Abstract
Background
The aim of this study was to establish a culture method for mouse dendritic cells (DCs) in vitro and observe their morphology at different growth stages and their ability to induce the proliferation of T lymphocytes.
Material/Methods
Granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin-4 (IL-4) were used in combination to induce differentiation of mouse bone marrow (BM) mononucleocytes into DCs. The derived DCs were then assessed for morphology, phenotype, and function.
Results
The mouse BM-derived mononucleocytes had altered cell morphology 3 days after induction by GM-CSF and IL-4 and grew into colonies. Typical dendrites appeared 8 days after induction. Many mature DCs were generated, with typical dendritic morphology observed under scanning electron microscopy. Expression levels of CD11c, a specific marker of BM-derived DCs, and of co-stimulatory molecules such as CD40, CD80, CD86, and MHC-II were elevated in the mature DCs. Furthermore, the mature DCs displayed a strong potency in stimulating the proliferation of syngenic or allogenic T lymphocytes.
Conclusions
Mouse BM-derived mononucleocytes cultured in vitro can produce a large number of DCs, as well as immature DCs, in high purity. The described in vitro culture method lays a foundation for further investigations of anti-tumor vaccines.
MeSH Keywords: Cells, Cultured; Dendritic Cells; T-Lymphocytes
Background
Tumor vaccines take advantage of tumor cells or antigens on tumor cells to evoke specific cellular or humoral immune reactions, modulate the immune function of the body, and eliminate the tumor. Dendritic cells (DCs) are the most powerful specialized antigen-presenting cells in the body. They are recognized as the initiator of immunity because of their ability to activate the naïve T-cells. DCs also play an important role in anti-tumor immunity as they can stimulate antigen-specific immune reactions, known as immunogenicity. DCs can be cultured in vitro and then be used in immunotherapy in vivo, and they may also be amplified in vivo with supplementation of cytokines or other factors such as Flt-3 [1]. A DC-based tumor vaccine as an approach to immunotherapy has attracted great attention in biomedical research, and is recognized as one of the most promising approaches for targeted anti-tumor therapies [2–5]. DCs are distributed throughout the body in very low abundance, accounting for less than 1% of peripheral white blood cells in animals and humans. In this study, mononuclear cells were isolated from mouse bone marrow (BM) and induced differentiation into DCs in vitro. The morphology of the cells was observed, and the ability to promote the proliferation of syngenic or allogenic T lymphocytes was analyzed. Our study provides a foundation for further investigations of anti-tumor vaccines.
Material and Methods
Material
The major materials employed in this study were recombinant murine granulocyte-macrophage colony stimulating factor (GM-CSF) (PeproTech, USA), interleukin-4 (IL-4) (PeproTech, USA), RPMI-1640 complete culture medium (supplemented with 10% fetus bovine serum (FBS) and antibiotics) (Hyclone, USA), MTT (Sigma, USA), fluorescence-labeled rat anti-mouse monoclonal CD11c, CD80, CD86, CD40, MHC-II, and the IgG isotypes (eBioscience, USA). The major equipment used in this study were a flow cytometer with analyzing software (Beckman, USA), an inverted optical microscope TH4-200 (Olympus, Japan), an incubator HEPA CLASS100 (Thermo, USA), a low-speed bench-top centrifuge BR4 (Jouan, France), a microplate reader (Bio-Rad, USA), nylon columns for isolating splenocytes, and culture dishes.
The experimental animals were C57BL/6 (H-2b) and BALB/c (H-2d) inbred mice, male, 6–8 weeks old, and weighing 18–20 g, purchased from the Animal Center of Xinjiang Medical University. The experiment was approved by the Animal Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (Approval Number: IACUC-2015721013).
