Abstract
Polymorphonuclear leukocytes (PMNs) are innate immune cells whose principal function is to migrate from the blood to sites of inflammation, where they exert crucial anti-infectious and immunomodulatory effects. However, dysregulated migration of PMNs into mucosal epithelial tissues is characteristic of chronic inflammatory disorders, including inflammatory bowel disease. Carbohydrate-mediated binding interactions between PMN Lewis glycans and endothelial glycan-binding proteins are critical for initial migration of PMN out of the vasculature. However, the role of Lewis glycans during transepithelial migration (TEM) has not been well characterized. Herein, we show that antibody blockade of Lewis X (Lex) displayed as terminal glycan residues on the PMN surface blocks chemotaxis and TEM while enhancing PMN-adhesive interactions with intestinal epithelia. Unexpectedly, targeting of subterminal Lex residues within glycan chains had no effect on PMN migration or adhesive interactions. There was increased surface expression of Lex on PMN after TEM, and blockade of terminal Lex regulated post-migratory PMN functions, increasing PMN phagocytosis and the surface mobilization of azurophilic (CD63, myeloperoxidase, and neutrophil elastase) and specific (CD66b and lactoferrin) granule markers. These findings suggest that terminal Lex represents a potential target for regulating PMN trafficking and function in inflamed mucosa. Furthermore, given its abundant expression on migrating PMN, Lex may be a rational target for modulating inflammation in diseases where dysregulated PMN influx is associated with host tissue damage.
Recruitment of polymorphonuclear neutrophils (PMNs) from the blood into the tissues is a critical part of the innate immune response triggered by infection or inflammation.1 Trafficking of PMN requires complex interactions between PMN-expressed ligands and tissue- and cell-specific receptors.2, 3, 4, 5, 6, 7, 8 It is well documented that some ligand-receptor recognition interactions during PMN extravasation are controlled by post-translational glycosylation modifications. Glycosylation modifies protein function, through both steric influences and the generation of specific lectin-binding glycan motifs.9, 10 For example, P-selectin glycoprotein ligand 1 is a heavily glycosylated PMN-expressed protein that regulates PMN rolling along the vascular endothelium during inflammatory responses in vivo.11 The glycans of P-selectin glycoprotein ligand 1 have been extensively studied, and several key glycan modifications (including α1,3 fucosylation, α2,3 sialylation, and β1,4 galactosylation) have been identified as being required for mediating PMN capture and rolling.12, 13, 14 Endothelial P- and E-selectin contain binding sites for PMN fucose-containing glycans, including sialyl Lewis X [sLex; Neu5Acα2-3Galβ1-4(Fucα1-3)GlcNAcβ-R] and the related glycan Lewis X [Lex; Galβ1-4(Fucα1-3)GlcNAcβ-R].9, 15, 16 Furthermore, E-selectin is also known to interact with specific glycans on the PMN glycoproteins leukosialin and CD44.17, 18
Although the role of glycosylation in mediating key steps in PMN transendothelial migration is well accepted, much less is known about the role of glycans once PMNs have exited the circulation and undergo the process of migration into epithelial-lined organs, such as the lungs or intestine. Interestingly, altered expression of epithelial glycans and glycoproteins during murine colitis19, 20 and in the inflamed mucosa of humans with active irritable bowel disease21, 22, 23, 24 has been reported. Furthermore, many of the proteins reported to be involved in regulating PMN transepithelial migration (TEM), including intercellular adhesion molecule 1, CD55, CD11b/CD18, and CD47, are extensively glycosylated.7, 8, 25 In addition, epithelial receptors for CD11b/CD18, although currently uncharacterized, include fucosylated glycoproteins.26
We recently reported that specific blockade of sialyl Lewis A [sLea; Neu5Acα2-3Galβ1-3(Fucα1-4)GlcNAcβ-R] residues present on the epithelial glycoprotein CD44v6 resulted in inhibition of PMN TEM by blocking detachment of migrating PMNs from the apical surface of inflamed intestinal epithelium.21, 22 It was also reported that targeting of related epithelial Lewis glycans, including sialyl Lewis C (sLec; Neu5Acα2-3Galβ1-3GlcNAcβ-R), had no effect on PMN TEM, thus highlighting remarkable selectivity and specificity of terminal glycans on PMN function.22
Despite the fact that binding interactions between PMN-expressed Lewis glycans and vascular endothelial lectins are a key step during PMN extravasation,16, 27, 28, 29 the role of Lewis glycans in regulating PMN TEM and PMN function in general is not well understood. It is known that PMNs abundantly express the Lewis glycan Lex. In addition, several PMN glycoprotein carriers for Lex, including CD11b/CD18 and carcinoembryonic antigen–related cell adhesion molecule 1,30 have been identified. However, the biological function of PMN-expressed Lex has yet to be characterized.
Herein, we demonstrate that specific engagement of Lex (when it is displayed terminally at the reducing ends of glycan chains) blocks PMN chemotaxis and TEM and increases PMN-adhesive interactions with epithelium. In addition to effects on PMN trafficking, targeting of terminal Lex also increased PMN phagocytosis and degranulation.
Materials and Methods
Antibodies and Reagents
Monoclonal antibodies (mAbs) against CD66b, CD63, Lex [H198 (IgM) and W6D3 (IgG)], as well as fluorescein isothiocyanate (FITC)–conjugated anti-Lex mAbs (W6D3 and H198), FITC-conjugated anti-CD66b mAb, FITC-conjugated anti-sLex mAb, FITC-conjugated anti-CD63 mAb, FITC-conjugated anti-CD11b mAb, and FITC-conjugated IgG and IgM isotype control mAbs were purchased from BD Biosciences (Franklin Lakes, NJ). Human TruStain FcX (Fc Receptor Blocking Solution) was purchased from Biolegend (San Diego, CA). The anti-Lex IgG mAb (F8A1.1) was isolated as described previously.31 Abs to Leb [Fucα1-2Galβ1-3(Fucα1-4)GlcNAcβ-R] and Ley [Fucα1-2Galβ1-4(Fucα1-3)GlcNAcβ-R], FITC-conjugated anti-lactoferrin mAb, anti-neutrophil elastase mAb, and anti-IgM and anti-IgG isotype control mAbs were purchased from Abcam (Cambridge, MA). The anti-CD11b mAb CBRM1/29 has been characterized elsewhere.32 BCECF, AM [2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester), 1 μm FITC-conjugated carboxylate FluoSpheres (505/5150), Zenon Alexa Fluor 488 Rabbit IgG staining kit, and To-Pro3-iodide were purchased from Invitrogen (Carlsbad, CA). N-formyl-l-methionyl-leucyl-l-phenylalanine (fMLF), polyhydroxyethylmethacrylate, and latrunculin B were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant human IL-8 was purchased from R&D Systems (Minneapolis, MN). Lactoferrin and myeloperoxidase (MPO) enzyme-linked immunosorbent assay kits were purchased from EMD Millipore (Darmstadt, Germany) and R&D Systems. Neutrophil elastase enzyme-linked immunosorbent assay kits and FITC-conjugated anti-MPO Ab were purchased from eBioscience (San Diego, CA).
Cell Culture and PMN Isolation
Cultures of T84 intestinal epithelial cells (IECs; passages 63 to 68) were grown as previously described.33 Human dermal microvascular endothelial cells (passages 4 to 9) were purchased from Promocell (Heidelberg, Germany) and grown according to the manufacturer's instructions. PMNs were isolated from whole blood obtained from normal human volunteers, with approval from the Emory University (Atlanta, GA) Institutional Review Board on human subjects, by using a previously described density gradient centrifugation technique.21 Briefly, PMNs were resuspended in Hanks' balanced salt solution with 10 mmol/L HEPES, pH 7.4, and without Ca2+ or Mg2+ at a concentration of 5 × 107 cells/mL. PMNs isolated in this way were 97% pure and >95% viable and were used for all assays within 2 hours of blood draw.
Analysis of mAb Specificity by Glycan Microarray Assay
The anti-Lex mAbs H198 and W6D3 were submitted to the Consortium for Functional Glycomics (http://www.functionalglycomics.org; last accessed June 6, 2014) at 25 μg/mL, and screened for glycoepitope binding using the version 5.1 glycan array. This glycan microarray contains 610 individual glycans printed on activated glass slides, representing a library of known natural and synthetic mammalian glycans, in replicates of six. After washing, binding of Abs to specific glycan epitopes was detected with Alexa-Fluor-488–labeled goat anti-mouse IgG (Life Technologies, Grand Island, NY), as described previously.22, 34 The relative fluorescent units of the bound Ab-glycan complexes were detected on a Perkin-Elmer (Houston, TX) ProScanArray 4 laser scanner and quantified using ImaGene software version 9.0 (Biodiscovery, El Segundo, CA).
Immunoblotting and Protein Purification
PMN cell lysates for immunoblotting were prepared with the following lysis buffer (20 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% TX-100, 1 mmol/L Na3VO4, and 1 mmol/L phenylmethylsulfonyl fluoride) supplemented with 10% mammalian tissue protease inhibitor cocktail (Sigma-Aldrich). PMN cell lysates were boiled in SDS-PAGE sample buffer under reducing conditions, and then subjected to SDS-PAGE, followed by transfer to polyvinylidene difluoride under standard conditions. Membranes were blocked with 4% milk powder or 3% bovine serum albumin and incubated with 1 μg/mL of indicated mAb. Primary Ab binding was detected using appropriate horseradish peroxidase–linked secondary Abs (Jackson Immunoresearch Laboratories, West Grove, PA). Data are representative of PMNs isolated from five independent donors. Functionally active human CD11b/CD18 was purified from PMNs by LM2/1 immunoaffinity chromatography, as described previously.32, 35
PMN Immunostaining
Isolated PMNs were fixed with 3.7% paraformaldehyde and, where indicated, permeabilized with 0.5% Triton-X (Sigma-Aldrich). After blocking with 3% bovine serum albumin (Sigma-Aldrich), PMNs were incubated with 10 μg/mL relevant Abs. After washing, PMNs were incubated with a FITC-labeled secondary Ab (Thermo Fisher Scientific, Grand Island, NY) and mounted in ProLong antifading embedding solution (Thermo Fisher Scientific). Nuclei were visualized by staining with TO-PRO-1 (Thermo Fisher Scientific). Images shown are representative of PMNs from at least three independent PMN donors, with multiple images captured per donor.
