Abstract
Study Objective
To characterize the properties and natural history of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor (statin)-associated muscle-related adverse effects (MAEs).
Design
Patient-targeted postmarketing adverse-effect surveillance approach coupling survey design with an open-ended narrative.
Setting
University-affiliated health care system.
Subjects
Three hundred fifty-four patients (age range 34–86 yrs) who self-reported muscle-related problems associated with statin therapy.
Measurements and Main Results
Patients with perceived statin-associated MAEs completed a survey assessing statin drugs and dosages; characteristics of the MAEs; time course of onset, resolution, or recurrence; and impact on quality of life (QOL). Cases were assessed for putative drug adverse-effect causality by using the Naranjo adverse drug reaction probability scale criteria and were evaluated for inclusion in groups for which mortality benefit with statins has been shown. Patients reported muscle pain (93%), fatigue (88%), and weakness (85%). Three hundred patients (85%) met literature criteria for probable or definite drug adverse-effect causality. Ninety-four percent of atorvastatin usages (240/255) generated MAEs versus 61% of lovastatin usages (38/62, p<0.0001). Higher potency statins reproduced MAEs in 100% of 39 rechallenges versus 73% (29/40) with lower potency rechallenges (p<0.01). Time course of onset after statin initiation varied (median 14 wks); some MAEs occurred after long-term symptom-free use. Recurrence with rechallenge had a significantly shorter latency to onset (median 2 wks). The MAEs adversely affected all assessed functional and QOL domains. Most patients with probable or definite MAEs were in categories for which available randomized controlled trial evidence shows no trend to all-cause mortality benefit with statin therapy.
Conclusion
This study complements available information on the properties and natural history of statin-associated MAEs, affirming dose dependence and strong QOL impact. The data indicating a dose-dependent relationship between MAE risk and recurrence suggest lower potency statins or discontinuation may bear consideration for ameliorating symptoms.
Keywords: 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, statins, drug adverse effects, myalgia, myopathy, muscle, quality of life
The 3-hydroxy-3-methylglutaryl coenzyme-A reductase inhibitors (statins) are a widely prescribed drug class and include the best-selling prescription drug in the world, atorvastatin.1–3 Although statins are generally well tolerated and have documented cardiovascular benefits in many persons, the most commonly reported adverse effects are muscle-related problems (i.e., muscle pain, weakness, and/or fatigue),4, 5 which can occur in the absence of creatine kinase (CK) level elevation.6, 7 Blinded N-of-1 crossover biopsy data indicate that muscle-related problems with normal or minimally elevated CK levels reflect a (partially) reversible mitochondrial myopathy.6
The medical literature has described muscle-related adverse effects (MAEs) with cholesterol-lowering agents8–12 but has most commonly focused on severe cases entailing rhabdomyolysis.13–16 The properties, natural history, drug-dose relationship, and impact of nonrhabdomyolytic statin-associated MAEs have received somewhat less attention.6, 7, 10, 17–22
Patient attributions of adverse effects to drugs have been reported to be reliable.23 This case series capitalizes on that finding, by using an expanded, patient-directed, survey-based post-marketing adverse-effect surveillance approach to preliminarily examine the characteristics, time course, functional impact, and quality-of-life (QOL) impact of nonrhabdomyolytic MAEs associated with statins. The importance of characterizing these MAEs is underscored by the prevalence of statin use and frequency of muscle-related problems among the reported adverse effects of statins.4, 24 Also, a previous study identified a lack of physician awareness of and receptivity to patient reports of adverse effects of statins that extend to nonrhabdomyolytic MAEs,25 reinforcing the need for more information on statin-associated MAEs. Demonstration of the concept and potential added value of an expanded, patient-targeted surveillance approach is timely in the face of attention to limitations in existing postmarketing surveillance systems, including physician-targeted surveillance.26
Methods
Patients in this study were selected from a subset of patients who participated in the University of California, San Diego (UCSD) Statin Effects Study.25 Recruitment of patients for that study was passive, analogous to physician-targeted postmarketing surveillance, and most patients learned about the study through the Internet or media. Patients with perceived statin-associated adverse effects were asked to complete a survey on general statin adverse effects. Patients reporting MAEs—specifically, muscle pain, weakness, and/or fatigue associated with statin use—were asked to complete a second survey directly targeting MAEs. This report is based on those patients who completed the second survey. Study methods and surveys were approved by the UCSD institutional review board, and all patients gave written informed consent.
The MAE survey instrument comprised a semi-structured survey with 55 items (additional items pertained for those patients citing more than one statin), as well as an open-ended narrative. Information was elicited on patient characteristics, drug(s), dose(s), lipid levels, MAE character (pain, weakness, fatigue) and severity, MAE time course of onset and recovery relative to statin usage, effect of statin change or discontinuation, recurrence with statin reinitiation, reported physician interaction in relation to the possible MAE, and functional as well as QOL impact. Data on physician interactions have been published elsewhere.25
Causality of Muscle-Related Adverse Effects
Cases were assessed for adherence to published presumptive Naranjo adverse drug reaction probability scale adverse-effect causality criteria,27 which uses a system of positive and negative causality points with a score of 9 or higher indicating definite adverse-effect causality, 5–8 probable, 1–4 possible, and 0 or lower doubtful causality. Causality assignments for statin-associated MAEs were generated conservatively. Functionally, cases deemed probable were limited to those patients who discontinued the drug (or reduced the dose) and experienced improvement. Cases deemed definite must have, in addition to recovery, undergone a statin rechallenge that produced symptom recurrence.
