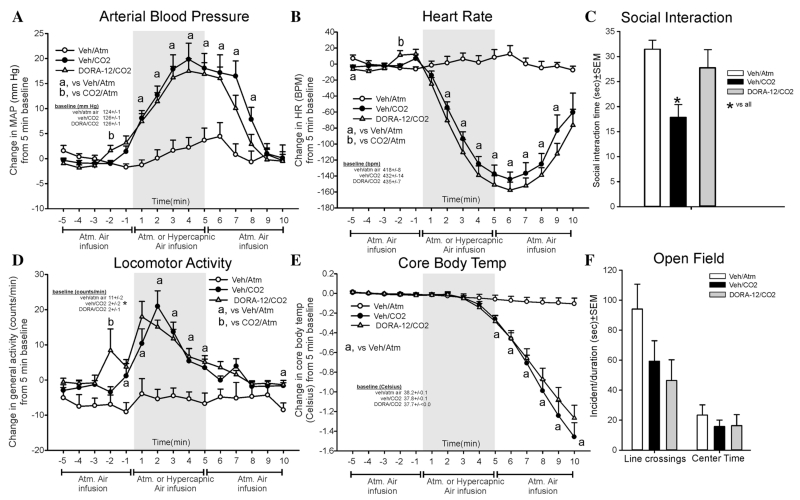
A robust CO2-induced pressor (F(28,336) = 7.0, P < .001, n = 9/group, Fig. 3a) and bradycardia (F(28,336) = 20.0, P < .001, n = 9/group, Fig. 3b) response was not attenuated by DORA-12. The increase in locomotor activity following CO2 was not altered by DORA-12 (F(28,336) = 3.3, P < .001, Fig. 3d). There were differences noted at baseline (F(2,24) = 9.4, P < .001), where the veh/CO2 and DORA-12/CO2 group has lower baseline activity (see table of baseline data in Fig. 3d) than the veh/atm. air group that had abnormally high baseline activity. For CBT, a robust CO2-induced response was not altered by DORA-12 (F(28,336) = 28.5, P < .001, n = 9/group, Fig. 3e). There were no significant baseline differences noted for MAP, HR, or CBT.
Figure 3. Line graphs indicate the effects of systemic oral gavage of 30 mg/kg of DORA-12 on 20% CO2-induced changes (from 5 min baseline) in (a) MAP (mm Hg), (b) HR (beats/min, BPM), (d) general locomotor activity (counts/min), and decreased CBT (Celsius) in freely moving rats surgically implanted with radiotelemetry probes. There were no significant differences in baseline MAP, HR, or CBT (see legends on figures with baselines ± SEM). However, there were differences noted in the baseline general locomotor activity (see baseline legend in Fig. 3D). Gray shaded area represents the 5 min CO2 or atmospheric air challenge, where atmospheric air was also infused prior to and after for 5 min (also indicated at bottom of x-axis). Bar graphs indicate the effects of systemic oral gavage of 30 mg/kg of DORA-12 on 20% CO2-induced changes in (c) SI in the SI test, and (f) line crossings and center time in the open field test. *, Between subjects differences using a Fisher’s LSD post hoc test that was protected with a repeated measures ANOVA and an ANOVA at each time point.
The CO2-induced reduction in SI behaviors (indicating anxiety state) were attenuated by DORA-12 (F(2,27) = 6.5, P = .005, n = 10/group, one probe malfunctioned so there is an n = 9/group for telemetry, Fig. 3c). In the open field test, there was no effect detected for line crossing (F(2,27) = 0.4, P = .641) or center time (F(2,27) = 2.8, P = .078, Fig. 3f).
EXPERIMENT 4: EFFECTS OF SB334867 ON CO2-INDUCED RESPONSES
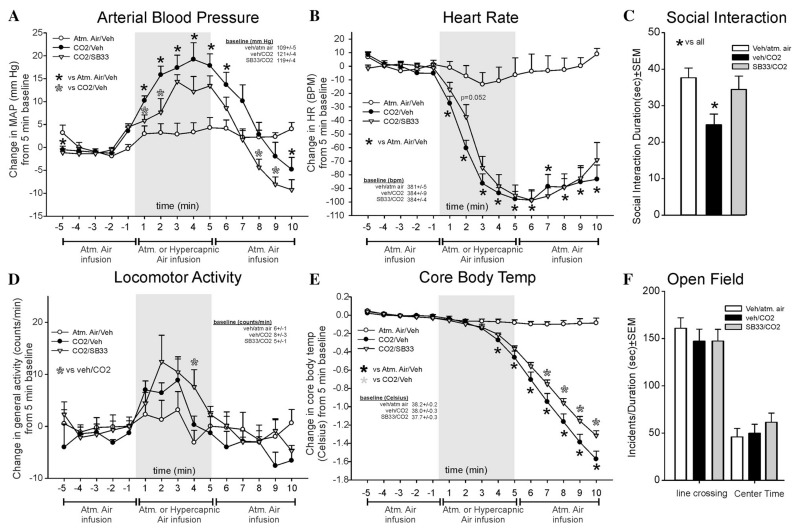
SB334867 attenuated CO2-induced pressor responses (F(28,266) = 8.5, P < .001, n = 8,7,7, Fig. 4a), but did not significantly alter CO2-induced bradycardia responses at any time point (CO2 effect, F(28,266) = 18.5., P < .001, Fig. 4b). For locomotor activity, the SB334867/CO2 group was significantly higher at one time point than the vehicle/CO2 group (F(28,266) = 1.7., P = .020, Fig. 4d). SB334867 significantly attenuated the CO2-induced decrease in CBT (F(28,266) = 65.5, P < .001, Fig. 4e). There were no differences noted at baseline for MAP, HR, activity, or CBT. MAP, HR, and activity r values were consistently two standard deviations from the mean for a rat in two groups so they were removed from analyses.