PMN Transmigration and Chemotaxis Assay
For transmigration experiments, T84 IECs were grown on collagen-coated, permeable, 0.33-cm2 polycarbonate filters (5-μm pore size; Costar Corp., Corning, NY), as described previously.21, 33, 36 All epithelial migration experiments were performed in the physiologically relevant basolateral-to-apical direction (ie, inverted monolayers), in the presence of a chemotactic gradient of 100 nmol/L fMLF. For migration experiments, 1 × 106 PMNs pretreated with 10 μg/mL indicated Abs were added to the upper chambers of Transwell inserts, and migration was measured at 37°C for 1 hour. Transmigrated PMNs were quantified by assaying for the PMN azurophilic marker MPO, as published previously.36 Briefly, Triton X-100 was added to the lower reservoir of the Transwell (final concentration, 0.5%), and the pH was adjusted to 4.2 with citrate buffer. For each sample, color development was assayed at 405 nm on a microtiter plate reader after mixing equal parts of sample and a solution containing 1 mmol/L 2,2'-azino-di-(3-ethyl)dithiazoline sulfonic acid and 10 mmol/L H2O2 in 100 nmol/L citrate buffer, pH 4.2. The assay was standardized with known dilutions of the same PMN used in each experiment and was linear in the range used (0.05 × 106 to 1 × 106 PMNs). The percentage migration represents the percentage of 1 × 106 PMNs added (for each sample) to the upper portion of the Transwell system that have migrated into the bottom chamber of the Transwell. For PMN chemotaxis assays, PMNs were incubated with 10 μg/mL indicated Abs before migration across collagen-coated, permeable, 0.33-cm2 polycarbonate filters to 100 nm fMLF or 100 nmol/L IL-8, which was assessed by measurement of MPO, as described above. Migration of PMNs across human dermal microvascular endothelial cell monolayers was performed in the apical-to-basolateral direction with a 10 nmol/L fMLF gradient, as described previously.21, 37 For migration experiments, 1 × 106 PMNs were added to the upper chambers of Transwell inserts and migration levels were assessed at 37°C after 1 hour. Transmigrated PMNs were quantified by colorimetric enzyme activity assay specific for the PMN azurophilic marker MPO, as described above, for TEM and chemotaxis assays.
PMN Adhesion Assays
PMN adhesion to confluent T84 IECs was measured directly using modifications of previous protocols.25, 38 Briefly, T84 IECs were washed free of media before BCECF-labeled PMNs (5 × 105), preincubated with 10 μg/mL anti-Lex mAbs (H198 or W6D3), 10 μg/mL isotype-matched control mAbs (IgM and IgG), or 10 μg/mL functionally inhibitory anti-CD11b/CD18 mAbs (CBRM1/29), as a positive control for inhibition of PMN-epithelial adhesion, were added. Epithelial monolayers were then centrifuged at 50 × g for 5 minutes, to uniformly settle PMNs, before adhesion in the presence of 10 nmol/L fMLF was allowed to proceed for 1 hour at 37°C. Next, IEC monolayers were gently washed with Hanks buffer, and fluorescence intensity (excitation, 485 nm; emission, 530 nm) was measured on a fluorescent plate reader. Adherent PMN numbers were determined from standard curves generated by serial dilution of known numbers of BCECF-AM–labeled PMNs. Data are means ± SEM from five healthy PMN donors.
Flow Cytometry and Phagocytosis Assay
For flow cytometry analyses, non-stimulated PMNs or PMNs stimulated with 10 nmol/L fMLF were blocked in 3% bovine serum albumin with Human TruStain FcX (Fc Receptor Blocking Solution; Biolegend) before incubation with 10 μg/mL of FITC-conjugated anti-Lex mAbs (W6D3 or H198), 10 μg/mL FITC-conjugated IgG or IGM isotype-matched control mAb, or 10 μg/mL FITC-conjugated anti-CD11b mAbs. After primary Ab incubation, PMNs were washed and fixed in 3.7% paraformaldehyde, and analyzed by flow cytometry. For analysis of Lex expression after PMN TEM, nonmigrated PMNs and PMNs that had migrated across T84 IEC monolayers into wells coated with polyhydroxyethylmethacrylate were collected before blocking and incubation with FITC-conjugated Abs against Lex or sLex, as described above. Flow cytometric analysis was performed using a FACScan (Becton Dickinson, Franklin Lakes, NJ) equipped with an argon ion laser tuned at a 488-nm wavelength. For phagocytosis assays, PMNs were incubated with 10 μg/mL anti-Lex mAbs (W6D3 or H198) or 10 μg/mL IgG or IgM isotype control mAbs and FITC-conjugated FluoSpheres at a ratio of 1:100 (PMN/FluoSpheres; Thermo Fisher Scientific) in the presence of 10 nmol/L fMLF for 30 minutes at 37°C. Uptake of FluoSpheres by PMNs was assessed by measurement of fluorescence by flow cytometry. For immunofluorescence analyses, PMNs were mounted in Pro-Long anti-fade embedding solution (Thermo Fisher Scientific), and phagocytosis was assessed by confocal and phase microscopy at a magnification of ×100. Images are representative of PMNs isolated from three to five independent donors, with multiple images captured for each donor.
PMN Degranulation Assay
PMNs were isolated, as described above, and incubated with 10 μg/mL anti-Lex mAbs (W6D3 or H918) or 10 μg/mL matched isotype control (IgG or IgM) mAbs for 30 minutes at 37°C. As a positive control for degranulation, PMNs were pretreated with 1.25 μmol/L latrunculin B for 5 minutes, followed by stimulation with 5 μmol/L fMLF for 10 minutes. After indicated incubations, PMNs were washed with PBS-EDTA (Lonza, Basel, Switzerland) and blocked in Human TruStain FcX (Fc Receptor Blocking Solution) before incubation with FITC-conjugated mAbs against CD63, CD66b, MPO, and lactoferrin. Anti–neutrophil elastase mAbs were labeled with Zenon Alexa Fluor 488 (according to the manufacturer's instructions; Thermo Fisher Scientific) before incubation with PMNs, as described for FITC-conjugated mAbs. After primary mAb incubation, PMNs were fixed overnight using BD LyseFix PhosFlow (BD Bioscience, San Jose, CA) before data acquisition using a BD LSR II Flow Cytometer (BD Bioscience). Data are representative of PMNs isolated from three to five independent donors. For detection of secreted proteins, PMNs were stimulated, as described above, and cell-free supernatants were measured for released lactoferrin, elastase, and MPO by enzyme-linked immunosorbent assay.
EM Analysis
For EM analyses, PMNs were incubated with anti-Lex mAbs (W6D3 or H198) or isotype control mAbs, as described above, before PMNs in suspension were fixed with 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.4) overnight at 4°C. Next, cells were post-fixed with 1% osmium tetroxide and dehydrated in an ethanol series up to 100%, infiltrated with epoxy resin before embedding in pure resin. Ultrathin sections were cut into sections (70 nm thin) on a Leica Ultracut S ultramicrotome (Buffalo Grove, IL), and cells were counterstained with 5% aqueous uranyl acetate, followed by 2% lead citrate. Ultrathin sections were examined on a JEOL JEM-1400 transmission electron microscope (Tokyo, Japan) equipped with a Gatan US1000 charge-coupled device camera (Pleasanton CA).
Data Analysis
Statistical differences were determined by two-factor analysis of variance using PRISM 5 for Mac OSX version 5.0a 1992-1998 (GraphPad Software, Inc., La Jolla, CA). Values are expressed as the means and SEM from at least three separate experiments.
