Abstract
Objective
Farletuzumab is a humanized monoclonal antibody that binds to folate receptor alpha, over-expressed in epithelial ovarian cancer (EOC) but largely absent in normal tissue. Previously, carboplatin plus pegylated liposomal doxorubicin showed superior progression-free survival and an improved therapeutic index compared with carboplatin/paclitaxel in relapsed platinum-sensitive EOC. This study assessed safety of farletuzumab/carboplatin/pegylated liposomal doxorubicin in women with platinum-sensitive recurrent EOC.
Methods
This multicenter, single-arm study enrolled patients with platinum-sensitive EOC in first or second relapse for treatment with weekly farletuzumab 2.5 mg/kg plus carboplatin AUC5–6 and pegylated liposomal doxorubicin 30 mg/m2 every 4 weeks for 6 cycles. Subsequently, maintenance with single-agent farletuzumab 2.5 mg/kg once weekly or farletuzumab 7.5 mg/kg once every three weeks continued until progression. The primary objective was to assess the safety of farletuzumab/carboplatin/pegylated liposomal doxorubicin.
Results
Fifteen patients received a median of 12.0 cycles (range, 3–26) of farletuzumab as combination therapy or maintenance, for a median of 45.0 weeks (range 9–95). Farletuzumab/carboplatin/pegylated liposomal doxorubicin was generally well tolerated, with no farletuzumab-related grades 3–4 adverse events. The most commonly reported adverse events were associated with combination chemotherapy: fatigue (73.3%), nausea (46.7%), and neutropenia (40%). Ten patients had grade ≥3 adverse events, most frequently neutropenia and fatigue. No cardiac toxicity was seen. Best overall responses (RECIST) were a complete response for one patient, partial responses for 10 patients, and stable disease for four patients.
Conclusions
Farletuzumab plus carboplatin/pegylated liposomal doxorubicin in women with platinum-sensitive EOC demonstrated a safety profile consistent with that of carboplatin plus pegylated liposomal doxorubicin.
Keywords: Ovarian cancer, Platinum-sensitive relapse, Pegylated liposomal doxorubicin, Farletuzumab, Monoclonal antibody therapy
1. Introduction
Farletuzumab (FAR) is a humanized monoclonal antibody that binds to folate receptor alpha, known to be overexpressed in epithelial ovarian cancer (EOC) but largely absent in normal tissue [1–4]. In preclinical studies, FAR has exhibited immune-effector mediated effects via antibody-dependent cell cytotoxicity (ADCC) and complement-dependent cytotoxicity, and single-agent anti-tumor activity in xenograft models of ovarian cancer, as well as synergistic effects with chemotherapeutic agents [5,6]. The combination of carboplatin and paclitaxel has long been utilized as a preferred treatment regimen for platinum-sensitive EOC. This regimen was used in a Phase 2 study of FAR in patients with EOC who had experienced first relapse, with the combination of carboplatin/paclitaxel/FAR found to be active as well as well tolerated [7]. Recent studies have shown that FAR enhances type 2 cell death in tumor cells and that the combination of combination of these immune-effector cellular signaling pathway most likely result in tumor growth suppression and toxicity [8].
Recent studies have suggested that the combination of carboplatin and pegylated liposomal doxorubicin (PLD) may be the preferred regimen than carboplatin/paclitaxel for platinum-sensitive recurrent EOC [9–11]. In a randomized Phase 3 noninferiority study [9] of carboplatin plus PLD versus carboplatin plus paclitaxel in relapsed platinum-sensitive ovarian cancer, the carboplatin/PLD combination demonstrated noninferiority with the comparator in terms of progression-free survival (PFS) (11.3 months versus 9.4 months; P = 0.005) and lower rates of severe and long-lasting neuropathy. The benefit of carboplatin/PLD over carboplatin/paclitaxel was noted to persist in analysis of patients who relapsed between 6 and 12 and 6–24 months [11,12]. Toxicities were more common with carboplatin/paclitaxel and included neutropenia, neuropathy, and hypersensitivity reactions. Interestingly, carboplatin/PLD was associated with a substantially reduced incidence of platinum-associated hypersensitivity reactions in this study. It should be noted that the safety profile of FAR consists of infrequent and mild drug hypersensitivity adverse events (AEs) and rare interstitial pulmonary changes. No adverse interaction with chemotherapy was expected.