Distribution of Statin Use
Numbers citing MAEs with each statin were tallied absolutely as well as relative to the number citing use of each statin. Relative reporting frequencies were compared qualitatively (i.e., by rank) to reported relative sales of each statin and to relative statin potencies.
Comparison of Drugs by Potency
When comparing the rate of problems, we examined the fraction of trials producing MAEs (among those patients selected for experiencing statin-associated MAEs) as a function of cholesterol-lowering drug potency by using the χ2 test and tests of trend. For comparing drugs, a caveat is in order. Rosuvastatin is more potent than atorvastatin per milligram; however, in as-used doses, atorvastatin may be more potent on average: atorvastatin 80 mg is a widely accepted and advocated dose, but warnings by the United States Food and Drug Administration expressly caution against higher doses of rosuvastatin (e.g., 40 mg).28 Due to its withdrawal from the market, cerivastatin equivalencies were not considered.
Calculation of Dose Equivalencies
Statin dose equivalencies for each drug and dose were based on published drug and dose equivalencies for expected potency of cholesterol reduction and are as follows: rosuvastatin 2.5 mg ≈ atorvastatin 5 mg ≈ simvastatin 10 mg ≈ lovastatin 20 mg ≈ pravastatin 20 mg ≈ fluvastatin 40 mg.29, 30 These are only approximate; for instance, in our 1000-person randomized controlled trial, simvastatin 20 mg/day led to a 49-mg/dl reduction in low-density lipoprotein cholesterol (LDL) versus a 40-mg/dl decrease with pravastatin 40 mg/day (p<0.001).31
Dose-Response Effect of Statin Potency
Dose equivalencies that use within-patient rechallenge data were used in assessing whether evidence supported a dose-response effect. For patients who experienced a presumptive MAE that improved with discontinuation of the statin, and later restarted a statin (or if no time to recovery was taken, improved with switch of statin), we assessed the proportion in which recurrence occurred as a function of whether the expected potency of the rechallenge statin was higher than, approximately equivalent (similar) to, or lower than that of the index statin, or whether rechallenge was with a nonstatin cholesterol-lowering agent. Irrespective of potency category, a minimum 2-month period of the rechallenge agent was required for “nonrecurrence with rechallenge” to be designated. The χ2 tests and tests of trend were performed to evaluate whether relative potency of the rechallenge drug related to the likelihood of recurrence with rechallenge.
Time Course of Muscle-Related Adverse Effects
The reported time course for onset of MAEs, for recovery after discontinuation of statin use (first MAE), and for recurrence of MAEs with statin rechallenge were explored by using descriptive statistics (mean, median, range). Differences in time to onset and recovery for first MAE versus recurrence with rechallenge were assessed with paired t tests for patients who provided time to onset and/or recovery for both an index MAE and recurrence with rechallenge. A p value of less than 0.05 was considered to indicate a statistically significant difference.
Functional and Quality-of-Life Impact
The subjective impact of MAEs on six areas of everyday muscle-related activity and eight domains relevant to QOL was appraised on a visual analog scale ranging from −10 (“maximally worse”) to +10 (“maximally better”) and was analyzed with nonparametric sign tests. Visual analog scales were selected because of their favorable psychometric properties for subjective outcomes.32, 33 They circumvent concerns regarding QOL instruments, which sum scores on specific questions and thereby may weight QOL considerations differently from patients’ weightings.34 Although patients were selected for reporting MAEs, benefits as well as harm to QOL domains were elicited.
Results
Patient Characteristics
Three hundred sixty-four male and female adult patients completed the MAE survey between January 2002 and June 2008. Six patients were excluded because of incomplete or inconsistent reported data; two patients were excluded because muscle-related symptoms were not attributed to cholesterol-lowering drugs (e.g., previously existing ailments); and two patients reported symptoms primarily consisting of peripheral neuropathy, which was not the focus of this analysis. The remaining 354 patients were included in the analysis. Table 1 summarizes patient characteristics. Male and female patients were similarly represented. Patients were pre-dominantly Caucasian and were generally well educated, with 86% of all patients having attended or graduated from college.
Table 1.
Patient Characteristics
| Characteristic | All Patients (n=354 | Probable or Definite Causality Group (n=300)a | p Value |
|---|---|---|---|
| Mean ± SD (range)
| |||
| Age (yrs)b | 63 ± 11 (34–86) | 63 ± 11 (34–86) | >0.99 |
| Total cholesterol (mg/dl)c | |||
| At baseline | 243 ± 52 (116–451) | 247 ± 49 (126–451) | —d |
| During treatment | 180 ± 39 (102–300) | 181 ± 39 (102–300) | —d |
| Decrease with treatment | 63 ± 45 (–65–202) | 66 ± 44 (–65–202) | — |
|
| |||
| Percentage of Patients Reporting Datab
| |||
| Male | 53 | 52 | 0.77 |
| Caucasian | 94 | 95 | 0.54 |
| Married | 76 | 78 | 0.54 |
| Some college education | 86 | 84 | 0.50 |
| Muscle-related problems incurred with statin use | |||
| Pain | 93 | 94 | 0.66 |
| Fatigue | 88 | 88 | >0.99 |
| Weakness | 85 | 85 | >0.99 |
| Not taking statin therapy (at time of survey) | 66 | 67 | 0.39 |
This group represents eligible patients for the Naranjo27 probable or definite categories (300 [85%] of 354 patients).