Results
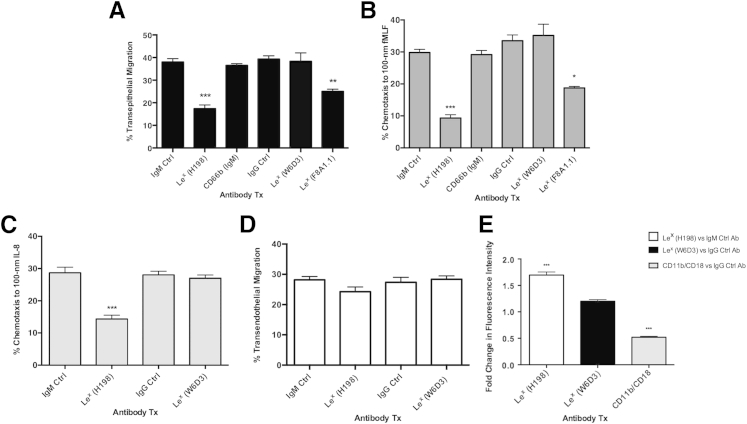
Previous reports have identified a role for PMN glycans (including Lex and sLex) interacting with E- and P-selectins during PMN transendothelial migration.27, 28, 29, 39, 40 However, the role of Lex during TEM has not been studied. When PMNs were treated with the anti-Lex mAb H198, TEM across T84 IECs to a gradient of the bacterial peptide fMLF was significantly reduced relative to PMNs treated with an isotype-matched control Ab or treated with a second isotype-matched binding control anti-CD66b mAb (P < 0.001) (Figure 1A). Similarly, PMN chemotaxis across collagen to fMLF (P < 0.001) (Figure 1B) or IL-8 (P < 0.001) (Figure 1C) was also inhibited by H198 targeting of Lex relative to treatment of PMNs with an isotype-matched or binding IgM control Ab. In contrast to results with H198, targeting of Lex with a second mAb to Lex, W6D3, did not have any significant effect on PMN TEM or PMN chemotaxis (Figure 1, A–C). However, a third anti-Lex mAb (IgG-F8A1.1) significantly inhibited both PMN TEM (P < 0.01) (Figure 1A) and chemotaxis to fMLF (P < 0.05) (Figure 1B) relative to treatment of PMNs with an isotype-matched control Ab and relative to treatment of PMNs with W6D3 (noninhibitory, anti-Lex IgG mAb). To determine whether the inhibition of PMN TEM downstream of targeting of Lex had an epithelial-specific component, the effect of Lex engagement on PMN transmigration across endothelial cells was next assessed. In contrast to effects on PMN TEM and chemotaxis, targeting of Lex with H198 had no effect on PMN transendothelial migration (Figure 1D). In addition, targeting of PMN Lex by H198, but not W6D3, significantly increased PMN adhesion to intestinal epithelial cells relative to isotype-matched binding controls (anti-IgG and anti-IgM isotype controls) (Figure 1E).
Figure 1.
Targeting of Lewis X (Lex) blocks polymorphonuclear leukocyte (PMN) transepithelial migration and increases PMN adhesive interactions. A: Epithelial cells were cultured to confluency on porous polycarbonate filters (Transwell). Human PMNs incubated with 10 μg/mL anti-Lex monoclonal antibodies (mAbs; H198, W6D3, and F8A1.1), isotype-matched control (Ctrl) mAbs (anti-IgG and anti-IgM), or anti-CD66b mAb as an anti-IgM binding control, were placed in the upper chamber of Transwell filters and induced to migrate into the 100-nm N-formyl-l-methionyl-leucyl-l-phenylalanine (fMLF)–containing lower Transwell chamber in the physiologically relevant basolateral-to-apical direction. Numbers of migrated PMNs were quantified by myeloperoxidase (MPO) assay, as described in Materials and Methods. Human PMNs incubated with 10 μg/mL anti-Lex mAbs (H198, W6D3, and F8A1.1) or isotype control mAbs (anti-IgG and anti-IgM) were added to the top of collagen-coated Transwell filters and induced to migrate into a lower chamber containing 100-nm fMLF (B) or 100-nm IL-8 (C). PMN chemotaxis was quantified by MPO assay (as described in Materials and Methods). D: Human PMNs incubated with 10 μg/mL anti-Lex mAbs (H198 or W6D3) or isotype control mAbs (anti-IgG and anti-IgM) were added to confluent human dermal microvascular endothelial cell (HDMEC) monolayers. PMNs were allowed to migrate for 1 hour in response to a 10 nmol/L gradient of fMLF. The number of migrated PMNs was quantified by MPO assay (as described in Materials and Methods). E: BCECF [2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester]–labeled PMNs were pretreated with 20 μg/mL H198, W6D3, CBRM1/29 (as a positive control for inhibition of PMN adhesion to T84 IECs), or isotype-matched control mAbs (anti-IgG or anti-IgM) before addition to confluent T84 intestinal epithelial cell monolayers. After 2 hours, nonadherent PMNs were removed by gentle washing, and adherent PMNs were lysed by addition of Triton-X. Adherence was measured by quantifying fluorescence at 485 nm. Data shown are fold change in fluorescence intensity for treatment (Tx) with anti-Lex mAbs (H198 or W6D3) or anti-CD11b/CD18 mAb versus isotype-matched control mAbs. Data depict means ± SEM (A–C and E). N = 5 independent donors (A); N = 3 independent donors (B–E). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Abs to Lex Recognize Different Presentations of Lex Glycan Structures
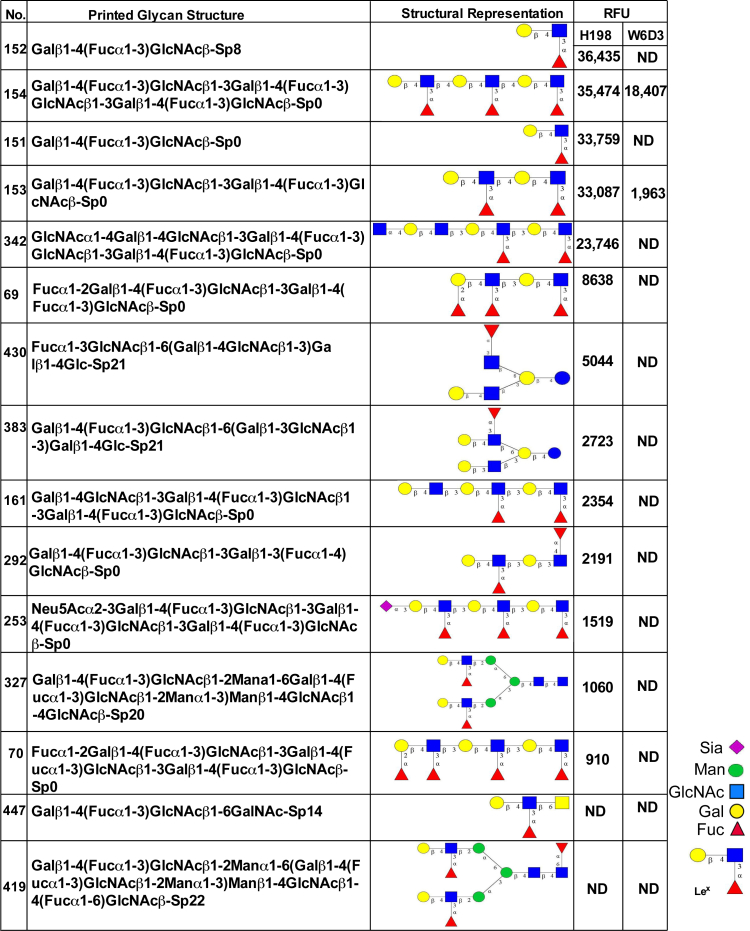
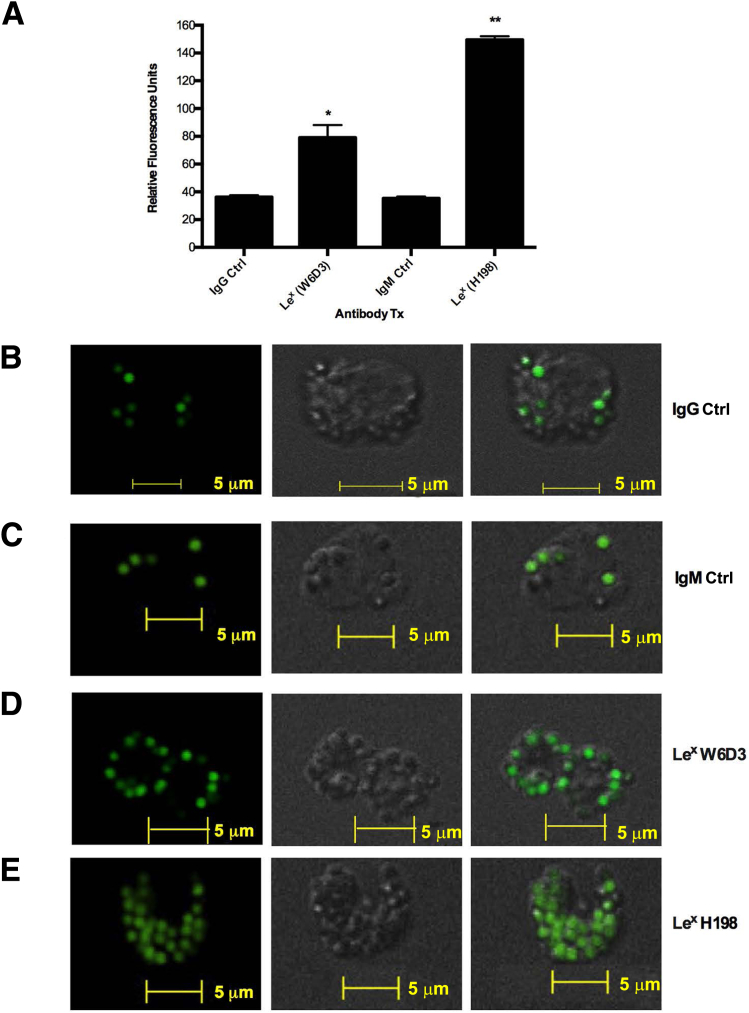
Given the surprising finding that different Abs to the glycan epitope Lex had discrete effects on PMN migratory and adhesive functions, we next decided to confirm specific Ab recognition of Lex, compare relative Ab glycan-binding specificities, and identify specific Lex presentations recognized by the anti-Lex mAbs used in this study. W6D3 (IgG) and H198 (IgM) were assayed for binding to a panel of 610 mammalian glycan structures on the glycan microarray of the Consortium for Functional Glycomics (Figure 2). Glycan-binding affinities were then compared with the known glycan-binding affinities for F8A1.1 (a second anti-Lex IgG), which have been recently published (Supplemental Figure S1).31 H198 bound to glycans expressing Galβ1-4(Fucα1-3)GlcNAc (Lex), in which the Lex structure is expressed in a terminal nonreducing position (glycans 152, 154, 151, and 153) (Figure 2). It was also determined that H198 recognized glycans containing only the simple trisaccharide Lex structure (glycans 152 and 151) (Figure 2) and glycans containing the terminal Lex determinant in repeating poly-Lex structures (glycans 154 and 153). In addition, H198 recognized glycans containing only the internal Lex-like sequence (glycans 161 and 342) (Figure 2 and Supplemental Figure S1).
