Abstract
The Janus kinase (JAK) system is involved in numerous cell signaling processes and is highly expressed in cardiac tissue. The JAK isoform JAK2 is activated by numerous factors known to influence cardiac function and pathologic conditions. However, although abundant, the role of JAK2 in the regulation or maintenance of cardiac homeostasis remains poorly understood. Using the Cre-loxP system, we generated a cardiac-specific deletion of Jak2 in the mouse to assess the effect on cardiac function with animals followed up for a 4-month period after birth. These animals had marked mortality during this period, although at 4 months mortality in male mice (47%) was substantially higher compared with female mice (30%). Both male and female cardiac Jak2-deleted mice had hypertrophy, dilated cardiomyopathy, and severe left ventricular dysfunction, including a marked reduction in ejection fractions as assessed by serial echocardiography, although the responses in females were somewhat less severe. Defective cardiac function was associated with altered protein levels of sarcoplasmic reticulum calcium-regulatory proteins particularly in hearts from male mice that had depressed levels of SERCA2 and phosphorylated phospholamban. In contrast, SERCA2 was unchanged in hearts of female mice, whereas phosphorylated phospholamban was increased. Our findings suggest that cardiac JAK2 is critical for maintaining normal heart function, and its ablation produces a severe pathologic phenotype composed of myocardial remodeling, heart failure, and pronounced mortality.
The Janus kinase (JAK) family of tyrosine kinases plays a key role in cellular signaling in response to a variety of stimuli.1, 2 Although four mammalian subtypes exist, JAK2 represents the predominant subtype found in the cardiac cell. Activation of JAKs, including JAK2, such as that occurring after cytokine administration, is associated with JAK dimerization3 and phosphorylation of tyrosines (Tyr1007/Tyr1008) without which JAK2 cannot be activated. A large number of biologically active compounds particularly important to the cardiovascular system, including angiotensin II acting on the AT1 receptor,4 platelet-activating factor,5 bradykinin B2 receptor agonists,6 and opioid receptor agonists,7 activate JAK2 in the heart. Reactive oxygen species and hyperglycemic conditions have been reported to activate JAK2 in aortic smooth muscle cells and cardiac myocytes and to enhance the ability of other factors to stimulate JAK2, including angiotensin II and endothelin-1.8, 9, 10, 11, 12, 13
The ability of a wide range of factors to stimulate JAK2/STAT activity in the heart suggests that this system plays multifaceted roles in cardiac homeostasis and pathologic conditions. For example, there is increasing evidence that JAK2/STAT activation is important for cardioprotection in hearts subjected to ischemia and reperfusion particularly with respect to reduction in infarct size, findings based principally on pharmacologic inhibition of the JAK2/STAT pathway.14, 15 There is also evidence that JAK2/STAT activation may mediate the cardioprotective effect of both early and delayed ischemic preconditioning.14, 15 Although these studies suggest a salutary cardioprotective role of JAK2 in the ischemic myocardium, others have proposed opposite roles. For example, Mascareno et al16 reported that angiotensin II–induced apoptosis in cultured adult cardiomyocytes is associated with JAK2 kinase activation concomitant with activation of BAX, caspase-1, and caspase-2 activity. Moreover, apoptosis and proapoptotic signals are inhibited by the JAK2 inhibitor tyrphostin AG490.16 JAK2 inhibition has also been reported to protect the rat heart against ischemic and reperfusion injury17 and leptin-induced mitochondrial transition pore opening.18 Therefore, when taken together, these results suggest that JAK2 activity may be beneficial or deleterious, depending on the nature of insult or the experimental condition.
It appears, therefore, that JAK2 plays important but multifaceted roles in cardiovascular regulation under normal and pathologic conditions. However, the overall importance of endogenous JAK2 to heart function is poorly understood, and a potential reason is the absence of animal models with a cardiac-specific Jak2 ablation. Global Jak2 deletion is embryonically lethal due to impaired erythropoiesis.19 We therefore successfully generated a cardiac-specific deletion of Jak2 in mice to obtain further insights into its role in cardiac function.
Materials and Methods
Experimental Animals
All protocols for the use of animals were performed in accordance with the University of Western Ontario animal care guidelines. These protocols conform to the guidelines of the Canadian Council on Animal Care (Ottawa, ON, Canada) and the Guide for the Care and Use of Laboratory Animals.20 The study has been approved by the University of Western Ontario Council on Animal Care (protocol 2013–031).
Generation of cJAK2-KO Mice
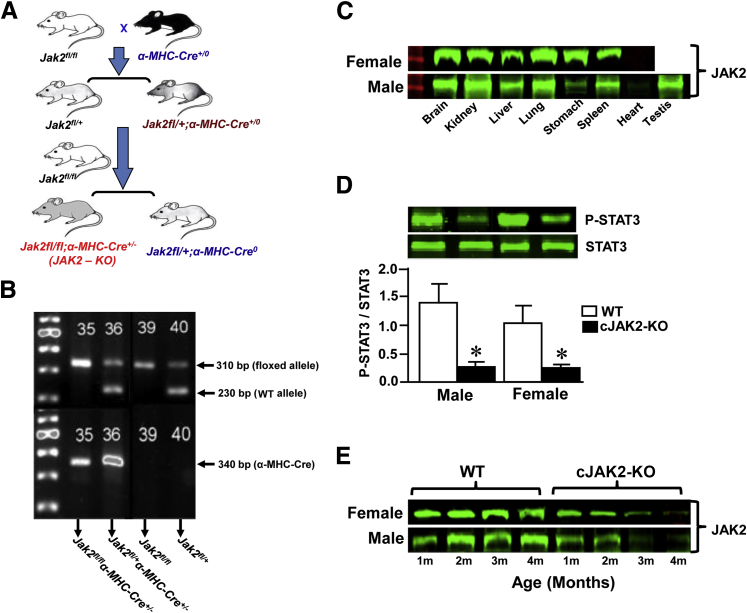
Cardiac-specific Jak2 knockout (cJAK2-KO) mice (C57BL/6J background) were generated by using the Cre-loxP system to disrupt the Jak2 gene in the heart. Jak2 conditional knockout (JAK2fl/fl;α-MHC-Cre+/0) mice were generated as described and characterized previously.21 Cardiac α-MHC-Cre transgenic mice (α-MHC-Cre+/0) were a generous gift from Dr. Qingping Feng (University of Western Ontario, ON, Canada). The specific breeding strategy that was used to generate homozygous JAK2f/lfl and JAK2−/− mutants (JAK2fl/fl;α-MHC-Cre+/0) is summarized in Figure 1A. Homozygous JAKfl/fl mice were mated to heterozygous JAKfl/+;α-MHC-Cre+/0 mice to finally generate a heart-specific knockout of Jak2 (Jak2fl/fl;α-MHC-Cre+/0).