In view of a recent increase in the use of carboplatin plus PLD in patients with platinum-sensitive EOC, a Phase 1b study of FAR plus carboplatin and PLD was undertaken to assess the safety of this triple-agent combination in this disease context.
2. Methods
2.1. Study population
Each participant provided written informed consent before initiating study procedures. All enrolled patients were greater than 18 years old and had histologically- or cytologically-confirmed, platinum-sensitive EOC (including primary peritoneal or fallopian tube malignancies) with relapse as defined by Gynecologic Cancer InterGroup (GCIG) CA-125 criteria or protocol-specific modified (to reflect current practices in the medical oncology community and nuances specific to ovarian cancer) Response Evaluation Criteria in Solid Tumors (RECIST) v.1.0 for 6 months or longer after completion of first- or second-line platinum chemotherapy. All had a Karnofsky Performance Status at least 70%. Patients were required to have the following laboratory and clinical results within two weeks prior to study day 1: absolute neutrophil count (ANC) ≥1.5 × 109 cells/L; platelet count ≥100 × 109 cells/L; hemoglobin ≥9 g/dL; creatinine ≤1.5 × upper limit of normal (ULN); bilirubin ≤ 1.5 × ULN; aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALK-P) <2.5 × ULN.
Women with known central nervous system (CNS) tumor involvement, other active malignancy, clinically significant cardiac disease, active serious systemic disease or infection, evidence of immune or allergic reaction or documented antidrug antibodies (ADAs) after prior monoclonal antibody therapy were excluded from participation.
2.2. Study design and treatment
This was a multicenter, open-label Phase 1b study with 2.5 mg/kg intravenous (IV) FAR in combination with carboplatin and PLD to assess the safety of this drug regimen in patients with platinum-sensitive EOC. The primary objective of this study was to assess the safety of FAR/carboplatin/PLD in this patient population. Hematology, clinical chemistries, urine, and left ventricular ejection fraction (LVEF) were monitored on Day 1, Week 1 of every 4-week cycle. Tumor assessment (using RECIST v.1.0) was performed every other cycle. Secondary objectives included assessment of response and PFS and the pharmacokinetic effect of FAR on chemotherapy (not reported here).
Study patients received carboplatin AUC5–6 IV and PLD 30 mg/m2 IV on Day 1 of an every 4-week combination treatment cycle. An ANC of 1.5 × 109 cells/L was required for retreatment with chemotherapy. If toxicity due to carboplatin or PLD occurred, doses could be reduced or delayed according to institutional guidelines. If chemotherapy was discontinued without disease progression, the investigator could elect to continue the patient on single-agent FAR until disease progression. Following completion of approximately 6 cycles with FAR/carboplatin/PLD therapy, patients who had not progressed began maintenance treatment with single-agent FAR 2.5 mg/kg once weekly in 4-week cycles until disease progression. A protocol amendment based on new pharmacokinetic data subsequently changed the maintenance therapy administration to single-agent FAR 7.5 mg/kg once every three weeks. Disease response and progression free survival was assessed utilizing modified RECIST v1.0 based upon computed tomography (CT) scan or magnetic resonance imaging (MRI) findings and by CA-125 levels (i.e., CA-125 ≥ 2 × upper limit of normal documented on 2 occasions).
All patients were premedicated prior to FAR infusion with acetaminophen 650 mg by mouth and, optionally, diphenhydramine 25 mg to 50 mg IV or equivalent per clinic routine. In the event of a drug hypersensitivity reaction believed to be associated with FAR, patients were premedicated for subsequent infusions with antipyretic and histamine receptor blocking medications per clinic routine. Prophylactic antiemetics were used for carboplatin and PLD according to usual practice at each site.
All documents pertaining to study design, informed consent, and patient information received Institutional Review Board approval in accordance with the Declaration of Helsinki before the study began.
2.3. Safety and efficacy evaluations
Safety assessments consisted of monitoring and recording all AEs and serious AEs; performance of history and physical examinations; regular monitoring of hematology, blood chemistry, and urine laboratory values (prior to treatment on Day 1, Week 1 of cycle 1); and monitoring with ECHO or MUGA at baseline and every third cycle during combination therapy, then every fourth cycle during maintenance therapy.
Efficacy evaluations by modified RECIST v1.0 were performed at screening, every second cycle during combination treatment, every third cycle during maintenance, and at the study exit visit. Patients who discontinued prior to disease progression for any other reason (e.g., intolerable AE) were followed radiographically until documented disease progression or initiation of a new anticancer treatment occurred. As feasible, follow-up scans were obtained every 3 months; CT or MRI scans were read locally.
