Abstract
Ethnopharmacological relevance
In Central America, most Maya women use ethnomedicines for all aspects of their reproductive cycle including menstruation, pregnancy and menopause. However, very few of these plants have been documented, collected and tested in appropriate pharmacological assays to determine possible safety and efficacy. The aim of this work was to provide an overview of information on the ethnomedical uses, ethnopharmacology, chemistry and pharmacological research for medicinal plants used for women’s reproductive health in Guatemala, with a special emphasis on the Q’eqchi Maya of the Lake Izabal region, to demonstrate therapeutic potential and support future research in the field.
Materials and Methods
Reviews of the ethnobotanical, ethnomedical and ethnopharmacological literature were performed for thirty plants collected in the Lake Izabal region of Guatemala and used by the Q’eqchi Maya for treatment of reproductive health issues were performed up to and including July 2015 using multiple databases, library searches for abstracts, books, dissertations, and websites.
Results and Conclusions
Review of the published research confirms that many of the plants used by Q’eqchi Maya women for the management of reproductive health issues have pharmacological activities, including analgesic, anti-inflammatory, estrogenic, progestagenic and/or serotonergic effects, that support the use of these plants and provide plausible mechanisms of action for their traditional uses. Furthermore, a new serotonin agonist, 9, 10- methylenedioxy-5, 6-Z-fadyenolide was isolated, thereby demonstrating an untapped potential for drug discovery. However, to date much of the pharmacological assays have been in vitro only, and few in vivo studies have been performed. Considering the large percentage of the Maya population in Guatemala that use traditional medicines, there remains a significant lack of pharmacological and toxicological data for these plants. Future research should focus on the safety and efficacy of medicinal plants using in vivo preclinical studies and clinical trials, as well as chemical analysis. Since medicinal plants from the Piperaceae are most commonly used as traditional medicines by the Q’eqchi Maya women, and new bioactive compounds have been identified from Piper species, investigations of commonly used plants from this family would be an appropriate place to start. Data generated from such studies would contribute to Guatemala’s national effort to promote a complementary relationship between traditional Maya medicine and public health services.
Keywords: cyclooxygenase, dysmenorrhea, estrogenic, Maya, pregnancy, progestagenic, serotonin, menopause
Graphical Abstract

1.0 Introduction
1.1 Past research in women’s reproductive health in Mexico and Central America
Although much of our understanding of attitudes, symptoms and treatments that are associated with the female reproductive cycle, including menstruation, pregnancy and menopause has been derived from studies performed primarily in the U.S. and Europe, among homogeneous groups of Caucasian, middle-class, well-educated women (Lock and Kaufert, 2001), a growing number of ethnobotanical studies have recently been published on plants used for reproductive health in the tropics (De Boer et al., 2014; De Giselle, 2014; Kamatenesi-Mugisha et al., 2007; Michel et al., 2006; 2007; 2010; 2012 and Ososki et al., 2002). Data from cross-cultural research studies indicates that the attitudes, symptoms and treatment choices of women vary considerably depending on their geographical location, environment, health status and specific cultural paradigms that impact women’s health (Avis et al., 2001; Michel et al., 2006; Rasor and Adler, 1999). For example, research studies of menstrual health in Latin America suggest that women experience a lower incidence of menstrual symptoms as compared with their US female counterparts (Pawlowski, 2004; Severy et al., 1993). Another large cross-cultural investigation of menstruation conducted among 14 different cultural groups in 10 countries found a that 23–34% of women in developing countries, including Mexico, had reduced rates of menstrual symptoms (including dysmenorrhea, bloating, psychological changes) (Severy et al., 1993). In addition, a 2004 study among Maya women (n = 177) in the Yucatan revealed a low (28%) prevalence of menstrual pain, and the only variable found to be significantly related to the dysmenorrhea was the age at which the women gave birth to her first child (Pawlowski, 2004). These data differ significantly from the high prevalence (up to 80%) of somatic symptoms and premenstrual mood changes among US women (Sundell et al., 1990; Smith and Schiff, 1993). Reasons for these differences are unknown but presumably diet, lifestyle and the use of ethnomedicines all appear to be involved and impact overall women’s health in these countries.
In terms of menopause, the limited number of studies investigating the attitudes, symptoms and treatment choices in Latin America report more positive attitudes and less symptomology as compared to their U.S. and European counterparts (Beyene, 1986; Canto-de-Cetina et al., 1988; Leon et al., 2007; Martin et al., 1993; Malacara et al., 2002; Sievert and Espinosa-Hernandez, 2003), yet, overall, knowledge about menopause among Latin American women is lacking (Dulon Perez et al., 2013; Leon et al., 2007). One frequently cited study involved menopausal Maya women (n = 78) from Chichimila, Mexico (Martin et al., 1993). The results of this study revealed that, although follicle stimulating hormone (FSH) and luteinizing hormone (LH) levels were as high in menopausal Maya women as those in U.S. postmenopausal women, none of these women reported having vasomotor symptoms that are usually associated with such hormonal changes (Martin et al., 1993). Another study among 107 Maya women in the Yucatan also found no symptomology related to menopause other than the cessation of menstruation (Beyene, 1986). Interestingly, bone mineral density, an important intermediate outcome and risk factor for fracture, was also found to be lower in women in both Mexican studies as compared with U.S. postmenopausal women, yet there was purportedly no observed osteoporosis in the Mexican women (Beyene, 1986; Martin et al., 1993). Again diet, lifestyle and the use of traditional plant based medicines have been suggested as reasons for these discrepancies.
Reviews of the Latin American literature over the past 25 years shows a rapid growth in scientific research on natural products, particularly medicinal plants (Calixto, 2005). However, very few of these ethnomedical studies have focused on women’s reproductive health, while, those that have, have concentrated specifically on herbs used to enhance fertility or terminate pregnancy (abortifacients) (Arenas and Moreno Azorero, 1977; Browner, 1985, Conway and Slocumb, 1979; Southam et al., 1983). In general, the daily use of plant-based medicines (ethnomedicines) by women in Latin America continues to be based primarily on empirical knowledge and there is little or no scientific or medical information available for the safety and efficacy of these botanical medicines (Bodeker et al., 2005; Calixto, 2005; Michel et al., 2006; Van Adel et al., 2014).
1.2 Guatemala as a site for ethnomedical research in women’s reproductive health
Guatemala is located at the northern end of the Central American (CA) isthmus and is inhabited by over 14 million people (Beyene and Martin, 2001). Guatemala is one of the regions with the greatest cultural and biological diversity in the world (Nations et al., 1988). Unfortunately, the habitats that support such diversity are being lost at an alarming rate. In 1980, 41.9% of the country’s land area was covered by forests, in 1990 that figure was reduced to 33.8% and then to 26.3 % in 2000 (Nations et al., 1988). In addition to its environmental consequences, such losses will also have a serious impact on the diverse Guatemalan population, 60% of whom live in rural areas and rely on subsistence agricultural subsistence and the extraction of natural resources for their survival (Girón and Caceres, 1994).
Economically, Guatemala is one of the poorest countries in CA, yet is among one of the richest nations in terms of indigenous peoples and lifestyles, traditional medical knowledge and biological resources (Caceres, 1996). In fact, Guatemala is the most ethnically diverse country in CA, with over 20 groups of ethno-linguistic Maya origin, making up almost 40% of the population (CIA, 2012). The majority of these populations live in extreme poverty, within remote rural areas that have little access to modern healthcare facilities or practitioners (PAHO, 2012). Being both a women and indigenous, Maya women are the most marginalized and oppressed populations in Guatemala. While some Maya women receive some formal prenatal care, it is estimated that ~70% of childbirths among Maya women are attended at home, of which ~75% are attended by midwives, and 5.5% get no medical assistance at all (PAHO, 2012). Furthermore, Guatemala’s population growth is due to a very high individual expected birth rate. Less than 50% of all married women use contraception, and these women are expected to have at least five births during their lifetime, the highest number of the seven Central American countries (Hughes, 2004; Schooley et al., 2009). Higher birth rates are influenced by religious beliefs that do not support family planning, as well as sociopolitical concerns for the survival of the indigenous people (Schooly et al., 2009). Thus, most Maya births occur at home and attended are exclusively by a traditional birth midwife, and Maya women rely almost exclusively on herbal remedies for all stages of their pregnancy (Hughes, 2004; Orellana, 1987; Schooley et al., 2009).
In terms of menopause, the first report from Guatemala was a qualitative exploration of attitudes and symptoms associated with menopause among 27 Quiché, Tzutujil, and Cakchiquel Maya women in the Guatemalan highlands (Stewart, 2003). Similar to reports from Maya women in Mexico, these women had a very positive attitude about their entrance into menopause. However, these women reported experiencing menopausal symptoms similar to those of U.S. women including hot flashes, night sweats, moodiness and menstrual irregularities. Although the specifics of these therapies were not given, treatment for these symptoms included steam baths, lower abdominal massage by midwives and herbal medicines. No Western-style treatment, such as hormone therapy was sought to manage menopausal symptoms (Stewart, 2003). A large percentage of the Maya population in Guatemala continue to rely on traditional medical practices and medicinal plants as their primary source of healthcare (Caceres, 1996).
Thus, because of the high indigenous populations and lifestyles, and the significant use of herbal medicines by the Maya population, as well as a lack of scientific studies of the safety and efficacy of these herbs, Guatemala is an excellent site for ethnomedical studies, and such studies are sorely needed. Moreover, due to the extensive use of herbal therapies by women, there is a critical need for studies that assess the safety and efficacy of plants used to treat reproductive health issues..
2.0 Review of the ethnobotanical and ethnomedical literature for Guatemala
The most comprehensive treatment of native Guatemalan plants, as well as their medical uses during colonial times is recorded in the “Recordacion Florida”, which is a compilation of 60 medicinal plants, written by Francisco Antonio de Fuentes y Guzman in the late seventeenth century (Villatoro 1996a,b,c). Other significant works on the subject include that of Fray Francisco Ximenez written in 1772, and entitled “Historia Natural del Reino de Guatemala”. In the 18th century, a scientific re-classification of New Spain, Mexican and Guatemalan flora was performed by Martin Sessé and Jose Mariano Mociño that was based on the system of Carl Linnaeus (Sprague, 1929). Beginning in the 1930s, the interest in traditional Maya medicine slowly increased providing many publications on the subject of medical practice and medicinal plants, including the seminal work of Ralph Roys on Maya ethnobotany (Roys, 1931).
Beginning in the 1960’s and 70’s the Instituto Indigenista Nacional (National Indigenous Institute) also published a series of papers and books covering various aspects of traditional Maya medicine and medicinal plants in Mesoamerica (Adams, 1952; Carlson and Eachus, 1978). In addition, the historical review by Guerra (1969) provides an excellent account of the diverse medical techniques used by the Maya.
Since the 1970’s there have been a number of studies conducted on the ethnobotany and ethnomedicine of the Maya, with a focus primarily on the Maya of Mexico, Belize and the western highlands of Guatemala (Arvigo and Balick, 1993, 1998; Berlin and Berlin, 1996; 1998; Heinrich et al., 1998; Orellana, 1987). In 1974 Mellen published “El Uso de las Plantas Medicinales en Guatemala” (Medicinal Plant Use in Guatemala) that describes the evolution of plants used as medicines in Guatemala, the role of traditional healers (curanderos) and midwives, and a list of over 100 medicinal plants commonly used in Guatemala (Mellen, 1974). In 1981 Morton described over 150 medicinal plant families in her “Atlas of Medicinal Plants of Middle America” (Morton, 1981). In 1984, Elfriede Pöll a prominent botanist in Guatemala, published “Plantas Comestibles y Toxicas de Guatemala” (Edible and Toxic Plants of Guatemala), in which 63 plants are described giving their scientific name, botanical description, use and geographic distribution (Poll, 1984: 2006). A well cited book about Maya medicine in Guatemala was written in 1987 by Rebecca Orellana and entitled “Indian Medicine in Highland Guatemala: the Pre-Hispanic and Colonial Periods”. Orellana provides a thorough overview of Maya cosmology and beliefs about the origin of disease as well as the types of healers employed during pre-Hispanic and Colonial times followed by a description of 157 medicinal plant species, including their taxonomy, uses, chemical constituents, and known activities (Orellana, 1987). Others such as Villatoro, Comerford and Caceres produced a number of valuable publications that included not only the use of medicinal plants, but a very complete and culturally relevant description of the history of Maya medicine, Maya cosmology and methods to integrate traditional Maya medicine in the official system of healthcare of Guatemala (Caceres, 1996; Comerford, 1996; Villatoro, 1996a–c). Over the past 20 years, the number of published articles in international journals of ethnobotany and ethnopharmacology has also increased, although many of these studies were conducted among Maya communities in Mexico or Belize (Amiguet et al., 2005; Ankli, 2000; Ankli et al., 2002; Arnason et al., 1980; Cruz & Andrade-Cetto, 2015; Heinrich et al., 1992; Heinrich et al., 1998; Kufer et al., 2005).
2.1 Ethnobotanical literature ofthe Q’eqchi
The Q’eqchi are currently the third largest Maya population in Guatemala and occupy the largest geographic area of any other ethnolinguistic group in the country (Instituto Nacional de Estadistica, 2002; Michel et al., 2012). Like all Maya communities, the Q’eqchi of the eastern lowlands (Izabal) maintain a rich tradition of medical beliefs and practices that include the use of the native flora to treat a variety of illnesses. Compared with the numerous ethnographic and ethnobotanical studies on the Q’eqchi from the highlands (Cabarrus, 1998; Carlson and Eachus, 1978; Coe, 1996, 1999; Collins, 2001), few studies have focused on the lowland Q’eqchi (Amiguet et al., 2005; Arnason et al., 1980; Comerford, 1996) and, prior to our work (Caceres et al., 2014; Michel et al., 2006; 2007; 2010; 2012) there were no other known ethnobotanical studies of the Q’eqchi from the eastern lowlands of Livingston, Izabal.
In 1977, Erwin Paul Dieseldorff published “Las Plantas Medicinales del Departamento de Alta Verapaz” (“Medicinal Plants of the Department of Alta Verapaz”), where he describes over 60 medicinal plants used in the Alta Verapaz region (Dieseldorff, 1977). He also includes the descriptions of three main types of healers used by the Q’eqchi people: the ilonel (the Q’eqchi term for healer), aj tul (the Q’eqchi word for brujo or “witch” who performs rituals to produce both good and bad outcomes) and the aj ke (the diviner who diagnoses and advises the ill). Other important works published on the Q’eqchi during this time include Carter’s “New Lands Old Traditions” (1969), and “El Mundo Kekchi de la Verapaz” (“The Kekchi World of Alta Verapaz”), “La Cosmovision K’eckchi en el Proceso de Cambio” (“Q’eqchi Cosmovision in the Process of Change”) (Cabarrus, 1974), and the various publications of Richard Wilson (Wilson, 1972; Wilson, 1993; Wilson, 1994, 1995b, 1995a), with the most extensive being “Maya Resurgence in Guatemala: Q’eqchi Experiences” (Wilson, 1995a). Considering the considerable ethnographic attention the Q’eqchi have received, it is surprising that there are so few publications on the medical ethnobotany of the Q’eqchi, almost all of which are focused exclusively on the life and customs of the Q’eqchi of Alta Verapaz (Carlson and Eachus, 1978; Collins, 2001; Wilson, 1995b). Although ethnographic works on the Q’eqchi mention the intimate relationship between Q’eqchi identity and the environment and flora that surround them (Carbarrus 1998; Wilson, 1993), only the work of Dieseldorff (1977) provides a list of medicinal plants used by the Q’eqchi, and Pesek et al. (2009) describes ethnobotanical surveys and spacial modeling of Q’eqchi medicinal plants in the Maya mountains of Belize. Additional published studies described the ethnobotany of the Q’eqchi of Southern Belize and documented the traditional medical beliefs and medicinal plants of the Q’eqchi in the same bioregion as the Q’eqchi of Livingston, Lake Izabal, Guatemala (Amiguet et al., 2005; De Gezelle, 2014; Heinrich et al., 1998). All other publications that provide detailed information on the medical ethnobotany of the Q’eqchi are limited exclusively to undergraduate thesis written within Guatemala. All of these are written in Spanish and none of them has been published outside of Guatemala. Furthermore, as with the majority of previous studies on the Q’eqchi, all of them focus on the Q’eqchi of Alta Verapaz. Consequently, our knowledge of the rich medical beliefs and practices of the Q’eqchi of the eastern lowlands (Lake Izabal), as well as the biological and chemical properties of the tropical medicinal plants that they use is extremely limited.