Figure 1.
Breeding strategy and characterization of cardiac Janus kinase 2 (JAK2)–deleted mice. A: Generation of a cardiac-specific knockout of Jak2−/− (cJAK2-KO) mice. Homozygous Jak2fl/fl mice were mated to heterozygous Jak2 floxed mice expressing α-MHC-Cre to finally generate a cardiac-specific knockout of Jak2 (Jak2fl/fl;α-MHC-Cre+/0) mice. B: Results of allele-specific PCR assay verifying the presence of two homozygous floxed alleles (Jak2fl/fl) and heterozygous Cre allele from tail biopsy specimens. This assay yielded a 230-bp PCR fragment without the loxP site [wild-type (WT) allele] and a band of 310 bp in size with the loxP site (floxed allele) and a band of 340 bp with Cre positive. C:α-MHC-Cre+/0–mediated cardiac-specific deletion of Jak2 in various tissues by Western blot analysis in 4-month-old cJAK2-KO male and female mice to confirm the selective absence of expression of JAK2 protein only in the heart but its presence in other tissues. D: Detection of the phosphorylated STAT3 (p-STAT3) in 3-month-old WT and cJAK2-KO male and female mice by Western blotting. Representative blots from WT and cJAK2-KO male and female mice are shown. Bars represent the quantified ratios of p-STAT3/STAT3 from each group. E:α-MHC-Cre+/0–mediated cardiac-specific deletion of Jak2 in an age-dependent manner by Western blot analysis in 1 to 4-month-old WT and cJAK2-KO male and female mice hearts. Data are expressed as means ± SEM. n = 6. ∗P < 0.05 from respective values for WT mice. m, months.
Genotyping Analysis by PCR
Genomic DNA was prepared from tail biopsy specimens harvested from 21-day-old mice by incubating overnight at 65°C in the buffer that consisted of 50 mmol/L Tris-HCl pH 8.0, 100 mmol/L EDTA, 100 mmol/L NaCl, 1% SDS, and 0.4 mg/mL of proteinase K. Proteinase K was inactivated by boiling the samples at 95°C for 10 minutes. Genotyping was determined through allele-specific PCR analysis (Figure 1B). By using the primers 5′-ATTCTGAGATTCAGGTCTGAGC-3′ (forward) and 5′-CTCACAACCATCTGTATCTCAC-3′ (reverse), we confirmed the correct insertion of the outermost loxP site into the endogenous JAK2. α-MHC-Cre expression was confirmed by amplifying with 5′-ATGTCCAATTTACTGACCG-3′ (forward) and 5′-CGCCGCATAACCAGTGAAAC-3′ (reverse) primers. The PCR reaction medium consisted of 10× PCR buffer (Sigma-Aldrich, St. Louis, MO), 0.2 mmol/L dNTPs, 1 μmol/L of each primer, 1 U of Jumpstart RED Taq DNA polymerase (Sigma-Aldrich), and 3 μL of genomic DNA. PCR conditions to amplify the genes were 30 seconds at 94°C followed by annealing at 58°C for 30 seconds and elongation at 72°C for 1 minute for 30 cycles. All PCR bands were confirmed by being easily distinguishable on a 3% agarose gel.
Echocardiography
Mice were anesthetized with isoflurane (Baxter Corporation, Mississauga, ON, Canada) at a concentration of 5% (induction) and 1.0% (maintenance) in 100% oxygen. Each animal was placed on a heating platform, and the chest was shaved using a chemical hair remover (Nair; Church & Dwight Canada Corp, Mississauga, ON, Canada). Warmed ultrasound gel (National Therapy Products, Woodbridge, ON, Canada) was applied to the thorax surface to optimize the visibility of the cardiac chambers. Echocardiography (M-model, two-dimensional, and Doppler) was performed using a Vevo 770 ultrasound system (Visualsonics, Toronto, ON, Canada).
Tissue Processing and Histological Staining
Hearts from each genotype were excised and fixed in 4% paraformaldehyde overnight at 4°C, subsequently dehydrated, and embedded in paraffin. Hearts from longitudinal or transverse sections sliced at 5-μm thickness were fixed on Superfrost Plus glass slides (Fisher Scientific, Toronto, ON, Canada). The sections were deparaffinated and stained with hematoxylin and eosin followed by a graded dehydrated alcohol and then mounted by Cytoseal XYL (Thermo Scientific, Waltham, MA). The images were visualized under a microscope and analyzed with Scope Image software version 9.0 (United Scope Inc., Kitchener, ON, Canada).