Methods
Isolation of mononucleocytes from mouse BM
C57BL/6 mice were killed by cervical dislocation and disinfected in 75% ethanol for 5 min. The tibias and femurs were removed under sterile conditions, then soaked in RPMI-1640 medium supplemented with 1% FBS. Both ends of the bone were cut off with scissors, and the needle of a 1-mL syringe was inserted into the bone cavity to rinse the BM out of the cavity into a sterile culture dish with RPMI-1640 medium. The cell suspension in the dish was collected and centrifuged at 1000 rpm for 5 min, and the supernatant was discarded. The cell pellet was resuspended with Tris-NH4Cl red blood cell (RBC) lysis buffer to lyse the RBCs. Following the second centrifugation, the supernatant was discarded and the pelleted cells were washed with PBS and collected.
Induced culture of BM-derived DCs (BMDCs)
The cells, suspended in RPMI-1640 medium supplemented with 10% FBS, were distributed into 24-well plates at a density of 1×106 cell/mL/well. Subsequently, GM-CSF and IL-4 were added into the medium to a final concentration of 20 ng/mL and 10 ng/mL, respectively. The cells were cultured at 37ºC in an incubator containing 5% CO2. The culture medium was replaced 12 h later to remove the unattached cells and cell debris, then the fresh medium was supplemented with GM-CSF and IL-6. On day 7, the semi-suspended cells and loosely attached cells were collected by gently pipetting the medium against the plate. The cells were plated into 6-well plates for an additional incubation with lipopolysaccharide (LPS) for 24 h, and BMDCs were obtained.
Morphological observation under optical and electron microscopy
Cellular morphological changes were observed every day under an inverted optical phase-contrast microscope. The cells were collected on day 8, washed with PBS, and centrifuged at 900 rpm for 5 min. The cells were fixed with 2% glutaraldehyde for 2 h at 4ºC, and washed with PBS twice. The washed cells were further fixed with osmic acid for 1 h at 4ºC, and washed with PBS twice. The washed cells were dehydrated in graded ethanol, incubated with acetic acid, dried at the CO2 critical point, and vacuum evaporated. The cell surface structure was observed under a scanning electron microscope.
Flow cytometric analysis of DC phenotypes
The cells were collected after days 4, 6, and 8, washed with PBS, and divided into several fractions of 5×105 cells/100 μL. Each sample was measured in triplicate. PE-labeled anti-CD11c and anti-CD80, PE-Cy5-labeled anti-CD86, and FITC-labeled anti-CD40 and anti-MHC-II were added into the suspension to a final concentration of 5 μg/mL and incubated for 30 min at 4ºC in the dark. The cells were washed with PBS twice and analyzed by flow cytometry. Fluorescence-labeled IgG isotypes were used as the control.
Mixed lymphocyte reaction (MLR)
The effect of DCs on the proliferation of syngenic or allogenic T lymphocytes was assessed by the MTT method. The spleens of C57BL/6 and BALB/c mice were dissected under sterile conditions to make splenocyte suspensions. The splenocytes were suspended in RPMI-1640 complete culture medium and passed through a nylon column. The cells were incubated at 37ºC for 1 h in an atmosphere of 5% CO2. The purified T-cells, as the reactive cells, were adjusted to a concentration of 2×106 cells/mL, and 100 μL of the suspension was plated into each well of a 96-well plate. The DCs were collected on days 1, 4, 6, and 8, and were adjusted to a concentration of 2×106 cells/mL. The cells were treated with 25 ng/mL mitomycin C to transition them into stimulating cells. The reactive cells and the stimulating cells were mixed in different ratios (100:1, 40:1, 20:1, or 10:1) and co-cultured in 96-well plates at 37ºC for 72 h in an atmosphere of 5% CO2. This experiment was performed with 5 replicates, and wells containing only T-cells were used as the control. MTT solution (20 μL; 5 mg/ml) was added to each well for an additional 4 h incubation. Thereafter, 150 μL of DMSO was added to each well to dissolve the crystals completely by shaking the plate for 10 min. The optical density (D) at 570 nm was measured by a microplate reader, and the stimulation index (SI) was calculated as follows: SI=D of sample/D of control.