Other ethnobotanical explorations of medicinal plants in the same tropical bioregion as the Q’eqchi of Livingston, Izabal include the work of Rosita Arvigo and Michael Balick in Belize (1998b), a survey of medicinal plant use among the Garifuna of Livingston (Giron et al., 1991) and the extensive efforts of TRAMIL (Traditional Medicine for the Islands) to record the ethnopharmacology of the Caribbean region (Robineau, 1991; 2014; Robineau and Soejarto, 1996). Data from the ethnobotanical field interviews of TRAMIL are combined with a thorough review of the safety and efficacy of each plant. Results of this on-going endeavor have been disseminated in a variety of formats and include a widespread book entitled the Vegetal Caribbean Pharmacopeia, as well as pamphlets, videos, music and dance performances and community meetings.
2.2 Ethnomedical and women’s health investigations of the Q’eqchi in Guatemala
The only known published work concerning women’s reproductive health in Guatemala was the qualitative study by Stewart (2003) of attitudes and symptoms of menopause among Maya women in the Guatemalan highlands. While this study detailed the attitudes, symptoms and some of the treatments that the Maya women used for treatment of these symptoms, very little information on the actual treatments was included, and no herbal treatments were documented, collected or tested in pharmacological and toxicological assays. Thus, the important information relating to the safety and efficacy of herbal therapies used routinely by the Maya women was lacking.
In our ethnomedical work with the Q’eqchi Maya women of Livingston, Guatemala, we investigated the symptoms and attitudes, as well as the herbal treatments associated with women’s reproductive health and menopause (Caceres et al., 2014; Michel et al., 2006; 2007; 2010; 2012). Data for these investigations were obtained through participant observation, semi-structured interviews, focus groups and plant walks with 50 Q’eqchi community members from the state of Izabal, municipality of Livingston, including five midwives, five traditional male healers and eight postmenopausal women (Michel et al., 2006). The results of these investigations demonstrated that the Q’eqchi Maya women in this region have their own cultural perceptions of women’s health that impact their attitudes, symptoms and treatment choices during the menopausal transition. In focus group sessions with the Q’eqchi women, we found that women’s reproductive health issues are considered cultural taboos by the Q’eqchi. Many women reported suffering from additional hardships when their spouse misinterpreted menopausal symptoms (vaginal dryness, sexual disinterest) as infidelity (Michel et al., 2006). Seven of the eight postmenopausal women interviewed had one or more symptoms during the menopausal transition, including headaches, anxiety, muscular pain, depression, and hot flashes. These results differ from previous studies in Mexico (Beyene, 1986; Martin et al., 1993), but are similar to the results of menopausal research conducted among other Maya groups from the highlands of Guatemala (Stewart 2003). Although the Q’eqchi did not use a specific term for “hot flash”, three Q’eqchi women used the expression “baja presion” or a “lowering of blood pressure” to explain symptoms of profuse sweating followed by chills, heart palpitations, and emotional instability. The Q’eqchi Maya mentioned a number of herbal remedies to treat menopausal symptoms (Michel et al., 2006).
In terms of ethnomedical treatments for women’s reproductive health, numerous plant species have been, and continue to be, used by Maya women and traditional healers in Guatemala (Michel et al., 2007). In our investigations, 48 medicinal plants used to treat conditions related to pregnancy, childbirth, menstruation, and menopause were documented under a Memorandum of Agreement between the University of Illinois at Chicago and the University of San Carlos, Guatemala City (Michel et al., 2007). The plants described by the Q’eqchi for their use in treating women’s reproductive health ailments included 48 different plant species belonging to 26 plant families (Michel et al., 2007). Of these 48 plants, 31 were collected near Livingston, Guatemala by Dr. Joanna Michel, of which 30 were identified by botanists and taxonomists at the Herbarium in the University of San Carlos, Guatemala and then confirmed in Chicago in the herbarium at the Field Museum and included in Table 1.
TABLE 1.
List of 30 plant species used by Q’eqchi Maya women in the Lake Izabal region of Guatemala for treating reproductive health issues and collected by Dr. Joanna Michel (modified from Michel, 2006; Michel et al., 2007). Data on ethnomedical uses by other groups in Central America and other countries as they pertain to women’s reproductive health are included. Biological and chemical information, and toxicity data are included where known.
| Species/Voucher Number | Q'eqchi Name | Part Usedb | Medicinal Use | Uses by other groups/countries | Biological and chemical data as it relates to uses for women’s health | Toxicology |
|---|---|---|---|---|---|---|
| Arachnothryx stachyoidea (Donn.Sm.) Borhidi.[syn. Rondeletia stachyoidea Donn. Smith] (Rubiaceae); JM41 | Kandel Che | Lf | Reposition womb, insomnia, “witchcraft” | None found. | Ethanol extracts of the leaves weakly bound to the serotonin receptors in vitro (Michel et al., 2007). | None found. |
| Cecropia peltata L. (Cecropiaceae); JM26 | Guarumo | Lf | Expel placenta, lower womb, insomnia, nerves | Menstrual problems, to increase labor contractions (Caceres 1996; De Giselle, 2014). | Ethanol extracts bound to the serotonin receptors in vitro (Michel et al., 2007). Phytochemical analysis shows the presence of chlorogenic acid and isoorientin (Costa et al., 2011; Valdez et al., 2011). | Pre-clinical topical application showed no toxicity in Sprague Dawley rats (Shivanandra Nayak, 2006). |
| Cissampelos tropaeolifolia DC. (Menispermeaceae); JM39 | Bejuco de ombligo Xchup I el |
Lf | Release of placenta | Used to induce labor and to speed up prolonged labor by the Q’eqchi in Belize (De Giselle, 2014). C. pareira L., a related species is used in other countries as an emmenagogue (Lewis and Elvin-Lewis, 2003). | Ethanol leaf extracts bound to the serotonin receptors (Michel et al., 2007); a 95% ethanol extract was antispasmodic and relaxed the uterus in rats, alkaloids such as warifteine, methyl-warifteine, berberine, hayatin and hayatidin found in other species of the genus (Semwal et al., 2014). | No experimental data found, but potentially an abortifacient (Michel, 2006). |
| Citrus aurantium L. (Rutaceae); JM27 | Naranja agria | Lf | Nerves, insomnia, body aches, body sweats | Used throughout Central America to treat nervous conditions, insomnia, body aches, and body sweats (Caceres 1996). | Ethanol extracts bound to the estrogen receptors, reduce bone loss and decrease serum lipids in mice (Chiba et al., 2003). Flavonoid glycosides and flavones are present in the leaf extracts (Park et al., 1983. | None found. |
| Clidemia setosa (Triana) Gleason (Melastomataceae); JM50 | Ixq Q’een | Lf | Fertility regulation Promote fertility in females, male contraceptive | Menstrual hemorrhages (Duke and Veasquez, 1994). | None found. | None found. |
| Clidemia petiolaris (Schltdl. & Cham.) Schltdl. ex Triana (Melastomataceae); JM63 | Xa bol q’een |
Lf | Postpartum hemorrhaging, fertility regulationc , blood clots | None found. | None found. | None found. |
| Crossopetalum parviflorum (Hemsl.) Lundell [syn. Crossopetalum eucymosum (Loes. & Pitt.) Lundell] (Celastraceae); JM13 | Ra Mox | Lf | Cold body, body aches | None found. | None found. | None found. |
| Dioscorea bartletti Morton (Dioscoreaceae); JM08 | Cocolmeca wild yam, xchup ixim qotz | Rh | Stagnant blood, anemia | Used to treat labor pains, dysmenorrhea, ovarian pain and vaginal cramping, and menopause (Rodriguez Martinez 1987; Ososki et al., 2002). Used as a female contraceptive by the Q’eqchin in Belize (De Giselle, 2014). | Extracts of the rhizome contain diosgenin, and have estrogenic effects when taken orally (Hidgon et al., 2001). | None found. |
| Henriettea cuneata (Standl.) Gleason (Melastomataceae); JM100 | Ixq Q’een | Lf | Fertility regulation | None found. | Ethanol extracts of the leaves bound to the serotonin receptors 5HT1A and 5 (Michel et al., 2007). | Overuse may cause uterine hemorrhage (Michel, 2006). |
| Hibiscus rosa-sinensis L. (Malvaceae); JM06 | Utz Uj’ | Lf, Fl | Postpartum hemorrhaging, infertility, nerves | Used as an emmenagogue, abortifacient, labor inducement, dysmenorrhea, and for menstrual disorders (Caceres 1996; Lans, 2007). Used to treat excessive menstruation by Q’eqchi in Belize (De Giselle, 2014). | Extracts of the flowers had mild estrogenic effects in mice and rats (Murthy et al., 1997); Ethanolic leaf extracts bound to the 5-HT1A and 5 receptors in vitro (Michel et al., 2007). Antioxidant effects (Chen et al., 2003). Presence of anthocyanins, flavonoids, flavonoid glycosides and phenolics have been reported (Agarwal and Shinde, 1967; Chen et al., 2003; Salib et al., 2011). | Methanol extracts of the leaves induced liver and kidney toxicity at a dose of 800 mg/kg administered to mice for 14 days, but 400 mg/kg showed no toxicity (Nath and Tadav, 2014). |
| Hyptis verticillata Jacq. (Lamiaceae); JM05 | Verbena, chu pim | Lf | Release placenta, and the treatment of erratic menses that are associated with menopause | Oral contraceptive, pain reliever, anti- inflammatory (Dominguez and Alcorn, 1985; Heinrich et al., 2004). Used to treat primary amenorrhea (De Giselle, 2014). | Antiinflammaory activities, hormone modulating effects (Picking et al., 2013). Leaf extracts bound to the 5HT7 receptor in vitro (Michel et al., 2007). Rosemarinic acid and sideritoflavone (Heinrich et al., 2004). Lignans, terpenes, flavonoids, and alkaloids (Picking et al., 2013). | A crude extract showed no sub-chronic toxicity (Picking et al., 2013). |
| Justicia breviflora (Nees) Rusby (Acanthaceae); JM37a | Rax Pim | Lf | Menorrhagia, postpartum hemorrhagec | None found | Leaf extracts bound strongly to the serotonin receptors in vitro (Michel et al., 2007). J. pectoralis had anti-inflammatory and estrogenic effects (Locklear et al., 2010; Telefo et al., 2002). Flavones and alkaloids found in other Justicia species. | None found. |
| Justicia fimbriata (Nees) V.A.W. Graham (Acanthaceae); JM16 | Numay Pim | Lf | Insomnia, excess body heat, inflammation | None found. | None found. | None found. |
| Mimosa pudica L. (Mimosaceae); JM57 | Dormilona sleeping prickle |
Lf | Insomnia, fertility | Used to treat anxiety, agitation and insomnia (Bum et al., 2011). The root used in childbirth and for infertility (Lans, 2007). Used to treat symptoms of menopause, uterine fibroids and insomnia by Q’eqchi of Belize (De Giselle, 2014). | Antiinflammatory effects in vitro and in animal models, efects due to the presence of flavanones (Patel et al., 2015). Anxiolytic effects in mice (Bum et al., 2011). Antifertility effects and prolongs the estrous cycle and disturbs the secretion of gonadotropin hormones in albino mice (Ganguly et al., 2007). | None found. |
| Momordica charantia L. (Cucurbitaceae); JM59 | Sorosi | Lf | Dysmenorrhea, amenorrhea | Abortifacient, contraceptive, dysmenorrhea (Caceres 1996). Contains triterpenes, sterols, saponins (Robineau 1991; 2014). Gynecological aid in Togo (Beloin et al., 2005). Used by Q’eqchi in Belize to treat symptoms of menopause, amenorrhea, and as a contraceptive (De Giselle, 2014). Also used for “suspended menses” in Dominican Republic (Ososki et al., 2002). | Several experimental studies demonstrated abortifacient properties (Grover & Yadav, 2004) | Two proteins isolated from the raw fruit demonstrated abortifacient properties (Basch et al., 2003). |
| Neurolaena lobata (L.) R. Br. Ex Cass (Asteraceae); JM31 | Kamank, jackass bitters, gan mank | Lf | Dysmenorrhea, vaginal infection | Gonorrhea. Inflammations and nervousness, labor pains (Caceres 1996; Coe and Anderson 1996). Used as birth control and for delayed menstruation by Q’eqchi in Belize (De Giselle, 2014). | Leaf extracts had analgesic and anti-inflammatory effects in rats (Gracioso et al., 1998). Leaf extracts bound to the serotonin receptor 5HT7 (Michel et al., 2007). Anti-inflammatory effects attributed to sesquiterpene lactones (McKinnon et al., 2014; Walshe-Roussel et al., 2013). | Acute oral toxicity of an ethanol extract was in mice was > 5.0 g/kg; oral and intraperitoneal acute and sub-acute toxicity in mice was >500 mg/kg (Cáceres et al., 1998) |
| Ocimum campechianum Mill. [syn. Ocimum micranthum Willd.] (Lamiaceae); JM04 | Obej', albahaca benq | Lf | Dysmenorrhea, vaginal hemorrhaging | Emmenagogue (Gupta et al., 1979). Used to treat heavy menstruation, dysmenorrhea and postpartum hemorrhages by Q’eqchi in Belize (De Giselle, 2014). | Contains essential oils and alkaloids (Coe and Anderson, 1996; Pino Benitez et al., 2009; Sacchetti et al., 2004).). Contains flavonoids and sesquiterepene lactones (Caceres 1996). | No cytotoxicity was found by the Artemia salina bioassay (Coe et al., 2010). |
| Piper jacquemontianum Kunth. [syn. Piper aeruginosibaccum Trelease] (Piperaceae); JM25 | Ampom | Lf | Anemia, body aches | Nervous conditions (Barrett, 1994). Used to treat hot flashes in Belize (De Giselle, 2014). | Contains essential oils rich in linalool (Cruz et al., 2011). Ethanol extracts of the leaves bound to the serotonin receptors in vitro (Michel et al., 2007); the same extract bound to the estrogen and progesterone receptor and enhanced the expression of estrogen related genes (Caceres et al., 2014). Dichloromethane extract exhibited antioxidant activity (Cáceres et al., 2012a). | An ethanol extract induced toxicity in brine shrimp (Artemia salina) and Thamnocephalus platyurus (Mayorga et al., 2010). |