2.4. Anti-drug antibodies
Patients were monitored for the presence of ADA at screening, Day 1 of each combination treatment cycle, Day 1 of every third once-weekly maintenance cycle (or every other maintenance cycle during FAR dosing every 3 weeks), study exit visit, and 30 days post final dose. Associated FAR serum concentration and occurrence of a drug hypersensitivity AE at the time of a positive ADA were identified. The validated assay used for ADA analysis was estimated at 0.5 ng/mL based on detection of a FAR-specific monoclonal IgG positive control used as a surrogate ADA; the sensitivity was well below the FDA minimum expected concentration of 250–500 ng/mL [13].
2.5. Statistical analysis
The target sample size for this study was 15 patients. The sample size for this study, 15 patients, was determined to be sufficient to adequately address the primary objective. No statistical comparisons were performed that would require a minimum sample size for the study.
Safety analyses were performed on all patients who received at least one dose of combination treatment. Safety data were summarized using descriptive statistics. For overall response and CA-125 response, the number (percentage) of patients who responded was obtained and an exact 2-sided 95% confidence interval (CI) was constructed based on Clopper-Pearson methodology [14]. For PFS, duration of response, and time to response, the median was estimated using Kaplan-Meier methodology [15]. The 2-sided 95% CI for the median was constructed using the methodology of Brookmeyer and Crowley [16]. Two-sided 95% CIs for estimates of these endpoints at selected time points were calculated using the log-log transformation [17].
3. Results
3.1. Patient disposition
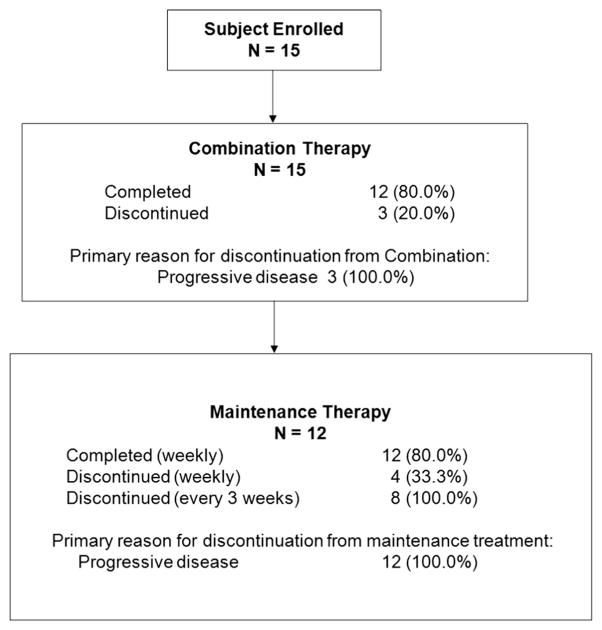
This study was conducted at three centers in the United States between 18 May 2010 and 14 Aug. 2012. Fifteen patients were enrolled and received at least one dose of FAR (Fig. 1). Carboplatin was dosed at AUC5 in 8 patients and AUC6 in 7 patients. Patient demographics and baseline characteristics are presented in Table 1. The median age was 62.0 years (range 47–82). All patients were Caucasian. All but one patient (with fallopian tube cancer) had a primary diagnosis of ovarian cancer.
Fig. 1.
Disposition of subjects across combination and maintenance treatment periods.
Table 1.
Patient demographics and baseline disease characteristics.
| All subjects
|
|
|---|---|
| (N = 15) | |
| Age, median (range), years | 62.0 (47–82) |
| Age group, n (%) | |
| 18–< 65 years | 10 (66.7) |
| ≥65 years | 5 (33.3) |
| Race white, n (%) | 15 (100) |
| Ethnicity not Hispanic or Latino, n (%) | 15 (100) |
| Karnofsky performance status, n (%) | |
| 100% | 3 (20.0) |
| 90% | 12 (80.0) |
| Stage of disease, n (%) | |
| IIC | 3 (20.0) |
| IIIA | 1 (6.7) |
| IIIC | 8 (53.3) |
| IV | 3 (20.0) |
| Histologic subtype, n (%) | |
| Serous | 10 (66.7) |
| Adenocarcinoma NOS | 5 (33.3) |
| Primary site, n (%) | |
| Fallopian tube | 1 (6.7) |
| Ovary | 14 (93.3) |
| Relapse, n (%) | |
| First relapse | 12 (80) |
| Second relapse | 3 (20) |
| Length of first remission, a mean (SD), months | 21.6 (13.47) |
| Length of first remission, a n (%), months | |
| 6 to <12 | 6 (40.0) |
| 12 to <18 | 1 (6.7) |
| 18 to 24 | 3 (20.0) |
| >24 | 5 (33.3) |
Length of first remission is based on the platinum-based chemotherapy used prior to study entry and is defined as the period of time (in months) from the date of last dose of platinum-based chemotherapy until date of first relapse by RECIST or GCIG.