Figure 2.
H198, but not W6D3, recognizes terminal Lewis X (Lex) epitopes. Monoclonal antibody (mAb) H198 (25 μg/mL) and mAb W6D3 (25 μg/mL) were incubated with an array of 610 glycan structures (Consortium for Functional Glycomics version 5.1 glycan microarray) and detected with Alexa Fluor-488–labeled anti-mouse IgG. ND, not detectable (<500 RFUs); RFU, relative fluorescence unit; Sp, spacer.
Consistent with these findings, anti-Lex mAb F8A1.1 has been shown to bind strongly to glycans containing the Lex determinant in a terminal nonreducing position (glycans 151 to 154) (Figure 2).33 However, unlike H198, F8A1.1 showed no binding to glycans containing only the internal Lex sequence (glycans 161 and 342). In contrast, W6D3 recognized internal Lex motifs on glycan chains composed of multiple repeating Lex determinants (glycans 153 and 154) (Figure 2), but failed to recognize Lex in terminal nonreducing positions.
Interestingly, H198, but not F8A1.1 or W6D3, was able to bind glycans with a terminal Lex linked to internal Lea (glycan 292) (Figure 2), suggesting that the Galβ1-3 linkage blocks the binding of both W6D3 and F8A1.1, but not H198. However, none of the Abs tested bound to the Lex trisaccharide on a core-2 O-glycan (glycan 447) (Figure 2), suggesting that β6 linkage of Lex to GalNAc is not optimal for Ab recognition of the Lex motif. In addition, there was no Ab recognition of Lex in a terminal position when it was β2 linked to mannose, as in N-glycans (glycan 419) (Figure 2). Taken together, these results highlight the restricted and differential recognition of discrete Lex glycan structures by specific anti-Lex Abs. More important, there was no recognition (by any of the anti-Lex Abs) of sialylated Lewis glycans, such as sLex and nonsialylated Lewis glycans in which galactose is β1,3 linked to GlcNAc [Lewis A, Lea, Galβ1-3(Fucα1-4)GlcNAc]. These data demonstrate that there are remarkable differences in the binding specificities between anti-Lex mAbs H198, W6D3, and F8A1.1 (Supplemental Figure S1) that correspond to differential functional effects on PMN trafficking. More important, only mAbs that are capable of recognizing and binding to the Lex determinant when it is presented in terminal nonreducing positions are capable of blocking PMN TEM and chemotaxis (Figure 1).
PMNs Express Terminal and Internal Lex But Not Leb or Ley
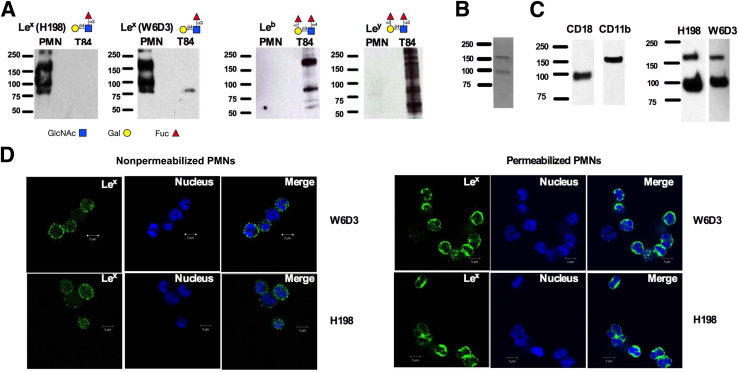
Having demonstrated the differing binding specificities of Lex mAbs for terminal and nonterminal Lex glycans, we next assessed the expression of these different Lex presentations in human PMNs. Lex was detected by immunoblot with both H198 and W6D3 (Figure 3A), suggesting expression of both terminal and internal Lex by human PMNs. Lex was also detected in human PMNs using F8A1.1 (Supplemental Figure S2A). In addition, parallel immunoblotting analyses failed to demonstrate expression of the related glycans Lewis b (Leb) or Lewis y (Ley) in human PMNs (Figure 3A). Analyses of T84 intestinal epithelial cells, on the other hand, demonstrated expression of both Leb and Ley. Epithelial Lex expression was not detected using H198 (Figure 3A) or F8A1.1 (Supplemental Figure S2). However, a faint protein band was detected in T84 protein lysates by W6D3, again highlighting differing binding specificities of these three anti-Lex mAbs (Figure 3A and Supplemental Figure S1).
Figure 3.
Polymorphonuclear leukocytes (PMNs) express Lewis X (Lex) but not Lewis B (Leb) or Lewis Y (Ley). A: Protein lysates were prepared from human PMNs or T84 intestinal epithelial cells and immunoblotted with monoclonal antibodies (mAbs) against Lex (W6D3 and H198), Leb, or Ley. B: CD11b/CD18 purified from human PMNs was run on a gel and stained with Coomassie Blue. C: CD11b/CD18, purified from human PMNs, was immunoblotted with mAbs against CD11b, CD18, or Lex (H198 or W6D3). D: Nonpermeabilized and permeabilized PMNs were stained with 10 μg/mL H198 or 10 μg/mL W6D3 (anti-Lex Abs). Nuclear staining with To-Pro is also shown. Glycan localization was determined by confocal microscopy analysis. N = 3 immunoblots from three independent blood donors (A); N = 3 blood donors in the en face plane of section (D). Scale bar = 5 μm (D). Original magnification, ×100 (D).
It has previously been reported that CD11b/CD18 on human PMNs is decorated with Lex.41 Analysis of CD11b/CD18 (purified from human PMNs by LM2/1 immunoaffinity) by gel electrophoresis revealed two protein bands consistent in size with the CD18 and CD11b subunits of human CD11b/CD18 (Figure 3B). Furthermore, immunoblotting of purified CD11b/CD18 with anti-Lex mAbs (H198 and W6D3) confirmed expression of terminal and subterminal Lex on both the CD11b and CD18 subunits (Figure 3C). Expression of nonterminal and terminal presentations of Lex by PMNs was confirmed by immunofluorescence staining, demonstrating surface and intracellular expression of PMN Lex by W6D3 and H198 (Figure 3D).
Expression of Lex on PMNs Increases after PMN TEM
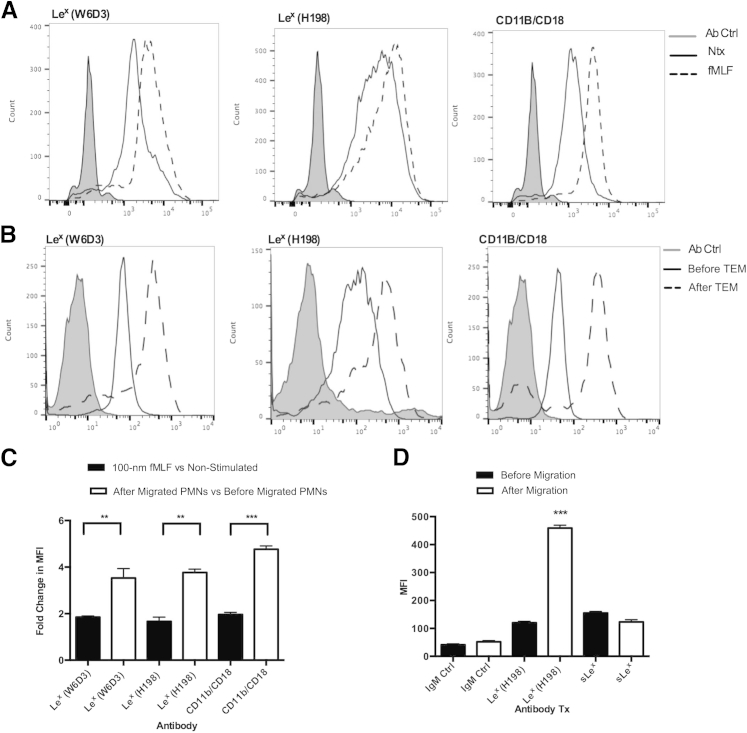
During PMN TEM, activation and mobilization of internal pools of receptors and granules occur. We therefore examined changes in the surface expression of PMN Lex after activation and after PMN TEM. As a positive control for PMN activation, changes in the surface expression of CD11b/CD18 (Mac-1) were also determined. An increase in the expression of Lex (detected by both W6D3 and H198) was observed in PMNs after stimulation with the bacterial formyl peptide fMLF (Figure 4A). In addition, analysis of expression of Lex on PMNs, both before and after TEM across T84 IECs, revealed a significant approximately threefold increase in Lex expression in the post-migrated PMNs (Figure 4, B and C), suggesting that there is increased expression of both terminal and nonterminal Lex glycans on PMNs during mucosal inflammation. In addition, the increase in surface expression of PMN Lex was significantly greater after PMN TEM relative to the increase seen after stimulation of PMNs with fMLF (P < 0.01) (Figure 4C).
Figure 4.