Figure 4. Line graphs indicate the effects of systemic intraperitoneal injections of 30 mg/kg of the SORA1 SB334867 on 20% CO2-induced changes (from 5 min baseline) in (a) MAP (mm Hg), (b) HR (beats/min, BPM), (d) general locomotor activity (counts/min), and decreased CBT (Celsius) in freely moving rats surgically implanted with radiotelemetry probes. There were no significant differences in baseline MAP, HR, locomotor activity, or CBT (see legends on figures with baselines ± SEM). Gray shaded area represents the 5 min CO2 or atmospheric air challenge, where atmospheric air was also infused prior to and after for 5 min (also indicated at bottom of x-axis). Bar graphs indicate the effects of systemic i.p. injections of 30 mg/kg of the SORA1 SB334867 on 20% CO2-induced changes in (c) SI in the SI test, and (f) line crossings and center time in the open field test. *, Between subjects differences using a Fisher’s LSD post hoc test that was protected with a repeated measures ANOVA and an ANOVA at each time point.
The CO2-induced reduction in SI behaviors (indicating anxiety state), was blocked by SB334867 (F(3,31) = 4.2, P = .014, n = 8/group, Fig 4c). In the open field test, no effect was seen for line crossing (F(2,21) = 0.4, P = .661) or center time (F(2,21) = 0.7, P = .495, n = 8/group, Fig. 4f).
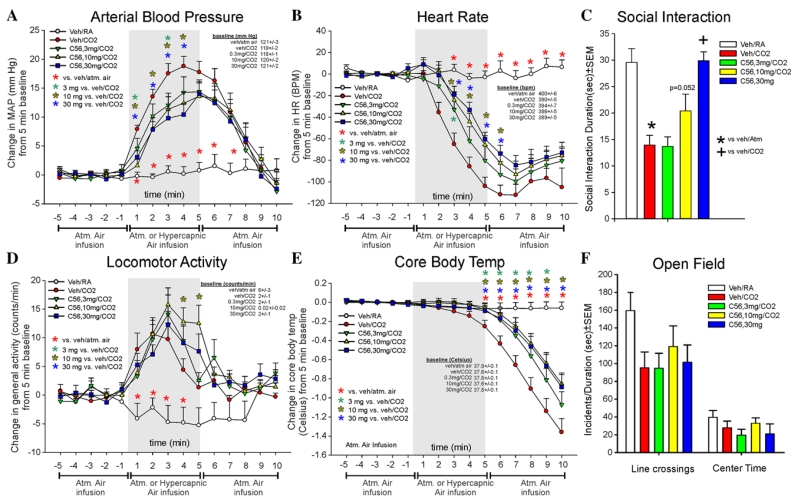
EXPERIMENT 5: EFFECTS OF COMPOUND 56 ON CO2-INDUCED RESPONSES
Compound 56 attenuated CO2-induced pressor responses (F(56,700) = 5.3, P < .001, n = 11/group, Fig. 5a), and significantly altered CO2-induced bradycardia responses (F(56,700) = 11.6., P < .001, n = 11/group, Fig. 5b). For locomotor activity, Compound 56 did not attenuate CO2-induced increases (CO2 effect, F(56,700) = 3.8, P < .001, n = 11/group, Fig. 5d). Compound 56 significantly attenuated the CO2-induced decrease in CBT (F(56,700) = 10.9, P < .001, n = 11/group, Fig. 5e). There were no differences noted at baseline for MAP, HR, activity, or CBT.
Figure 5. Line graphs indicate the effects of systemic subcutaneous injections of 3, 10, and 30 mg/kg of a SORA1 (compound 56: C65), on 20% CO2-induced changes (from 5 min baseline) in (a) MAP (mm Hg), (b) HR (beats/min, BPM), (d) general locomotor activity (counts/min), and decreased CBT (Celsius) in freely moving rats surgically implanted with radiotelemetry probes. There were no significant differences in baseline MAP, HR, locomotor activity, or CBT (see legends on figures with baselines ± SEM). Gray shaded area represents the 5 min CO2 or atmospheric air challenge, where atmospheric air was also infused prior to and after for 5 min (also indicated at bottom of x-axis). Bar graphs indicate the effects of systemic subcutaneous injections of 3,1,0 and 30 mg/kg of the SORA1 (compound A) on 20% CO2-induced changes in (c) SI in the SI test, and (f) line crossings and center time in the open field test. *, Between subjects differences using a Fisher’s LSD post hoc test that was protected with a repeated measures ANOVA and an ANOVA at each time point.