3.2. Safety
Fifteen patients received a median of 12.0 cycles (range, 3–6) of FAR as a component of combination therapy or as maintenance monotherapy, 6 cycles (range, 3–9) during combination therapy and 6 cycles (range, 3–26) during maintenance. The median length of exposure to FAR was 45 weeks (range 9–95 weeks). Table 2 presents all related AEs that occurred in more than one patient. During combination treatment, the most common AEs were fatigue (73.3%), nausea (46.7%), and neutropenia (40%). For single-agent maintenance (once weekly), the most common AEs were peripheral edema (16.7%) and cough (16.7%). During single-agent maintenance (once every three weeks), the most common AE was urinary tract infection (25%). Ten patients on combination treatment reported Grade 3 or higher AEs, the most common being neutropenia (5 patients) and fatigue (2 patients). One patient experienced Grade 3 palmar-plantar erythrodysesthesia (PPE). Six patients (40%) experienced AEs that interrupted or delayed the administration of chemotherapy. The most frequent AEs that delayed or interrupted chemotherapy were neutropenia and PPE.
Table 2.
Treatment-related adverse events that occurred in more than one subject.
| MedDRA system organ class/preferred terma | FAR/carboplatin/PLD (N = 15)
|
|
|---|---|---|
| FAR-related n (%)b | Chemotherapy-related n (%)b | |
| Blood and lymphatic system disorders | ||
| Neutropenia | 6 (40.0) | |
| Anemia | 2 (13.3) | |
| Gastrointestinal disorders | ||
| Nausea | 5 (33.3) | |
| Diarrhea | 3 (20.0) | |
| Vomiting | 3 (20.0) | |
| Constipation | 2 (13.3) | 2 (13.3) |
| General disorders and administration site conditions | ||
| Fatigue | 2 (13.3) | 10 (66.7) |
| Mucosal inflammation | 3 (20.0) | |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 3 (20.0) | |
| Musculoskeletal and connective tissue disorders | ||
| Pain in extremity | 2 (13.3) | |
| Nervous system disorders | ||
| Hypoesthesia | 3 (20.0) | |
| Dysgeusia | 2 (13.3) | |
| Neuropathy peripheral | 2 (13.3) | |
| Skin and subcutaneous tissue disorders | ||
| Palmar-plantar erythrodysesthesia syndrome | 5 (33.3) | |
| Rash | 5 (33.3) | |
| Alopecia | 4 (26.7) | |
FAR = farletuzumab, PLD = pegylated liposomal doxorubicin.
Subjects may have more than one Preferred Term within a given System Organ Class.
For each Preferred Term: n = number of subjects; % = percentages are based on the number of subjects who received FAR/carboplatin/PLD.
All 15 patients who received combination treatment reported 103 chemotherapy-related AEs. The most frequently reported chemotherapy-related AEs were fatigue (66.7%), neutropenia (40%), nausea (33.3%), PPE (33.3%), rash (33.3%), and alopecia (26.7%). These percentages were comparable to those seen with carboplatin/PLD only. Two patients (13.3%) experienced 2 drug hypersensitivity AEs, hyperhidrosis and pruritus. Because of the development of ADAs, these were considered FAR related. Both events occurred during combination treatment. Three of the 15 patients (20%) receiving FAR/carboplatin/PLD reported 11 AEs that were considered (investigator judgment) related to FAR, the most frequent being constipation and fatigue (2 patients each [13.3%]); during maintenance, there were no AEs considered at least possibly related to FAR.