Limited to patients who reported data for these characteristics (≥ 86% of patients in each category).
Limited to patients for whom cholesterol values at baseline and during treatment were available (160 of 354 total patients; 133 patients in the probable or definite subgroup); the value during treatment is that associated with the first onset of muscle-related problems.
Baseline and during treatment total cholesterol paired t test: all patients p<2×10−39; probable or definite subgroup p<2×10−35.
Among all patients, 93% cited muscle pain associated with statin use, 88% muscle fatigue, and 85% muscle weakness. Two-hundred thirty-two patients (66%) reported cessation of statin therapy after experiencing muscle-related symptoms; of these, 174 patients (75%) cited some recovery on discontinuation of the statin, with 66 patients (28%) citing complete recovery of their muscle-related symptoms with discontinuation. Based on the Naranjo criteria,27 which seek to categorize causality of adverse effects, 106 patients (30%) satisfied presumptive literature criteria for definite causality of statin-associated MAEs, 194 patients (55%) probable causality, 54 patients (15%) possible causality, and 0 patients doubtful (we excluded patients with known other causes of muscle-related symptoms). The total patient group and probable or definite subgroup were found to be demographically similar (Table 1).
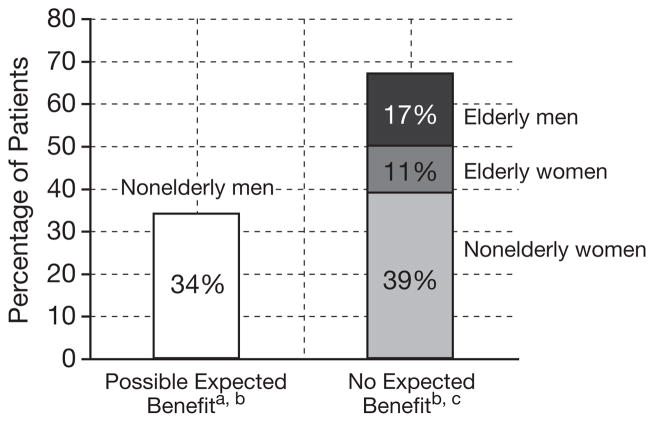
Figure 1 presents characteristics of the probable or definite subgroup as a function of age and sex, in comparison to published studies.35–38 Age and sex are characteristics that help define groups that have shown (or have not shown) all-cause mortality and morbidity benefit from statins.39–41 Even in studies focused on patients at high cardiovascular risk, patients who are female (Scandinavian Simvastatin Survival Study [4S]35, Long-term Intervention with Pravastatin in Ischaemic Disease [LIPID] study36) and those aged 70 years or older (Prospective Study of Pravastatin in the Elderly at Risk [PROSPER]37) have shown no trend to survival benefit. Middle-aged men have generally shown no trend to survival benefit or all-cause morbidity benefit (by the available proxy, serious adverse events) if not with high cardiovascular risk or high C-reactive protein level (Air Force/Texas Coronary Athero-sclerosis Prevention Study [AFCAPS/TEXCAPS]38), but we erred conservatively and counted all men younger than 70 years as having prospect of net benefit. Thus, the findings overstate the fraction of those with MAEs in whom benefit might have been expected.
Figure 1.
Expectation of overall mortality benefit from statins among patients with muscle-related adverse effects in the probable or definite causality subgroup as a function of age and sex (data were available for 290 of the 300 patients).
a“Expected benefit” uses outcomes that balance net risks and benefits from the use of cholesterol-lowering drugs (overall mortality, all-cause serious morbidity).
bExpected benefit of statin therapy in middle-aged men is expected to be overstated in this figure. Middle-aged men without high risk for cardiovascular disease (Air Force/Texas Coronary Atherosclerosis Prevention Study [AFCAPS]38) have shown no trend toward overall mortality or morbidity or serious adverse events benefit.
cWomen (Scandinavian Simvastatin Survival Study [4S]35 and Long-term Intervention with Pravastatin in Ischaemic Disease [LIPID]36) and those older than 70 years (Prospective Study of Pravastatin in the Elderly at Risk [PROSPER]37), even those at high risk for cardiovascular disease, have shown no trend to overall mortality benefit.
Distribution and Relation of Statin Use to Muscle-Related Adverse Effect
Table 2 outlines the distribution of statin drug use reported by patients, which parallels statin prescribing patterns in the United States1–3 based on both percentage of patients prescribed each drug and percentage of trials involving each drug (many patients were receiving sequentially more than one statin over time, or received the same statin more than once). The fraction of trials for each drug that resulted in MAEs is shown in Table 3. The percentage of trials resulting in muscle-related symptoms correlated with the potency of the statin used. For instance, 94% of atorvastatin trials resulted in MAEs compared with 61% of lovastatin trials (p<0.0001).