The CO2-induced reduction in SI behaviors was attenuated by the 10 mg/kg dose of Compound 56 (F(4,50) = 12.1, P < .001, n = 11/group, Fig. 5c). In the open field test, no effect was noted for line crossings (F(4,50) = 1.9, P = .128, n = 11/group, Fig. 5f) or center time (F(4,50) = 1.1, P = .363, n = 11/group, Fig. 5f).
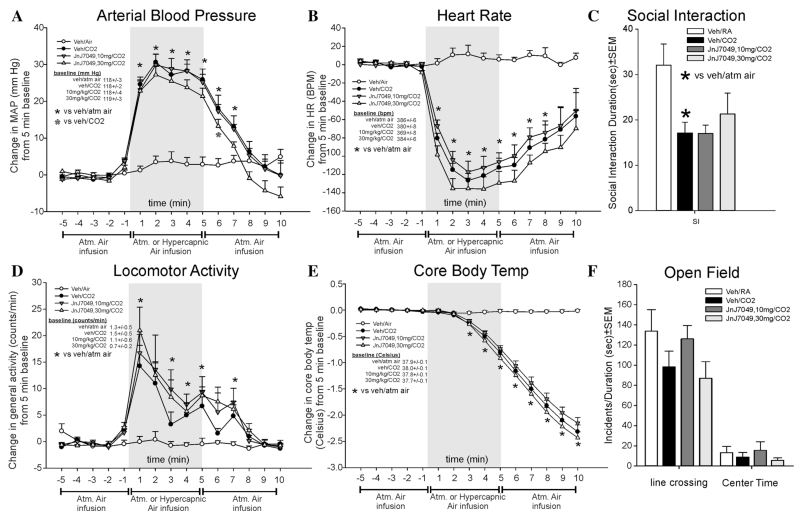
EXPERIMENT 6: EFFECTS OF JNJ7049 ON CO2-INDUCED RESPONSES
JnJ7049 attenuated CO2-induced pressor responses only at one time point (F(42,366) = 13.5, P <.001, n = 7/group, Fig. 6a), and did not significantly alter CO2-induced bradycardia (CO2 effect, F(42,366) 11.5, P < .001, Fig. 6b). JnJ7049 did not significantly=alter CO2-induced locomotor activity (F(42,366) = 2.9, P < .001, Fig. 6d). JnJ7049 did not alter CO2-induced effects (CO2 effect, F(42,366) = 90.9, P < .001, Fig. 6e). There were no differences noted at baseline for MAP, HR, activity, or CBT.
Figure 6. Line graphs indicate the effects of systemic subcutaneous injections of 10, and 30 mg/kg of a SORA2 (JnJ10397049, on 20% CO2-induced changes (from 5 min baseline) in (a) MAP (mm Hg), (b) HR (beats/min, BPM), (d) general locomotor activity (counts/min), and decreased CBT (Celsius) in freely moving rats surgically implanted with radiotelemetry probes. There were no significant differences in baseline MAP, HR, locomotor activity, or CBT (see legends on figures with baselines ± SEM). Gray shaded area represents the 5 min CO2 or atmospheric air challenge, where atmospheric air was also infused prior to and after for 5 min (also indicated at bottom of x-axis). Bar graphs indicate the effects of systemic subcutaneous injections of 10 and 30 mg/kg of the SORA2 on 20% CO2-induced changes in (c) SI in the SI test, and (f) line crossings and center time in the open field test. *, Between subjects differences using a Fisher’s LSD post hoc test that was protected with a repeated measures ANOVA and an ANOVA at each time point.
The CO2-induced reduction in SI behaviors (indicating anxiety state) was not altered by JnJ7049 (F(3,24) = 3.9, P = .022, n = 8/group, Fig 6c). In the open field test, no effect was=noted on line crossings (F(3,24) = 1.7, P = .186) or center time (F(3,24) = 0.6, P = .629, Fig. 6f).