| Piper auritum HBK. (Piperaceae); JM03 | Ob'el, cowfoot, pata de vaca | Lf | Dysmenorrhea, galactagogue | Rheumatism, ovarian pain, emmenagogue (Giron et al., 1991; Gupta et al., 1996). Used by Q’eqchi in Belize as a contraceptive, to treat postpartum hemorrhage, reduce infection and pain postpartum (De Giselle, 2014). | Uterine stimulating effects in rats (Caceres et al., 1995). Ethanol extracts of the leaves bound to the serotonin receptors in vitro (Michel et al., 2007); Contains essential oils, sesquiterepenes, flavone and alkaloids. | Tincture showed no cytotoxicity and LD50 in mice was 1,802 mg/kg (Lagarto Parra et al., 2001). |
| Piper sanctum (Miq.) Schltdl. ex C.DC. [syn. Piper diandrum C.DC] (Piperaceae); JM45 | Nin qui ru chaq’ q'een | Lf | Body aches, reposition womb | P. sanctum used as an abortifacient (Lozoya, 1976). | Essential oils, terpenes, piperolides present (Mata et al., 2004). Contains isoquinoline alkaloids, beta sitosterol (Mata et al., 2004) | May be abortifacient (Michel, 2006). |
| Piper hispidum Sw. (Piperaceae); JM32 | Puchuq | Lf | Dysmenorrhea, amenorrhea, body aches | Used to treat pain and regulate menstruation (Coe and Anderson, 1996; Duke and Vasquez, 1994). Contains flavonones, chalcones, alkaloids (Coe and Anderson, 1996). | Ethanol extracts of the leaves bound to the serotonin receptors in vitro (Michel et al., 2007); Essential oils, benzoic acids, butenolides present (Michel et al., 2006; 2010; Peno Benitez et al., 2009). | None found. |
| Piper tuerckheimii C. DC. (Piperaceae); JM14 | Caite de Diablo | Lf | Inflammation, “loss of senses”, eclampsia, contraceptive | Used to treat heavy menstruation and menstrual cramps by Q’eqchi in Belize (De Giselle, 2014). | Extracts of the leaves had displacement activities in the GABA-T and GABAA-BZD bioassays (Awad et al., 2009). | None found. |
| Psychotria poeppigiana Müll. Arg. [syn. Cephaelis tomentosa (Aubl.) Vahl. ] (Rubiaceae) JM33 | Ak Pere Tzo”, peren pim | Lf | Postpartum hemorrhaging | Abortifacient, (Coe and Anderson, 1996). Used to treat primary and secondary amenorrhea, heavy menstruation and hot flashes by Q’eqchi in Belize (De Giselle, 2014). | Ethanol extracts of the leaves bound to the serotonin receptors in vitro (Michel et al., 2007); Contains alkaloids, phenols and flavonoids (Coe and Anderson, 1996). | No experimental data found but potentially an abortifacient (Coe and Anderson, 1996; Michel, 2006). |
| Scoparia dulcis L. (Schrophulariaceae); JM58 | “Como Escobillo” | Lf | Labor pains | Used for gynecological conditions (Barret, 1994; Coe and Anderson, 1996. | Anti-inflammatory and anti-pain effects (Gupta et al., 2009). In vitro serotonin effects (Hasrat et al., 1997); Sedative effects in rats (Ahmed et al., 2001). Contains alkaloids, terpenes, phenolic compounds (Ahmed et al., 2001; Hayashi et al., 1988; 1990). Also betulinic acid, diterpenes, flavonids (de Freitas et al., 2015; Fuentes et al., 2015). | None found. |
| Senna reticulata (Willd.) H.S.Irwin & Barneby. [syn. Cassia reticulata Willd.] (Fabaceae); JM30 | Barajo | Lf | Dysmenorrhea | Treatment of menstrual pain (Coe and Anderson, 1996) | Phytochemical investigation (Messmer et al., 1968). The anthroquinones-chrysophanol, physcion, aloe-emodin, 1,3,8- trihydroxy- anthraquinone, 3- methoxy-1,6,8- trihydroxyanthraquinone, emodin, chrysophanol- 10,10 'bianthrone, and the triterpenes α and β-amirin, the steroids β - sitosterol and stigmasterol, as well as the flavonoid kaempferol anthraquinones were isolated from the wood (dos Santos and Silva, 2008). An aqueous extract showed antioxidant activity and high polypohenol content (Lizcano et al., 2010) | None found |
| Sida rhombifolia L. (Malvaceae); JM94 | Mesbel, mes’b’eel | Lf | Used to reduce labor pains, burning urine | Used to treat gonorrhea, as an emmenagogue, galactagogue, sedative and analgesic (Caceres 1996; Duke and Vasquez 1994). Used to treat dysmenorrhea (Manfred, 1947). Used to speed labor and expel placenta by Q’eqchi in Belize (De Giselle, 2014). | Anti-implantation activities and stimulated rat uterus (Sattawongsakul, 1980). Antiinflammatory and antioxidant effects in vivo (Narendhirakannaet al., 2012) Contains alkaloids, flavonols, sterols, tannins, polypohenols and saponins (Assam et al., 2010). | No acute toxicity was demonstrated in mice (Assam et al., 2010). |
| Smilax domingensis Willd. (Smilacaceae); JM60 | Chub Ixim, Cocolmeca | Rh | Night sweats associated with menopause (Michel et al., 2007). | Used to treat sexual impotence, rheumatism, physical weakness (Penna et al., 2003). Night sweats and hot flashes in Costa Rica (Doyle et al., 2009); leucorrhea and venereal diseases (Cáceres et al., 2012b). | Ethanol extract bound to the estrogen receptor and had in vitro estrogen agonist activity in MCF-7 cells (Doyle et al., 2009). Contains sarsasapogenin, smilasaponin, sarsaparilloside, sitosterol and others (Chen 1999). Anthocyanins, flavonoids and saponins (Cáceres et al., 2012b). Antioxidant activity (Cáceres et al. 2012c) | No subchronic toxicity (2 g/kg, 90 says) was demonstrated (García- Gonzalez et al., 2008) |
| Solanum americanum Miller (Solanaceae); JM72 | Hierba Mora | Lf | Anemia, dysmenorrhea | Used to treat cervical cancer (Nellis, 1997). | Anti-inflammatory activity in cervix uteri (Nogueiras et al., 2010). Solasonione, solasodine, solamargine, tigotenine (Nogueiras et al., 2010). | Alpha-solamargine isolated from the fresh fruit showed a dose- mortality relationship with a median lethal dose of 42 mg/kg body weight intraperitoneally in rats (Al Chami et al., 2003). |
| Zebrina pendula Schnizl. (Commelinaceae); JM106 | Cha cha | Lf | Dysmenorrhea | Used to treat Gonorrhea, amenorrhea (Caceres, 1996; Asprey and Thorton, 1955). | Anti-inflammatory activity in vitro (Feng et al, 1962). | None found. |
| Zingiber officinale Roscoe (Zingiberaceae); JM01 | Xinxibeer | Rh | Dysmenorrhea, night sweats | Dysmenorrhea, rheumatism, menstrual irregularities (Arvigo and Balick, 1993). | Serotonergic effects (Marles et al., 1992; Penna et al., 2003). Contains essential oils, gingerols and other phenolics with antioxidant and anti- inflammatory activities (Park et al., 1998). Antiinflammatory, and analgesic effects (Ali et al., 2008) | Generally considered as safe, no toxic or teratogenic effect were demonstrated (Ali et al., 2008) |
Fl = Flower, Lf = Leaf, Rh = Rhizome
Voucher herbarium collection number (Joanna Michel collection #)
When combined with other herbs
Detailed documentations of collection sites, ethnomedical uses, preparation and administration of the ethnomedicine, as well as healing concepts were recorded for each of the plant species (Michel, 2006). Voucher specimens were collected were deposited in the herbaria both in Guatemala and the USA. Portions of 100–500 g of the used plant part were collected and dried in a solar herb dryer. The dried plant materials were hand milled and approximately 100 g of each sample was extracted at the University of San Carlos laboratory with 500 ml of 95% ethanol. Aqueous extracts of some plant materials were prepared from dried plant materials in Chicago. One of the issues with ethnopharmacology and the testing of medicinal plants is the problem of solvents. Preparation of the ethnomedicines using fresh plant materials as an infusion or decoction tend to be common practice, however aqueous extracts contain high amounts of sugars, that may facilitate the growth of microbes. Thus the ideal situation would allow for collection, identification and pharmacological testing on site, to prevent contamination of aqueous plant extracts. However, since this is not feasible in the jungle, often alcohols such as ethanol or methanol for are used for extraction to ensure that bacteria or fungi do not contaminate the plant extracts when they are sent out of the country for testing. The plant extracts from the Q’eqchi Maya were then sent to Chicago and tested in a wide range of bioassays pertinent to women’s reproductive health.
3.0 Estrogenic and progestagenic effects of medicinal plants used in Guatemala
In female reproduction, estrogen (E2) and progesterone (P4) are the key ovarian hormones produced by the developing ovulatory follicle. These sex hormones are involved in all stages of female development and the reproductive cycle including ovulation, menstruation, pregnancy and finally the menopause. During ovulation, the serum levels of these hormones rise from the mid-follicular phase to facilitate the development of the dominant follicle (Berga and Naftolin, 2012). Androgens produced in the follicle theca cells are converted into estrogens by the granulosa cells and then the estrogens are secreted into the follicular fluid. Both E2 and P4 then act consecutively to regulate the concentrations of their respective receptors within the cell, ultimately initiating gene transcription. Numerous events prepare the endometrium for implantation, however in the absence of pregnancy, a decline in P4 levels leads to menstruation and cyclic repair (Critchley et al., 2001).
In terms of ovarian follicle ageing and reproductive senescence, a decline in specific functions of oocytes and granulosa cells (GC), as well as general cellular dysfunction, including a reduction in mitrochondrial activity and energy failure induces GCs apoptosis that triggers follicular atresia (Wei et al., 2015; Matsuda et al., 2012; 2006). Since GCs are the main source of estradiol, as well as progesterone synthesis in the ovary, a reduction in their number and function leads to reduced levels of endogenous E2 and P4 and hence, menopausal symptoms (Wei et al., 2015). Thus, both E2 and P4 are essential to the female reproductive cycle and any medicinal herbs used for treatment of reproductive health should be screened for E2 or P4 activities.
E2 and P4 binding assays were performed as described in detail in our previously published reports (Doyle et al., 2009; Locklear et al., 2010; Michel, 2006; Michel et al., 2006; 2007). Competitive binding assays were performed to determine if Maya plant extracts displaced the binding activity of radiolabeled (³H) estradiol or (³H) progesterone to bind to the estrogen (ER) or progesterone (PR) receptor, respectively. Such activity would indicate the presence of chemical constituents within the plant that had binding affinity for either receptor, and further suggest that the plant may have E2 and/or P4 activities. Of 20 plant extracts tested, 12 bound to both the ER α and β, and 11 bound to the PR (Table 2). These results suggest that these extracts may act as both estrogen and progesterone agonists in vitro. Since premenstrual syndrome (PMS), dysmenorrhea, fertility, perimenopause and the menopause are all associated with alterations circulating estrogen and progesterone levels, it is logical to hypothesize that traditional therapies used to treat these disorders may have estrogenic or progestagenic effects. Estrogens and progestins are used as hormonal therapy for the symptomatic management of dysmenorrhea, menopause and PMS symptoms (Nelson, 2008). Estrogens, either alone or in combination with progestins are clinically effective treatments for the relief of dysmenorrhea, vasomotor symptoms and have also been shown to relieve vaginal atrophy, prevent the loss of bone mineral density, and reduce fracture risk, including clinical fractures of the vertebrae and hip (Cirigliano, 2008, Gold et al., 2004). In cases of women with an intact uterus, it is necessary to give estrogens in combination with progesterone to avoid the development of endometrial hyperplasia and endometrial cancer (Gold et al., 2004). Thus, this suggests that the extracts used by the Q’eqchi for female reproductive issues have a plausible mechanism of action.
Table 2.
Ethanol extracts of Q’eqchi medicinal plants that bound to both the estrogen and progesterone receptors (concentrations 50 μg/ml). Methods as described previously (Michel, 2006; Michel et al., 2006; 2008; 2010; Doyle et al., 2009). Table prepared and modified from Michel, 2006). Plant families, voucher number and species author citations are presented in Table 1.
| Ethanol extract of Species | Plant Part | % binding ER α (mean ± SD) | % binding ER β (mean ± SD) | % binding to Progesterone receptor (mean ± SD) |
|---|---|---|---|---|
| A. stachyoidea | Leaves | 31 ± 3.3 | 36 ± 4.2 | 2 ± 0.5 |
| C. peltata | Leaves | 61 ± 9.8 | 57 ± 6.1 | 34 ± 4.0 |
| C. tropaeolifolia | Leaves | 46 ± 3.1 | 88 ± 9.1 | 34 ± 2.7 |
| C. tomentosa | Leaves | 48 ± 5.5 | 52 ± 4.9 | 15 ± 2.0 |
| H. rosa-sinensis | Leaves | 43 ± 4.9 | 38 ± 4.0 | 38 ± 6.2 |
| H. cuneata | Leaves | 56 ± 4.4 | 55 ± 5.6 | 43 ± 3.3 |
| P. jacquemontianum | Leaves | 49 ± 3.9 | 48 ± 5.1 | 39 ± 4.4 |
| P. auritum | Leaves | 49 ± 4.3 | 42 ± 3.2 | 29 ± 1.1 |
| P. hispidum | Leaves | 49 ± 2.6 | 76 ± 4.5 | 49 ± 4.3 |
| P. tuerckheimii | Leaves | 41 ± 4.4 | 47 ± 5.0 | 45 ± 3.6 |
| S. domingensis | Rhizome | 67 ± 6.1 | 69 ± 3.4 | 32 ± 2.8 |
Lf = Leaf; extract 5:1 in 95% ethanol
Of the plants tested, S. domingensis, P. hispidum Sw. and C. peltata L. were selected for further follow-up due to their active binding at ERα and ERβ. The median inhibitory concentration (IC50’s) of these extracts at ERα and ERβ are presented in Tables 3 and 4 respectively.
TABLE 3 and 4.