Table 2.
Distribution of Statin Drug Use Relative to U.S. Prescribing Patterns
| Drug | Percentage of Patients Who Tried the Drug (n=351)a | Percentage of Trials Involving the Drug (n=617)b | Rank Order Tried | U.S. Prescribing Pattern
|
|
|---|---|---|---|---|---|
| Rank Orderc | Percent Used | ||||
| Atorvastatin | 67 | 41 | 1 | 1 | 54 |
| Simvastatin | 38 | 24 | 2 | 2 | 22 |
| Pravastatin | 20 | 13 | 3 | 3 | 11 |
| Lovastatin | 16 | 10 | 4 | 4 | 4 |
| Rosuvastatin | 11 | 6 | 5 | — | — |
| Cerivastatin | 7 | 4 | 6 | — | — |
| Fluvastatine | 3 | 2 | 7 | 5 | — |
Percentages sum to greater than 100% because many patients tried more than one drug (n=351 because 3 patients in the study reported using only nonstatin cholesterol-lowering drugs).
A different “trial” is defined as a transition to a different statin drug or dose, with or without a period of statin withdrawal, that caused new muscle-related symptoms or a change in severity.
Prescribing order excludes cerivastatin (withdrawn from the U.S. market) and rosuvastatin (recently introduced to the U.S. market) and was determined from rank order of prescribing in the United States from 2001–2007.
Percent use excludes cerivastatin, rosuvastatin, and fluvastatin, and was determined from number of dispensed prescriptions in the United States from 2001–2005 (years for which data were available).42
A limited number of patients reported the use of fluvastatin (n=11).
Table 3.
Fractions of Drug Trials That Resulted in Muscle-Related Adverse Effects Correlated with Drug Potency
| Drug | No. of Trials Involving the Drug | No. (%) of Trials That Resulted in Muscle-Related Adverse Effectsa, b | Rank Order Potencyc, d |
|---|---|---|---|
| Current statins | |||
| Atorvastatin | 255 | 240 (94) | 1, 2e |
| Rosuvastatin | 40 | 32 (80) | 1, 2e |
| Simvastatin | 148 | 114 (77) | 3 |
| Pravastatin | 78 | 52 (67) | 4 |
| Lovastatin | 62 | 38 (61) | 4 |
| Fluvastatin | 11 | 9 (82)f | 5 |
| Other lipid-lowering agents | |||
| Cerivastating | 23 | 17 (74)f | — |
| Ezetimibe | 29 | 13 (45)f | — |
| Simvastatin-ezetimibe | 16 | 15 (94)f | — |
| Niacin | 14 | 3 (21)f | — |
| Gemfibrozil | 10 | 5 (50)f | — |
| Fenofibrate | 4 | 4 (100)f | — |
If the patient switched to a new drug without time off between drugs, the second drug is reported here as a new trial if the patient reported a change in severity of the muscle-related symptoms. Only the drug that was used during the onset of muscle-related symptoms is reported here if subsequent drugs used did not result in a change of symptoms because it is not possible to determine if persistent symptoms reflected lack of resolution (index drug) versus reproduction of symptoms (new drug[s]).
The percentages represent trials by those who experienced muscle-related symptoms and are not indicative of trials of general statin users.
The p values for potency are as follows: atorvastatin vs lovastatin, p<0.0000001; atorvastatin vs lovastatin + pravastatin, p<0.0000001; atorvastatin + rosuvastatin vs lovastatin + pravastatin, p<0.0000001; atorvastatin vs simvastatin, p<0.0000002; atorvastatin + rosuvastatin vs simvastatin, p=0.000003; simvastatin vs lovastatin + pravastatin, p=0.01.
The test of trend p values are as follows: atorvastatin + rosuvastatin vs simvastatin vs lovastatin + pravastatin p<0.0000001; atorvastatin vs rosuvastatin + simvastatin vs lovastatin + pravastatin p<0.0000001; atorvastatin vs simvastatin vs lovastatin + pravastatin p<0.0000001.
Although rosuvastatin is more potent than atorvastatin per milligram, it is not as commonly prescribed or used at high doses. Whereas an 80-mg dose of atorvastatin is commonly used and widely advocated, a 40-mg dose of rosuvastatin has been discouraged by the U.S. Food and Drug Administration.28
These percentages are based on a small number of trials.
Cerivastatin was withdrawn from the U.S. market.
Dose Response
For patients who noted recovery while discontinuing the statin or with a switch to another statin or to a nonstatin cholesterol-lowering drug, the instances in which MAEs recurred was calculated for patients who reported rechallenging with an expected higher or lower potency statin or with a nonstatin cholesterol-lowering drug (Table 4). “Effective dose” was calculated by using current literature on approximate statin dose equivalencies based on expected potency (cholesterol reduction).29, 30, 43–45
Table 4.