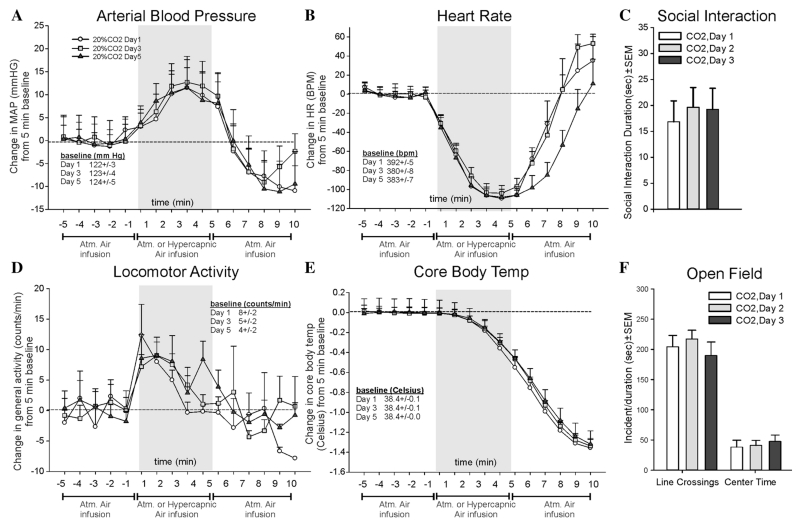
EXPERIMENT 7: EFFECTS OF REPEATED HYPERCARBIC GAS EXPOSURE ON BEHAVIOR AND PHYSIOLOGY
Repeated CO2 challenges on three different days did not alter MAP (F(30,225) = 0.5, P = .994, n = 6,6,6, Fig. 7a), HR (F(30,225) = 1.7, P = .014, Fig. 7b), SI (F(2,15) = 0.2, P = .865, n = 6/day, Fig 7c), activity (F(30,225) = 0.7., P = .823, Fig. 7d), CBT (F(30,225) = 0.3, P = 1.00, Fig. 7e), or open field test (center time F(2,15) = 0.2, P = .802, line crossings F(2,15) = 0.5, P = .600, Fig. 7f) responses to CO2. There were no differences noted at baseline for MAP, HR, activity, or CBT.
Figure 7. Line graphs indicate the effects of repeated 20% CO2 (day 1, 3, and 5) on changes (from 5 min baseline) in (a) MAP (mm Hg), (b) HR (beats/min, BPM), (d) general locomotor activity (counts/min), and decreased CBT (Celsius) in freely moving rats surgically implanted with radiotelemetry probes. There were no significant differences in baseline MAP, HR, locomotor activity, or CBT (see legends on figures with baselines ± SEM). Gray shaded area represents the 5 min CO2 or atmospheric air challenge, where atmospheric air was also infused prior to and after for 5 min (also indicated at bottom of x-axis). Bar graphs indicate the effects of repeated 20% CO2-induced changes in (c) SI in the SI test, and (f) line crossings and center time in the open field test.
DISCUSSION
Here, we initially determined if systemically pretreating rats with a benzodiazepine would attenuate panic-associated responses evoked by a 5-min exposure to 20% CO2, normoxic gas infusion. The initial doses were chosen based on a previous study showing that the 0.5 mg/kg dose is anxiolytic.[46] Lorazepam blocked CO2-induced pressor response (with a minimal effect on bradycardia), and attenuated decreases in core temperature. However, these doses appeared sedative (evidenced by decreased locomotion and decreases in SI). In the subsequent experiment, 0.1 and 0.3 mg/kg lorazepam were used, which attenuated CO2-induced anxiety in the SI test at both doses, and had minimal effects on autonomic and locomotor responses. Although benzodiazepines are currently the most effective fast-acting treatments for PAs;[47–49] they have significant safety concerns, including sedation, dependence, and addiction. Despite these issues, these data provide further validity of this panic provocation model by demonstrating responsiveness to an established treatment (postdictive validity).
We then inhibited both OX receptors with DORA-12 in the CO2 model, which attenuated anxiety-associated behaviors in the SI test, but did not alter CO2-induced pressor, bradycardia, or thermal responses. Although DORAs in general have been shown to promote sleep in various species (see review in[50]), at the doses used here there were no obvious sedating effects noted in locomotor activity assessed with radiotelemetry or in an open field test.[51] Yet, soporific effects would be predicted with repeated use and in situations with increasing sleep pressure. Thus, globally inhibiting orexin activity with DORAs may be effective in treating symptoms associated with anxiety disorders with less somnolescent side effects as benzodiazepines.
We then assessed a highly selective SORA1 to assess OX1R involvement. Compound 56 attenuated anxiety-associated behavior in the SI test at the highest dose and approached significance at a lower dose (P < .052), consistent with a previous study using SB334867,[30] and in an experiment replicated herein with SB334867. More importantly, both doses of Compound 56 also attenuated CO2-induced pressor, bradycardia, and hypothermic responses, without any apparent sedative effects. Although OX2R antagonists alone both induce and prolong sleep in rodents,[45,52] SORA1s have been shown to have little effect on sleep-wake states.[53] This is an important new finding and demonstrates a marked difference in putative clinical benefits between the SORA1 and benzodiazepines. These data demonstrate that at nonsedating anxiolytic doses, benzodiazepines are not particularly effective in blocking panic responses. Thus, to obtain panicolytic effects, benzodiazepines have to be taken in high doses that disrupt motor coordination and cause sedation. In sharp contrast to this scenario, the SORA1 appears effective in blocking panic-associated symptoms at doses that are anxiolytic but nonsedating. This could provide the SORA1s a major therapeutic advantage in the treatment of PD.