Polymorphonuclear leukocyte (PMN) expression of Lewis X (Lex) increases after transepithelial migration (TEM). A: Changes in surface expression of Lex on PMNs were analyzed by flow cytometry in non-stimulated (Ntx) PMNs and PMNs exposed to 100-nm N-formyl-l-methionyl-leucyl-l-phenylalanine (fMLF) using fluorescein isothiocyanate (FITC)–conjugated anti-Lex monoclonal antibodies (mAbs; H198 and W6D3). The extent of PMN activation was assessed by changes in surface expression of CD11b/CD18. B: Levels of surface expression of Lex and CD11b/CD18 were assessed before and after PMN TEM by flow cytometry using FITC-conjugated anti-Lex mAbs (H198 and W6D3) and a FITC-conjugated anti-CD11b mAb. C: Quantification of changes in surface expression of Lex (detected with FITC-conjugated H198 or W6D3) after PMN stimulation with fMLF or after PMN TEM. Black bars represent changes in Lex surface expression in fMLF-stimulated PMNs relative to non-stimulated PMNs. White bars represent changes in Lex expression in PMNs after relative to before migration. D: Levels of surface expression of Lex and sLex were assessed before and after PMN TEM by flow cytometry using a FITC-conjugated anti-Lex mAb (H198) and a FITC-conjugated anti-sLex mAb. N = 5 independent blood donors for PMNs. ∗∗P < 0.01, ∗∗∗P < 0.001. Ctrl, control; MFI, mean fluorescence intensity.
It has been reported that PMN activation results in the release of glycan-modifying enzymes, including sialidases that can convert sLex into Lex.42 Thus, we examined the levels of PMN surface expression of sLex before and after TEM. As can be seen in Figure 4D, there were no significant changes in surface sLex expression after TEM, suggesting that increases in PMN Lex expression were not because of conversion of sLex to Lex. In addition, there was no decrease in fMLF-stimulated PMN Lex surface expression in the presence of 100 nmol/L sialidase inhibitor zanamirvir (data not shown). Confirmation of specific recognition of sLex (and lack of cross recognition of Lex) for the anti-sLex Ab (CD15s) was performed using the Consortium for Functional Glycomics version 5.1 glycan microarray (Supplemental Figure S2B).
Engagement of Lex Increases PMN Phagocytosis
Given the increase in the expression of Lex on PMNs after migration across model intestinal epithelial monolayers, the potential role of Lex in regulating other important PMN functions was next assessed. Flow cytometric and immunofluorescence analyses demonstrated that engagement of PMN Lex by H198 increased the uptake of fluorescent microspheres by PMNs relative to incubation of PMNs with an isotype-matched control Ab (P < 0.01) (Figure 5, A, C, and E). Targeting of subterminal internal Lex determinants by W6D3 also increased the uptake of fluorescent microspheres relative to an isotype-matched control Ab (P < 0.05) (Figure 5, A, B, and D). Interestingly, there was a significant increase in the phagocytosis induced by targeting of both terminal and nonterminal Lex with H198 compared with just targeting the nonterminal Lex with W6D3 (P < 0.05) (Figure 5A), even when the IgG mAb was used at five times the concentration of H198 (data not shown), once again highlighting the importance of terminal Lex glycan determinants in modulating PMN function.
Figure 5.
Engagement of polymorphonuclear leukocyte (PMN) Lewis X (Lex) increases PMN phagocytosis. PMNs were incubated with 10 μg/mL anti-Lex monoclonal antibodies (mAbs; H198 and W6D3) or isotype-matched binding control (Ctrl) mAbs in the presence of fluorescein isothiocyanate (FITC)–conjugated 1-μm FluoSpheres, at a ratio of 1:100, PMN/FluoSpheres. A: Fluorescent microsphere phagocytosis/uptake was quantified by measuring changes in cell fluorescence by flow cytometry. PMN phagocytosis of FluoSpheres after incubation with an IgG isotype-matched control mAb (B), an IgM isotype-matched mAb (C), or anti-Lex mAbs (W6D3 and H198; D and E, respectively) was confirmed by confocal microscopy analysis. PMNs are shown in bright field with 1-μm FluoSpheres in green. Data depict means ± SEM (A). N = 3 (A). ∗P < 0.05, ∗∗P < 0.01. Original magnification, ×100 (B–E).
Engagement of Lex Increases PMN Granule Marker Surface Expression But Does Not Trigger Complete Degranulation
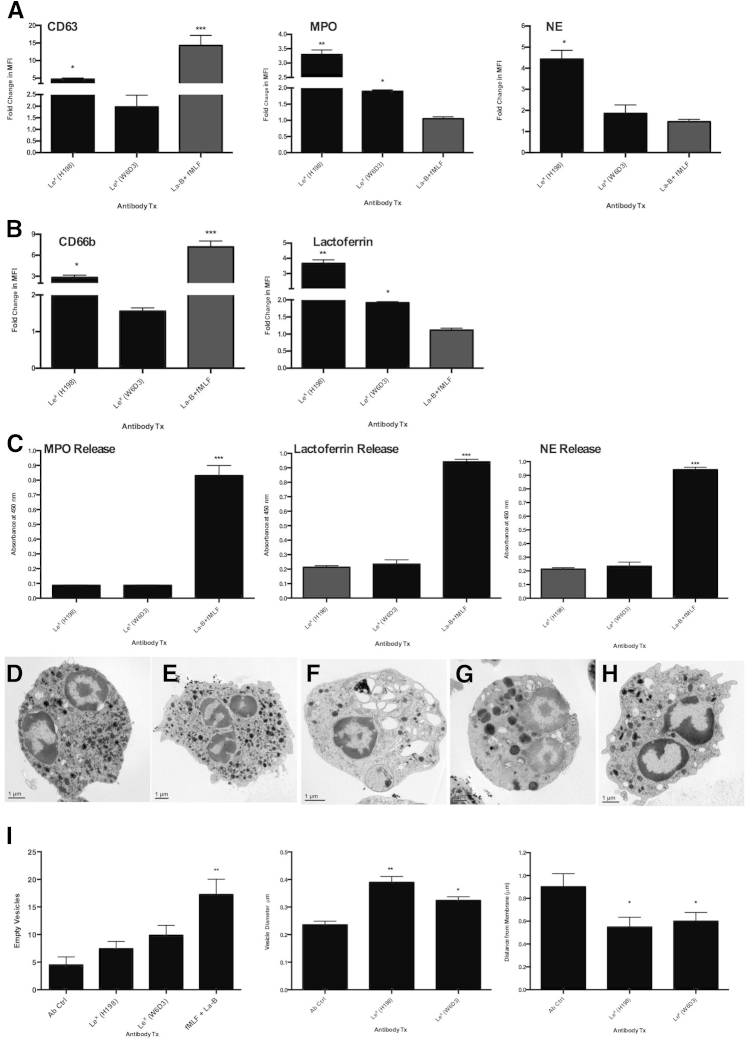
To determine effects of Lex engagement on PMN degranulation, PMNs were incubated with H198 or W6D3 (or isotype-matched binding control Abs) before examination of changes in surface expression of CD66b and lactoferrin, or CD63, NE, and MPO (as markers of specific and azurophilic granules, respectively). As a positive control, PMNs were treated with the actin-depolymerizing agent latrunculin B, followed by stimulation with the bacterially derived peptide fMLF before measurement of changes in expression of degranulation markers (Figure 6, A and B). An increase in surface CD63, NE, and MPO expression was seen after targeting of Lex by both H198 and W6D3 relative to isotype-matched Ab controls (Figure 6A). In addition, targeting of Lex with H198 or W6D3 increased PMN surface expression of CD66b and lactoferrin (Figure 6B), suggesting movement of specific granule markers to the cell surface downstream of engagement of PMN Lex. More important, the increase in surface expression of specific and azurophilic granule contents was greater after targeting of terminal and nonterminal Lex with H198 compared with the targeting of only internal Lex glycans with W6D3 (P < 0.01 and P < 0.05) (Figure 6, A and B).
Figure 6.
Engagement of polymorphonuclear leukocyte (PMN) Lewis X (Lex) increases surface expression of granule markers. A and B: PMNs were treated with 10 μg/mL anti-Lex monoclonal antibodies (mAbs; H198 or W6D3) or isotype-matched control mAbs for 30 minutes at 37°C before assessment by flow cytometry of surface expression levels of CD66b, CD63, NE, myeloperoxidase (MPO), and lactoferrin using fluorescein isothiocyanate (FITC)–conjugated anti-CD66b, anti-CD63, anti–neutrophil elastase, anti-MPO, and anti-lactoferrin mAbs. Data shown are fold-change in mean fluorescence intensity (MFI) comparing treatment (Tx) with anti-Lex mAbs with treatment with isotype-matched control mAbs. C: PMNs were pretreated with 10 μg/mL H198, W6D3, or isotype control Abs before supernatants were removed and examined for levels of MPO, lactoferrin, or NE by enzyme-linked immunosorbent assay. As a positive control for degranulation, PMNs were treated with 1.25 μmol/L latrunculin B (La-B) + 5 μmol/L N-formyl-l-methionyl-leucyl-l-phenylalanine (fMLF). PMNs were incubated with 10 μg/mL IgG ctrl Ab (D), IgM ctrl Ab (E), 1.25 μmol/L La-B + 5 μmol/L fMLF (F), 10 μg/mL anti-Lex mAb (H198; G), or 10 μg/mL anti-Lex (W6D3; H) before morphological analysis of degranulation was performed by EM. I: The number of empty vesicles, vesicle diameter, and distance from the center of vesicles to the cell membrane were calculated. Data depict means ± SEM (I). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
However, despite the observed increases in surface expression of specific and azurophilic granule markers, targeting of PMN Lex with H198 or W6D3 did not result in any appreciable release of MPO, NE, or lactoferrin (Figure 6C). These data suggest that the increased detection of granule markers at the PMN cell membrane, triggered by targeting of specific Lex glycans, does not directly correlate with complete degranulation and release of granule contents into the surrounding media. In support of these data, examination of changes in PMN granule distribution by electron microscopy demonstrated that there was not full degranulation and release of granule contents after engagement of both terminal and subterminal Lex by H198 (Figure 6G). However, there did appear to be an increase in granule diameter, suggesting granule fusion downstream of Lex engagement by H198 and movement of granules toward the cell membrane (Figure 6I). Similarly, EM analyses revealed that targeting of PMN Lex by W6D3 did not trigger release of granule contents (Figure 6H). In addition, targeting of subterminal Lex also resulted in some redistribution of granules away from the center of the cell and toward the cell membrane (Figure 6I). However, there was less granule redistribution than was observed downstream of engagement of terminal and subterminal Lex by H198 (Figure 6, G and I). As expected, treatment of PMNs with latrunculin B and fMLF resulted in robust release of granule contents (P < 0.001) (Figure 6, F and I) compared to nonactivated PMN (Figure 6, D and E). These data indicate that targeting of PMN-expressed Lex increases mobilization of PMN granules toward the cell surface but does not result in complete degranulation and release of specific and azurophilic granule contents into the extracellular space.