Western Blot Analysis
Mouse hearts and tissues were washed with phosphate-buffered saline and homogenized using 200 μL of lysis buffer that contained protease inhibitor cocktail followed by centrifugation at 10,000 × g for 5 minutes at 4°C. The supernatant was transferred to a fresh tube, and the protein concentration was determined by the Bradford protein assay method (Bio-Rad Laboratories, Mississauga, ON, Canada). Thirty to fifty micrograms of protein were resolved on 6%, 10%, or 15% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were blocked in 5% milk for 1 hour and incubated with primary antibodies for JAK2, p-STAT3, STAT3, SERCA2, calsequestrin2, phosphorylated phospholamban, phospholamban, ryanodine receptor, or actin (1:500; Santa Cruz Biotechnology, Dallas, TX) overnight at 4°C followed by incubation with secondary antibody conjugated to IRDye 800 (1:5000; LI-COR Biosciences, Lincoln, NE) for 1 hour. The bound complex was then detected using the Odyssey Clx Infrared Imaging System (Li-COR; Mandel Scientific, Guelph, ON, Canada). The images were analyzed for density using the Odyssey Application Software (Li-COR Image Studio version 3.14).
RNA Isolation, Reverse Transcription, and Real-Time PCR Analysis
RNA was extracted using Tri reagent (Sigma-Aldrich) according to the manufacturer's instructions. RNA (1 μg) was used to synthesize the first strand of cDNA using M-MLV reverse transcriptase according to the manufacturer's protocol and was used as a template in the PCR reactions. The expression of atrial natriuretic peptide, α-skeletal actin, or tumor protein, translationally-controlled 1 (TPT1) RNA (loading internal control) was determined in 10-μL reaction volumes using EvaGreen qPCR Mastermix (Applied Biological Materials, Richmond, BC, Canada). Fluorescence was measured and quantified using a CFX96 Real-Time system (Bio-Rad Laboratories). Amplification was performed using the following primers: α-skeletal actin forward: 5′-CACGGCATTATCACCAACTG-3′ and reverse: 5′-CCGGAGGCATAGAGAGACA-G-3′ (221 bp); ANP forward: 5′-CTGCTAGACCACCTGGAGGA-3′ and reverse: 5′-AAGCTGT-TGCAGCCTAGTCC-3′ (320 bp); and TPT1 RNA forward: 5′-GGAGGGCAAGATGGTCAGTA-3′ and reverse: 5′-AGGCCTCTTTTGTGAAGCTG-3′ (170 bp). PCR conditions to amplify the genes were 30 seconds at 94°C followed by annealing at the gene-specific temperature (58°C) for 20 seconds and elongation at 72°C for 30 seconds. Melting curve analysis revealed a single-product formation for each gene amplified. The number of gene copies was calculated from a standard curve consisting of 5 serial dilutions ranging from 1 to 10−5. Expression was normalized using the housekeeping gene TPT1 RNA as an internal control.
Statistical Analysis
The data were analyzed by a one-way analysis of variance, and group differences were detected using a Student-Newman-Keuls post hoc test. P < 0.05 was considered significant.
Results
Mice were genotyped through allele-specific PCR analysis (Figure 1B). We confirmed the correct insertion of the outermost loxP site into the endogenous JAK2 locus by using the specific primers as mentioned in Materials and Methods. The amplification yielded a 230-bp PCR fragment without the loxP site [wild-type (WT) allele] and a band of 310-bp in size with the loxP site [floxed Jak2 allele (Jak2fl/fl)]. The specific primers for the α-MHC-Cre transgene amplified a product of 340 bp. α-MHC-Cre–mediated recombination produced a deleted Jak2 allele. The presence or absence of JAK2 protein expression was also verified from hearts in Jak2−/− and Jak2+/+ mice and other tissues in Jak2−/− mice (Figure 1C). Furthermore, hearts from mice with the Jak2 deletion had markedly reduced phosphorylated STAT3 protein expression, although total STAT3 was unchanged (Figure 1D). α-MHC-Cre+/0–mediated cardiac-specific deletion of Jak2 was clearly evident in 4-month-old cJAK2-KO male and female mice hearts.
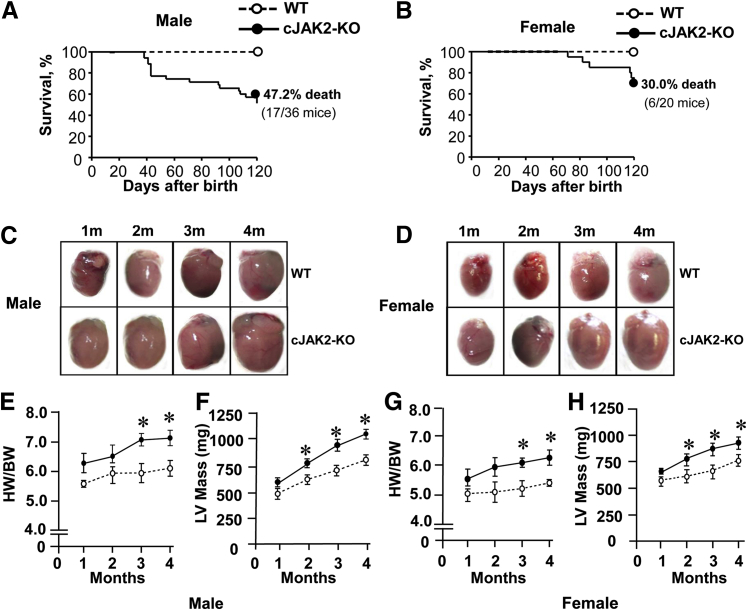
We monitored the survival rates of cJAK2-KO mice to determine cardiac morphologic conditions in surviving animals. Cardiac-specific Jak2 deletion was associated with a progressive reduction in survival, which was particularly prominent in male mice (Figure 2A) and markedly less so in female animals (Figure 2B) with postnatal mortality after 4 months of 47.2% and 30% in male and female mice, respectively. Moreover, mortality was not observed in female mice until at least 2 months of age. Hearts from both male and female mice exhibited marked hypertrophy as illustrated visually (Figure 2, C and D) and by both quantitative analysis of heart weights and left ventricular mass from echocardiography analyses (Figure 2, E–H). Although hearts tended to be larger as early as 1 month after birth, significant increases in heart tissues were generally observed after 2 or 3 months. Another important distinction between hearts from male and female is the rate and magnitude of change in left ventricular mass during the 4-month period, which was substantially greater in male (77%) (Figure 2F) versus female animals (46%) (Figure 2H).