In a final experiment, we screened a highly selective SORA2 (JnJ10397049) to assess OX2R involvement in CO2-induced panic responses. In this experiment, the SORA2 did not alter any panic-associated behavioral or physiological response to CO2. Similar to the DORA experiment, there was no obvious sedating effects noted in locomotor activity. Collectively, these studies suggest that the anxiolytic effects of the SORA1s (Compound 56 and SB334867) and DORA are occurring through actions at the OX1R. Evidence in support of this hypothesis is that we have systemically administered less selective SORA1s (i.e., SB334867 and SB408124) and observed panicolytic effects in experiments using panicogenic challenges (e.g., “suffocation”-associated stimuli [CO2],[30] sodium lactate,[22] and anxiogenic/panicogenic drugs[31]). Pretreating rats with a SORA1 (SB334867) also attenuated cellular activation in panic/fear circuitry associated with PAs (e.g., periaqueductal gray, amygdala, and BNST, see reviews[54–56]) in rats challenged with the panicogenic compound FG-7142, a partial inverse benzodiazepine agonist.[31]
The relevance of CO2 to panic symptoms may not be immediately apparent, but subtle increases in CO2 in the blood (i.e., hypercapnia) that occurs from hypoventilation, or restricted breathing, results in acidosis in peripheral and central brain structures, which initially increases respiration to help “blow off” excess CO2 to prevent life-threatening acidosis [see review[57]]. If CO2 levels continue to rise, a feeling of “suffocation” and panic occurs that produces additional behaviors (e.g., urge to remove constrictive clothing, or escape restricted space), and physiologic responses that includes marked cardiovascular and thermoregulatory responses. For instance, having healthy human subjects inhale 20% CO2 for 20 s elicits all cardinal symptoms of a PA (e.g., catastrophic fear accompanied by cardiovascular and thermoregulatory responses) in 25% of subjects, and approximately 75% experience some significant cognitive fear or autonomic response.[36] A fairly unique feature of PD is that concentrations of CO2 that normally are insufficient to produce panic responses in healthy subjects (typically less than 5–7% CO2 concentrations) provoke PAs in the majority of PD subjects. Specifically, exposing PD subjects to 5% CO2 provoked a PA in approximately 50% of PD patients, but only increased nervousness and dyspnea in healthy subjects.[14] Overall, this suggests that some PAs could be provoked by subtle CO2-related cues (pCO2, and peripheral and central acidosis), which signal suffocation. This is further supported from a recent meta-analysis showing that higher baseline hyperventilation and rates of sighing are associated with PD, and suggests that PAs may be a result of an overreaction to increases in arterial CO2.[58] Finally, the cardiovascular profile seen here in rodents with the 20% CO2 challenge (i.e., pressor and bradycardia response) appears to be similar to cardiovascular responses in healthy humans that have inhaled a single breath of 35% CO2 that produces an increase in systolic blood pressure that is accompanied by a bradycardia and fear,[59] and this cardiovascular profile does appear during some PAs.[60]
CO2 easily crosses the blood–brain barrier to interact with central chemoreceptive systems.[61,62] Although there are specialized CO2/H+ chemosensory neurons that respond to subtle changes in CO2 that are located in respiratory medullary regions,[57] OX neurons have also been shown to be excited by CO2/H+,[63] and show increased cellular c-Fos responses to 10[64] and 20%[30] CO2, which is consistent with their involvement in CO2-mediated panic responses. Furthermore, prepro-OX knockout mice have blunted respiratory responses to 5–10% hypercarbic gas exposure during wake periods, and injecting wild-type mice with the SORA1 SB334867 attenuates CO2-induced respiratory responses.[65] We have also shown that pretreating rats with SB334867 attenuates CO2-induced pressor responses and anxiety/fear-associated behaviors without altering respiratory responses during hypercarbic gas infusions.[34] This provides some additional clinical insights into subjects with episodes of hypercapnia (e.g., patients with chronic obstructive pulmonary disease (COPD), bronchitis, or asthma) who have impaired breathing that is significantly comorbid with severe anxiety and sympathetic arousal, which can make the management of these symptoms difficult. Currently, the treatment of anxiety associated with conditions such as COPD with traditional anxiolytics, such as benzodiazepine drugs, is unsafe due to significant respiratory depression, cognitive disruption, addiction, and other peripheral motor side effects. Therefore, SORA1s may represent a novel anxiolytic treatment for these respiratory conditions. Furthermore, SORA1s also reduce hypertensive responses due to hypercapnia, which may also be exacerbated by the use of sympathomimetics and bronchodilators.
Overall, the data presented here demonstrate that a 20% CO2 challenge produces consistent anxiety-like behaviors in the SI test and robust autonomic responses that are similar to physical symptoms associated with PAs. The anxiolytic effects of the benzodiazepine (used as a positive control) provides further postdictive validity for the 20% CO2 challenge increasing anxiety/panic states, and also provides further support to SORA1s and DORAs as potential novel anxiolytic compounds. Our data further suggest that SORA1s may be particularly well suited as antianxiety and antipanic agents with lower sedative properties.