Statistical analysis
Statistical analysis of the data was performed using software SPSS 17.0. The data are expressed as χ̄±s. Inter-group differences are analyzed by one-way analysis of variance (ANOVA), and P<0.05 indicated a statistically significant difference.
Results
Morphological observation
Morphological observation by inverted microscope
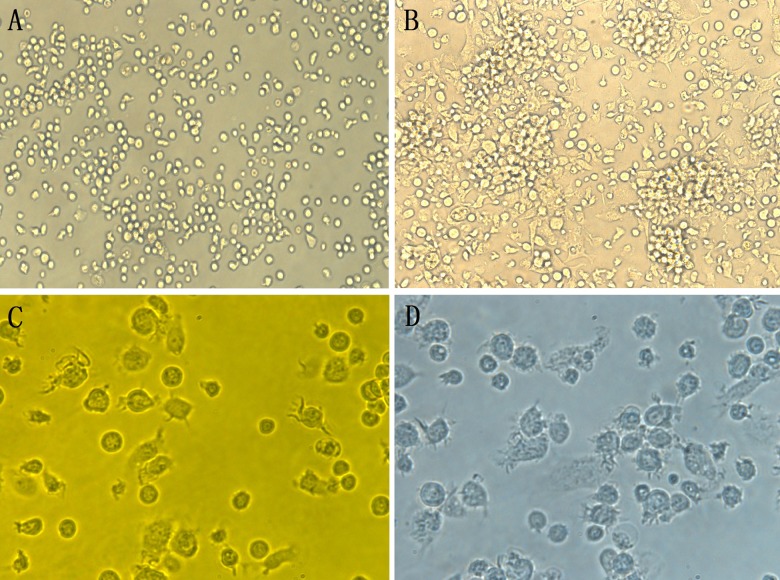
Approximately 2.5×107 to 3.5×107 BM mononucleocytes were obtained from each mouse, and the cells adhered to the plate after 3–4 h. After 24 h of recombinant murine granulocyte-macrophage colony stimulating factor (rmGM-CSF) and recombinant murine interleukin-4 (rmIL-4) induction, some of the cells adhered to the plate and many others suspended in the culture medium, as observed under inverted phase-contrast microscope. Colonies appeared after 72 h. The number of cells also significantly increased and the cell volume enlarged. After the number of adherent cells gradually decreased over time, a few suspended cells with dendritic protrusions were first observed. On day 5, suspended cells with dendritic protrusions increased gradually. On day 7, the suspended cells began to aggregate and the protrusions elongated. Following 24-h incubation with LPS, the colonies dispersed, the cells were evenly distributed in the suspension, and typical dendrites were observed (Figure 1).
Figure 1.
Morphological characteristics of BMDCs at different culture times. (A) Mononucleocytes after 24-h induction (×200); (B) Mononucleocytes after 3-day induction (×200); (C) Mononucleocytes after 5-day induction (×400); (D) Mononucleocytes after 8-day induction (×400).
Scanning electron microscopy
Under a scanning electron microscope, the surfaces of DCs were observed to be rough and intact. The dendritic structure was obvious and the microvilli protuberances were clear and gauze-like. There were many folds and irregular protrusions, some with irregular spherical expansion at the ends, typical of the morphology of DCs (Figure 2).
Figure 2.
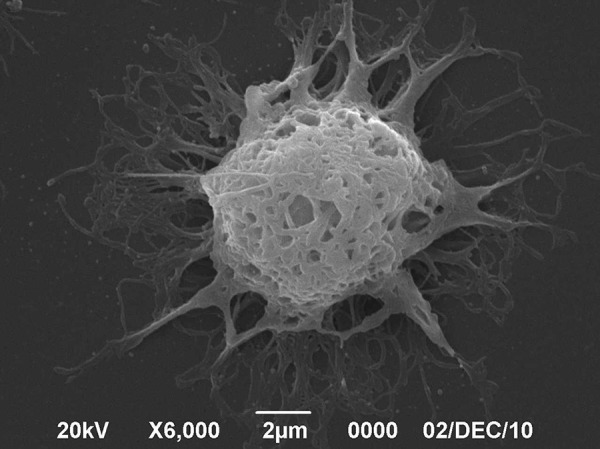
BMDC morphology scanning electron microscopy (SEM).