Figure 2.
Cardiac-specific Janus kinase 2 (JAK2) deletion produces mortality associated with the development of left ventricular hypertrophy. Open circles indicate wild-type (WT) mice; closed circles, cardiac-specific knockout of Jak2−/− (cJAK2-KO) mice. A and B: Survival curves in male and female mice during the 4-month postnatal period. C and D: Representative images of hearts during the 4-month period. E–H: Quantified data for heart weight to body weight ratios (HW/BW) and left ventricular (LV) mass for both male and female animals. HW/BW values were obtained by gravimetric measurements, whereas LV mass was calculated with echocardiography. Data are expressed as means ± SEM. n = 7 to 9. ∗P < 0.05 from respective values for WT mice.
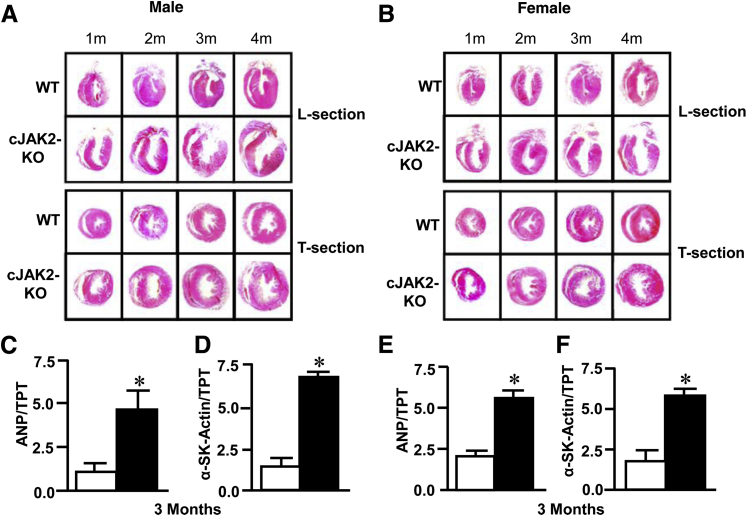
We further assessed hypertrophy in 3-month-old animals histologically and by determining gene expression of molecular markers associated with the hypertrophic response. Histologic examination of hearts at each month revealed that these animals principally had an eccentric form of hypertrophy with marked left ventricular dilation compared with wild-type animals (Figure 3, A and B). Hypertrophy was further documented by determining gene expression of ANP and α-skeletal actin, which were both markedly significantly elevated in cJAK2-KO male and female mice (Figure 3, C–F).
Figure 3.
Cardiac-specific Janus kinase 2 (JAK2) deletion reveals histological and molecular evidence of hypertrophy. White bars indicate wild-type (WT) mice; black bars, cardiac-specific knockout of Jak2−/− (cJAK2-KO) mice. A and B: Longitudinal (L) and transverse (T) 5-μm sections stained with hematoxylin and eosin during the 4-month postnatal period of both male and female mice. C–F: Cardiac gene expression levels on atrial natriuretic peptide (ANP) and α-skeletal actin (α-SK-Actin) determined by quantitative RT-PCR in the hearts of 3-month-old mice. Histologic images depict left ventricular hypertrophy with marked dilatation in mice carrying cardiac JAK2 deletion. Hypertrophy was further found in hearts of these animals by increased expression of ANP and α-SK-Actin. Data are expressed as means ± SEM. n = 7 to 9. ∗P < 0.05 from hearts of wild-type (WT) mice. m, months.
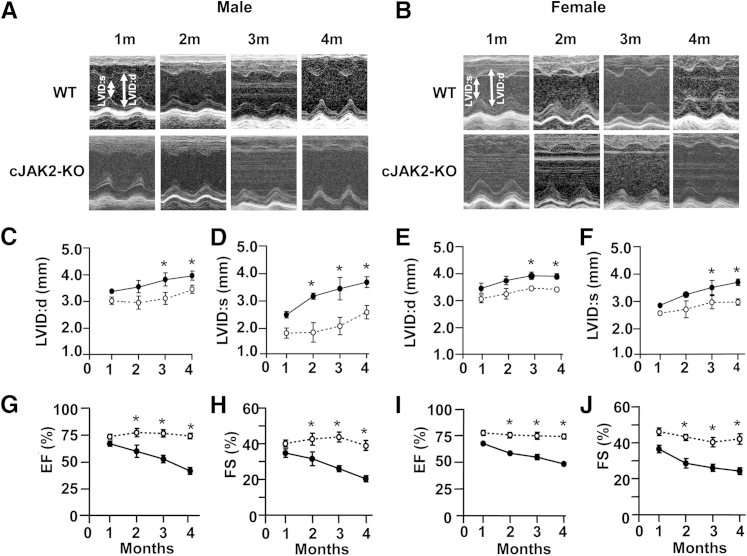
Further assessment of the cardiac phenotype in cJAK2-KO mice was determined by performing Doppler and M-mode serial echocardiography during the 4-month postnatal period. M-mode analyses revealed that cJAK2-KO mice had progressive significantly enhanced left ventricular diastolic and systolic dimensions. This finding was significant as early as 2 months after birth (Figure 4, A, C, and D) although significant elevations in hearts from female mice occurred only at 3 and 4 months (Figure 4, B, E, and F).