Acknowledgments
Contract grant sponsor: Janssen Research and Development, LLC; Contract grant sponsor: Indiana University Simon Cancer Center Basic Science Pilot; Contract grant number: 23-87597; Contract grant sponsor: NIA, NIH K; Contract grant number: 1K01AG044466; Contract grant sponsor: Indiana CTSI; Contract grant number: UL1 RR025761; Contract grant sponsor: NIMH; Contract grant numbers: R01 MH52619 and R01 MH65702.
Footnotes
Conflict of interest. Philip L. Johnson received a research grant from Janssen Research and Development, LLC, to conduct the SORA1 experiments. Within the last 3 years Anantha Shekhar received research grants from Johnson & Johnson and Eli Lilly & Co. for conducting preclinical studies that are unrelated to the present paper. Philip L. Johnson and Anantha Shekhar also have a patent filed for the use of orexin receptor antagonists in the treatment of anxiety. Christopher J. Winrow and John J. Renger are full-time employees of Merck & Co., Inc., and may have received stock and/or stock options from Merck & Co., Inc. Pascal Bonaventure is a full-time employee of Janssen Research and Development, LLC.
REFERENCES
- 1.Hess WR, Brugger M. Das subkortikake Zenrrumder affektriven Abwehrreaktion. Helv Physiol Acta. 1943;1:33–52. [Google Scholar]
- 2.Anderson JJ, DiMicco JA. Effect of local inhibition of gamma-aminobutyric acid uptake in the dorsomedial hypothalamus on extracellular levels of gamma-aminobutyric acid and on stress-induced tachycardia: a study using microdialysis. J Pharmacol Exp Ther. 1990;255(3):1399–1407. [PubMed] [Google Scholar]
- 3.Samuels BC, Zaretsky DV, DiMicco JA. Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J Physiol. 2002;538(Pt 3):941–946. doi: 10.1113/jphysiol.2001.013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shekhar A, DiMicco JA. Defense reaction elicited by injection of GABA antagonists and synthesis inhibitors into the posterior hypothalamus in rats. Neuropharmacology. 1987;26(5):407–417. doi: 10.1016/0028-3908(87)90020-7. [DOI] [PubMed] [Google Scholar]
- 5.Shekhar A, Hingtgen JN, DiMicco JA. GABA receptors in the posterior hypothalamus regulate experimental anxiety in rats. Brain Res. 1990;512(1):81–88. doi: 10.1016/0006-8993(90)91173-e. [DOI] [PubMed] [Google Scholar]
- 6.Soltis RP, DiMicco JA. Hypothalamic excitatory amino acid receptors mediate stress-induced tachycardia in rats. Am J Physiol. 1992;262(4 Pt 2):R689–R697. doi: 10.1152/ajpregu.1992.262.4.R689. [DOI] [PubMed] [Google Scholar]
- 7.Rasche D, Foethke D, Gliemroth J, Tronnier VM. Deep brain stimulation in the posterior hypothalamus for chronic cluster headache. Case report and review of the literature. Schmerz. 2006;20(5):439–444. doi: 10.1007/s00482-005-0462-3. [DOI] [PubMed] [Google Scholar]
- 8.Wilent WB, Oh MY, Buetefisch C, et al. Mapping of microstimulation evoked responses and unit activity patterns in the lateral hypothalamic area recorded in awake humans. Technical note. J Neurosurg. 2011;115(2):295–300. doi: 10.3171/2011.3.JNS101574. [DOI] [PubMed] [Google Scholar]
- 9.Wilent WB, Oh MY, Buetefisch CM, et al. Induction of panic attack by stimulation of the ventromedial hypothalamus. J Neurosurg. 2010;112(6):1295–1298. doi: 10.3171/2009.9.JNS09577. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association DSM-V . Diagnostic and Statistical Manual—Fifth Edition (DSM-V) American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- 11.Johnson PL, Shekhar A. An animal model of panic vulnerability with chronic disinhibition of the dorsomedial/perifornical hypothalamus. Physiol Behav. 2012;107(5):686–698. doi: 10.1016/j.physbeh.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorman JM, Papp LA, Coplan JD, et al. Anxiogenic effects of CO2 and hyperventilation in patients with panic disorder. Am J Psychiatry. 1994;151(4):547–553. doi: 10.1176/ajp.151.4.547. [DOI] [PubMed] [Google Scholar]
- 13.Pitts FN, Jr., McClure JN., Jr. Lactate metabolism in anxiety neurosis. N Engl J Med. 1967;277(25):1329–1336. doi: 10.1056/NEJM196712212772502. [DOI] [PubMed] [Google Scholar]
- 14.Woods SW, Charney DS, Goodman WK, Heninger GR. Carbon dioxide-induced anxiety. Behavioral, physiologic, and biochemical effects of carbon dioxide in patients with panic disorders and healthy subjects. Arch Gen Psychiatry. 1988;45(1):43–52. doi: 10.1001/archpsyc.1988.01800250051007. [DOI] [PubMed] [Google Scholar]