Discussion
Glycans and PMN Trafficking
Although PMN migration into the tissues is essential to host immune defense against invading microbes, dysregulated PMN trafficking has been implicated in the pathology of many chronic inflammatory conditions, including rheumatoid arthritis, myocardial reperfusion injury, chronic obstructive pulmonary disease, and irritable bowel disease.43 PMN recruitment to sites of mucosal inflammation is governed by complex and sequential interactions between adhesion molecules and their counterligands. It has been well documented that PMN capture from flowing blood is dependent on interactions between endothelial C-type lectins and PMN-expressed carbohydrates, including sLex.15, 16 However, little is known about the role of PMN-expressed glycans in PMN function after diapedesis.
Targeting of PMN Lex Blocks TEM
Herein, we have shown that Ab-mediated ligation of PMN Lex blocks PMN chemotaxis (to IL-8 and fMLF) and PMN TEM. Interestingly, targeting of PMN Lex with another anti-Lex mAb (W6D3) had no effect on PMN chemotaxis or TEM. To exclude the possibility that observed functional effects were because of differences in Ab isotype (IgM versus IgG), the effects of a second bonafide anti-Lex mAb IgG (F8A1.1) on PMN migration were studied. Similar to what was observed for H198, F8A1.1 targeting of Lex reduced PMN chemotaxis and TEM, suggesting that Ab-mediated effects on PMN trafficking are active effects that are downstream of specific engagement of Lex by select anti-Lex mAbs. In addition, these data demonstrate that not all mAbs to the Lex epitope have functional effects on PMN trafficking.
In the 1970s, several mAbs that recognized Lex were discovered and designated anti-CD15 by the international leukocyte typing workshop.44 Although classified by a single CD number, CD15 Abs are known to show some heterogeneity in their biological function.45 Specifically, Ab engagement of PMN Lex has been reported to induce CD11b/CD18-dependent homotypic PMN aggregation.45, 46 Such Ab-mediated aggregation was found to be an active process that was selective in terms of anti-Lex mAb specificity, required divalent cations, and was both temperature and energy dependent.45 In support of the role of Lex in PMN-adhesive interactions, it has been previously demonstrated that desialyation of PMNs and the resultant exposure of surface Lex epitopes result in an enhanced PMN capacity for aggregation and adherence to substrata.47 Similarly, in the current study, we demonstrate that engagement of terminally expressed PMN Lex by H198, but not W6D3, enhanced adhesive interactions between PMNs and human IECs. Taken together, these data demonstrate that discrete differences in glycan-binding specificity account for the divergence in functional effects of anti-Lex mAbs on PMN adhesion and migration.
Glycan Specificity of Anti-Lex mAbs
Recent advances in glycan microarray technology have resulted in an explosion of new insights into the specificity and affinity of the binding interactions between glycan-binding proteins (including Abs) and their glycan ligands. Therefore, using this newly expanded glycan array technology from the Consortium for Functional Glycomics (containing >600 mammalian glycan structures), we analyzed the exquisite glycan-binding specificities of two anti-Lex mAbs (H198 and W6D3) and compared results with the known glycan-binding affinities of the anti-Lex mAb F8A1.1.31 Similar to F8A1.1, we observed that H198, but not W6D3, binds Lex that is displayed in a terminal nonreducing position. Interestingly, all mAbs tested recognize Lex on glycans containing multiple repeating Lex structures. Because W6D3 failed to recognize terminally expressed Lex, these findings demonstrate that Lex-mediated effects on PMN function are highly dependent on how Lex is displayed in the overall glycan backbone. Because mAb H198, but not F8A1.1 or W6D3, recognized Lex connected to an internal Lea, it is likely that a β1-3 linkage to Gal prohibits binding of F8A1.1 and W6D3, but not H198. The wider recognition of presentations of Lex-containing glycans by H198 could explain the increased inhibitory effect of H198 on chemotaxis and TEM compared with lesser inhibition observed with F8A1.1. Interestingly, there was no Ab recognition of Lex displayed in a terminal position when the linkage to mannose was in a β2 conformation (glycan 419) (Figure 2). Taken together, these results highlight the restricted and differential recognition of discrete Lex glycan structures by specific anti-Lex Abs. More important, no anti-Lex Ab showed any significant binding to sialylated Lewis glycans, such as sLex, or to related Lewis glycans, including Lea. These data demonstrate that there are remarkable differences in the binding specificities between different so-called anti-Lex Abs. Furthermore, these data also indicate that not all Lex structures are equally accessible to different ligands nor are equally effective in transmitting stimulatory signals that can regulate PMN function.
Expression of Terminal and Subterminal Lex Glycans by PMNs
Given the different functional effects observed after ligation of terminal and subterminal Lex glycans, we next examined expression of Lex-containing glycoproteins in human PMNs using mAbs H198 and W6D3. Despite recognizing disparate presentations of Lex, Western blots of PMN lysates with both Abs produced similar protein banding profiles. These findings indicate that PMN glycoproteins decorated with Lex contain mixed-type glycans, where some have terminal Lex with or without subterminal Lex glycans. Furthermore, the relative molecular masses of proteins labeled by H198 and W6D3 (180, 100, and 80 kDa) are consistent with those previously observed in Western blots of PMNs using anti-CD15/Lex Abs.39
It has been previously reported that PMN adhesion molecules, including CD11b/CD18 (as well as carcinoembryonic antigen–related cell adhesion molecule 1), express the CD15 antigen.41 However, the specific presentations of Lex displayed by these PMN proteins have not been characterized. Herein, we confirm that CD11b/CD18, isolated from human PMNs, contains Lex, and we further demonstrate that both the CD18 and CD11b subunits of human Mac-1 are extensively decorated with Lex in terminal and subterminal presentations. Because PMN activation and migration are associated with increased surface expression of adhesion molecules (including CD11b/CD18), we examined the expression of terminal and subterminal Lex after stimulation with fMLF and after TEM. We observed an increase in the surface expression of Lex detected by H198 and W6D3 under both conditions, with the greatest increase in surface expression of Lex observed after TEM. To determine whether the increase in surface expression was because of the up-regulation of internal pools of glycosylated proteins, rather than activation of endogenous sialidases that convert sLex to Lex,48 we performed additional flow cytometric analyses. We determined that there was no significant loss of surface sLex expression after PMN stimulation with fMLF or following TEM. In addition, increases in Lex expression were seen in the presence of the sialidase inhibitor zanamivir, suggesting that increases in the surface expression of Lex are most likely because of translocation of glycoproteins from intracellular stores to the cell surface and not secondary to sialidase-mediated conversion of sLex into Lex.
Role of PMN Lex in Phagocytosis and Degranulation
The host inflammatory response to infection requires that PMNs migrate into the tissues where they can engulf and destroy invading microorganisms. Therefore, having demonstrated increased expression of Lex on the surface of PMNs that have migrated across an epithelial barrier, we next investigated if this Lex could be targeted to alter PMN phagocytosis of 1-μm fluorescent microspheres. Selective targeting of internal Lex with W6D3 resulted in significantly increased uptake of microspheres; however, the stimulatory effect was more pronounced with targeting of terminal and subterminal Lex by mAb H198. Such findings add more support to the importance of how Lex is displayed with respect to modulation of PMN function. In support of a role for Lex in regulating PMN phagocytosis, it has been recently reported that PMNs from patients with common variable immunodeficiency have reduced expression of Lex and reduced levels of phagocytosis and degranulation.49
On arrival at sites of infection, PMNs respond to pathogen stimulation by release of antimicrobial proteins and potent inflammatory mediators that are stored within cytoplasmic granules.50 mAb-mediated engagement of Lex by W6D3 revealed that specific targeting of nonterminal Lex resulted in a small increase in the surface expression of CD66b, lactoferrin, CD63, NE, and MPO, markers of specific and azurophilic granules, respectively. In a manner analogous to that observed for phagocytosis, targeting of both terminal and nonterminal Lex with H198 resulted in a more robust and significant increase in the surface expression of both specific and azurophilic granule markers, demonstrating once again the importance of terminal Lex glycans in modulating neutrophil function. Despite increased surface expression of PMN granule markers, there was no significant release of NE, MPO, or lactoferrin detected. These observations suggest that engagement of Lex results in an incomplete degranulation response with mobilization of granule contents to the PMN surface but without release of granule contents into the surrounding milieu. Electron microscopic analyses of Lex-stimulated PMNs revealed enlarged granules that were diminished in number and relocalized to closer to the plasma membrane. These data suggest that Lex can be targeted to mobilize PMN granules to the cell surface, with H198 targeting of both terminal and internal Lex once again having the more robust effect on PMN function.