Figure 4.
Cardiac-specific Janus kinase 2 (JAK2) deletion reveals left ventricular abnormalities as determined by serial echocardiographic measurements in hearts from male and female mice. All parameters were determined from serial echocardiography images obtained at monthly intervals in isoflurane-anesthetized mice. Left ventricular internal dimensions during diastole (LVID:d) and systole (LVID:s) were calculated from M-mode images as illustrated in A and B with quantified data for these parameters shown in C–F. G–J: Left ventricular ejection fractions (EFs) and fractional shortening (FS) determined from serial echocardiography images obtained at monthly intervals in anesthetized mice. EF and FS were calculated from left ventricular diastolic and systolic volumes and internal diameters, respectively, and obtained by M-mode. Open circles indicate wild-type (WT) mice; closed circles, cardiac-specific knockout of Jak2−/− (cJAK2-KO) mice. Data are expressed as means ± SEM. n = 7 to 9. ∗P < 0.05 from hearts of wild-type (WT) mice. m, months.
In addition to alterations in left ventricular dimensions, cJAK2-KO mice also had severe progressive left ventricular dysfunction as manifested by reduced ejection fractions, systolic shortening, stroke volumes, and cardiac output compared with WT mice (Figure 4, G–J). Both male and female mice exhibited a significant decline in left ventricular function over time, although in general the response was more pronounced in males with respect to some parameters. For example, the ejection fraction in male cJAK-2 KO mice was reduced to 33% (Figure 4G), although female mice had a mean ejection fraction of 49% at the 4-month period (Figure 4I). A relatively similar pattern of responses was observed with respect to fractional shortening, another important index of left ventricular systolic performance (Figure 4, H and J).
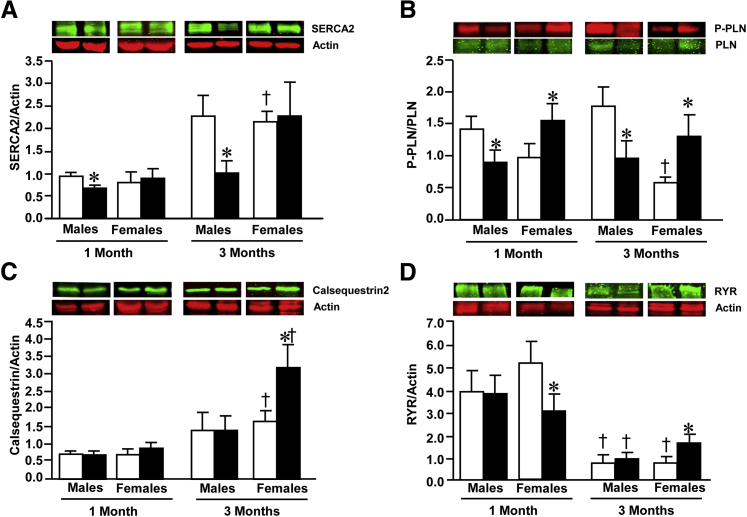
To better understand the basis for defective left ventricular function in cJAK2-KO mice and the increased severity seen in male mice, we determined the expression of key sarcoplasmic reticulum proteins involved in cardiac excitation-contraction coupling, namely, SERCA2, calsequestrin, phosphorylated phospholamban, and the ryanodine receptor. These results are summarized in Figure 5 and reveal a number of age-related changes in these proteins and different pattern of changes in hearts of males versus female subjects due to Jak2 ablation. SERCA2 protein levels were significantly increased in hearts of 3-month-old animals but not in hearts of male cJAK-2-KO mice with values significantly lower from WT controls, an effect also observed in hearts from 1-month-old animals (Figure 5A). Phosphorylated phospholamban protein levels were initially depressed at 1 month of age and increased at 3 months in hearts of both male and female cJAK2-KO mice (Figure 5B), whereas with respect to calsequestrin, protein expression levels significantly increased only in hearts of 3-month-old female cJAK2-KO mice (Figure 5C). Lastly, we also examined the protein expression of the sarcoplasmic reticulum ryanodine receptor. The protein levels of ryanodine receptor were markedly reduced in hearts from all 3-month-old animals when compared with values seen at 1 month of age (Figure 5D). Protein levels were unaffected by Jak2 ablation in hearts from male mice, whereas hearts from female mice had a significant reduction in ryanodine receptor protein levels, whereas a significant increase was seen at 3 months, when compared with WT controls.
Figure 5.
Cardiac-specific Janus kinase 2 (JAK2) deletion reveals alterations in sarcoplasmic reticulum calcium regulatory proteins, including SERCA2 (A), phosphorylated phospholamban (P-PLN) (B), calsequestrin (C), and the ryanodine receptor (RYR) (D), Each panel shows quantified data expressed as a ratio to actin, used as a loading control, and representative Western blots. White bars indicate wild-type (WT) mice; black bars, cardiac-specific knockout of Jak2−/− (cJAK2-KO) mice. Data are expressed as means ± SEM. n = 6 to 8. ∗P < 0.05, from respective wild-type (WT) mice; †P < 0.05, respective value from 1-month-old animals.