Dose Dependency of Recurrence of Muscle-Related Symptoms on Rechallenge
| Expected Potency of Rechallenge Drug | No. of Rechallenge Trialsa | No Recurrence | Recurrence | Recurrence Rate (%)b |
|---|---|---|---|---|
| Statin | ||||
| Higher | 39 | 0 | 39 | 100 |
| Similar | 20 | 5 | 15 | 75 |
| Lower | 40 | 11 | 29 | 73 |
| Nonstatin cholesterol-lowering drug | 27 | 16 | 11 | 41 |
Data are limited to patients who reported recovery and dose rechallenge data (126 trials). Patients were excluded if there was insufficient time (< 2 mo) to note and report recovery.
A “rechallenge” is defined as any trial of a cholesterol-lowering drug after a period of noted recovery either while discontinuing the drug or after a change in the drug. If no time to recovery was taken, the severity of symptoms rated on an analog scale was compared. Trials that produced more severe symptoms were noted as a recurrence and those that produced less severe symptoms were noted as no recurrence with rechallenge. In those switching from simvastatin to the same dose of simvastatin as part of the ezetimibe-simvastatin combination, the latter was deemed a “higher” dose because of the addition of the ezetimibe.
The test of trend p values are as follows: four separate levels, p=0.0000003; three levels (higher, similar, lower), p<0.002; two levels (higher + similar, lower), p<0.02; two levels (higher, lower), p<0.0003; two levels (higher + similar, lower + nonstatin), p=0.00003.
Recovery with discontinuation or change in statin was reported for 172 “trials.” The recurrence of MAEs with rechallenge was strongly dose dependent. In all trials, rechallenging with a statin of higher expected potency (for at least 2 mo with the new agent), patients reexperienced MAEs (100% recurrence). A smaller, but still substantial, fraction of rechallenges with a lower expected potency statin (73%), or rechallenges with a nonstatin cholesterol-lowering drug (41%), exhibited recurrence (75% of rechallenges with a similar potency statin showed recurrence; however, potency equivalencies are inexact.) Thus, recurrence of MAEs was strongly dose dependent, and the difference was highly significant (tests of trend p<0.001).
Time Course
Time course to onset of statin-associated MAEs, recovery, and recurrence with rechallenge, using as the index case the first trial of statin use resulting in MAEs, showed high individual variation (Table 5). Median reported time to initial onset was 14 weeks (mean ± SD 55 ± 95 wks, reflecting the skewed distribution). Median time to first suggestion of improvement with discontinuation was 2 weeks. Cases that arose long into statin usage were reported to the database; these cases also abated or resolved with statin discontinuation and recurred with rechallenge, suggesting that these cases too were statin-associated.
Table 5.
Time Course to Muscle-Related Adverse-Effect Onset and Recovery for Trials of Statins That Resulted in Muscle-Related Adverse Effects
| Time Course for Trials of Statins | No. of Patients Responding | Time to Onset or Recovery (wks)
|
||
|---|---|---|---|---|
| Median | Mean ± SD | Rangea | ||
| First trials resulting in MAEsb | ||||
| Time to MAE onset after starting the statin | 301 | 14 | 55 ± 95 | 0.14–520 |
| Time to first noted recovery after statin discontinuation | 189 | 2 | 8 ± 16 | 0.14–144 |
| Time to maximal recovery after statin discontinuation | 149 | 8 | 29 ± 64 | 0.14–624 |
| Time to complete recovery after statin discontinuation (confined to those reporting complete recovery) | 70 | 4 | 14 ± 19 | 0.14–96 |
| Index vs rechallenge trial resulting in MAEsb | ||||
| Time to onset, first occurrence, in those with rechallenge datac | 98 | 12 | 43 ± 78 | 0.14–520 |
| Time to onset after first rechallengec | 98 | 2 | 15 ± 42 | 0.14–286 |
| Time to recovery, first occurrence, in those with rechallenge datad | 56 | 2 | 6 ± 11 | 0.14–48 |
| Time to recovery after first rechallenged | 56 | 2 | 12 ± 39 | 0.14–286 |
| Rechallenge trials resulting in MAEs | ||||
| Time to first noted recovery after first statin rechallenge discontinued | 72 | 2 | 8 ± 18 | 0.14–104 |
| Time to maximal recovery after first statin rechallenge discontinued | 62 | 10 | 27 ± 43 | 0.14–208 |
| Time to complete recovery after first statin rechallenge discontinued (confined to those reporting complete recovery) | 33 | 4 | 9 ± 14 | 0.14–78 |
MAEs = muscle-related adverse effects.
0.14 wk = 1 day.
Limited to patients with time course data for both index and rechallenge MAEs.
Paired t test for index MAE onset vs first rechallenge onset, p=0.0014.
Paired t test for index MAE recovery vs first rechallenge recovery, p=0.24.
For patients who underwent rechallenge with statins and had recurrence of MAEs (not considering lipid-lowering dose and/or drug), time to recurrence with rechallenge was significantly shorter (median 2 wks) than time to onset of first MAE after starting the index statin (median 14 wks, p=0.0015, paired t test). The median time to first noted improvement with discontinuation after rechallenge (2 wks) did not differ from median time to first noted improvement with discontinuation for the index MAEs. However, the mean time to improvement after the rechallenge statin (mean ± SD 12 ± 39 wks) was materially longer than the mean time to first noted recovery after the index statin (6 ± 11 wks; although the variance was high, this did not reach significance, p=0.240 paired t test). This suggests that some patients with impaired or prolonged recovery may have experienced further recovery delays with rechallenge.