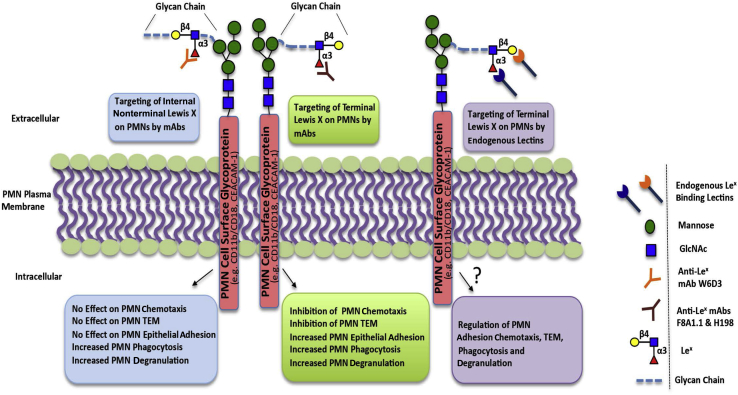
The findings in this study reveal how specific engagement of PMN Lex can differentially modulate key PMN functions, including adhesion, chemotaxis, TEM, phagocytosis, and mobilization of antimicrobial granules (Figure 7). These Lex-mediated changes in PMN function are analogous to previous reports describing PMN priming with both conditions, resulting in mobilization of intracellular granules to the plasma membrane, increased phagocytosis, and changes in PMN-adhesive interactions.51, 52, 53, 54 In addition, these glycan-mediated effects on PMN function are physiologically important because mAbs that selectively bind to carbohydrate structures likely mimic natural lectins and thus afford the possibility of understanding functional consequences of interactions between PMN surface glycan determinants and their physiological ligands. In addition, given its abundant expression on migrating PMNs, selective targeting of Lex represents a potential therapeutic target for modulating inflammation in diseases where dysregulated PMN influx is associated with host tissue destruction.
Figure 7.
Schematic figure summarizing the functional effects of targeting terminal versus nonterminal polymorphonuclear leukocyte (PMN) Lewis X (Lex) and comparing monoclonal antibody (mAb) engagement of Lex with targeting of PMN Lex by endogenous glycan-binding lectins. CEACAM-1, carcinoembryonic antigen–related cell adhesion molecule 1; TEM, transepithelial migration.
Footnotes
Supported by NIH grants DK079392 and DK072564 (C.A.P.) and HL085607 and P41GM103694 (R.D.C.), NIHS10 RR025679 01 (EM Microscope), and the Crohn's & Colitis Foundation of America Career Development Award (J.C.B.).
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2015.10.015.
Contributor Information
Jennifer C. Brazil, Email: brazilj@med.umich.edu.
Charles A. Parkos, Email: cparkos@med.umich.edu.
Supplemental Data
Figure showing important glycan similarities and differences for the anti-Lewis X monoclonal antibodies H198, W6D3, and F8A1.1.
Protein lysates were prepared from human polymorphonuclear leukocytes (PMNs), and T84 intestinal epithelial cells were immunoblotted for Lewis X using F8A1.1. A: Data represent three immunoblots. B: Monoclonal antibody CD15s (25 μg/mL) was incubated on an array with 610 glycan structures (version 5.1 Consortium for Functional Glycomics glycan array microarray) and binding was detected with Alexa Fluor-488–labeled anti-mouse IgG. ND, not detectable (<RFU); RFU, relative fluorescence unit; Sp, spacer.
References
- 1.Smith J.A. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 2.Woodfin A., Voisin M.B., Imhof B.A., Dejana E., Engelhardt B., Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood. 2009;113:6246–6257. doi: 10.1182/blood-2008-11-188375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 4.Imhof B.A., Dunon D. Basic mechanism of leukocyte migration. Horm Metab Res. 1997;29:614–621. doi: 10.1055/s-2007-979112. [DOI] [PubMed] [Google Scholar]
- 5.Blake K.M., Carrigan S.O., Issekutz A.C., Stadnyk A.W. Neutrophils migrate across intestinal epithelium using beta2 integrin (CD11b/CD18)-independent mechanisms. Clin Exp Immunol. 2004;136:262–268. doi: 10.1111/j.1365-2249.2004.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y., Buhring H.J., Zen K., Burst S.L., Schnell F.J., Williams I.R., Parkos C.A. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J Biol Chem. 2002;277:10028–10036. doi: 10.1074/jbc.M109720200. [DOI] [PubMed] [Google Scholar]
- 7.Parkos C.A., Colgan S.P., Diamond M.S., Nusrat A., Liang T.W., Springer T.A., Madara J.L. Expression and polarization of intercellular adhesion molecule-1 on human intestinal epithelia: consequences for CD11b/CD18-mediated interactions with neutrophils. Mol Med. 1996;2:489–505. [PMC free article] [PubMed] [Google Scholar]
- 8.Parkos C.A., Colgan S.P., Liang T.W., Nusrat A., Bacarra A.E., Carnes D.K., Madara J.L. CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J Cell Biol. 1996;132:437–450. doi: 10.1083/jcb.132.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperandio M., Gleissner C.A., Ley K. Glycosylation in immune cell trafficking. Immunol Rev. 2009;230:97–113. doi: 10.1111/j.1600-065X.2009.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marth J.D., Grewal P.K. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008;8:874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J. 2006;273:4377–4389. doi: 10.1111/j.1742-4658.2006.05437.x. [DOI] [PubMed] [Google Scholar]
- 12.Asano M., Nakae S., Kotani N., Shirafuji N., Nambu A., Hashimoto N., Kawashima H., Hirose M., Miyasaka M., Takasaki S., Iwakura Y. Impaired selectin-ligand biosynthesis and reduced inflammatory responses in beta-1,4-galactosyltransferase-I-deficient mice. Blood. 2003;102:1678–1685. doi: 10.1182/blood-2003-03-0836. [DOI] [PubMed] [Google Scholar]
- 13.Ellies L.G., Sperandio M., Underhill G.H., Yousif J., Smith M., Priatel J.J., Kansas G.S., Ley K., Marth J.D. Sialyltransferase specificity in selectin ligand formation. Blood. 2002;100:3618–3625. doi: 10.1182/blood-2002-04-1007. [DOI] [PubMed] [Google Scholar]
- 14.Homeister J.W., Thall A.D., Petryniak B., Maly P., Rogers C.E., Smith P.L., Kelly R.J., Gersten K.M., Askari S.W., Cheng G., Smithson G., Marks R.M., Misra A.K., Hindsgaul O., von Andrian U.H., Lowe J.B. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 15.Beauharnois M.E., Lindquist K.C., Marathe D., Vanderslice P., Xia J., Matta K.L., Neelamegham S. Affinity and kinetics of sialyl Lewis-X and core-2 based oligosaccharides binding to L- and P-selectin. Biochemistry. 2005;44:9507–9519. doi: 10.1021/bi0507130. [DOI] [PubMed] [Google Scholar]
- 16.Foxall C., Watson S.R., Dowbenko D., Fennie C., Lasky L.A., Kiso M., Hasegawa A., Asa D., Brandley B.K. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. J Cell Biol. 1992;117:895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katayama Y., Hidalgo A., Chang J., Peired A., Frenette P.S. CD44 is a physiological E-selectin ligand on neutrophils. J Exp Med. 2005;201:1183–1189. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones A.T., Federsppiel B., Ellies L.G., Williams M.J., Burgener R., Duronio V., Smith C.A., Takei F., Ziltener H.J. Characterization of the activation-associated isoform of CD43 on murine T lymphocytes. J Immunol. 1994;153:3426–3439. [PubMed] [Google Scholar]
- 19.Brown S.J., Miller A.M., Cowan P.J., Slavin J., Connell W.R., Moore G.T., Bell S., Elliott P.R., Desmond P.V., d'Apice A.J. Altered immune system glycosylation causes colitis in alpha1,2-fucosyltransferase transgenic mice. Inflamm Bowel Dis. 2004;10:546–556. doi: 10.1097/00054725-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Fu J., Wei B., Wen T., Johansson M.E., Liu X., Bradford E., Thomsson K.A., McGee S., Mansour L., Tong M., McDaniel J.M., Sferra T.J., Turner J.R., Chen H., Hansson G.C., Braun J., Xia L. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121:1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brazil J.C., Lee W.Y., Kolegraff K.N., Nusrat A., Parkos C.A., Louis N.A. Neutrophil migration across intestinal epithelium: evidence for a role of CD44 in regulating detachment of migrating cells from the luminal surface. J Immunol. 2010;185:7026–7036. doi: 10.4049/jimmunol.1001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brazil J.C., Liu R., Sumagin R., Kolegraff K.N., Nusrat A., Cummings R.D., Parkos C.A., Louis N.A. alpha3/4 Fucosyltransferase 3-dependent synthesis of Sialyl Lewis A on CD44 variant containing exon 6 mediates polymorphonuclear leukocyte detachment from intestinal epithelium during transepithelial migration. J Immunol. 2013;191:4804–4817. doi: 10.4049/jimmunol.1301307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinzaki S., Kuroki E., Iijima H., Tatsunaka N., Ishii M., Fujii H., Kamada Y., Kobayashi T., Shibukawa N., Inoue T., Tsujii M., Takeishi S., Mizushima T., Ogata A., Naka T., Plevy S.E., Takehara T., Miyoshi E. Lectin-based immunoassay for aberrant IgG glycosylation as the biomarker for Crohn's disease. Inflamm Bowel Dis. 2013;19:321–331. doi: 10.1097/MIB.0b013e318280eade. [DOI] [PubMed] [Google Scholar]