In vitro binding of ethanol extracts from S. domingensis, P. hispidum and C. peltata to the ERα and ERβ. IC50 values were determined by a non-linear, least squares regression analysis. Inhibition constants (Ki) were calculated using the equation of Cheng and Prusoff (Cheng and Prusoff 1973) using the observed median inhibitory concentration (IC50) of the tested compound, the concentration of radio-ligand employed in the assay, and the historical values for the KD of the ligand obtained experimentally. The Hill coefficient (nH), defining the slope of the competitive binding curve, was calculated using Data Analysis Toolbox. Hill coefficients significantly different than 1.0, may suggest that the binding displacement does not follow the laws of mass action with a single binding site. Tables prepared using modified data from Michel, 2006; Michel et al., 2007).
| Table 3. | ||||
|---|---|---|---|---|
| Species/Voucher Number | Plant Part | IC 50 ERα μg/ml | Ki | nH |
| C. peltata | Leaves | 50.0 | None | None |
| P. hispidum | Leaves | 50.3 | 14.4 | 0.798 |
| S. domingensis | Rhizomes | 28.9 | 8.27 | 1.15 |
| Table 4. | ||||
|---|---|---|---|---|
| Species/Voucher Number | Plant Part | IC 50 ERβ μg/ml | Ki | nH |
| C. peltata | Leaves | 31.5 | 6.49 | 0.89 |
| P. hispidum | Leaves | 51.5 | 10.6 | 1.16 |
| S. domingensis | Rhizomes | 38.5 | 7.94 | 1.33 |
3.1 ER reporter and endogenous gene assays
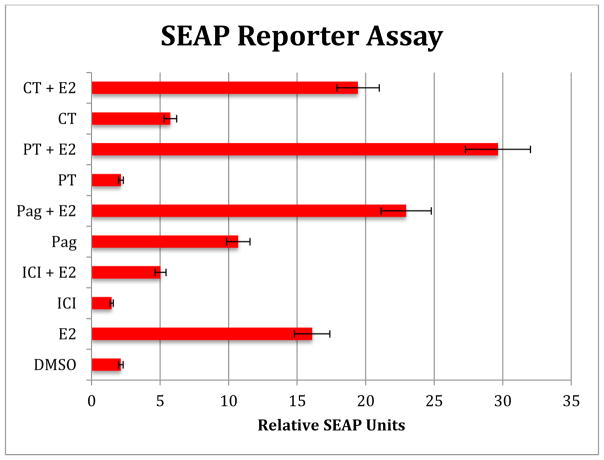
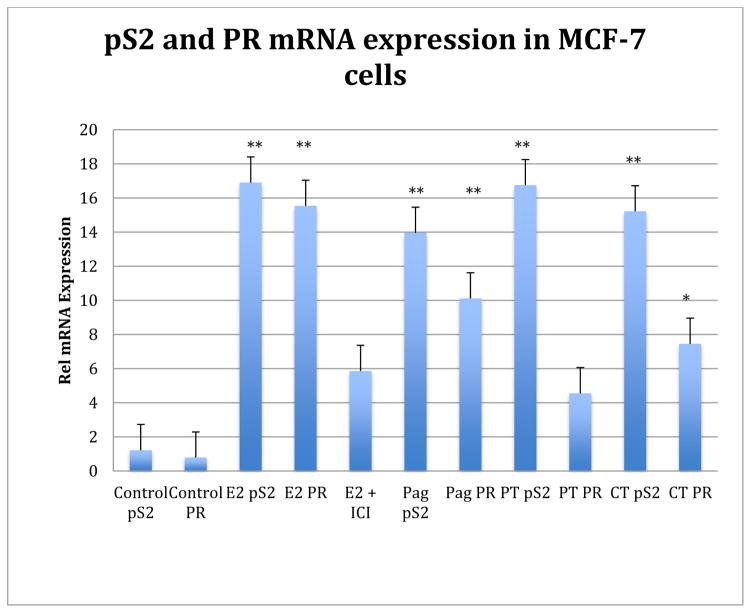
Of the 27 different plant families used by the Q’eqchi Maya women for reproductive health, some of the most prominent were the Piperaceae and Menispermiaceae (Michel et al., 2007). Extracts from Piper and Cissampelos species bound to both the estrogen and progesterone receptors indicating potential agonist effects (Table 2, modified from Michel et al., 2006Table 2, modified from Michel et al., 2008; 2010; Doyle et al., 2008). Active extracts from P. jacquemontianum, P. auritum, P. tuerckheimii and C. tropaeolifolia were then tested in functionalized cell based bioassays including a SEAP reporter gene and by qPCR of ER-responsive gene expression in MCF-7 cells (Figure 1, Caceres et al., 2014; Cullen et al., 1995; Doyle et al., 2008). In the SEAP reporter gene assay, P. jacquemontianum (20 μg/ml) was estrogenic and enhanced E2 effects in MCF-7 cells (Cullen et al., 1995; Mahady et al., 2014). P. tuerckheimii was not estrogenic alone but significantly enhanced the effects of E2 on SEAP reporter gene expression (Figure 1). In the endogenous gene assays, both P. jacquemontianum and P tuerckheimii altered mRNA expression of E2 responsive genes in MCF-7 breast cancer cells (Figure 2). C. tropaeolifolia (20 μg/ml) was also active (Figure 2). In vitro data from cell-based assays indicates that these extracts not only bind to the ER, but that they also have a functional effect in that they act by either up-regulating the expression of ER-related genes (direct estrogenic effects) or by down-regulating the expression of ER-related genes (anti-estrogenic effects) in breast cancer cells. Thus, by regulating the hormone profile, the extracts could potentially treat PMS, dysmenorrhea, menorrhagia, and infertility. However, while these data support their use by the Q’eqchi Maya women in Guatemala for reproductive health disorders, is important to recognize that they have not been tested as yet in animal models or in clinical trials, and in vivo data in animal and human studies would be needed before any conclusions can be made (Caceres et al., 2014). The other issues are the possible potentiation of breast cancer or estrogen-responsive cancers, but these issues would need to be resolved using appropriate animal models. Plant extracts that have an anti-estrogenic effect have a possible use as a chemotherapeutic agent for treating estrogen responsive cancers. Thus, these plants species would be good candidates for drug-discovery of novel anti-estrogens for cancer chemotherapy.
Figure 1.
SEAP reporter activity of P. tuerckheimii (PT), P. jacquemontianum (Pag) and Cissampelos tropaeofolia (CT). Methanol extracts of P. aeruginosibaccum (Pag) was estrogenic alone and also enhanced the SEAP activity of E2. P. tuerckheimii (PT) was not estrogenic alone, but interestingly, strongly enhance the activity of E2 in MCF-7 cells. C. tropaeolifolia DC. (CT, 20 μg/ml) was weakly estrogenic alone and also enhanced the SEAP activity of E2 in MCF-7 cells. Secreted embryonic alkaline phosphatase (SEAP) is a reporter widely used to study promoter activity or gene expression. It is a truncated form of human placental alkaline phosphatase (PLAP) by deletion of the GPI anchor. SEAP supplies and kits were obtained from InVivoGen (San Diego, CA) and performed as described by the manufacturer and methods were as previously described (Patel et al., 2014; Cullen et al., 1995).
Figure 2.
In vitro expression of ER-responsive endogenous pS2 and PR genes in MCF-7 cells. Methanol extracts of P. jacquemontianum (Pag) and P. tuerckheimii (PT) altered mRNA expression of E2 responsive genes in MCF-7 cells. C. tropaeolifolia DC. (CT, 20 μg/ml) was also active. All three enhance the expression of pS2 and P. aeruginosibaccum significantly increased the expression of PR. Statistics were performed using one way ANOVA followed by Tukey’s multiple comparison test.*P < 0.05; **P < 0.01. Methods and data were as previously described (Doyle et al., 2008; Patel et al., 2014).
4.0 Serotonergic effects of medicinal plants used for women’s reproductive health in Guatemala
In addition to the estrogen receptor, distribution studies have indicated that there is a high abundance of serotonin receptors in the brain that have been associated with the symptoms of PMS and menopause (Medina et al., 2007). Serotonin (5-HT) is involved in various physiological and pathological processes via its interaction with 14 distinct receptor subtypes, grouped in seven classes of receptors (5-HT1-7) on the basis of amino acid sequence, pharmacology, and signal transduction pathways. The 5-HT7 receptor is a G-protein coupled receptor with seven trans-membrane domains (Medina et al., 2007; Michel et al 2007). 5-HT7 receptors are found particularly in the hippocampus, thalamus and hypothalamus, areas of the brain associated with the symptoms of PMS and menopause (Medina et al., 2007). The presence of 5-HT7 in the hypothalamus also correlates with its involvement in thermoregulation and endocrine function (Medina et al., 2007). Therefore along with compounds that mimic E2 and P4, agonists of 5-HT7 may be useful for the treatment of menopause and other women’s health disorders such as PMS. Of the Q’eqchi Maya medicinal plants collected, 20 extracts were tested in several serotonin receptor-binding assays (Michel et al., 2007). Of the extracts tested, 15 bound to the various serotonin receptors (Table 5). Eleven species were found to bind to the 5-HT1A receptor, with activity defined as greater than 50% binding at a concentration of 50 μg/mL (Michel et al., 2007). At 81% P. poeppigiana showed the strongest binding affinity for 5-HT1A. Five species displayed significant binding to the 5-HT5A receptor with C. tropaeolifolia (94%), P. poeppigiana (78%), P. hispidum (76%), and H. rosa-sinensis L. (70%) being the most active extracts. Fifteen species also bound to the 5-HT7 receptor. P. hispidum (96%), N. lobata (86%), P. auritum (83%), and H. verticilata (89%) showed the greatest binding affinity.
Table 5.
Ethanol extracts from Q’eqchi Maya plant species that bound to the serotonin receptors when tested at 50–100 μg/ml. Results and methods are described in Michel et al., 2006; 2008; 2010.
| Species | Plant Part | 5HT1A % bindinga | 5HT5A % bindinga | 5HT7 % bindingb |
|---|---|---|---|---|
| Arachnothryx stachyoidea | Leaves | 51 | 37 | 26 |
| Cecropia peltata | Leaves | 62 | 38 | 81 |
| Cissampelos tropaeolifolia | Leaves | 51 | 94 | 34 |
| Clidemia petiolaris | Leaves | NT | NT | 44 |
| Clidemia setosa | Leaves | NT | NT | 47 |
| Henriettea cuneata | Leaves | 55 | 43 | NT |
| Hibiscus rosa-sinensis | Leaves | 61 | 70 | NT |
| Hyptis verticillata | Leaves | NT | NT | 89 |
| Justicia fimbriata | Leaves | 48 | 28 | 17 |
| Neurolaena lobata | Leaves | NT | NT | 86 |
| Piper auritum | Leaves | 66 | 68 | 83 |
| Piper hispidum | Leaves | 63 | 76 | 96 |
| Piper jacquemontianum | Leaves | NT | NT | 82 |
| Piper tuerckheimii | Leaves | NT | NT | 72 |
| Psychotria poeppigiana | Leaves | 81 | 78 | 67 |
| Senna reticulata | Leaves | NT | NT | 48 |
| Smilax domingensis | Rhizome | 27 | 2 | 16 |
Tested at a concentration of 50 μg/ml.
Tested at a concentration of 100 μg/ml.
NT = not tested.
It has further been suggested that 5-HT7 receptor-selective ligands may prove to be therapeutically useful for the treatment of psychiatric disorders, as well as disorders of circadian rhythms and insomnia, such as seen many menopausal women (Medina et al., 2007). Thus, these extracts may therefore represent novel therapeutic alternatives for the treatment of those disorders in which disturbances in circadian rhythms and sleep architecture are thought to be contributory factors. Finally, there is evidence to suggest that the 5-HT7 receptor may play a role in other CNS disorders including, anxiety, cognitive disturbances and also migraine probably via both peripheral and central mechanisms (Medina et al., 2007). Considering the fact that many women suffer from anxiety, depression and sleep disorders during menopause, it is very possible that the Q’eqchi Maya women use these plants for these purposes.
In addition to serotonergic effects,, many of the collected plant extracts also inhibited the activity of cyclooxygenase-2 (COX-2) (Table 5; Michel, 2006). COX-2 is a pro-inflammatory enzyme that produces acute inflammation via the eicosanoids and cytokines. The expression of COX-2 by neutrophils results in the synthesis and secretion of inflammatory prostaglandin E2, which is associated with inflammation and pain (Locklear et al., 2010). In terms of women’s health, COX-2 is up-regulated in PMS and is associated with the pain of dysmenorrhea, a problem that has a high prevalence in Latin America, and many Maya women report the use of many plants for the treatment of body aches, labor pains, and dysmenorrhea (Table 1). Using the methodology described previously (Locklear et al., 2010), thirteen of the plant extracts tested inhibited the activity of COX-2 (Table 6; Michel, 2006), suggesting a potential use for the treatment of dysmenorrhea, and other inflammatory conditions. As can be seen from Table 1, many of these plants used by the Q’eqchi Maya women for these conditions inhibited COX2, thereby indicating that the plant extracts have in vitro anti-inflammatory effects.
Table 6.
Cyclooxygenase-2 is a pro-inflammatory enzyme responsible for inflammation and pain that has been associated with dysmenorrhea, PMS and menopause. The data presented in this table are a list of Q’eqchi medicinal plant extracts that inhibited the activity of COX2 in vitro. (Modified from Michel 2006; methods Locklear et al., 2010).
| Ethanol extract of plant species | Plant Part | % inhibition of COX-2 activity at 25 μg/ml |
|---|---|---|
| A. stachyoidea | Leaves | 100 |
| C. peltata | Leaves | 103 |
| C. tropaeolifolia | Leaves | 101 |
| H. cuneata | Leaves | 102 |
| H. rosa-sinensis | Leaves | 94 |
| J. breviflora | Leaves | 100 |
| J. fimbriata | Leaves | 98 |
| P. auritum | Leaves | 101 |
| P. hispidum | Leaves | 99 |
| P. poeppigiana | Leaves | 102 |
| S. domingensis | Rhizome | 97 |
5.0 ER and serotonin agonist compounds isolated from Piper hispidum
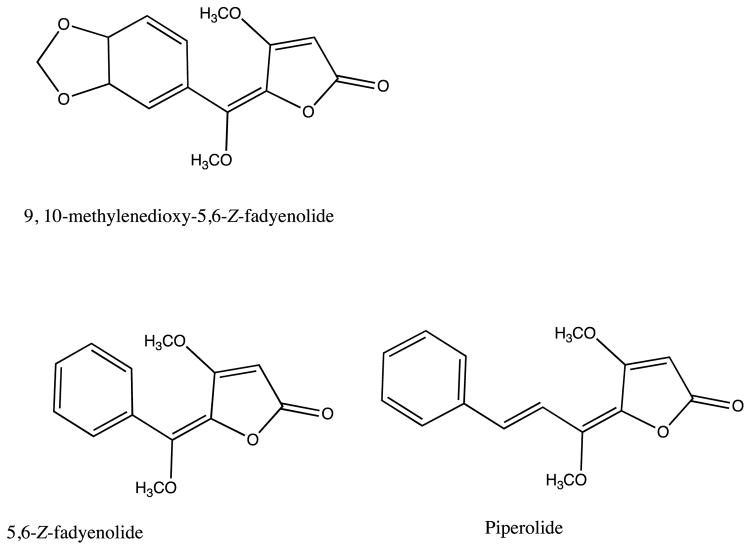
The genus Piper (Piperaceae) has over 1000 species of herbs, scrubs, small trees and hanging vines distributed throughout both hemispheres (Caceres and Kato, 2014). Species belonging to this genus are included in the Indian Ayurvedic system of medicine, as well as Maya traditional medicine in Central America (Michel et al., 2010). Extracts of P. hispidum leaves were shown to bind to the ER and PR, as well as the serotonin receptors 5-HT1A, 5-HT5A and 5-HT7. A bioassay-guided fractionation resulted in the isolation of active compounds that were identified as the butenolides 1–3 (Figure 3). The 9, 10- methylenedioxy-5, 6-Z-fadyenolide (1) was identified as a new compound, while 5, 6-Z-fadyenolide (2) and piperolide (3) are known compounds that had previously not been reported in P. hispidium (Michel et al., 2010). Compounds 1–3 were tested in the 5-HT1A and 5-HT7 receptor binding assays. Only compound 1 had binding affinity for the two subtypes of receptors with an IC50 of 16.1 and 8.3 μM to 5-HT1A and 5-HT7, respectively. Using GTP shift assays, compound 1 was found to be a partial agonist of the 5-HT7 receptor (Michel et al., 2010). These activities are consistent with the Q’eqchi traditional use of this plant for the treatment of disorders associated with the female reproductive cycle. One of the intrinsic problems related to in vitro testing of plant extracts is that in some cases the in vitro results do not translate well into in vivo or human studies. Therefore, in vivo studies are needed to confirm these activities.