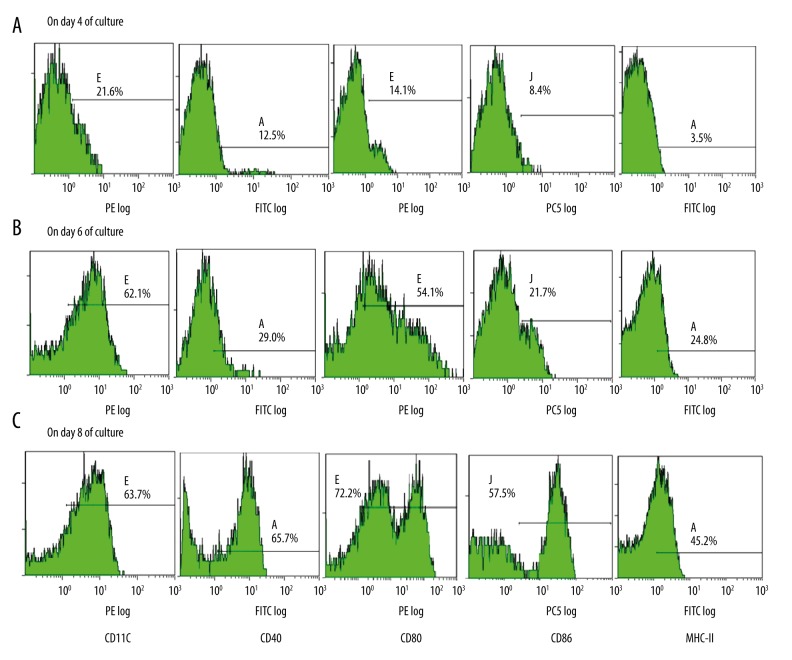
Flow cytometric analysis of DC phenotype
On day 4 of incubation, before maturation, the ratio of CD11c+ cells was 21.6%, and the expression levels of cell surface MHC-II family molecules and the co-stimulatory molecules CD40, CD80, and CD86 were very low. On day 8, the ratio of CD11c+ cells was 63.7%, and the expression of CD40, CD80, CD86, and MHC-II was high. The expression of CD11c, a specific surface marker of BMDCs, indicates that mouse BM mononucleocytes cultured in vitro can generate DCs in relatively high purity. After LPD-induced maturation, the ratios of CD40+, CD80+, CD86+, and MHC-II+ cells on day 8 were (58.0±7.0)%, (70.2±1.8)%, and (46.9±1.8)%, respectively, and these ratios significantly increased compared with the corresponding ratios on day 6 (P<0.01), which were (29.2±1.3)%, (70.2±1.8)%, and (46.9±1.8)%, respectively. However, the expression of CD11c was not significantly different between day 8 and day 6 [(61.57±1.87)% vs. (60.80±1.14)%, P>0.05] (Figure 3).
Figure 3.
Flow cytometric analysis of the expression of BMDC surface antigens at different culture times. (A) CD11c 21.6%, CD40 12.2%, CD80 14.1%, CD86 8.4%, MHC-II 3.5%; (B) CD11c 62.1%, CD40 29.0%, CD80 54.1%, CD86 21.7%, MHC-II 24.8%, (C) CD11c 63.7%, CD40 65.7%, CD80 72.2%, CD86 57.5%, MHC-II 45.2%.