- 15.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 17.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130(Pt 6):1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson AV, Samson WK. The orexin/hypocretin system: a critical regulator of neuroendocrine and autonomic function. Front Neuroendocrinol. 2003;24(3):141–150. doi: 10.1016/s0091-3022(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 21.Johnson PL, Molosh A, Fitz SD, et al. Orexin, stress, and anxiety/panic states. Prog Brain Res. 2012;198:133–161. doi: 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson PL, Truitt W, Fitz SD, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16(1):111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heydendael W, Sengupta A, Beck S, Bhatnagar S. Optogenetic examination identifies a context-specific role for orexins/hypocretins in anxiety-related behavior. Physiol Behav. 2014;130:182–190. doi: 10.1016/j.physbeh.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flores A, Valls-Comamala V, Costa G, et al. The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology. 2014;39(12):2732–2741. doi: 10.1038/npp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson PJ, Fitz SD, Molosh A, Shekhar A. Pre-exposure to panicogenic hypercapnia stimulus enhances acquisition and delays extinction of conditioned fear by an orexin 1 receptor mechanism. Society for Neuroscience; New Orleans, LA: 2012. [Google Scholar]
- 26.Sears RM, Fink AE, Wigestrand MB, et al. Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proc Natl Acad Sci USA. 2013;110(50):20260–5. doi: 10.1073/pnas.1320325110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcus JN, Aschkenasi CJ, Lee CE, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 28.Trivedi P, Yu H, MacNeil DJ, et al. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438(1–2):71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 29.Ishii Y, Blundell JE, Halford JC, et al. Satiety enhancement by selective orexin-1 receptor antagonist SB-334867: influence of test context and profile comparison with CCK-8S. Behav Brain Res. 2005;160(1):11–24. doi: 10.1016/j.bbr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Johnson PL, Samuels BC, Fitz SD, et al. Activation of the orexin 1 receptor is a critical component of CO2-mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacology. 2012;37(8):1911–1922. doi: 10.1038/npp.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson PJ, Samuels BC, Fitz SD, et al. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol Behav. 2012;107(5):733–742. doi: 10.1016/j.physbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McElhinny CJ, Jr., Lewin AH, Mascarella SW, et al. Hydrolytic instability of the important orexin 1 receptor antagonist SB-334867: possible confounding effects on in vivo and in vitro studies. Bioorg Med Chem Lett. 2012;22(21):6661–6664. doi: 10.1016/j.bmcl.2012.08.109. [DOI] [PubMed] [Google Scholar]
- 33.Gotter AL, Webber AL, Coleman PJ, et al. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev. 2012;64(3):389–420. doi: 10.1124/pr.111.005546. [DOI] [PubMed] [Google Scholar]
- 34.Johnson PL, Fitz SD, Hollis JH, et al. Induction of c-Fos in “panic/defence”-related brain circuits following brief hypercarbic gas exposure. J Psychopharmacol. 2011;25(1):26–36. doi: 10.1177/0269881109353464. [DOI] [PubMed] [Google Scholar]
- 35.Ziemann AE, Allen JE, Dahdaleh NS, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139(5):1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forsyth JP, Eifert GH, Canna MA. Evoking analogue subtypes of panic attacks in a nonclinical population using carbon dioxide-enriched air. Behav Res Ther. 2000;38(6):559–572. doi: 10.1016/s0005-7967(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 37.Johnson PL, Hollis JH, Moratalla R, et al. Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J Psychopharmacol. 2005;19(4):327–341. doi: 10.1177/0269881105053281. [DOI] [PubMed] [Google Scholar]
- 38.Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52(4):701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- 39.Shekhar A, Katner JS. Dorsomedial hypothalamic GABA regulates anxiety in the social interaction test. Pharmacol Biochem Behav. 1995;50(2):253–258. doi: 10.1016/0091-3057(94)00307-5. [DOI] [PubMed] [Google Scholar]
- 40.Cox CD, Breslin MJ, Whitman DB, et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methy l-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem. 2010;53(14):5320–5332. doi: 10.1021/jm100541c. [DOI] [PubMed] [Google Scholar]
- 41.Gotter AL, Roecker AJ, Hargreaves R, et al. Orexin receptors as therapeutic drug targets. Prog Brain Res. 2012;198:163–188. doi: 10.1016/B978-0-444-59489-1.00010-0. [DOI] [PubMed] [Google Scholar]
- 42.Gotter AL, Winrow CJ, Brunner J, et al. The duration of sleep promoting efficacy by dual orexin receptor antagonists is dependent upon receptor occupancy threshold. BMC Neurosci. 2013;14:90. doi: 10.1186/1471-2202-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonaventure P, Yun S, Johnson PL, et al. A selective orexin-1 receptor antagonist attenuates stress induced hyperarousal without hypnotic effects. J Pharmacol Exp Ther. 2015;352(3):590–601. doi: 10.1124/jpet.114.220392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lebold TP, Bonaventure P, Shireman BT. Selective orexin receptor antagonists. Bioorg Med Chem Lett. 2013;23(17):4761–4769. doi: 10.1016/j.bmcl.2013.06.057. [DOI] [PubMed] [Google Scholar]