Figure 3.
Structures of new and known butenolides isolated from the leaves of P. hispidum (Michel et al., 2006; 2010).
6.0 Recent studies in the field
In recent studies, eight medicinal plants used to treat menopause in Guatemala were collected or re-collected and extracted including Plantago major L. (Plantaginaceae), Wigandia urens (Ruiz & Pavón) Kunth. (Boraginaceae), Hamelia patens Jacq. (Rubiaceae), S. domingensis, Pimenta guatemalensis (Lundell) Lundell (Myrtaceae), Phlebodium pseudoaureum (Cav.) Lellinger (Polypodiaceae), C. peltata and Euphorbia lancifolia Schldt (Euphorbiaceae) (Caceres et al., 2014). The plant extracts were tested in a wide range of bioassays including ER α and β and PR binding assays, and functional assays, serotonin (5-HT1A) binding assays. Active extracts were further tested in cell-based SEAP reporter gene assays in transiently transfected MCF-7 cells and endogenous gene expression in MCF-7 cells (Caceres et al., 2014). Ethanol extracts from five plants bound both to ERα and β (C. peltata, P. major, Wigandia urens, S. domingensis and P. guatemalensis with an IC50 range 28.9–51.5 μg/ml); three plants bound to the PR receptor (S. domingensis, P. major, P. guatemalensis) and two bound to the 5HT1A receptor (H. patens and C. peltata; Caceres et al., 2014). Interestingly, in a recent study from Mexico it was reported that ethanol extracts from H. patens were not toxic in rat models, but had potent antinociceptive effects when administered to rats in doses of 100–200 mg (Alonso-Castro et al., 2015).
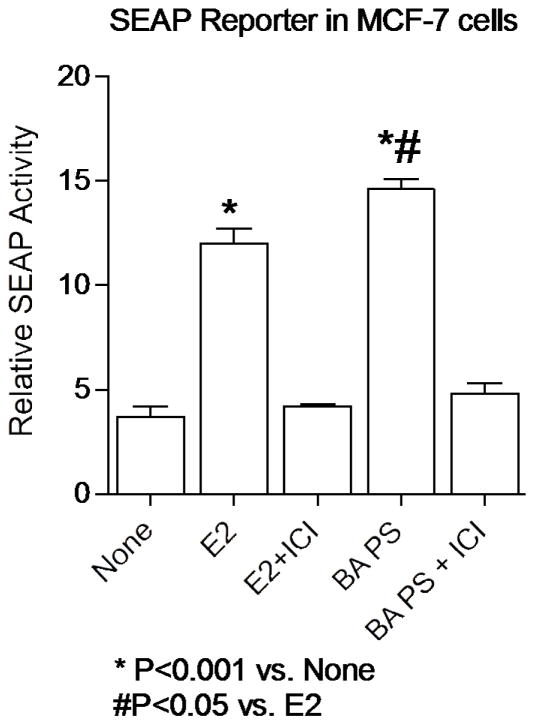
In vitro treatment of MCF-7 cells with the S. domingensis ethanol extracts (20 μg/ml) induced SEAP reporter gene expression (using methods published in Cullen et al., 1995) in transiently transfected MCF-7 cells, indicating both estrogen agonist and antagonist effects. Smilax domingensis extracts (20 μg/ml) enhanced the expression of an ERE reporter gene, and the expression of endogenous PR mRNA in MCF-7 cells (Caceres et al., 2014; Doyle et al., 2008). P. pseudoaureum and E. lancifolia were not active in any of the test assays (Caceres et al., 2014). Leaves and fruit of Brosimum alicastrum Swingle (Moraceae), a large evergreen canopy tree were collected in Guatemala (Patel et al., 2014). The fruit of the tree is also known as “Mayan bread fruit” or breadnut, and are used for the treatment of menopause and osteoporosis, and are thought to have hormone-like activities. Methanol extracts of the fruit displaced 3H-estradiol binding from the estrogen receptors (ERα and ERβ, EC50 48 and 49 μg/ml) (Patel et al., 2014). Methanol extracts of the fruit pulp including seeds, and an extract of the leaves (20 μg/ml) induced a SEAP reporter gene in transiently transfected MCF-7 cells, which was inhibited by the ER antagonist ICI 182,780 indicating estrogenic effects were mediated through the ER (Patel et al., 2014). Conversely, a methanol extract of the fruit pulp alone was not estrogenic as compared with vehicle controls, and reduced SEAP reporter gene activity in a concentration dependent manner, suggesting potential anti-estrogenic effects (Figure 4). Thus, in keeping with the reported traditional uses to treat menopause and osteoporosis by the Maya, B. alicastrum extracts have at least in vitro pharmacological activities that support these traditional uses.
Figure 4.
In vitro SEAP reporter gene activity of Brosimum alicastrum. Methanol extracts of the fruit pulp and seeds (BAPS, 20 μg/ml) were estrogenic alone and enhanced the SEAP activity in MCF-7 breast cancer cells. The activity of BAPS was shown to be ER dependent as when MCF-7 cells were treated with BAPS and ICI 182780 (an ER antagonist that promotes degradation of the ER), the activity of the extract was significantly reduced demonstrating that the ER was necessary for this activity. Results and methods were previously reported (Cullen et al., 1995; Patel et al., 2014).
7.0 Summary and Future Perspectives
7.1 Efficacy of medicinal plants used by the Q’eqchi Maya women
The purpose of this review was to provide an overview of the pharmacological support for the traditional uses of medicinal plants used by the Q’eqchi Maya from the Lake Izabal region of Guatemala for the treatment of women’s reproductive health disorders. In general, Maya women in Guatemala regularly use medicinal plants to treat women’s health concerns, yet pharmacological data on their safety and efficacy are largely absent. Review of the literature reveals that plants used by the Q’eqchi for reproductive health exhibited significant in vitro estrogen and progesterone effects, suggesting potential use for the treatment of PMS and menopause; serotonin agonist activities indicating potential use for the treatment of anxiety, depression, PMS, insomnia and menopause; COX2 inhibition indicating a potential use for treating inflammation and pain as seen in dysmenorrhea and labor pains. Thus, the experimental pharmacology supports many of the medicinal plants used by the Q’eqchi for women’s reproductive health, and also provides a plausible mechanism of action.
7.2 Correlation of plant species used by Q’eqchi Maya and other groups
Literature reviews of many of the medicinal plants used for reproductive health by the Q’eqchi Maya in Livingston shows that many of these species are also used traditionally in other Central American countries and cultural groups. One example is C. peltata (Table 1), also known as Guarumo, or Trumpet Tree. The leaves of this plant are used by the Q’eqchi Maya to expel the placenta after childbirth, lower the womb, and to treat nervousness and insomnia. Other uses among the Maya of Belize and Guatemala and the Paya of Honduras include the use of an infusion of the leaves of this species as a bath for rheumatism, and as a tea to treat nervous conditions, menstrual problems, and to ease the pain of childbirth (Avis, 2001; Caceres, 1996;[ Cosminsky, 1982; Comerford, 1996; Jiang and Xu, 2003; Lentz, 1989). A recent study of traditional medicine for women’s health issues among the Q’eqchi of the Toledo District of Belize documented the use of these leaves to quicken labor, and to treat a difficult labor, when combined with other species (De Gezelle, 2014). In vitro data have shown that extracts of C. peltata leaves bound to both the estrogen receptors and the progesterone receptor, indicating potential hormonal activity that would be useful in the management of menstrual problems and for the management of menopause. In addition the extracts from Cecropia bound to the serotonin receptors 1A and 7, as well as inhibited the activity of COX-2 indicating its benefits in the treatment of nervousness, anxiety, insomnia and pain, all as cited by the Q’eqchi for its traditional use. However, the majority of this work was in vitro and the safety of the extracts was not assessed and no toxicological studies were performed.
7.3 Piperaceae is the most commonly used plant family by the Q’eqchi Maya
Of all of the plant families recorded, Piperaceae was the family most often mentioned by the Q’eqchi of Livingston, Guatemala for the treatment of women’s health ailments (Michel et al., 2006). Interestingly, ethnopharmacological studies with the Q’eqchi of Belize also found Piperaceae to be the most common plant family used for the treatment of neurological and mental health conditions (Bourbonnais-Spear et al., 2005), and women’s health conditions (De Gezelle, 2014). Traditional medicinal uses of Piper species relevant to this paper that have been supported by in vitro and/or in vivo activity include the use of Piper betle L. as an anti-fertility agent (Adhikary et al., 1989); Piper methysticum G. Forst. and Piper chaba Hunter to relieve depression and anxiety (Pittler and Ernst, 2000; Taufiq-Ur-Rahman et al., 2005); and Piper kadsura (Choisy) Ohwi and P. chaba Hunter for aches, pains and inflammation (Chiou et al., 2003; Taufiq-Ur-Rahman et al., 2005).
All of the Piper extracts tested in an earlier study were active in the ER and weakly active in the PR assays; had good activity in all serotonin bioassays, and strongly inhibited the activity of COX-2 in vitro. In the cellular reporter gene assays and endogenous gene assays, all Piper species tested either was directly estrogenic, or enhanced the activity of the positive control, estradiol, all suggesting estrogen agonist effects. Extracts of P. hispidum were found to act as agonists of both the ER and 5-HT7 receptors, and a new natural product, 9, 10-methylenedioxy-5,6-Z-fadyenolide, was found to be a 5-HT7 agonist (Michel et al., 2010). Piper hispidum, known as puchuq by the Q’eqchi, is used as a tea to treat dysmenorrhea, amenorrhea, menopause and body aches, but is also commonly known as cordoncillo in Nicaragua where it is used to treat aches and pains (Coe and Anderson, 1996; Michel et a., 2010), and in Peru to regulate menstruation (Coe and Anderson, 1996; Michel 2006; Michel et al., 2007). Thus, the in vitro pharmacology fully supported these traditional uses of this plant, and the identification of novel chemical constituents indicates that the use of ethnomedicine and ethnopharmacology can to facilitate drug discovery programs.
7.4 Lack of in vivo, human and toxicological studies
Some criticisms of the reviewed work include the fact that most of the bioassays were performed in vitro, and animal studies need to be performed to confirm these works. Also, no direct cytotoxicity studies were performed, however there was no cytotoxicity observed in the MCF cells treated with the plant extracts. While the absence or presence of activity observed from in vitro bioassays does not necessarily correspond directly to in vivo efficacy, or suggest they are safe for human consumption, in vitro assays are a good starting point for most pharmacological studies and can often suggest mechanisms of action (Farnsworth, 1998). Other concerns include the reports that some plants are used to treat amenorrhea (maybe acting as an abortifacient), fertility and labor pains, suggesting a direct effect on the uterus or ovaries, or maybe substances that are toxic to the women or fetus (Farnsworth et al., 1975). Thus, in vivo studies to assess safety (toxicity) and efficacy are essential before plants can be recommended for use. Nevertheless these data do suggest that plants used traditionally to treat women’s health complaints by the Q’eqchi Maya of Livingston Guatemala have a plausible mechanism of action and therefore do warrant further animal and human studies.
7.5 Issues of extraction methods for pharmacological studies and drug discovery
Other issues with the studies include the extraction methods that use organic solvents versus traditional infusing or boiling plants in water. Also, investigating individual plants versus the common Q’eqchi practice of using herbal combinations, somewhat similar to traditional Chinese medicine formulas. In terms of extraction procedures it is extremely difficult to use aqueous extractions (boiling or otherwise) due to the lack of facilities for large-scale lyophilization to prevent the extracts from being contaminated with microbes. Extractions with ethanol or methanol are usually a low cost method that are polar enough to extract out compounds that would be seen in aqueous extracts, without the excess of sugars that would be present in aqueous extracts and that would promote microbial contamination of the extracts. Another consideration that is especially important for the isolation and characterization of the chemical constituents, and subsequent pharmacological testing of the extract is to be able to use a solvent that allows for evaporation at low temperatures to prevent the degradation of potential active compounds. Solvent selection is critical if such studies are to be further pursued for drug discovery, as it has been shown that extraction of plant materials with aqueous-organic solvents (such as 80–95% ethanol or methanol) is more effective and efficient means of obtaining high compound yielding extracts (Sultana et al., 2009).
7.6 Ethnomedicine is an important part of overall healthcare
For most indigenous peoples in Guatemala and other countries, healers and ethnomedicines still represent the frontline of primary healthcare for these populations. In Guatemala up to 65% of the population is indigenous, and up to 80% rely on medicinal plants as their primary source of medical treatment (Caceres, 1996; Villatoro, 1996a; WHO, 2005). Thus, pharmacological and chemical investigations to determine safety and efficacy of ethnomedicines and traditional healing methods are critically important for the health of not only indigenous women, but also the public health of indigenous communities as a whole. Furthermore, traditional medical knowledge coupled with ethnomedical and pharmacological investigations should be used in public health efforts to determine factors that may contribute to poor health, as well as to identify ways to incorporate safe and effective traditional medical practices into the health care of these communities. This approach can also supply new leads for drug discovery as many of the medicinal plants used by the Maya have never been tested scientifically, nor has much of the knowledge of traditional Maya medicines been documented or published. Thus, documentation, as well as chemical and pharmacological studies could contribute significantly to the overall health of the Q’eqchi Maya, but also may impact other cultures where new therapies are developed.
7.7 Conclusion and future priorities
The results of the reviewed works suggest that there is a basic scientific basis supporting the traditional use of these plants by the Q’eqchi Maya women from the Lake Izabal region of Guatemala. However, the work does not cover all of the Q’eqchi Maya, nor does it address the traditional medicines of the other Maya groups within Guatemala. Thus, some future research priorities should include a collection and compilation of these data, along with investigations of the quality, safety and efficacy of these medicinal products, particularly where they impact pregnancy. Since the Piperaceae is the family most often utilized by the Q’eqchi, it would be a good place to start, and where possible, priority should be given to animal and human studies. Since the Ministry of Health in Guatemala has been proactive in recognizing traditional Maya medicines, data from such studies would significantly contribute to Guatemala’s national effort to promote a complementary relationship between traditional Maya medicine and public health services. Implementing such an approach as part of national public health efforts would not only be more effective and less costly than trying to provide everyone with Western medicine, but would likely be much more culturally acceptable for all involved.
Acknowledgments
We would like to express our deep gratitude and appreciation to Q’eqchi communities in Izabal, Asociacion Ak’Tenamit and the Universidad de San Carlos. This publication was supported in part by Grant Number R21-AT02381 from the National Center for Complementary and Integrative Health (previously NCCAM) at NIH, and a Fulbright Fellowship to JLM. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or NIH.