Assessment of BMDC-induced T-cell proliferation and activation by MLR
In the syngenic mouse T-cells, the SIs of various groups of DCs were significantly different when the ratio of reactive cells to stimulating cells was 10:1 (P<0.05). When the ratio of reactive cells to stimulating cells was 20:1 or 40:1, the SI of day 8 DCs was significantly different than that of other groups of DCs (P<0.05), among which the SIs were not significantly different (P>0.05). The SIs were not significantly different among all groups when the ratio of reactive cells to stimulating cells was 100:1 (P>0.05) (Table 1).
Table 1.
Stimulation index of DCs at different culture times and ratios of the stimulation of proliferation of syngenic mouse T lymphocytes (χ̄±s, n=5).
| DC Culture time (d) | Reactive cells: Stimulating cells | |||
|---|---|---|---|---|
| 100:1 | 40:1 | 20:1 | 10:1 | |
| 1 | 1.11±0.16 | 1.09±0.14* | 1.17±0.12* | 1.12±0.13*,#,$ |
| 4 | 1.10±0.08 | 1.24±0.10* | 1.22±0.16* | 1.89±0.29*,# |
| 6 | 1.28±0.13 | 1.26±0.09* | 1.70±0.38* | 3.04±0.38* |
| 8 | 1.71±0.39 | 2.12±0.21 | 3.13±0.44 | 4.36±0.34 |
Compared with 8d DCs,
P<0.05; compared with 6d DCs;
P<0.05; compared with 4d DCs,
P<0.05.
In the allogenic mouse T-cells, the SIs were not significantly different between day 4 and day 1 DCs (P>0.05) when the ratio of reactive cells to stimulating cells was 10:1, whereas the SIs of the other groups were significantly different at this ratio (P<0.05). When the ratio was 20:1, the SI of day 8 DCs was significantly different from that of other groups of DCs (P<0.05), among which the SIs were not significantly different (P>0.05). When the ratio was 40:1, the SIs were significantly different between day 8 and day 1 DCs (P<0.05), while the SIs of the other groups were not significantly different (P>0.05). The SIs were not significantly different among all groups when the ratio of reactive cells to stimulating cells was 100:1 (P>0.05) (Table 2).
Table 2.
Stimulation index of DCs at different culture times and ratios of the stimulation of proliferation of allogenic mouse T lymphocytes (χ̄±s, n=5).
| DC Culture time (d) | Reactive cells: Stimulating cells | |||
|---|---|---|---|---|
| 100:1 | 40:1 | 20:1 | 10:1 | |
| 1 | 1.07±0.10 | 1.02±0.14* | 1.11±0.17* | 1.14±0.09*,# |
| 4 | 1.08±0.12 | 1.18±0.11 | 1.16±0.15* | 1.39±0.21*,# |
| 6 | 1.06±0.11 | 1.24±0.60 | 1.51±0.36* | 2.27±0.20* |
| 8 | 1.44±0.35 | 1.66±0.30 | 2.84±0.40 | 4.02±0.15 |
Compared with 8d DCs,
P<0.05; compared with 6d DCs,
P<0.05.
Discussion
DCs were first isolated from mouse spleen by Steinman [6] but DC precursors can now be isolated from multiple tissues and induced to differentiate into functional DCs [7–11]. The biological characteristics of DCs from different sources or at different differentiation stages are quite distinct [12]. BM has become a major source of DCs due to its easy accessibility and abundance of DC precursors.
In the present study, hematopoietic precursor cells were obtained from the BM of C57BL/6 mice, and BMDCs were induced by rmGM-CSF and rmIL-4 in vitro. The induced BMDCs were then characterized by morphological observation, flow cytometric analysis of phenotype, and functional MLR tests. Typical features of mature DCs were observed on day 8 of the culture. Morphologically, the cells were round with irregular dendritic protrusions on the surface. An average of 4–5×106 BMDCs could be obtained from each mouse. Phenotypically, the flow cytometry results showed that the immature DCs on day 4 expressed low levels of CD11c, MHC-II family molecules, and co-stimulatory molecules. On day 6, DCs expressed high CD11c (62.1%), and moderate levels of MHC-II family molecules and co-stimulatory molecules. Mature DCs on day 8 expressed high levels of CD11c, MHC-II family molecules and co-stimulatory molecules. As CD11c is a specific marker of DCs and is expressed at especially high levels in splenocyte-derived DCs, the high ratio (63.7%) of CD11c+ mature DCs on day 8 indicates a relatively high purity of BMDCs obtained by this method.