- 24.Furr A.E., Ranganathan S., Finn O.J. Aberrant expression of MUC1 mucin in pediatric inflammatory bowel disease. Pediatr Dev Pathol. 2010;13:24–31. doi: 10.2350/08-06-0479.1. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence D.W., Bruyninckx W.J., Louis N.A., Lublin D.M., Stahl G.L., Parkos C.A., Colgan S.P. Antiadhesive role of apical decay-accelerating factor (CD55) in human neutrophil transmigration across mucosal epithelia. J Exp Med. 2003;198:999–1010. doi: 10.1084/jem.20030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zen K., Liu Y., Cairo D., Parkos C.A. CD11b/CD18-dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. J Immunol. 2002;169:5270–5278. doi: 10.4049/jimmunol.169.9.5270. [DOI] [PubMed] [Google Scholar]
- 27.Larkin M., Ahern T.J., Stoll M.S., Shaffer M., Sako D., O'Brien J., Yuen C.T., Lawson A.M., Childs R.A., Barone K.M. Spectrum of sialylated and nonsialylated fuco-oligosaccharides bound by the endothelial-leukocyte adhesion molecule E-selectin. Dependence of the carbohydrate binding activity on E-selectin density. J Biol Chem. 1992;267:13661–13668. [PubMed] [Google Scholar]
- 28.Larsen E., Palabrica T., Sajer S., Gilbert G.E., Wagner D.D., Furie B.C., Furie B. PADGEM-dependent adhesion of platelets to monocytes and neutrophils is mediated by a lineage-specific carbohydrate, LNF III (CD15) Cell. 1990;63:467–474. doi: 10.1016/0092-8674(90)90443-i. [DOI] [PubMed] [Google Scholar]
- 29.Larsen G.R., Sako D., Ahern T.J., Shaffer M., Erban J., Sajer S.A., Gibson R.M., Wagner D.D., Furie B.C., Furie B. P-selectin and E-selectin: distinct but overlapping leukocyte ligand specificities. J Biol Chem. 1992;267:11104–11110. [PubMed] [Google Scholar]
- 30.Stocks S.C., Albrechtsen M., Kerr M.A. Expression of the CD15 differentiation antigen (3-fucosyl-N-acetyl-lactosamine, LeX) on putative neutrophil adhesion molecules CR3 and NCA-160. Biochem J. 1990;268:275–280. doi: 10.1042/bj2680275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandalasi M., Dorabawila N., Smith D.F., Heimburg-Molinaro J., Cummings R.D., Nyame A.K. Development and characterization of a specific IgG monoclonal antibody toward the Lewis x antigen using splenocytes of Schistosoma mansoni-infected mice. Glycobiology. 2013;23:877–892. doi: 10.1093/glycob/cwt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balsam L.B., Liang T.W., Parkos C.A. Functional mapping of CD11b/CD18 epitopes important in neutrophil-epithelial interactions: a central role of the I domain. J Immunol. 1998;160:5058–5065. [PubMed] [Google Scholar]
- 33.Parkos C.A., Delp C., Arnaout M.A., Madara J.L. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heimburg-Molinaro J., Song X., Smith D.F., Cummings R.D. Preparation and analysis of glycan microarrays. Curr Protoc Protein Sci. 2011;ch 12:Unit12. doi: 10.1002/0471140864.ps1210s64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diamond M.S., Staunton D.E., de Fougerolles A.R., Stacker S.A., Garcia-Aguilar J., Hibbs M.L., Springer T.A. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18) J Cell Biol. 1990;111:3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colgan S.P., Parkos C.A., Delp C., Arnaout M.A., Madara J.L. Neutrophil migration across cultured intestinal epithelial monolayers is modulated by epithelial exposure to IFN-gamma in a highly polarized fashion. J Cell Biol. 1993;120:785–798. doi: 10.1083/jcb.120.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruyninckx W.J., Comerford K.M., Lawrence D.W., Colgan S.P. Phosphoinositide 3-kinase modulation of beta(3)-integrin represents an endogenous “braking” mechanism during neutrophil transmatrix migration. Blood. 2001;97:3251–3258. doi: 10.1182/blood.v97.10.3251. [DOI] [PubMed] [Google Scholar]
- 38.Louis N.A., Campbell E., Colgan S.P. Model systems to investigate neutrophil adhesion and chemotaxis. Methods Mol Biol. 2007;412:257–270. doi: 10.1007/978-1-59745-467-4_17. [DOI] [PubMed] [Google Scholar]
- 39.Kerr M.A., Stocks S.C. The role of CD15-(Le(X))-related carbohydrates in neutrophil adhesion. Hist Jahrb. 1992;24:811–826. doi: 10.1007/BF01046353. [DOI] [PubMed] [Google Scholar]
- 40.Fukuda M., Spooncer E., Oates J.E., Dell A., Klock J.C. Structure of sialylated fucosyl lactosaminoglycan isolated from human granulocytes. J Biol Chem. 1984;259:10925–10935. [PubMed] [Google Scholar]
- 41.Skubitz K.M., Snook R.W., 2nd Monoclonal antibodies that recognize lacto-N-fucopentaose III (CD15) react with the adhesion-promoting glycoprotein family (LFA-1/HMac-1/gp 150,95) and CR1 on human neutrophils. J Immunol. 1987;139:1631–1639. [PubMed] [Google Scholar]
- 42.Feng C., Zhang L., Almulki L., Faez S., Whitford M., Hafezi-Moghadam A., Cross A.S. Endogenous PMN sialidase activity exposes activation epitope on CD11b/CD18 which enhances its binding interaction with ICAM-1. J Leukoc Biol. 2011;90:313–321. doi: 10.1189/jlb.1210708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss S.J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 44.Gooi H.C., Thorpe S.J., Hounsell E.F., Rumpold H., Kraft D., Forster O., Feizi T. Marker of peripheral blood granulocytes and monocytes of man recognized by two monoclonal antibodies VEP8 and VEP9 involves the trisaccharide 3-fucosyl-N-acetyllactosamine. Eur J Immunol. 1983;13:306–312. doi: 10.1002/eji.1830130407. [DOI] [PubMed] [Google Scholar]
- 45.Stockl J., Majdic O., Rosenkranz A., Fiebiger E., Kniep B., Stockinger H., Knapp W. Monoclonal antibodies to the carbohydrate structure Lewis(x) stimulate the adhesive activity of leukocyte integrin CD11b/CD18 (CR3, Mac-1, alpha m beta 2) on human granulocytes. J Leukoc Biol. 1993;53:541–549. doi: 10.1002/jlb.53.5.541. [DOI] [PubMed] [Google Scholar]
- 46.Stocks S.C., Kerr M.A. Stimulation of neutrophil adhesion by antibodies recognizing CD15 (Le(X)) and CD15-expressing carcinoembryonic antigen-related glycoprotein NCA-160. Biochem J. 1992;288(Pt 1):23–27. doi: 10.1042/bj2880023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cross A.S., Wright D.G. Mobilization of sialidase from intracellular stores to the surface of human neutrophils and its role in stimulated adhesion responses of these cells. J Clin Invest. 1991;88:2067–2076. doi: 10.1172/JCI115536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakarya S., Rifat S., Zhou J., Bannerman D.D., Stamatos N.M., Cross A.S., Goldblum S.E. Mobilization of neutrophil sialidase activity desialylates the pulmonary vascular endothelial surface and increases resting neutrophil adhesion to and migration across the endothelium. Glycobiology. 2004;14:481–494. doi: 10.1093/glycob/cwh065. [DOI] [PubMed] [Google Scholar]
- 49.Casulli S., Coignard-Biehler H., Amazzough K., Shoai-Tehrani M., Bayry J., Mahlaoui N., Elbim C., Kaveri S.V. Defective functions of polymorphonuclear neutrophils in patients with common variable immunodeficiency. Immunol Res. 2014;60:69–76. doi: 10.1007/s12026-014-8555-7. [DOI] [PubMed] [Google Scholar]
- 50.Mocsai A., Zhou M., Meng F., Tybulewicz V.L., Lowell C.A. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 51.Nieminen J., St-Pierre C., Sato S. Galectin-3 interacts with naive and primed neutrophils, inducing innate immune responses. J Leukoc Biol. 2005;78:1127–1135. doi: 10.1189/jlb.1204702. [DOI] [PubMed] [Google Scholar]
- 52.Wright H.L., Moots R.J., Bucknall R.C., Edwards S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 2010;49:1618–1631. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- 53.Rainard P., Riollet C., Poutrel B., Paape M.J. Phagocytosis and killing of Staphylococcus aureus by bovine neutrophils after priming by tumor necrosis factor-alpha and the des-arginine derivative of C5a. Am J Vet Res. 2000;61:951–959. doi: 10.2460/ajvr.2000.61.951. [DOI] [PubMed] [Google Scholar]
- 54.Volk A.P., Barber B.M., Goss K.L., Ruff J.G., Heise C.K., Hook J.S., Moreland J.G. Priming of neutrophils and differentiated PLB-985 cells by pathophysiological concentrations of TNF-alpha is partially oxygen dependent. J Innate Immun. 2011;3:298–314. doi: 10.1159/000321439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure showing important glycan similarities and differences for the anti-Lewis X monoclonal antibodies H198, W6D3, and F8A1.1.
Protein lysates were prepared from human polymorphonuclear leukocytes (PMNs), and T84 intestinal epithelial cells were immunoblotted for Lewis X using F8A1.1. A: Data represent three immunoblots. B: Monoclonal antibody CD15s (25 μg/mL) was incubated on an array with 610 glycan structures (version 5.1 Consortium for Functional Glycomics glycan array microarray) and binding was detected with Alexa Fluor-488–labeled anti-mouse IgG. ND, not detectable (<RFU); RFU, relative fluorescence unit; Sp, spacer.