Abbreviations
- COX-2
Cyclooxygenase 2
- E2
Estradiol
- ERα,ERβ
estrogen receptor alpha and beta
- ERE
estrogen responsive element
- GC
granulosa cells
- 5-HT
Serotonin
- 5-HT1-7
Serotonin receptors 1-7
- IC50
median inhibitory concentration
- Ki
Inhibition constant
- MCF-7
human breast cancer cells
- mRNA
messenger ribonucleic acid
- nH
Hill coefficient
- PMS
premenstrual syndrome
- P4
progesterone
- PR
progesterone receptor
- qPCR
quantitative polymerase chain reaction
- SEAP
Secreted alkaline phosphatase assay
- TRAMIL
Traditional Medicine for the Islands
Footnotes
8.0 Contribution of the authors
All authors contributed to the writing of this review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joanna L Michel, Department of Medical Education, College of Medicine, University of Illinois at Chicago, Chicago, IL, 60612.
Armando Caceres, School of Biological Chemistry, Faculty of Chemical Science and Pharmacy, Universidad de San Carlos, Guatemala City, Guatemala.
Gail B Mahady, Department of Pharmacy Practice, College of Pharmacy, University of Illinois at Chicago, Chicago, IL 60612, USA.
10.0 References
- Adams R. Un análisis de las creencias y prácticas médicas en un pueblo indigena de Guatemala. Instituto Nacional Indigenista. 1952;17:15–32. [Google Scholar]
- Adhikary P, Banerji J, Chowdhury D, Das AK, Deb CC, Mukherjee SR, Chatterjee A. Antifertility effect of Piper betle Linn. Extract on ovary and testis of albino rats. Indian Journal of Experimental Biology. 1989;27:868–870. [PubMed] [Google Scholar]
- Ahmed M, Shikha HA, Sadhu SK, Rahman MT, Datta BK. Analgesic, diuretic, and anti-inflammatory principle from Scoparia dulcis. Pharmazie. 2001;56:657–660. [PubMed] [Google Scholar]
- Al Chami L, Méndez R, Chataing B, O'Callaghan J, Usubillaga A, LaCruz L. Toxicological effects of alpha-solamargine in experimental animals. Phytotherapy Research. 2003;17(3):254–258. doi: 10.1002/ptr.1122. [DOI] [PubMed] [Google Scholar]
- Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical and toxicological properties of ginger (Zingiber officinale): A review of recent research. Food and Chemical Toxicology. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Alonso-Castro AJ, Balleza-Ramos S, Morales A, Morales J, Chavex M, Alverez C. Toxicity and antinociceptive effects of Hamelia patens. Revista Brasileira de Farmacognosia. 2015;25:170–176. [Google Scholar]
- Amiguet VT, Arnason JT, Maquin P, Cal V, Vindas PS, Poveda L. A consensus ethnobotany of the Q'eqchi Maya of Southern Belize. Economic Botany. 2005;59:29–42. [Google Scholar]
- Ankli A, Heilmann J, Heinrich M, Sticher O. Cytotoxic cardenolides and antibacterial terpenoids from Crossopetalum gaumeri. Phytochemistry. 2000;54:531–537. doi: 10.1016/s0031-9422(00)00144-8. [DOI] [PubMed] [Google Scholar]
- Ankli A, Heinrich M, Bork P, Wolfram L, Bauerfeind P, Brun R, Schmid C, Weiss C, Bruggisser R, Gertsch J, Wasescha M, Sticher O. Yucatec Mayan medicinal plants: evaluation based on indigenous uses. Journal of Ethnopharmacology. 2002;79:43–52. doi: 10.1016/s0378-8741(01)00355-5. [DOI] [PubMed] [Google Scholar]
- Arenas P, Moreno Azorero R. Plants used as means of abortion, contraception, sterilization and fecundation by Paraguayan indigenous people. Economic Botany. 1977;31:302–306. doi: 10.1007/BF02866880. [DOI] [PubMed] [Google Scholar]
- Arnason T, Uck F, Lambert J, Hebda R. Maya medicinal plants of San Jose Succotz, Belize. Journal of Ethnopharmacology. 1980;2:345–364. doi: 10.1016/s0378-8741(80)81016-6. [DOI] [PubMed] [Google Scholar]
- Asprey GF, Thornton P. Medicinal plants of Jamaica. III. West Indian Medical Journal. 1955;4:69–82. [PubMed] [Google Scholar]
- Assam JP, Dzoyem JP, Pieme CA, Penlap VB. In vitro antibacterial activity and acute toxicity studies of aqueous-methanol extract of Sida rhombifolia Linn. (Asteraceae) BMC Alternative & Complementary Medicine. 2010;10:40. doi: 10.1186/1472-6882-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvigo RD, Balick M. Rainforest remedies one hundred healing herbs of Belize. Lotus Press; Twin Lakes, Wisconsin: 1993. [Google Scholar]
- Arvigo RD, Balick M. Rainforest Remedies: One hundred healing herbs of Belize. 2. Lotus Press; Twin Lakes, Wisconsin: 1998. [Google Scholar]
- Avis NE, Stellato R, Crawford S, Bromberger J, Ganz P, Cain V, Kagawa-Singer M. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Social Science Medicine. 2001;52:345–356. doi: 10.1016/s0277-9536(00)00147-7. [DOI] [PubMed] [Google Scholar]
- Awad R, Ahmed F, Bourbonnais-Spear N, Mullally M, Ta CA, Tang A, Merali Z, Maquin P, Caal F, Cal V, Poveda L, Vindas PS, Trudeau VL, Arnason JT. Ethnopharmacology of Q'eqchi' Maya antiepileptic and anxiolytic plants: effects on the GABAergic system. Journal of Ethnopharmacology. 2009;125(2):257–264. doi: 10.1016/j.jep.2009.06.034. [DOI] [PubMed] [Google Scholar]
- Barrett B. Medicinal plants of Nicaragua's Atlantic coast. Economic Botany. 1994;48:8–20. [Google Scholar]
- Basch WE, Gabardi S, Ulbricht C. Bitter melon (Momordica charantia): A review of efficacy and safety. American Journal of Health System Pharmacy. 2003;60:356–359. doi: 10.1093/ajhp/60.4.356. [DOI] [PubMed] [Google Scholar]
- Berga S, Naftolin F. Neuroendocrine control of ovulation. Gynecology and Endocrinology. 2012;28(Suppl 1):9–13. doi: 10.3109/09513590.2012.651929. [DOI] [PubMed] [Google Scholar]
- Beloin N, Gbeassor M, Akpagana K, Hudson J, de Soussa K, Koumaglo K, Arnason JT. Ethnomedicinal uses of Momordica charantia (Cucurbitaceae) and relation to its phytochemistry and biological activity. Journal of Ethnopharmacology. 2005;96:40–55. doi: 10.1016/j.jep.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Berlin EA, Berlin B. Medical ethnobiology of the highland Maya of Chiapas Mexico. Princeton University Press; Princeton, New Jersey: 1996. [Google Scholar]
- Beyene Y. Cultural significance and physiological manifestations of menopause a biocultural analysis. Culture, Medicine and Psychiatry. 1986;10:47–71. doi: 10.1007/BF00053262. [DOI] [PubMed] [Google Scholar]
- Beyene Y, Martin MC. Menopausal experiences and bone density of Mayan women in Yucatan. American Journal of Human Biology. 2001;13(4):505–511. doi: 10.1002/ajhb.1082. [DOI] [PubMed] [Google Scholar]
- Bodeker G, Ong CK, Burford G, Shein K. WHO global atlas of traditional, complementary and alternative medicine. Kobe, Japan: World Health Organization; Geneva Switzerland: 2005. [Google Scholar]
- Bourbonnais-Spear N, Awad R, Maquin P, Cal V, Vindas PS, Poveda L, Arnason JT. Plant use by the Q’eqchi Maya of Belize in ethnopsychiatry and neurological pathology. Economic Botany. 2005;59:326–336. [Google Scholar]
- Boulet MJ, Oddens BJ, Lehert P, Vemer HM, Visser A. Climacteric and menopause in seven Southeast Asian countries. Maturitas. 1994;19:157–176. doi: 10.1016/0378-5122(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Browner CH. Plants used for reproductive health in Oaxaca, Mexico. Economic Botany. 1985;39(4):482–504. doi: 10.1007/BF02858757. [DOI] [PubMed] [Google Scholar]
- Bodeker G, Ong CK, Burford G, Shein K. WHO global atlas of traditional, complementary and alternative medicine. Kobe, Japan: World Health Organization; Geneva Switzerland: 2005. [Google Scholar]
- Bum EN, Soudi S, Ayissi ER, Dong C, Lakoulo NH, Maidawa F, Seke PF, Nanga LD, Taiwe GS, Dimo T, Njikam N, Rakotonirina A, Rakotonirina SV, Kamanyi A. Anxiolytic activity evaluation of four medicinal plants from Cameroon. African Journal of Traditional, Complementary, and Alternative Medicines. 2011;8(5 Suppl):130–139. doi: 10.4314/ajtcam.v8i5S.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabarrus CR. La cosmovision Q'eqchi en proceso de cambio. Guatemala: Cholsamaj; 1974. [Google Scholar]
- Cáceres A, Cruz SM, Gaitán I, Guerrero K, Álvarez LE, Marroquín MN. Antioxidant activity and quantitative composition of extracts of Piper species from Guatemala with potential use in natural product industry. Acta Horticulturae. 2012a;964:77–84. [Google Scholar]
- Cáceres A, Cruz SM, Martínez V, Gaitán I, Santizo A, Gattuso S, Gattuso M. Ethnobotanical, pharmacognostical, pharmacological and phytochemical studies on Smilax domingensis in Guatemala. Brazilian Journal of Pharmacognosy. 2012b;22:239–248. [Google Scholar]
- Cáceres A, Lange K, Cruz SM, Velásquez R, Lima S, Menéndez MC, Dardón R, Córdova D, González J. Assessment of antioxidant activity of 24 native plants used in Guatemala for their potential application in natural product industry. Acta Horticulturae. 2012c;964:85–92. [Google Scholar]
- Cáceres A, López B, González S, Berger I, Tada I, Maki J. Plants used in Guatemala for the treatment of protozoal infections. I. Screening of activity to bacteria, fungi and American trypanosomes of 13 native plants. Journal of Ethnopharmacology. 1998;62:195–202. doi: 10.1016/s0378-8741(98)00140-8. [DOI] [PubMed] [Google Scholar]
- Cáceres A, Menendez H, Mendez E, Cohobon E, Samayoa BE, Jauregui E, Peralta E, Carrillo G. Antigonorrheal activity of plants used in Guatemala for the treatment of sexually transmitted diseases. Journal of Ethnopharmacology. 1995;48:85–88. doi: 10.1016/0378-8741(95)01288-o. [DOI] [PubMed] [Google Scholar]
- Cáceres A. Plantas de Uso Medicinal en Guatemala. Guatemala: Editorial Universitaria: Universidad de San Carlos, Guatemala City, Guatemala; 1996. [Google Scholar]
- Cáceres A, Kato MJ. Importance of a multidisciplinary evaluation of Piper genus for development of new natural products in Latin America. International Journal of Phytocosmetics and Natural Ingredients. 2014;1:4. [Google Scholar]
- Cáceres A, Michel JL, Doyle BJ, Locklear TD, Mahady GB. Estrogenic and progestagenic effects of medicinal plants used for women’s reproductive health in Guatemala. Planta Medica. 2014;80:758. [Google Scholar]
- Calixto JB. Twenty-five years of research on medicinal plants in Latin America: a personal view. Journal of Ethnopharmacology. 2005;100:131–134. doi: 10.1016/j.jep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Canto-de-Cetina TE, Canto-Cetina P, Polanco-Reyes L. Survey of climacteric symptoms in semi-rural areas of Yucatan. Revista de Investigacion Clinica. 1988;50:133–135. [PubMed] [Google Scholar]
- Carlson R, Eachus F. El mundo espiritual de los Kekchies. XIII. Instituto Indigenista Nacional; 1978. pp. 37–73. [Google Scholar]
- Chen CC, Hsu JD, Wang SF, Chiang HC, Yang MY, Kao E, Ho YC, Wang CJ. Hibiscus sabdariffa extract inhibits the development of atherosclerosis in cholesterol-fed rabbits. Journal of Agriculture and Food Chemistry. 2003;51:5472–5477. doi: 10.1021/jf030065w. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor that causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochemical Pharmacology. 1973;2:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Chiba H, Uehara M, Wu J, Wang X, Masuyama R, Suzuki K, Kanazawa K, Ishimi Y. Nutrient metabolism-hesperidin, a Citrus flavonoid inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice. Journal of Nutrition. 2003;133:6–10. doi: 10.1093/jn/133.6.1892. [DOI] [PubMed] [Google Scholar]
- Chiou WF, Peng CH, Chen CF, Chou CJ. Anti-inflammatory properties of piperlactam S: modulation of complement 5a-induced chemotaxis and inflammatory cytokines production in macrophages. Planta Medica. 2003;69:9–14. doi: 10.1055/s-2003-37041. [DOI] [PubMed] [Google Scholar]
- CIA. The World Factbook. Cia.gov. Retrieved September 23, 2015.
- Cirigliano M. Bioidentical hormone therapy: a review of the evidence. Journal of Women’s Health (Larchmt) 2007;16:600–631. doi: 10.1089/jwh.2006.0311. [DOI] [PubMed] [Google Scholar]
- Coe FG, Anderson GJ. Screening of medicinal plants used by the Garifuna of Eastern Nicaragua for bioactive compounds. Journal of Ethnopharmacology. 1996;53:29–50. doi: 10.1016/0378-8741(96)01424-9. [DOI] [PubMed] [Google Scholar]
- Coe M. Breaking the Maya code. Thames & Hudson; New York: 1999. [Google Scholar]
- Coe FG, Parikh DM, Johnson CA. Alkaloid presence and brine shrimp (Artemia salina) bioassay of medicinal species of eastern Nicaragua. Pharmaceutical Biology. 2010;48:439–445. doi: 10.3109/13880200903168015. [DOI] [PubMed] [Google Scholar]
- Collins DA. From woods to weeds: Cultural and ecological transformations in Alta Verapaz, Guatemala. Tulane University; New Orleans: 2001. [Google Scholar]
- Comerford SC. Medicinal plants of two Mayan healers from San Andres, Peten, Guatemala. Economic Botany. 1996;50:327–336. [Google Scholar]
- Conway GA, Slocumb JC. Plants used as abortifacients and emmenagogues by Spanish New Mexicans. Journal of Ethnopharmacology. 1979;1:241–261. doi: 10.1016/s0378-8741(79)80014-8. [DOI] [PubMed] [Google Scholar]
- Cosminsky S. Midwifery across the generations: A modernizing midwife in Guatemala. Medical Anthropology. 2001;20:345–378. doi: 10.1080/01459740.2001.9966198. [DOI] [PubMed] [Google Scholar]
- Costa GM, Schenkel EP, Reginatto FH. Chemical and pharmacological aspects of the genus Cecropia. Natural Products Communications. 2011;6(6):913–920. [PubMed] [Google Scholar]
- Critchley HO, Kelly RW, Brenner RM, Baird DT. The endocrinology of menstruation-a role for the immune system. Clinical Endocrinology (Oxf) 2001;55(6):701–710. doi: 10.1046/j.1365-2265.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- Cruz EC, Andrade-Cetto A. Ethnopharmacological field study of the plants used to treat type 2 diabetes among the Cakchiquels in Guatemala. Journal of Ethnopharmacology. 2015;159:238–244. doi: 10.1016/j.jep.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Cruz SM, Cáceres A, Alvarez LE, Apel MA, Henriques AT. Chemical diversity of essential oils of 15 Piper species from Guatemala. Acta Horticulturae. 2012;968:39–46. [Google Scholar]
- Cruz SM, Cáceres A, Álvarez L, Morales J, Apel MA, Henriques AT, Salamanca E, Gimenez A, Vásquez Y, Gupta MP. Chemical composition of essential oil of Piper jacquemontianum and Piper variabile from Guatemala and bioactivity of the dichloromethane and methanol extracts. Brazilian Journal of Pharmacognosy. 2011;21:587–593. [Google Scholar]
- Cullen BR, Malim MH. Secreted placental alkaline phosphatase as a eukaryotic reporter gene. Methods in Enzymology. 1995;216:362–368. doi: 10.1016/0076-6879(92)16033-g. [DOI] [PubMed] [Google Scholar]
- de Freitas PL, Dias AC, Moreira VR, Monteiro SG, Pereira SR. Antimutagenic action of the triterpene betulinic acid isolated from Scoparia dulcis (Scrophulariaceae) Genetic and Molecular Research. 2015;14(3):9745–9752. [PubMed] [Google Scholar]
- de Gezelle J. Q’eqchí Maya Reproductive Ethnomedicine. Switzerland: Springer International Publishing; 2014. [Google Scholar]
- Dieseldorff P. Las Plantas Medicinales del Departamento de Alta Verapaz. Medicinal Plants of the Department of Alta Verapaz. 1977 http://uiclibrary.worldcat.org/title/las-plantas-medicinales-del-departamento-de-alta-verapaz/oclc/79946035.