Immature DCs undergo a series of changes after antigen uptake and processing and gain mature phenotypes and functions: (1) loss of phagocytic receptors; (2) upregulation of MHC-II family molecules and co-stimulatory molecules, including CD40, CD80, and CD86; (3) morphological changes; and (4) activation of antigen processing mechanism [13–16].
DC activates T-cells via 2 major signaling pathways: (1) interaction between T-cell receptor (II) and MHC-antigen complex; and (2) co-stimulatory signaling – the most typical example is the interaction between CD38 on T-cells and CD80/CD86 on DCs. These 2 pathways initiate acquired immune responses, including expansion of T-cell clones and differentiation into effector cells. A third signaling pathway is delivered to T-cells by DCs [17,18].
In this study, both mature DCs and immature DCs could stimulate T-cell proliferation, and the stimulatory potency was correlated with the ratio of reactive cells to stimulatory cells. When the ratio of reactive cells to stimulatory cells was 10:1, the stimulatory potency increased along with culture time and as the expression of the co-stimulatory molecules such as CD40, CD80, and CD86 increased.
The principle of MLR is that the MHC-II and co-stimulatory molecules expressed on the antigen-presenting cells (the stimulatory cells) can directly stimulate the proliferation of the reactive cells, and the stimulatory potency is associated with the expression levels of the co-stimulatory molecules. A higher level of expression of the co-stimulatory molecules is correlated with stronger potency in stimulating T-cell proliferation. The expression levels of MHC-II and co-stimulatory molecules are relatively high in mature DCs, and the expression of co-stimulatory molecules was relatively low in immature DCs. After sensitization by tumor antigens, the expression of MHC-II and co-stimulatory molecules is upregulated, resulting in enhanced function of tumor-specific cytotoxic T lymphocytes (CTL) [19,20]. Hence, the MLR result can reflect the antigen-presenting ability of BMDCs and their induced, specific anti-tumor immunity after sensitization by tumor-related antigens. LPS-stimulated DCs displayed an augmented potency in stimulating T-cell proliferation, suggesting that the antigen-presenting ability of the mature DCs significantly improved along with the increased expression of co-stimulatory molecules. In the stimulation of T-cells by DCs with various culture times, the SIs from the MLR tests changed in the same way for both syngenic and allogenic T-cells, implying that the DCs obtained in this study possessed antigen-presenting function. The change in the phenotype of the BMDCs along with the culture time was consistent with the MLR result: except for day 4 of culture, the SI to allogenic T lymphocytes was not significantly different from the SI on day 1 (P>0.05), even in the presence of abundant stimulating cells (reactive cells: stimulatory cells=10:1), whereas the SI to synergic T lymphocytes was significantly different from the SI on day 1 (P<0.05). These results suggest that the immature DCs on day 4 could not stimulate the proliferation of allogenic mouse T lymphocytes, but could exert a stimulatory effect on the proliferation of syngenic T lymphocytes.
Immature DCs possess strong phagocytic and antigen-processing abilities, but have a low potency to stimulate T-cell proliferation because they express low levels of, or even none of, MHC-II family molecules and co-stimulatory molecules (such as CD40, CD80, and CD86) on the surface. Following antigen capture, DC can undergo differentiation and maturation in the presence of some activating factors, such as LPS, IL-1b, and TNF-a. The levels of surface MHC-II family molecules or co-stimulatory molecules increase dramatically, leading to an enhanced DC-mediated immunity and an increased potency in T-cell stimulation, but a reduced ability for antigen capture. The co-stimulatory molecules upregulated in mature DCs also secrete IL-12, a T-cell differentiation factor, so that the mature phenotype is stabilized and the half-life of MHC-I and MHC-II family molecules is prolonged. Therefore, antigen presentation by mature DCs is more effective [21,22].