Function and Quality of Life
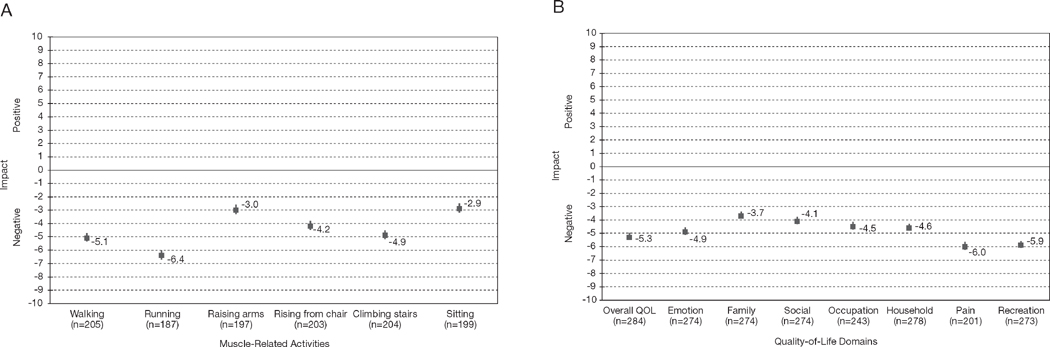
Patients used visual analog scales (ranging from −10 [“maximally worse”] to +10 [“maximally better”]) to rate how MAE symptoms affected six everyday muscle-related activities such as climbing stairs and sitting (Figure 2A). In the probable or definite adverse-effect category, the overall impact of MAEs on everyday muscle-related activity was strongly negative (p<0.000000001 for each domain, sign test). The domains most adversely affected were running (mean rating −6.4) and walking (mean −5.1).
Figure 2.
Negative impact of statin-associated muscle-related adverse effects on six everyday activities (A) and eight general quality-of-life (QOL) domains (B). Data indicate mean ratings of patients with probable or definite muscle-related adverse effects based on Naranjo criteria.27 Error bars indicate standard error, 2-sided p<0.000000001 for all domains. Some patients indicated positive values for both everyday muscle-related activity (26 patients) and general QOL domains (18 patients). Some patients responded with positive values to all QOL domains (everyday muscle-related activity [4 patients], general QOL [8 patients]) indicating possible misinterpretation of the visual analog scales. All other patients indicating positive values (everyday muscle-related activity [22 patients], general QOL [10 patients]) reported at least one or more other domains with negative impact on QOL.
Patients also assessed the impact of their muscle-related symptoms on eight general QOL domains (Figure 2B). Each assessed general QOL domain was negatively affected, with high statistical significance (p<0.0001, sign test), including overall QOL. The magnitude of effect was largest for pain (mean −6.0), recreation (mean −5.9), and overall QOL (mean −5.3), which is compatible with the character of the symptoms reported. Rarely, patients cited benefit to one or more QOL domains. On inquiry, some such patients thought that their health problems brought their family closer, thus improving family or social function.
The use of an open-ended narrative allowed patients to more fully elucidate the impact of statin-associated MAEs (Appendix 1). Many patients attributed a decrease in overall social and recreational activity to their symptoms, whereas others noted the impact it had on their occupation.
Appendix 1.
Patient Narratives Illustrating the Negative Impact on Quality-of-Life Domains
|
Discussion
To our knowledge, this study is the first to use patient-targeted postmarketing surveillance as an approach to characterize muscle-related problems associated with statins and to expand our understanding of statin-associated MAEs. Muscle-related adverse effects were reported with all prescribed statins, with most cases meeting existing literature criteria for probable or definite adverse-effect causality. Symptoms entailed pain, weakness, and/or fatigue, and commonly all three. “Trials” yielding MAEs correlated strongly with the potency of the statin in that trial. In addition, a powerful relationship was seen between the relative potency of the cholesterol-lowering agent and the recurrence of muscle-related symptoms with rechallenge. The time course for symptom onset was variable; however, time to onset for recurrence of MAEs with rechallenge was significantly shorter than time to onset for initial statin-associated MAEs. Findings agree with others’ observations that recovery from statin-associated MAEs is not universally complete.21, 22, 46 Visual analog scales as well as patient narratives indicated that symptoms had a significant and sometimes life-altering negative impact on function and QOL.
A key finding was the strong evidence for a dose-response relationship for MAEs across statins, concordant with published evidence for dose-related adverse-effect risk generally limited to single statin comparisons.8, 13, 16, 47 In our sample, patients with a statin-associated MAE who underwent rechallenge for at least 2 months with any statin at a higher expected potency reexperienced MAEs 100% of the time versus a significantly lower fraction who underwent rechallenge at lower expected potency. This suggests that the therapeutic potency of a statin may be a seminal determinant of vulnerable patients’ risk when selecting a statin. Of note, the strong dose-response relationship and consistent recurrence with higher potency statins add support for a causal link between MAEs and statin use, as well as internal validity of these data. This finding is unlikely to result from reporting bias, as even physicians are commonly not aware of these dose equivalencies; patients are expected to be even less so.