Four patients reported serious AEs during combination treatment, two with small bowel obstructions and one each with febrile neutropenia and venous thrombosis. One patient experienced 3 serious AEs of small bowel obstruction that were considered per investigator judgment to be related to chemotherapy. None of the reported serious events was related to FAR. Four of 15 patients (26.7%) tested positive for ADA at least once while on study. Potential immunogenic reactions to FAR occurred in two patients, one with grade 2 hyperdyrosis and one with grade 1 pruritus. Seven patients (46.7%) experienced Grade 3 laboratory abnormalities while receiving FAR/carboplatin/PLD: 6 patients with decreased neutrophils, one with increased alanine aminotransferase, and one with decreased white blood cell count. One patient experienced a Grade 3 increase in glucose while receiving maintenance treatment. No drug-induced liver events were identified. No cardiac toxicity, defined as a ≥ 15% decrease in LVEF from baseline or ≥5% from institutional lower limit of normal, was observed during chemotherapy or during FAR maintenance. All but 3 patients had the same LVEF at last study assessment as they had at screening. Three clinically not significant decreases were noted (14%, 10%, 27%); overall, the lowest value noted remained above the upper limit of normal for the site.
No patient discontinued treatment during the study for any reason other than disease progression, and no on-study deaths were reported.
3.3. Antitumor activity
Median radiologic PFS was 10.4 months (range 9.3–15.5); median CA-125 PFS was 17.7 months (range 11.1–20.3). Best overall responses by RECIST were a complete response for one patient, partial responses for 10 patients, and stable disease for four patients. Median duration of radiologic response was 8.4 months (range 6.9–13.6). Overall CA-125 response occurred in 12 patients (80.0%) with 50% response, 8 patients (53.3%) with 75% response, and 10 patients (66.7%) with complete normalization of CA-125. and no patient had a study remission length longer than the prior remission.
4. Discussion
While the standard treatment of platinum-sensitive EOC continues to be debulking surgery followed by paclitaxel/carboplatin, adding a third cytotoxic agent provided no improvement in either PFS or OS when investigated in a Phase 3 study of 4312 women with advanced stage EOC [18]. Recently, the combination of FAR plus carboplatin/taxane (paclitaxel or docetaxel) was associated with high response rates in patients with platinum-sensitive EOC at first relapse, based on results of a Phase 2 trial [7] and in a subset of patients in a subsequent placebo-controlled Phase 3 trial [19]. The Phase 2 study of the efficacy of FAR plus carboplatin/taxane found normalization of CA-125 in 80.9% of patients, an objective response rate of 75%, as well as increased duration of the progression-free interval (relative to the first response interval) in 21% of evaluable patients [7].
Though for many years carboplatin/paclitaxel has been regarded as the treatment of choice at time of relapse for patients with platinum-sensitive disease, recent clinical trial data have emerged to support the use of carboplatin/gemcitabine or carboplatin/PLD as an alternative to carboplatin/paclitaxel in this setting [9–11,20]. Carboplatin/PLD appears to be better tolerated and includes the benefit of a slight improvement in PFS [9]. Additionally, there are some aspects of this regimen that may be preferred by patients, including the lack of propensity for alopecia and psychological implications of receiving a new regimen (rather than retreatment with the original regimen, which ultimately resulted in recurrence). The use of carboplatin/PLD has increased since the aforementioned trials of FAR plus carboplatin/paclitaxel. In Europe, as well as in major academic centers in the US, a preference for carboplatin/PLD has evolved. However, in the US in general, a preference for carboplatin/paclitaxel remains. Accordingly, to assess the feasibility of adding FAR to the carboplatin/PLD combination, we undertook the Phase 1b safety study reported here in women with platinum-sensitive EOC. In this study, FAR in combination with carboplatin/PLD appeared to be generally well tolerated, with a safety profile consistent with that seen previously for FAR alone and in combination with the carboplatin/PLD regimen, where no additive toxicity was found [7]. The majority of AEs were those expected with the carboplatin/PLD combination chemotherapy backbone, including neutropenia and PPE. Compared with 103 chemotherapy-related AEs, there were 11 AEs that were considered by the investigators to be at least possibly FAR-related, the most common being constipation and fatigue at 2 reports each; however high-dose (10 mg/kg weekly) single-agent FAR did not show similar effects [21]. Although farletuzumab may be associated with fatigue, the incidence of fatigue in this study was low compared with the ~40% reported in other carboplatin/PLD studies [9,11]. No clinical safety concerns were noted after the maintenance dose was modified, from FAR 2.5 mg/kg every 4 weeks to 7.5 mg/kg every 3 weeks, per the protocol amendment. Although the efficacy data reported here are preliminary, given that this was a Phase 1b safety study, the CA-125 normalization rate of 67% and objective response rate of 73% (11 of 15) are encouraging for this study patient, which included patients in second as well as first platinum-sensitive relapse.