In our study, mature DCs (8d) could stimulate the proliferation of syngenic T-cells (1.71±0.39) even in a low abundance (reactive cells: stimulating cells=100:1). The stimulatory capacity was gradually enhanced as the number of DCs increased. By contrast, mature DCs could stimulate the proliferation of allogenic T-cells only in the presence of relatively abundant cells (reactive cells: stimulating cells <20:1).
Generally, the cells that display a typical dendritic morphology and express high level of MHC-II family molecules possess the ability to migrate to lymphoid organs and stimulate the proliferation and activation of naive T-cells and present a group of specific surface markers that are recognized as DCs [23,24].
Conclusions
In this study, we established a method to isolate, induce, and culture BMDCs. The induced DCs with a relatively high purity displayed dendritic morphology, expressed DC surface markers, and possessed biological functions. A relatively high purity of immature DCs was obtained on day 6 of culture, and these immature DCs had a potential ability to stimulate specific CTLs after sensitization by tumor antigens. Our study provides a foundation for further investigations of anti-tumor vaccines.
Footnotes
Declaration of interest
All authors have no conflict of interest regarding this paper.
Source of support: Departmental sources
References
- 1.Wang HL, Xu H, Lu WH, et al. In vitro and in vivo evaluations of human papillomavirus type 16 (HPV16)-derived peptide-loaded dendritic cells (DCs) with a CpG oligodeoxynucleotide (CpG-ODN) adjuvant as tumor vaccines for immunotherapy of cervical cancer. Arch Gynecol Obstet. 2014;289:155–62. doi: 10.1007/s00404-013-2938-1. [DOI] [PubMed] [Google Scholar]
- 2.Rosalia RA, Cruz LJ, van Duikeren S, et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials. 2015;40:88–97. doi: 10.1016/j.biomaterials.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 3.Jiang PL, Lin HJ, Wang HW, et al. Galactosylated liposome as a dendritic cell-targeted mucosal vaccine for inducing protective anti-tumor immunity. Acta Biomater. 2015;11:356–67. doi: 10.1016/j.actbio.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Lin W, Chen YL, Jiang L, Chen JK. Reduced expression of chemerin is associated with a poor prognosis and a lowed infiltration of both dendritic cells and natural killer cells in human hepatocellular carcinoma. Clin Lab. 2011;57:879–85. [PubMed] [Google Scholar]
- 5.Zheng C, Yu G, Wang H, et al. Meta-analysis of chemotherapy and dendritic cells with cytokine-induced killer cells in the treatment of non-small-cell lung cancer. Int J Clin Exp Med. 2015;8:14527–37. [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman RM, Cohn ZA. Pillars article: identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Immunol. 2007;178:5–25. [PubMed] [Google Scholar]
- 7.Andrés C, Plana M, Guardo AC, et al. HIV-1 reservoir dynamics after vaccination and antiretroviral therapy interruption are associated with dendritic cell vaccine-induced T cell responses. J Virol. 2015;89:9189–99. doi: 10.1128/JVI.01062-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruno L. Differentiation of dendritic cell subsets from mouse bone marrow. Methods Mol Biol. 2007;380:47–57. doi: 10.1007/978-1-59745-395-0_3. [DOI] [PubMed] [Google Scholar]
- 9.Alissafi T, Hatzioannou A, Ioannou M, et al. De novo-induced self-antigen-specific Foxp3+ regulatory T cells impair the accumulation of inflammatory dendritic cells in draining lymph nodes. J Immunol. 2015;194:5812–24. doi: 10.4049/jimmunol.1500111. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Zhang L, Lu B, et al. Role of dendritic cell-mediated abnormal immune response in visceral hypersensitivity. Int J Clin Exp Med. 2015;8:13243–50. [PMC free article] [PubMed] [Google Scholar]