Discussion
JAK-dependent signaling plays an important role in the regulation of cellular homeostasis in a large number of tissues in response to a variety of stimuli.1, 2 Although four mammalian JAK subtypes exist, JAK2 represents the predominant subtype found in the cardiac cell, which likely mediates cardiac function, especially under pathologic circumstances. Most of our current knowledge regarding JAK2 and its role in cardiac conditions arises from pharmacologic modulation of the JAK2 system. This is particularly evident during myocardial ischemia and reperfusion because there is increasing evidence that JAK2 activity is important for cardioprotection under these conditions.14, 15 Conversely however, as previously noted, a contributing role for JAK2 in cardiac conditions has also been proposed.16, 17, 18
A potential basis for the uncertainty of functions played by JAK2-dependent signaling in the heart may be, as previously suggested, the absence of cardiac-specific JAK2-KO animal models.2 Although global JAK2-KO mice have been generated, these animals die in mid-gestation principally because of the absence of erythropoiesis.19 To the best of our knowledge, this report represents the first cardiac-specific JAK2-KO mouse that has been generated and allows the determination of cardiac consequences. We confirm in the present study that Jak2 deletion was selectively restricted to the heart and that expression of JAK2 was maintained in all other tissues that were probed. Our results reveal that these mice rapidly develop a severe cardiac defect as manifested by progressive left ventricular dysfunction and early mortality, possibly due to lethal arrhythmias, although many of these effects, particularly mortality, were less pronounced in females.
There were two major phenotypical observations in mice with cardiac Jak2 deletion, namely, myocardial hypertrophy and heart failure, thereby suggesting that cardiac Jak2 deletion unmasks a relatively rapid and severe heart failure phenotype in the absence of insult. Cardiac dysfunction was evidenced by a progressive deterioration in left ventricular performance as assessed by serial echocardiography. Among these was a severe reduction in the ejection fraction and fractional shortening, especially in 4-month-old mice, a finding particularly more pronounced in hearts from male animals. Moreover, myocardial remodeling was evident from histologic assessments and the determination of left ventricular dimensions from M-mode serial echocardiography. These results suggest that JAK2 is an important contributor to the maintenance of normal cardiac function, and its disruption can then predispose the myocardium to undesirable consequences in the absence of any other insult.
STAT3 phosphorylation is an important component of JAK2 activation, and indeed cardiac-specific deletion of Stat3 in mice produces a heart failure phenotype; however, this was evident only in older animals (ie, >6 months of age).22 Our results reveal a markedly different cardiac phenotype than that seen with Stat3 ablation because heart failure and indeed mortality occurred at a much earlier age, indeed as early as 1 month after birth. These differences suggest that the consequences of cardiac Jak2 ablation extend beyond the inability to phosphorylate and activate only STAT3, which would not be surprising in view of the large number of substrates that serve as targets for JAK2. Thus, upstream deletion of Jak2 appears to produce more profound effects than those seen with Stat3 ablation.
The use of a Cre-LoxP system to generate cJAK2-KO mice may be problematic in view of the potential direct cardiac toxic effects produced by prolonged Cre expression. However, these toxic effects are relatively mild in 3-month-old α-MHC-Cre transgenic mice and are actually associated with increased ejection fractions at this age, possible due to compensatory left ventricular hypertrophy.23 In control studies we found no evidence of cardiac dysfunction in 4-month-old α-MHC-Cre mice (data not shown).
To obtain better insight into potential mechanisms of reduced contractility as a result of Jak2 deletion and differences between male and female cJAK2-KO mice, we determined levels of proteins involved in calcium regulation by the sarcoplasmic reticulum. Cardiomyocyte contractility is determined to a large degree by the interplay between factors responsible for calcium uptake by the sarcoplasmic reticulum (ie, SERCA2 regulated by phospholamban) and its release via the ryanodine receptor channel.24, 25 Many of these proteins are altered in clinical heart failure, including a marked reduction in SERCA2 levels,26 and it has been proposed that gene therapy aimed at increasing SERCA2 levels in the failing myocardium may represent a promising approach toward the treatment of heart failure by improving contractile function and diminishing the potential for arrhythmogenesis.24, 25 Of interest, in the present study, we found significant reductions in SERCA2 protein levels in hearts from cJAK2-KO male mice, although hearts from female animals were unaffected. SERCA2 activity is regulated by the protein phospholamban, which is an inhibitor of SERCA2 in its unphosphorylated state, whereas the inhibitory effect is prevented by phospholamban phosphorylation.27 Hearts from male cJAK2-KO mice exhibited reduced phosphorylated to unphosphorylated phospholamban ratios at both 1 and 3 months of age, whereas the opposite effect was observed in hearts from female animals. This may be an important finding as well because phospholamban mutations or deletion is believed to contribute to the development of cardiomyopathy and heart failure.27 We also determined the expression levels of calsequestrin, a sarcoplasmic reticulum calcium-binding protein, and the sarcoplasmic reticulum ryanodine receptor calcium release channel. The importance of these proteins to heart failure is not well understood, although a marked (20-fold) overexpression of cardiac calsequestrin produces a heart failure phenotype in mice.28 Although we observed a twofold elevation in calsequestrin levels in hearts from 3-month-old female cJAK2-KO mice, the relevance of this in relation to cardiac dysfunction is uncertain at present, especially because no changes in calcequestrin levels were observed in any of the other groups.
With respect to the ryanodine receptor, defective function rather than protein abundance likely contributes to the pathology of heart failure.29 Thus, when taken together, these findings reveal a clear association between defective left ventricular performance in hearts from male cJAK2-KO male mice and reduced SERCA2 function as evidenced by a reduction in SERCA2 protein levels coupled with a reduction in phosphorylated phospholamban protein abundance. The lack of SERCA2 changes seen in hearts from female animals and the increase in phosphorylated phospholamban protein expression may have represented a compensatory response resulting in reduced left ventricular dysfunction and mortality, the latter possibly due to reduced arrhythmogenesis. A cause-and-effect relationship between SERCA2/phospholamban and cardiac conditions in cJAK-KO mice was not established in this study. Moreover, the fact that cardiac dysfunction and mortality were still evident in hearts from cJAK2-KO female mice strongly implies that additional mechanisms underlie the deleterious consequences of cardiac Jak2 deletion.