A second key finding is that whereas adverse-effect cases commonly arise within weeks after drug initiation, new cases can continue to arise for statin-associated MAEs after successful symptom-free use. Muscle-related adverse effects occurring after prolonged successful statin use also reportedly resolved with drug discontinuation and recurred with rechallenge, suggesting that these cases were also causally influenced by the statin. Factors that could explain delayed onset of MAEs (new onset after prolonged statin tolerance) include the following: patient’s continual aging (age is a risk factor for statin-related adverse effects47); accrual of muscle or mitochondrial dysfunction until clinical thresholds are achieved with continued use47; lags in depletion of tissue vitamin D, coenzyme Q10, or other factors after reduction in blood lipid levels with statins48; and interposition of drugs, exposures, or conditions that affect statin metabolism or contribute to mitochondrial (or other statin-associated) vulnerability. In addition, evidence suggests that statins may be toxic to muscle satellite cells (i.e., the muscle stem cells that enable regeneration and repair after injury).49 Thus, symptoms may arise in some patients only after accrued muscle wear and tear without repair that surpasses a clinical threshold.50 (Mitochondrial effects are also well recognized to progress subclinically until clinical thresholds are achieved.) The significantly shorter latency with rechallenge is compatible with postulated mechanisms of the adverse effects of statins, including reduced levels of coenzyme Q10,48 partially reversible mitochondrial injury,7 and injury to muscle satellite cells.49, 50 The study findings suggest that although new development of MAEs taper with duration of time receiving statin therapy, there may be no time period of successful, symptom-free statin use that guarantees future freedom from statin-associated MAEs.
A third observation is that mean recovery time after statin rechallenge (12 wks) was double that of mean recovery time after first statin-associated MAE (6 wks). Although in our sample this difference did not achieve statistical significance, it suggests additional studies should examine whether recovery is further impaired with rechallenge in some patients.
A fourth key finding is that statin-associated MAEs significantly affected many domains of QOL. Walking and running were particularly affected, which is relevant to mortality because of the established benefits of exercise to health and QOL extending to cardiovascular disease,51, 52 peripheral arterial disease,53, 54 cognitive function,55 mood,56 bone density,57 all elements of metabolic syndrome,58 diabetes mellitus,59 as well as cancer60, 61 and all-cause mortality52, 62 in observational studies. General QOL domains were also significantly negatively impacted; among queried domains, pain was the most affected followed by recreational function. These data are buttressed by existing literature; it has been postulated that statins may interfere with muscle response to physical exertion,63 and in a clinical observational setting, a great number of patients who indicated MAEs noted that symptoms prevented even moderate exertion.24 Moreover, a previous study has shown a majority of professional athletes with familial hyperlipidemia could not tolerate statins because of MAEs.19
A relevant observation is that large numbers of statin-associated MAEs may affect patients for whom, available evidence indicates, benefits from statin therapy do not exceed risks. Based on objective outcomes that balance risks and benefits of statin therapy such as overall mortality and all-cause morbidity (by the available proxy, all-cause serious adverse events), 66% of the patients (examining the probable or definite subgroup) were female and/or elderly (≥ 70 yrs), groups for whom even patients at high risk for cardiovascular disease have shown no trend to net benefit from statins in randomized controlled trial data41 (4S35 and LIPID36 for women, PROSPER37 for elderly). Thirty-four percent were middle-aged men, a group for which some—those with cardiovascular disease and/or some risk characteristics—have prospects for net benefit. However, this may overstate those with prospects of benefit; men without such characteristics have shown no trend toward lower overall death rate or morbidity with statin use (AFCAPS38, 40). Thus, many individuals who experience muscle-related problems that may arise from statin therapy were in groups with no expectation of net health or survival benefit from statin therapy. The risk-benefit balance may bear consideration for determining whether statin rechallenge, in a patient who has experienced a putative adverse effect, is in fact merited.
A substantial literature review has linked mitochondrial mechanisms to statin-associated MAEs.47 Statins inhibit the mevalonate pathway, with products including coenzyme Q10 and heme A (as well as steroid hormones including testosterone, estrogens, and vitamin D); dose-dependent reductions can cause effects that serve to cause or amplify mitochondrial dysfunction.47 Muscles are highly aerobically dependent “post-mitotic” tissues, highly dependent on mitochondrial function and vulnerable to its alteration. Muscle-related symptoms and pathologic conditions reflect classic manifestations of mitochondrial dysfunction, potentially accounting for the high rate of MAEs relative to other statin-related adverse effects.64
The finding of dose dependence for nonrhabdomyolytic MAEs of statins within individuals parallels findings of dose dependence across populations for MAEs (e.g., rhabdomyolysis,15, 16, 65 CK elevation,8, 13 and non–CK-elevating muscle-related symptoms66). It is consistent with heightened risk of rhabdomyolysis cited with high doses of more potent statins on a per-milligram basis, including rosuvastatin,67–69 and, formerly, cerivastatin.