- Domingez XA, Alcorn JB. Screening of medicinal plants used by Huastec Mayans of northeast Mexico. Journal of Ethnopharmacology. 1985;13:139–156. doi: 10.1016/0378-8741(85)90002-9. [DOI] [PubMed] [Google Scholar]
- Dos Santos RN, Silva MGV. Constituintes químicos do caule de Senna reticulata Willd. (Leguminoseae) Quimica Nova. 2008;31:1979–1981. [Google Scholar]
- Doyle BJ, Frasor J, Perez A, Locklear TD, Mahady GB. Estrogenic and antiestrogenic activities of plant extracts from Costa Rica, used for the treatment of menopausal symptoms. Menopause. 2009;16(4):748–755. doi: 10.1097/gme.0b013e3181a4c76a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer H, Cotingting C. Medicinal plants for women’s healthcare in Southeast Asia: a meta-analysis of their traditional use, chemical constituents, and pharmacology. Journal of Ethnopharmacology. 2014;151:747–767. doi: 10.1016/j.jep.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Duke JA, Vásquez R. Amazonian ethnobotanical dictionary. CRC Press; Boca Raton, Fla: 1994. [Google Scholar]
- Dulón-Pérez A, Depiano E, Chedraui P, Monterrosa-Castro A. The menopause in Latin America. Maturitas. 2013;74:291–292. doi: 10.1016/j.maturitas.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Farnsworth NR. Safety, efficacy, and the use of medicinal plants. In: Akerele TRTO, editor. Medicinal plants: their role in health and biodiversity. Philadelphia: University of Pennsylvania Press; 1998. [Google Scholar]
- Farnsworth NR, Bingel AS, Cordell GA, Crane FA, Fong HS. Potential value of plants as sources of new antifertility agents I. Journal of Pharmaceutical Sciences. 1975;64:535–598. [PubMed] [Google Scholar]
- Feng PC, Haynes LJ, Magnus KE, Plimmer JR, Sherratt HS. Pharmacological screening of some West Indies medicinal plants. Journal of Pharmacy and Pharmacology. 1962;14:556–561. doi: 10.1111/j.2042-7158.1962.tb11139.x. [DOI] [PubMed] [Google Scholar]
- Fuentes RG, Toume K, Arai MA, Sadhu SK, Ahmed F, Ishibashi M. Scopadulciol, isolated from Scoparia dulcis, induces tumor necrosis factor-related apoptosis ligand resistance in AGS human gastric adenocarcinoma cells. Journal of Natural Products. 2015;78(4):864–872. doi: 10.1021/np500933v. [DOI] [PubMed] [Google Scholar]
- Ganguly M, Devi N, Mahanta R, Borthakur MK. Effect of Mimosa pudica root extract on vaginal estrous and serum hormones for screening of antifertility activity in albino mice. Contraception. 2007;76(6):482–485. doi: 10.1016/j.contraception.2007.08.008. [DOI] [PubMed] [Google Scholar]
- García-González M, Diaz C, Villabolos R. Estudio toxicológico y farmacológico de los extractos hidroalcohólicos de algunas especies de Smilax de Centroamérica. Revista de Fitoterapia. 2008;8:49–57. [Google Scholar]
- Girón LM, Caceres A. Estado de conservación de la flora útil de Guatemala. Plantas útiles amenazadas de la cuenca del Caribe; Santo Domingo, Dominican Republic: 1994. [Google Scholar]
- Girón LM, Freire V, Alonzo A, Caceres A. Ethnobotanical survey of the medicinal flora used by the Caribs of Guatemala. Journal of Ethnopharmacology. 1991;34:173–187. doi: 10.1016/0378-8741(91)90035-c. [DOI] [PubMed] [Google Scholar]
- Gold EB, Block G, Crawford S. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women's Health Across the Nation. American Journal of Epidemiology. 2004;159:1189–1199. doi: 10.1093/aje/kwh168. [DOI] [PubMed] [Google Scholar]
- Gracioso JP, Paulo MQ, Lima CA, Brito AR. Antinociceptive effect in mice of a hydroalcoholic extract of Neurolaena lobata (L.) R.Br. and its organic fractions. Journal of Pharmacy and Pharmacology. 1998;50:1425–1429. doi: 10.1111/j.2042-7158.1998.tb03370.x. [DOI] [PubMed] [Google Scholar]
- Grover JK, Yadav SP. Pharmacological actions and potential uses of Momordica charantia: A review. Journal of Ethnopharmacology. 2004;93:123–132. doi: 10.1016/j.jep.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Guerra F. Maya medicine. Medical History. 1964;8(1):31–43. doi: 10.1017/s0025727300029070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta MP, Arias TD, Correa M. Ethnopharmacognostic observation on Panamanian medicinal plants. Part I. Quarterly Journal of Crude Drug Research. 1979;17:115–130. [Google Scholar]
- Gupta SR, Nirmal SA, Patil RY, Asane GS. Anti-arthritic activity of various extracts of Sida rhombifolia aerial parts. Natural Products Research. 2009;23(8):689–695. doi: 10.1080/14786410802242778. [DOI] [PubMed] [Google Scholar]
- Hasrat JA, Pieters L, De Backer JP, Vauquelin G, Vlietinck AJ. Screening of medicinal plants from Suriname for 5-HT1A ligands: Bioactive isoquinoline alkaloids from the fruit of Annona muricata. Phytomedicine. 1997;4:133–140. doi: 10.1016/S0944-7113(97)80059-1. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Uchida K, Hayashi K, Niwayama S, Morita N. A cyctotoxic flavone from Scoparia dulcis L. Chemical and Pharmaceutical Bulletin (Tokyo) 1988;36:4849–4851. doi: 10.1248/cpb.36.4849. [DOI] [PubMed] [Google Scholar]
- Heinrich M, Barnes J, Gibbons S, Williamson E. Fundamentals of Pharmacognosy and Phytotherapy. Vol. 2004 London: Churchill Livingstone; 2004. [Google Scholar]
- Heinrich M, Ankli A, Frei B, Weimann C, Sticher O. Medicinal plants in Mexico: healer's consensus and cultural importance. Social Science Medicine. 1998;47:1859–1871. doi: 10.1016/s0277-9536(98)00181-6. [DOI] [PubMed] [Google Scholar]
- Heinrich M, Rimpler H, Barrera NA. Indigenous phytotherapy of gastrointestinal disorders in a lowland Mixe community (Oaxaca, Mexico): Ethnopharmacologic evaluation. Journal of Ethnopharmacology. 1992;36:63–80. doi: 10.1016/0378-8741(92)90062-v. [DOI] [PubMed] [Google Scholar]
- Higdon K, Scott A, Tucci M, Benghuzzi H, Tsao A, Puckett A, Cason Z, Hughes J. The use of estrogen, DHEA, and diosgenin in a sustained delivery setting as a novel treatment approach for osteoporosis in the ovariectomized adult rat model. Biomedical Sciences Instrumentation. 2001;37:281–286. [PubMed] [Google Scholar]
- Hughes J. Gender equity and indigenous women’s health in the Americas. [Accessed June 12, 2015];PAHO Report. 2004 www.paho.org.
- Kamatenesi-Mugisha M, Oryem-Origa H. Medicinal plants used in some gynaecological morbidity ailments in western Uganda. African Journal of Ecology. 2007;45:34–40. [Google Scholar]
- Kufer J, Förther H, Pöll E, Heinrich M. Historical and modern medicinal plant uses--the example of the Ch'orti' Maya and Ladinos in Eastern Guatemala. Journal of Pharmacy and Pharmacology. 2005;57(9):1127–1152. doi: 10.1211/jpp.57.9.0008. [DOI] [PubMed] [Google Scholar]
- Lagarto Parra A, Silva Yhebra R, Guerra Saldiñas I, Iglesias Buela L. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine. 2001;8:395–400. doi: 10.1078/0944-7113-00044. [DOI] [PubMed] [Google Scholar]
- Lans C. Ethnomedicines used in Trinidad and Tobago for reproductive problems. Journal of Ethnobiology and Ethnomedicine. 2007;3:13. doi: 10.1186/1746-4269-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz DL. Medicinal and other economic plants of the Paya of Honduras. Economic Botany. 1989;47:358–370. [Google Scholar]
- Leon P, Chedraui P, Hidalgo L, Ortiz F. Perceptions and attitudes toward the menopause among middle aged women from Guayaquil. Ecuador Maturitas. 2007;57:233–238. doi: 10.1016/j.maturitas.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Lewis WH, Elvin-Lewis MPF. Medical Botany: Plants affecting human health. New Jersey: John Wiley and Sons; 2003. [Google Scholar]
- Lino GS, Gomes PB, Lucetti DL, Diógenes JPI, Sousa FCF, Silva MGV, Viana GSB. Evaluation of antinociceptive and antiinflammatory activities of the essential oil (EO) of Ocimum micranthum Willd. from Northeastern Brazil. Phytotherapy Research. 2005;19:708–712. doi: 10.1002/ptr.1737. [DOI] [PubMed] [Google Scholar]
- Lizcano PJ, Bakkali F, Ruiz-Larrea MB, Ruiz-Sanz JI. Antioxidant activity and polyphenol content of aqueous extracts from Colombian Amazonian plants with medicinal use. Food Chemistry. 2010;119:1566–1570. [Google Scholar]
- Lock M, Kaufert P. Menopause, local biologies, and cultures of aging. American Journal of Human Biology. 2001;13:494–504. doi: 10.1002/ajhb.1081. [DOI] [PubMed] [Google Scholar]
- Locklear TD, Doyle BJ, Huang Y, Perez A, Frasor J, Mahady GB. Estrogenic and progestagenic effects of Justicia pectoralis, an herbal remedy used in Costa Rica for menopause and PMS. Maturitas. 2010;66:315–322. doi: 10.1016/j.maturitas.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoya X. Estado Actual del Conocimiento en Plantas Medicinales Mexicanas. Mexico City: Instituto para el Estudio de Plantas Medicinales; 1976. [Google Scholar]
- Mahady GB, Patel U, Michel JL, Caceres A. Extracts of Q’eqchi Mayan Piper and Cissampelos species alter reporter gene expression and endogenous gene expression in MCF-7 cells. Planta Medica. 2014;80:842. [Google Scholar]
- Malacara JM, Canto de Cetina T, Bassof S, Gonzalez N, Cacique L, Vera Ramirez ML, Nava LE. Symptoms at pre- and postmenopause in rural and urban women from three states of Mexico. Maturitas. 2002;43:11–19. doi: 10.1016/s0378-5122(02)00077-4. [DOI] [PubMed] [Google Scholar]
- Marles RJ, Kaminski J, Arnason JT, Pazos L, Heptinstall S, Fischer NH, Crompton CW, Kindack DG, Awang D. A bioassay for inhibition of serotonin release from bovine platelets. Journal of Natural Products. 1992;55:1044–1056. doi: 10.1021/np50086a003. [DOI] [PubMed] [Google Scholar]
- Manfred L. Siete Mil Recetas Botanicas a Base de Mil Trescientas Plantas. Buenos Aires; 1947. [Google Scholar]
- Martin MC, Block JE, Sanchez SD, Arnaud CD, Beyene Y. Menopause without symptoms: the endocrinology of menopause among rural Mayan Indians. American Journal of Obstetrics & Gynecology. 1993;168:1839–1846. doi: 10.1016/0002-9378(93)90699-j. [DOI] [PubMed] [Google Scholar]
- Mata R, Morales I, Perez O, Rivero-Cruz I, Acevedo L, Enrique-Mendoza I, Bye R, Franzblau S, Timmerman B. Antimycobacterial compounds from Piper sanctum. Journal of Ethnopharmacology. 2004;67:1961–1968. doi: 10.1021/np0401260. [DOI] [PubMed] [Google Scholar]
- Mayorga P, Pérez KR, Cruz SM, Cáceres A. Comparison of bioassays using the anostracan crustaceans Artemia salina and Thamnocephalus platyurus for plant extract toxicity screening. Brazilian Journal of Pharmacognosy. 2010;20:897–903. [Google Scholar]
- McKinnon R, Binder M, Zupkó I, Afonyushkin T, Lajter I, Vasas A, de Martin R, Unger C, Dolznig H, Diaz R, Frisch R, Passreiter CM, Krupitza G, Hohmann J, Kopp B, Bochkov VN. Pharmacological insight into the anti-inflammatory activity of sesquiterpene lactones from Neurolaena lobata (L.) R.Br. ex Cass. Phytomedicine. 2014;21(12):1695–1701. doi: 10.1016/j.phymed.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Medina RA, Sallander J, Benhamu B, Porras E, Campillo M, Pardo L, Lopez-Rodriguez ML. Synthesis of new serotonin 5-HT7 receptors ligand. Determinants of 5-HT7/5-HT1A receptor selectivity. Journal of Medicinal Chemistry. 2007;2:2384–2392. doi: 10.1021/jm8014553. [DOI] [PubMed] [Google Scholar]
- Mellen GA. El uso de plantas medicinales en Guatemala. Guatemala Indigena. 1974;9:119–129. [Google Scholar]
- Messmer WM, Farnsworth NR, Persinos GJ, Wilkes JD. Phytochemical investigation of the flowers of Cassia reticulata Willd. (Leguminosae) Journal of Pharmaceutical Sciences. 1968;57(11):1996–1998. doi: 10.1002/jps.2600571138. [DOI] [PubMed] [Google Scholar]
- Michel JL. PhD thesis. University of Illinois; Chicago: 2006. Medical ethnobotany of the Q’eqchi Maya: Perceptions and botanical treatments related to women’s health. [Google Scholar]
- Michel JL, Mahady GB, Caceres A, Soejarto DD. Attitudes and traditional medicine treatments for menopause in Guatemala. Social Sciences and Medicine. 2006;63:732–736. doi: 10.1016/j.socscimed.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel JL, Duarte RE, Caceres A, Yao P, Huang Y, Bolton J, Soejarto DD, Mahady GB. Q’eqchi Maya medicine for women’s health: In vitro evaluation of Guatemalan plants in estrogen and serotonin bioassay. Journal of Ethnopharmacology. 2007;114(1):92–101. doi: 10.1016/j.jep.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel JL, Yegao Chen, Hongjie Zhan, Huang Yue, Krunic A, Orjala J, Veliz M, Soejarto DD, Caceres A, Perez A, Mahady GB. Estrogenic and serotonergic butenolides from the leaves of Piper hispidum Swingle (Piperaceae) Journal of Ethnopharmacology. 2010;129:220–226. doi: 10.1016/j.jep.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel JL, Caceres A, Yao P, Huang Y, Bolton J, Soejarto DD, Mahady GB. Q’eqchi Maya medicine for women’s health: Guatemalan plants with estrogen and serotonin activities. Acta Horticulturae. 2012;964:235–250. [Google Scholar]
- Morton JF. Bahamas to Yucatan. Charles Thomas; Springfield, Illinois: 1981. Atlas of medicinal plants of Middle America. [Google Scholar]
- Murthy DRK, Reddy CM, Patil SB. Effect of benzene extract of Hibiscus rosa-sinensis on the estrous cycle and ovarian activity in albino mice. Biological and Pharmaceutical Bulletin. 1997;20:756–758. doi: 10.1248/bpb.20.756. [DOI] [PubMed] [Google Scholar]
- Narendhirakannan RT, Limmy TP. Anti-inflammatory and anti-oxidant properties of Sida rhombifolia stems and roots in adjuvant induced arthritic rats. Immunopharmacology Immunotoxicology. 2012;34(2):326–36. doi: 10.3109/08923973.2011.605142. [DOI] [PubMed] [Google Scholar]
- Nath P, Yadav AK. Acute and sub-acute oral toxicity assessment of the methanolic extract from leaves of Hibiscus rosa-sinensis L. in mice. Journal of Intercultural Ethnopharmacology. 2015;4(1):70–73. doi: 10.5455/jice.20141028021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nations JD, Houseal B, Ponciano I, Billy S, Godoy JC, Castro F, Miller G, Rose D, Rey M, Azurdia C. Biodiversity in Guatemala: biological diversity and tropical forests assessment. Centre for International Development and Environment. World Resources Institute; Washington, D.C: 1988. [Google Scholar]
- Nelson HD. Menopause. Lancet. 2008;371:760–770. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Nellis D. Poisonous Plants and Animals of Florida and the Caribbean. Pineapple Press; New York: 1997. [Google Scholar]
- Nogueiras C, Spengler I, Guerra JO, Ortiz Y, Torres S, García TH, Romeu CR, Regaklado EL, González TA, Perera WH, Lacaret R. Contribution to the phytochemical study and biological activity of plants of Cuban flora. Biotecnología Aplicada. 2010;27:314–318. [Google Scholar]
- Orellana SL. Indian Medicine in Highland Guatemala. University of New Mexico Press; Albuquerque, New Mexico: 1987. [Google Scholar]
- Ososki AL, Lohr P, Reiff M, Balick MJ, Kronenberg F, Fugh-Berman A, O'Connor B. Ethnobotanical literature survey of medicinal plants in the Dominican Republic used for women's health conditions. Journal of Ethnopharmacology. 2002;79:285–298. doi: 10.1016/s0378-8741(01)00376-2. [DOI] [PubMed] [Google Scholar]
- PAHO: Pan American Health Organization. Health situation in the Americas: Basic health indicators. 2012 http://new.paho.org.