Further detailed studies are necessary to adequately determine whether cJAK2 deficiency or defective cJAK2-dependent signaling contributes to clinical heart failure or other cardiac conditions. Of interest, recent evidence suggests that clinical end-stage heart failure is associated with defective cJAK2-dependent STAT3 phosphorylation; however, whether this represents a cause-and-effect relationship is uncertain.30 JAK2 inhibition is associated with an antineoplastic effect31, 32; therefore, another potential clinical implication of our finding may lie in the potential adverse effect associated with the use of JAK2 inhibitors that are being developed for the treatment of cancers.33 The possibility exists that tissue-nonspecific pharmacologic JAK2 inhibitors may need to be used with caution, particularly in patients with cardiac comorbidities. This concept has been reinforced by a recent study that found that endothelium-specific Jak2 ablation results in defective vascular response to vasodilatating agents, thus potentially additionally contributing to the cardiovascular burden.34
Conclusion
We have clearly found a heart failure phenotype in mice with cardiac-specific deletion of Jak2, suggesting that JAK2 preservation may be a potential therapeutic goal for the treatment of heart failure. Although further studies are required to delineate the underlying mechanisms for cardiac conditions in cJAK2-KO mice, the results presented here suggest that reduction in protein levels of both SERCA2 and phosphorylated phospholamban may contribute to this phenotype, at least in male mice. The results further suggest that reduced severity of contractile dysfunction and mortality in female cJAK2-KO mice may reflect the preservation of these key proteins after Jak2 ablation.
Acknowledgment
We thank Dr. Qingping Feng (University of Western Ontario, ON, Canada) for the gift of the α-MHC-Cre+/0 mice.
Footnotes
Supported by grant MPO 136782 from the Canadian Institutes of Health Research (M.K.) and Public Health Service grant CA117930 from the National Cancer Institute (K.-U.W.). M.K. holds a Canada Research Chair in Experimental Cardiology.
Disclosures: None declared.
References
- 1.Schindler C.W. JAK-STAT signaling in human disease. J Clin Invest. 2002;109:1133–1137. doi: 10.1172/JCI15644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurdi M., Booz G.W. JAK redux: a second look at the regulation and role of JAKs in the heart. Am J Physiol Heart Circ Physiol. 2009;297:H1545–H1556. doi: 10.1152/ajpheart.00032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss A., Schlessinger J. Switching signals on or off by receptor dimerization. Cell. 1998;94:277–280. doi: 10.1016/s0092-8674(00)81469-5. [DOI] [PubMed] [Google Scholar]
- 4.Paxton W.G., Heerdt L., Berk B.C., Delafontaine P., Bernstein K.E. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature. 1995;375:247–250. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- 5.Lukashova V., Chen Z., Duhé R.J., Rola-Pleszczynski M., Stanková J. Janus kinase 2 activation by the platelet-activating factor receptor (PAFR): roles of Tyk2 and PAFR C terminus. J Immunol. 2003;171:3794–3800. doi: 10.4049/jimmunol.171.7.3794. [DOI] [PubMed] [Google Scholar]
- 6.Lefler D., Mukhin Y.V., Pettus T., Leeb-Lundberg L.M., Garnovskaya M.N., Raymond J.R. Jak2 and Ca2+/calmodulin are key intermediates for bradykinin B2 receptor-mediated activation of Na+/H+ exchange in KNRK and CHO cells. Assay Drug Dev Technol. 2003;1:281–289. doi: 10.1089/15406580360545099. [DOI] [PubMed] [Google Scholar]
- 7.Gross E.R., Hsu A.K., Gross G.J. The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3β. Am J Physiol Heart Circ Physiol. 2006;291:H827–H834. doi: 10.1152/ajpheart.00003.2006. [DOI] [PubMed] [Google Scholar]
- 8.Mazière C., Conte M.A., Mazière J.C. Activation of JAK2 by the oxidative stress generated with oxidized low-density lipoprotein. Free Radic Biol Med. 2001;31:1334–1340. doi: 10.1016/s0891-5849(01)00649-9. [DOI] [PubMed] [Google Scholar]
- 9.Simon A.R., Rai U., Fanburg B.L., Cochran B.H. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;275:C1640–C1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 10.Amiri F., Venema V.J., Wang X., Ju H., Venema R.C., Marrero M.B. Hyperglycemia enhances angiotensin II-induced janus-activated kinase/STAT signaling in vascular smooth muscle cells. J Biol Chem. 1999;274:32382–32386. doi: 10.1074/jbc.274.45.32382. [DOI] [PubMed] [Google Scholar]
- 11.Banes-Berceli A.K., Ketsawatsomkron P., Ogbi S., Patel B., Pollock D.M., Marrero M.B. Angiotensin II and endothelin-1 augment the vascular complications of diabetes via JAK2 activation. Am J Physiol Heart Circ Physiol. 2007;293:H1291–H1299. doi: 10.1152/ajpheart.00181.2007. [DOI] [PubMed] [Google Scholar]
- 12.Modesti A., Bertolozzi I., Gamberi T., Marchetta M., Lumachi C., Coppo M., Moroni F., Toscano T., Lucchese G., Gensini G.F., Modesti P.A. Hyperglycemia activates JAK2 signaling pathway in human failing myocytes via angiotensin II-mediated oxidative stress. Diabetes. 2005;54:394–401. doi: 10.2337/diabetes.54.2.394. [DOI] [PubMed] [Google Scholar]
- 13.Shaw S., Wang X., Redd H., Alexander G.D., Isales C.M., Marrero M.B. High glucose augments the angiotensin II-induced activation of JAK2 in vascular smooth muscle cells via the polyol pathway. J Biol Chem. 2003;278:30634–30641. doi: 10.1074/jbc.M305008200. [DOI] [PubMed] [Google Scholar]