- 11.Tuettenberg A, Becker C, Correll A, et al. Immune regulation by dendritic cells and T cells – basic science, diagnostic, and clinical application. Clin Lab. 2011;57:1–12. [PubMed] [Google Scholar]
- 12.Sennikov SV, Obleukhova IA, Kurilin VV, et al. Features of functional activity of dendritic cells in tumor growth. Vopr Onkol. 2015;61:556–62. [PubMed] [Google Scholar]
- 13.Pletinckx K, Vaeth M, Schneider T, et al. Immature dendritic cells convert anergic nonregulatory T cells into Foxp3- IL-10+ regulatory T cells by engaging CD28 and CTLA-4. Eur J Immunol. 2015;45:480–91. doi: 10.1002/eji.201444991. [DOI] [PubMed] [Google Scholar]
- 14.Ryu SW, Han EC, Yoon J, Choi C. The mitochondrial fusion-related proteins Mfn2 and OPA1 are transcriptionally induced during differentiation of bone marrow progenitors to immature dendritic cells. Mol Cells. 2015;38:89–94. doi: 10.14348/molcells.2015.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi W, Hou X, Peng H, et al. MEK/ERK signaling pathway is required for enterovirus 71 replication in immature dendritic cells. Virol J. 2014;11:227. doi: 10.1186/s12985-014-0227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang AX, Chong N, Jiang Y, et al. Molecular characterization of antigen-peptide pulsed dendritic cells: immature dendritic cells develop a distinct molecular profile when pulsed with antigen peptide. PLoS One. 2014;9:e86306. doi: 10.1371/journal.pone.0086306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Lavender P, Watson J, et al. Stress-activated dendritic cells (DC) induce dual interleukin (IL)-15- and IL1b-mediated pathways, which may elicit CD4+ memory T cells and interferon (IFN)-stimulated genes. J Biol Chem. 2015;290:15595–609. doi: 10.1074/jbc.M115.645754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan PH, Tyrrell HE, Gao L, et al. Adiponectin receptor signaling on dendritic cells blunts antitumor immunity. Cancer Res. 2014;74:5711–22. doi: 10.1158/0008-5472.CAN-13-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong CY, Kim SY, Lee HJ, et al. A bacterial flagellin in combination with proinflammatory cytokines activates human monocyte-derived dendritic cells to generate cytotoxic T lymphocytes having increased homing signals to cancer. J Immunother. 2014;37:16–25. doi: 10.1097/CJI.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 20.Jung SH, Lee YK, Lee HJ, et al. Dendritic cells loaded with myeloma cells pretreated with a combination of JSI-124 and bortezomib generate potent myeloma-specific cytotoxic T lymphocytes in vitro. Exp Hematol. 2014;42:274–81. doi: 10.1016/j.exphem.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Q, Long J, Jia B, et al. Transforming growth factor-b1 deteriorates microrheological characteristics and motility of mature dendritic cells in concentration-dependent fashion. Clin Hemorheol Microcirc. 2014;56:25–40. doi: 10.3233/CH-121653. [DOI] [PubMed] [Google Scholar]
- 22.Morrison VL, James MJ, Grzes K, et al. Loss of beta2-integrin-mediated cytoskeletal linkage reprogrammes dendritic cells to a mature migratory phenotype. Nat Commun. 2014;5:5359. doi: 10.1038/ncomms6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ardavin C. Origin, precursors and differentiation of mouse dendritic cells. Nat Rev Immunol. 2003;3(7):582–90. doi: 10.1038/nri1127. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Zhang Y, Yin X, et al. Construction and evaluation of rats’ tolerogenic dendritic cells (DC) induced by NF-kB Decoy method. Afr Health Sci. 2014;14:626–33. doi: 10.4314/ahs.v14i3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]