- Park GL, Avery SM, Byer JL, Nelson DB. Identification of bioflavonoids from Citrus. Food Technology. 1983;37:98–105. [Google Scholar]
- Patel NK, Bairwa K, Gangwal R, Jaiswal G, Jachak SM, Sangamwar AT, Bhutani KK. 2'-Hydroxy flavanone derivatives as inhibitors of pro-inflammatory mediators: Experimental and molecular docking studies. Bioorganic Medicinal Chemistry Letters. 2015;25(9):1952–1955. doi: 10.1016/j.bmcl.2015.03.025. [DOI] [PubMed] [Google Scholar]
- Patel U, Doyle BJ, Locklear TD, Perez AL, Caceres A, Mahady GB. Mayan breadnut (Brosimum alicastrum Swartz) extracts have estrogen agonist/antagonist effects in vitro. Planta Medica. 2014;80:843. [Google Scholar]
- Pawlowski B. Prevalence of menstrual pain in relation to the reproductive life history of women from the Mayan rural community. Annals of Human Biology. 2004;31:1–8. doi: 10.1080/03014460310001602072. [DOI] [PubMed] [Google Scholar]
- Pelter A, Hänsel R. Epoxipiperolid from Piper sanctum. Zeitschrift f r Naturforschung B. 1972;27(10):1186–1190. doi: 10.1515/znb-1972-1017. [DOI] [PubMed] [Google Scholar]
- Penna SC, Medeiros MV, Aimbire FS, Faria-Neto HC, Sertie J, Lopes-Martinez RA. Anti-inflammatory effect of the hydroalcoholic extract of Zingiber officinale rhizomes on rat paw. Phytomedicine. 2003;10:381–385. doi: 10.1078/0944-7113-00271. [DOI] [PubMed] [Google Scholar]
- Pesek T, Abramiuk M, Garagic D, Fini N, Meerman J, Cal V. Sustaining plants and people: traditional Q'eqchi Maya botanical knowledge and interactive spatial modeling in prioritizing conservation of medicinal plants for culturally relative holistic health promotion. Ecohealth. 2009;6(1):79–90. doi: 10.1007/s10393-009-0224-2. [DOI] [PubMed] [Google Scholar]
- Picking D, Delgoda R, Boulogne I, Mitchell S. Hyptis verticillata Jacq: A review of its traditional uses, phytochemistry, pharmacology and toxicology. Journal of Ethnopharmacology. 2013;142:16–41. doi: 10.1016/j.jep.2013.01.039. [DOI] [PubMed] [Google Scholar]
- Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: systematic review and meta-analysis. Journal of Clinical Psychopharmacology. 2000;20:84–89. doi: 10.1097/00004714-200002000-00014. [DOI] [PubMed] [Google Scholar]
- Pino Benitez N, Meléndez León EM, Stashenko EE. Eugenol and methyl eugenol chemotypes of essential oil of species Ocimum gratissimum L. and Ocimum campechianum Mill. from Colombia. Journal of Chromatographic Sciences. 2009;47(9):800–803. doi: 10.1093/chromsci/47.9.800. [DOI] [PubMed] [Google Scholar]
- Pöll E. Plantas comestibles y tóxicas. Guatemala: Centro de Estudios Conservacionistas (CECON); 1984. [Google Scholar]
- Pöll E. Doce plantas tóxicas de Guatemala. Revista de la Universidad del Valle de Guatemala. 2006;15:80–89. [Google Scholar]
- Rasor N, Adler S. Attitudes toward menopause and aging across ethnic/racial groups. Psychosomatic Medicine. 1999;61:868–875. doi: 10.1097/00006842-199911000-00023. [DOI] [PubMed] [Google Scholar]
- Robineau L, Soejarto DD. TRAMIL: A research project on the medicinal plant resources of the Caribbean. In: Balick MJ, Elisabetsky E, Laird SA, editors. Medicinal Resources of the Tropical Forest: Biodiversity and its Importance to Human Health. Columbia University Press; New York, NY: 1996. [Google Scholar]
- Robineau LE. Towards a Caribbean ethno pharmacopoeia: Scientific research and popular use of medicinal plants in the Caribbean. Santo Domingo, Enda-Caribe: UNAH; 1991. [Google Scholar]
- Robineau L. Farmacopea Vegetal Caribeña. TRAMIL-Centro de Investigación Científica de Yucatán; Mérida: 2014. [Google Scholar]
- Rodriguez Martinez NR. Plantas alimenticias y medicinales. Editora Nani; Santiago, Republica Dominicana: 1987. [Google Scholar]
- Roys RL. The ethno-botany of the Maya. New Orleans, Louisiana: The Department of Middle American Research: Tulane University of Louisiana, Louisiana; 1931. [Google Scholar]
- Sacchetti G, Medici A, Maietti S, Radice M, Muzzoli M, Manfredini S, Braccioli E, Bruni R. Composition and functional properties of the essential oil of Amazonian basil, Ocimum micranthum Willd., Labiatae in comparison with commercial essential oils. Journal of Agriculture and Food Chemistry. 2004;52(11):3486–3491. doi: 10.1021/jf035145e. [DOI] [PubMed] [Google Scholar]
- Salib JY, Daniel EN, Hifnawy MS, Azzam SM, Shaheed IB, Abdel-Latif SM. Polyphenolic compounds from flowers of Hibiscus rosa-sinensis Linn. and their inhibitory effect on alkaline phosphatase enzyme activity in vitro. Zeitschrift fur Naturforschung C. 2011;66(9–10):453–459. doi: 10.1515/znc-2011-9-1003. [DOI] [PubMed] [Google Scholar]
- Satthawongsakul S. Studies on the uterine stimulant activity of Thai medicinal plants. Chulalongkorn University; Bangkok, Thailand: 1980. [Google Scholar]
- Schooley J, Mundt C, Wagner P, Fullerton J, O’Donnell M. Factors influencing health care-seeking behaviors among Mayan women in Guatemala. Midwifery. 2009;25:411–421. doi: 10.1016/j.midw.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Semwal DK, Semwal RB, Vermaak I, Viljoen A. From arrow poison to herbal medicine--the ethnobotanical, phytochemical and pharmacological significance of Cissampelos (Menispermaceae) Journal of Ethnopharmacology. 2014;155(2):1011–1028. doi: 10.1016/j.jep.2014.06.054. [DOI] [PubMed] [Google Scholar]
- Severy LJ, Thapa S, Askew I, Glor J. Menstrual experiences and beliefs: a multicountry study of relationships with fertility and fertility regulating methods. Women & Health. 1993;20:1–20. doi: 10.1300/J013v20n02_01. [DOI] [PubMed] [Google Scholar]
- Shivananda Nayak B. Cecropia peltata L (Cecropiaceae) has wound-healing potential: A preclinical study in a Sprague Dawley rat model. International Journal of Lower Extremity Wounds. 2006;5:20–26. doi: 10.1177/1534734606286472. [DOI] [PubMed] [Google Scholar]
- Sievert LL, Espinosa-Hernandez G. Attitudes towards menopause in relation to symptom experience in Puebla, Mexico. Women & Health. 2003;38:93–106. doi: 10.1300/J013v38n02_07. [DOI] [PubMed] [Google Scholar]
- Smith S, Schiff I, editors. Modern management of premenstrual syndrome. Norton Medical Books; New York: 1993. [Google Scholar]
- Southam L, Pedron N, Ponce-Monter H, Giron H, Estrada A, Lozoya X, Enriquez RG, Bejar E, Gallegos AJ. The zoapatle IV--toxicological and clinical studies. Contraception. 1983;27:255–265. doi: 10.1016/0010-7824(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Sprague TA. Sessé and Mociño’s Plantae Novae Hispaniae and Flora Mexicana. Bulletin of Miscellaneous Information (Royal Botanical Garden, Kew) 1929;1926(9):417–425. [Google Scholar]
- Stewart DE. Menopause in highland Guatemala Mayan women. Maturitas. 2003;44:293–297. doi: 10.1016/s0378-5122(03)00036-7. [DOI] [PubMed] [Google Scholar]
- Sultana B, Anwar F, Ashraf M. Extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–2180. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner J. The Natural History of Medicinal Plants. Timber Presss; Portland, Oregon, Timber Press: 2000. [Google Scholar]
- Sundell G, Milsom I, Andersch B. Factors influencing the prevalence and severity of dysmenorrhoea in young women. British Journal of Obstetrics & Gynaecology. 1990;97:588–594. doi: 10.1111/j.1471-0528.1990.tb02545.x. [DOI] [PubMed] [Google Scholar]
- Taufiq-Ur-Rahman M, Shilpi JA, Ahmed M, Faiz Hossain C. Preliminary pharmacological studies on Piper chaba stem bark. Journal of Ethnophamracology. 2005;99:203–209. doi: 10.1016/j.jep.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Telefo PB, Moundipa PF, Tchouanguep FM. Oestrogenicity and effect on hepatic metabolism of the aqueous extract of the leaf of Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus and Justicia insularis. Fitoterapia. 2002;73:472–478. doi: 10.1016/s0367-326x(02)00177-6. [DOI] [PubMed] [Google Scholar]
- Valdés AF, Mendiola Martínez J, Acuña Rodríguez D, Caballero Lorenzo Y, Scull Lizama R, Gutiérrez Gaitén Y. Antimalarial activity and cytotoxicity of hydroalcoholic extracts from six plant species used in Cuban traditional medicine. Revista Cubana de Medicina Tropical. 2011;63(1):52–57. Article in Spanish. [PubMed] [Google Scholar]
- Van Andel T, de Boer H, Barnes J, Vandebroek I. Medicinal plants used for menstrual disorders in Latin America, the Caribbean, sub-Saharan Africa, Southand Southeast Asia and their uterine properties: A review. Journal of Ethnopharmacology. 2014;155:992–1000. doi: 10.1016/j.jep.2014.06.049. [DOI] [PubMed] [Google Scholar]
- Villatoro E. El papel de la mujer en la atención obstetrica-pediátrica en Guatemala. Tradiciones de Guatemala. 1996a;45:69–83. [Google Scholar]
- Villatoro E. Tradiciones de Guatemala. Universidad de San Carlos de Guatemala Revista del Centro de Estudios Folklóricos; Guatemala City, Guatemala: 1996b. La Medicina Tradicional en Guatemala: Un Acercamiento Histórico. [Google Scholar]
- Villatoro E. La Medicina Tradicional en Guatemala: Un Acercamiento Historico. Universidad de San Carlos de Guatemala Revista del Centro de Estudios Folklóricos; Guatemala City, Guatemala: 1996c. [Google Scholar]
- Walshe-Roussel B, Choueiri C, Saleem A, Asim M, Caal F, Cal V, Rojas MO, Pesek T, Durst T, Arnason JT. Potent anti-inflammatory activity of sesquiterpene lactones from Neurolaena lobata (L.) R. Br. ex Cass., a Q'eqchi' Maya traditional medicine. Phytochemistry. 2013;92:122–127. doi: 10.1016/j.phytochem.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Wei M, Zheng SZ, Lu Y, Liu D, Ma H, Mahady GB. Herbal formula menoprogen alters insulin-like growth factor-1 and insulin-like growth factor binding protein-1 levels in the serum and ovaries of an aged female rat model of menopause. Menopause. 2015;22:1125–1133. doi: 10.1097/GME.0000000000000494. [DOI] [PubMed] [Google Scholar]
- Wilson MR. A highland Maya people and their habitat: The natural history, demography and economy of the K'ekchi. University of Oregon; 1972. [Google Scholar]
- Wilson R. Anchored communities: identity and history of the Maya-Q'eqchi'. Man. 1993;28:121. [Google Scholar]
- Wilson R. Ametralladoras y espiritus de la montaña. Cobán, Alta Verapaz: Centro Ak'Kutan; 1994. [Google Scholar]
- Wilson R. Maya resurgence in Guatemala: Q'eqchi experiences. Norman and London: University of Oklahoma Press; 1995a. [Google Scholar]
- Wilson R. Persona y comunidad Q'eqchi. Coban, Alta Vera Paz, Guatemala: Centro Ak'Kutan; 1995b. [Google Scholar]