- 45.Dugovic C, Shelton JE, Aluisio LE, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330(1):142–151. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- 46.Mar A, Spreekmeester E, Rochford J. Fluoxetine-induced increases in open-field habituation in the olfactory bulbectomized rat depend on test aversiveness but not on anxiety. Pharmacol Biochem Behav. 2002;73(3):703–712. doi: 10.1016/s0091-3057(02)00881-x. [DOI] [PubMed] [Google Scholar]
- 47.Ballenger JC, Burrows GD, DuPont RL, Jr., et al. Alprazolam in panic disorder and agoraphobia: results from a multicenter trial. I. Efficacy in short-term treatment. Arch Gen Psychiatry. 1988;45(5):413–422. doi: 10.1001/archpsyc.1988.01800290027004. [DOI] [PubMed] [Google Scholar]
- 48.Charney DS, Heninger GR. Noradrenergic function and the mechanism of action of antianxiety treatment. II. The effect of long-term imipramine treatment. Arch Gen Psychiatry. 1985;42(5):473–481. doi: 10.1001/archpsyc.1985.01790280055005. [DOI] [PubMed] [Google Scholar]
- 49.Tesar GE, Rosenbaum JF. Successful use of clonazepam in patients with treatment-resistant panic disorder. J Nerv Ment Dis. 1986;174(8):477–482. doi: 10.1097/00005053-198608000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Hoyer D, Jacobson LH. Orexin in sleep, addiction and more: is the perfect insomnia drug at hand? Neuropeptides. 2013;47(6):477–488. doi: 10.1016/j.npep.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Ramirez AD, Gotter AL, Fox SV, et al. Dual orexin receptor antagonists show distinct effects on locomotor performance, ethanol interaction and sleep architecture relative to gamma-aminobutyric acid-A receptor modulators. Front Neurosci. 2013;7:254, 1–11. doi: 10.3389/fnins.2013.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mang GM, Durst T, Burki H, et al. The dual orexin receptor antagonist almorexant induces sleep and decreases orexin-induced locomotion by blocking orexin 2 receptors. Sleep. 2012;35(12):1625–1635. doi: 10.5665/sleep.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dugovic C, Shelton JE, Yun S, et al. Orexin-1 receptor blockade dysregulates REM sleep in the presence of orexin-2 receptor antagonism. Front Neurosci. 2014;8(28):1–8. doi: 10.3389/fnins.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dresler T, Guhn A, Tupak SV, et al. Revise the revised? New dimensions of the neuroanatomical hypothesis of panic disorder. J Neural Transm. 2013;120(1):3–29. doi: 10.1007/s00702-012-0811-1. [DOI] [PubMed] [Google Scholar]
- 55.Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000;157(4):493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- 56.Johnson PL, Federici LM, Shekhar A. Etiology, triggers and neurochemical circuits associated with unexpected, expected, and laboratory-induced panic attacks. Neurosci Biobehav Rev. 2014;46(Pt 3):429–454. doi: 10.1016/j.neubiorev.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guyenet PG, Stornetta RL, Abbott SB, et al. Central CO2 chemoreception and integrated neural mechanisms of cardiovascular and respiratory control. J Appl Physiol. 2010;108(4):995–1002. doi: 10.1152/japplphysiol.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grassi M, Caldirola D, Vanni G, et al. Baseline respiratory parameters in panic disorder: a meta-analysis. J Affect Disord. 2013;146(2):158–173. doi: 10.1016/j.jad.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 59.Kaye J, Buchanan F, Kendrick A, et al. Acute carbon dioxide exposure in healthy adults: evaluation of a novel means of investigating the stress response. J Neuroendocrinol. 2004;16:1–9. doi: 10.1111/j.0953-8194.2004.01158.x. [DOI] [PubMed] [Google Scholar]
- 60.Massana J, Lopez Risueno JA, Masana G, et al. Subtyping of panic disorder patients with bradycardia. Eur Psychiatry. 2001;16(2):109–114. doi: 10.1016/s0924-9338(01)00547-8. [DOI] [PubMed] [Google Scholar]
- 61.Forster HV, Smith CA. Contributions of central and peripheral chemoreceptors to the ventilatory response to Co2/H+ J Appl Physiol. 2010;108(4):989–994. doi: 10.1152/japplphysiol.01059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fukuda Y, Sato A, Suzuki A, Trzebski A. Autonomic nerve and cardiovascular responses to changing blood oxygen and carbon dioxide levels in the rat. J Auton Nerv Syst. 1989;28(1):61–74. doi: 10.1016/0165-1838(89)90008-8. [DOI] [PubMed] [Google Scholar]
- 63.Williams RH, Jensen LT, Verkhratsky A, et al. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA. 2007;104(25):10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sunanaga J, Deng BS, Zhang W, et al. CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol. 2009;166(3):184–186. doi: 10.1016/j.resp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Deng BS, Nakamura A, Zhang W, et al. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103(5):1772–1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]