- 14.Hattori R., Maulik N., Otani H., Zhu L., Cordis G., Engelman R.M., Siddiqui M.A., Das D.K. Role of STAT3 in ischemic preconditioning. J Mol Cell Cardiol. 2001;33:1929–1936. doi: 10.1006/jmcc.2001.1456. [DOI] [PubMed] [Google Scholar]
- 15.Barry S.P., Townsend P.A., Latchman D.S., Stephanou A. Role of the JAK-STAT pathway in myocardial injury. Trends Mol Med. 2007;13:82–89. doi: 10.1016/j.molmed.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Mascareno E., Beckles D.L., Siddiqui M.A. Janus kinase-2 signaling mediates apoptosis in rat cardiomyocytes. Vascul Pharmacol. 2005;43:327–335. doi: 10.1016/j.vph.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Mascareno E., El-Shafei M., Maulik N., Sato M., Guo Y., Das D.K., Siddiqui M.A. JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. Circulation. 2001;104:325–329. doi: 10.1161/01.cir.104.3.325. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Abundis E., Rajapurohitam V., Haist J.V., Gan X.T., Karmazyn M. The obesity-related peptide leptin sensitizes cardiac mitochondria to calcium-induced permeability transition pore opening and apoptosis. PLoS One. 2012;7:e41612. doi: 10.1371/journal.pone.0041612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neubauer H., Cumano A., Müller M., Wu H., Huffstadt U., Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 20.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals . National Academies Press; Washington, DC, U.S: 1996. Guide for the Care and Use of Laboratory Animals, 7th Edition. [Google Scholar]
- 21.Krempler A., Qi Y., Triplett A.A., Zhu J., Rui H., Wagner K.U. Generation of a conditional knockout allele for the Janus kinase 2 (Jak2) gene in mice. Genesis. 2004;40:52–57. doi: 10.1002/gene.20063. [DOI] [PubMed] [Google Scholar]
- 22.Jacoby J.J., Kalinowski A., Liu M.G., Zhang S.S., Gao Q., Chai G.X., Ji L., Iwamoto Y., Li E., Schneider M., Russell K.S., Fu X.Y. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci U S A. 2003;100:12929–12934. doi: 10.1073/pnas.2134694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pugach E.K., Richmond P.A., Azofeifa J.G., Dowell R.D., Leinwand L.A. Prolonged Cre expression driven by the α-myosin heavy chain promoter can be cardiotoxic. J Mol Cell Cardiol. 2015;86:54–61. doi: 10.1016/j.yjmcc.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayward C., Banner N.R., Morley-Smith A., Lyon A.R., Harding S.E. The current and future landscape of SERCA gene therapy for heart failure: a clinical perspective. Hum Gene Ther. 2015;26:293–304. doi: 10.1089/hum.2015.018. [DOI] [PubMed] [Google Scholar]
- 25.Park W.J., Oh J.G. SERCA2a: a prime target for modulation of cardiac contractility during heart failure. BMB Rep. 2013;46:237–243. doi: 10.5483/BMBRep.2013.46.5.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasenfuss G., Meyer M., Schillinger W., Preuss M., Pieske B., Just H. Calcium handling proteins in the failing human heart. Basic Res Cardiol. 1997;92(Suppl 1):87–93. doi: 10.1007/BF00794072. [DOI] [PubMed] [Google Scholar]
- 27.MacLennan D.H., Kranias E.G. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y., Ferguson D.G., Sako H., Dorn G.W., 2nd, Kadambi V.J., Yatani A., Hoit B.D., Walsh R.A., Kranias E.G. Cardiac-specific overexpression of mouse cardiac calsequestrin is associated with depressed cardiovascular function and hypertrophy in transgenic mice. J Biol Chem. 1998;273:28470–28477. doi: 10.1074/jbc.273.43.28470. [DOI] [PubMed] [Google Scholar]
- 29.Belevych A.E., Radwański P.B., Carnes C.A., Györke S. ‘Ryanopathy’: causes and manifestations of RyR2 dysfunction in heart failure. Cardiovasc Res. 2013;98:240–247. doi: 10.1093/cvr/cvt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cambi G.E., Lucchese G., Djeokeng M.M., Modesti A., Fiaschi T., Faggian G., Sani G., Modesti P.A. Impaired JAK2-induced activation of STAT3 in failing human myocytes. Mol Biosyst. 2012;8:2351–2359. doi: 10.1039/c2mb25120e. [DOI] [PubMed] [Google Scholar]
- 31.Kirabo A., Park S.O., Majumder A., Gali M., Reinhard M.K., Wamsley H.L., Zhao Z.J., Cogle C.R., Bisht K.S., Keserü G.M., Sayeski P.P. The Jak2 inhibitor, G6, alleviates Jak2-V617F-mediated myeloproliferative neoplasia by providing significant therapeutic efficacy to the bone marrow. Neoplasia. 2011;13:1058–1068. doi: 10.1593/neo.111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baskin R., Park S.O., Keserű G.M., Bisht K.S., Wamsley H.L., Sayeski P.P. The Jak2 small molecule inhibitor, G6, reduces the tumorigenic potential of T98G glioblastoma cells in vitro and in vivo. PLoS One. 2014;9:e105568. doi: 10.1371/journal.pone.0105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenthal A., Mesa R.A. Janus kinase inhibitors for the treatment of myeloproliferative neoplasms. Expert Opin Pharmacother. 2014;15:1265–1276. doi: 10.1517/14656566.2014.913024. [DOI] [PubMed] [Google Scholar]
- 34.Yang P., Zhang Y., Pang J., Zhang S., Yu Q., He L., Wagner K.U., Zhou Z., Wang C.Y. Loss of Jak2 impairs endothelial function by attenuating Raf-1/MEK1/Sp-1 signaling along with altered eNOS activities. Am J Pathol. 2013;183:617–625. doi: 10.1016/j.ajpath.2013.04.007. [DOI] [PubMed] [Google Scholar]