This Phase 1b study assessed the safety and tolerability of FAR plus carboplatin/PLD as a first step toward the pursuit of this triple-agent regimen in future studies of EOC. Use of PLD as a replacement for paclitaxel, specifically in the context of FAR, is also of interest, given observations of immunosuppressive effects for paclitaxel in an ovarian cell line (stemming from an observed interference with interleukin-2-mediated immune system activation) [22], which could potentially interfere with the immune-mediated mechanism of actions of FAR; however, the clinical relevance of these preclinical findings is uncertain. Nonetheless, in view of increased use of carboplatin/PLD in the platinum-sensitive EOC population, additional investigation of this combination plus a third agent appears warranted. Formal assessment of efficacy was not feasible given the study limitation of sample size in this Phase 1 study. As supported by the favorable safety data described here, a randomized, placebo-controlled Phase 2 study is planned (MORAb-003-011 [NCT02289950]) to assess the efficacy of FAR in combination with carboplatin/PLD or carboplatin/paclitaxel (per investigator choice) in patients with platinum-sensitive EOC (low CA-125 at ≤3× upper limit of normal) at first relapse, with PFS as the primary endpoint.
In conclusion, we found that when FAR was combined with carboplatin/PLD for the treatment of platinum-sensitive EOC, the safety profile was consistent with that of the doublet of carboplatin/PLD. Further evaluation of FAR/carboplatin/PLD is underway.
HIGHLIGHTS.
Farletuzumab (FAR), a monoclonal antibody to folate receptor alpha, which is expressed in epithelial ovarian cancer (EOC).
FAR has shown activity against EOC in platinum-sensitive relapse when combined with carboplatin and a taxane.
Carboplatin in combination with pegylated liposomal doxorubicin (PLD) is a frequently used alterative regimen.
This safety study assessed the addition of FAR to carboplatin/PLD, with a view toward future larger studies.
This combination was generally well tolerated; adverse event profile was similar to that of carboplatin/PLD alone.
Acknowledgments
Medical writing assistance was provided by J.R. Foehl, PhD, Sr. Medical Writer, Morphotek, Inc. and Laurie Orloski, PharmD, Sr. Medical Writer, Pharmite, and funding by Morphotek, Inc.
Footnotes
Financial support: Funding for this study was provided by Morphotek, Inc., Exton, PA.
Neither this manuscript nor any similar manuscript, in whole or in part, other than the abstract listed below, is under consideration, in press, published, or reported elsewhere.
The results presented in this manuscript were previously presented at the ASCO 2011 Annual Meeting: Jelovac D. et al. Phase 1 safety study of Farletuzumab, Carboplatin and Pegylated Liposomal Doxorubicin (PLD) in subjects with platinum-sensitive epithelial ovarian cancer (EOC). J Clin Oncol 2011;29(suppl):abstr 5056.
Conflict of interest statements
Kenneth H. Kim: No conflicts to report.
Danijela Jelovac: No conflicts to report.
Deborah K. Armstrong: My institution received grant funding for this trial. Part of that funding included salary support for me.
Benjamin Schwartz: No conflicts to report.
Susan C. Weil: Employee of Morphotek.
Charles Schweizer: Employee of Morphotek.
Ronald D. Alvarez: Received grant support from Morphotek for this study.
References
- 1.Dainty LA, Risinger JI, Morrison C, et al. Overexpression of folate binding protein and mesothelin are associated with uterine serous carcinoma. Gynecol Oncol. 2007;105:563–570. doi: 10.1016/j.ygyno.2006.10.063. http://dx.doi.org/10.1016/j.ygyno.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 2.Weitman SD, Lark RH, Coney LR, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–3401. [PubMed] [Google Scholar]
- 3.Toffoli G, Cenigoi C, Russo A, et al. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997;74:193–198. doi: 10.1002/(sici)1097-0215(19970422)74:2<193::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Chen YL, Chang MC, Huang CY, et al. Serous ovarian carcinoma patients with high alpha-folate receptor had reducing survival and cytotoxic chemo-response. Mol Oncol. 2012;6:360–369. doi: 10.1016/j.molonc.2011.11.010. http://dx.doi.org/10.1016/j.molonc.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebel W, Routhier EL, Foley B, et al. Preclinical evaluation of MORAb-003, a humanized monoclonal antibody antagonizing folate receptor-alpha. Cancer Immun. 2007;7:6–13. [PMC free article] [PubMed] [Google Scholar]
- 6.Lin J, Spidel JL, Maddage CJ, et al. The antitumor activity of the human FOLR1-specific monoclonal antibody, farletuzumab, in an ovarian cancer mouse model is mediated by antibody-dependent cellular cytotoxicity. Cancer Biol Ther. 2013;14:1032–1038. doi: 10.4161/cbt.26106. http://dx.doi.org/10.4161/cbt.26106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong DK, White AJ, Weil SC, et al. Farletuzumab (a monoclonal antibody against folate receptor alpha) in relapsed platinum-sensitive ovarian cancer. Gynecol Oncol. 2013;129:452–458. doi: 10.1016/j.ygyno.2013.03.002. http://dx.doi.org/10.1016/j.ygyno.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Wen Y, Graybill WS, Previs RA, et al. Immunotherapy targeting folate receptor induces cell death associated with autophagy in ovarian cancer. Clin Cancer Res. 2015;21:448–459. doi: 10.1158/1078-0432.CCR-14-1578. http://dx.doi.org/10.1158/1078-0432.CCR-14-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol. 2010;28:3323–3329. doi: 10.1200/JCO.2009.25.7519. http://dx.doi.org/10.1200/JCO.2009.25.7519. [DOI] [PubMed] [Google Scholar]
- 10.Power P, Stuart G, Oza A, et al. Efficacy of pegylated liposomal doxorubicin (PLD) plus carboplatin in ovarian cancer patients who recur within six to twelve months: a phase II study. Gynecol Oncol. 2009;114:410–414. doi: 10.1016/j.ygyno.2009.04.037. http://dx.doi.org/10.1016/j.ygyno.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 11.Gladieff L, Ferraro A, De Rauglaudre G, et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in partially platinum-sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial. Ann Oncol. 2012;23:1185–1189. doi: 10.1093/annonc/mdr441. http://dx.doi.org/10.1093/annonc/mdr441. [DOI] [PubMed] [Google Scholar]
- 12.Markman M, Moon J, Wilczynski S, et al. Single agent carboplatin versus carboplatin plus pegylated liposomal doxorubicin in recurrent ovarian cancer: Final survival results of a SWOG (S0200) phase 3. Gynecol Oncol. 2010;116:323–325. doi: 10.1016/j.ygyno.2009.11.026. http://dx.doi.org/10.1016/j.ygyno.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Human and Murine, ADA Isotyping Multiplexes for Immunogenicity Testing of Protein Therapeutics Using the MT-ADA™. Assay, Genalyte, Inc; San Diego, CA: 2013. [Google Scholar]
- 14.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 17.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. John Wiley & Sons, Inc; 1980. [Google Scholar]
- 18.Bookman MA, Brady MF, Maguire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the gynecologic cancer InterGroup. J Clin Oncol. 2009;27:1419–1425. doi: 10.1200/JCO.2008.19.1684. http://dx.doi.org/10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vergote I, Armstrong D, Scambia G, et al. Phase 3 double-blind, placebo controlled study of weekly farletuzumab with carboplatin/taxane in subjects with platinum-sensitive ovarian cancer in first relapse. Oral Presentation at the 18th International Meeting of the European Society of Gynaecological Oncology; October 19–22, 2013; Liverpool, UK. [Google Scholar]
- 20.Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–4707. doi: 10.1200/JCO.2006.06.0913. http://dx.doi.org/10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 21.Konner JA, Bell-McGuinn KM, Sabbatini P, et al. Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: a phase I study. Clin Cancer Res. 2010;16:5288–5295. doi: 10.1158/1078-0432.CCR-10-0700. http://dx.doi.org/10.1158/1078-0432.CCR-10-0700. [DOI] [PubMed] [Google Scholar]
- 22.Javeed A, Ashraf M, Riaz A, et al. Paclitaxel and immune system. Eur J Pharm Sci. 2009;38:283–290. doi: 10.1016/j.ejps.2009.08.009. http://dx.doi.org/10.1016/j.ejps.2009.08.009. [DOI] [PubMed] [Google Scholar]