The finding of dose dependence is relevant in light of guideline revisions endorsing more aggressive statin use, targeting lower LDL levels.70 Resulting considerations are of particular importance for groups in whom mortality benefit from statins is not suggested by current randomized controlled trial literature, such as women, the elderly, and persons not at high risk for cardiovascular disease.39 It may bear note that higher versus lower potency statin comparisons in randomized controlled trials have failed to show mortality benefit with higher potency statin use (the sole identified exception was in the acute coronary syndrome setting).41, 71
Limitations
This study has limitations, including those common to most surveillance approaches. There is no defined base population or control group, precluding calculation of relative rates or risk ratios. However, only patients who sustained a putative adverse effect are relevant to the goal of characterizing and understanding an adverse effect, its natural history, relation to dose and/or drug, time course, recovery, and QOL impact. This study provides data that randomized controlled trials do not provide, including information on continued use after onset of adverse effect, recovery time from adverse effects, or effect of switching drugs with or without change in drug potency. Moreover, patients serve as their own controls when they undergo rechallenge with the possible causative agent.
Self-selection to participate may affect external generalizability of study findings, as with all studies involving volunteer subjects. Patient distribution may be skewed, as access to paper surveys occurred primarily through the Internet. Patients with mild problems may not be motivated to participate; and those with severe problems may be less able to participate—in this and other studies. However, the need to evaluate patients with minimal effects is limited; and more extensive literature exists for the most severe MAEs (e.g., entailing rhabdomyolysis); this study fills a significant gap in the literature for intermediate statin-related adverse effects. Moreover, this study provides data that may be more relevant to the general population, as randomized controlled trials have strict imposed patient-selection criteria that limit generalizability of findings.
The data rely on self-reporting, providing opportunity for recall and reporting bias. However, this limitation pervades all studies entailing questionnaires, and there is no basis to suppose its impact is greater here. Previous studies have shown that patient self-reporting of adverse effects can be a valuable and reliable tool.23, 72–75 Physician-targeted reporting is not inherently more reliable and has prospects for bias especially if the physician’s actions may have contributed to the adverse effect, and in addition cannot validly address subjective elements such as QOL impact.
Patients may be flawed in their inferences regarding statin causality of the adverse effect. However, 85% of eligible cases met literature-based criteria for definite or probable drug causality of the adverse effect. (This is expected to understate the fraction of probable or definite cases because some patients completed the survey within days of discontinuing the statin and had not yet had a chance for improvement while discontinuing the statin to allow for probable causality to be achieved.) Moreover, the data are internally validating: a strong relationship was seen between the statin potency and MAE risk; and uniform recurrence of symptoms in patients who underwent rechallenge with higher potency statins militates against a major contribution by incidental symptoms falsely ascribed to statins. This study does not focus on MAEs arising with nonstatin cholesterol-lowering drugs, although some patients also experienced problems while taking other classes of cholesterol-lowering drugs and common underlying mechanisms are likely.
Although postmarketing surveillance evaluations have limitations for certain important functions (they cannot provide rates and risk ratios), they do have advantages for assessment of the character and natural history of the adverse effect. They focus directly on the population with relevance to understanding the characteristics and impact of adverse effects, that is, those who have experienced adverse effects.
Conclusion
Findings from this study extend existing literature on MAEs. Information based on patient self-report suggests that MAEs can arise with all statins, are dose dependent, can develop (although less commonly) even well after statin initiation, have significant functional and QOL implications, and do not universally show full recovery. Many individuals exhibiting MAEs were in groups for whom there is no expected mortality benefit from statins. Physicians should be aware of statin-associated MAEs and their functional and QOL implications to enable more informed risk-benefit decisions, and to allow effective treatment modification when MAEs occur.
Acknowledgments
Funded by a Robert Wood Johnson Generalist Physician Faculty Scholar Award to Dr. Golomb; an Inamori Research Scholarship Fund of the Chancellor’s Associates Undergraduate Research award and the American Heart Association Undergraduate Research Program award to Ms. Cham; and kind contributions to the University of California–San Diego Statin Study Group research fund.
We thank John McGraw for assistance with recruitment and surveys; Joe Graedon, M.S., Theresa Graedon, Ph.D., and John McGuire for invaluable assistance with recruitment early in this study; Sabrina Koperski for outstanding administrative and editorial assistance; and Elaine Yang, who conducted an earlier analysis involving 100 patients. We appreciatively acknowledge help from Janis Ritchie and all members of the UCSD Statin Effects Study team.
Footnotes
For reprints, visit http://www.atypon-link.com/PPI/loi/phco.
Presented in part at the 43rd Annual Conference on Cardiovascular Disease Epidemiology and Prevention, Miami, Florida, March 5–8, 2003.
References
- 1.IMS Health. [Accessed April 19, 2002];US top ten products by prescriptions. Available from http://wwwimshealthcom/public/structure/dispcontent/1,2779,1343-1343-144004,00html.
- 2.IMS Health. [Accessed May 23, 2005];Lipitor leads the way in 2003. Available from http://wwwims-globalcom/insight/news_story/0403/news_story_040316htm.
- 3.IMS Health. [Accessed May 2, 2008];IMS global insights – IMS retail drug monitor. 2007 Dec; Available from http://wwwimshealthcom/web/content/0,3148,64576068_63872702_70260998_83746585,00html.
- 4.Scott RS, Lintott CJ, Wilson MJ. Simvastatin and side effects. N Z Med J. 1991;104:493–5. [PubMed] [Google Scholar]
- 5.Wierzbicki AS, Lumb PJ, Semra Y, Chik G, Christ ER, Crook MA. Atorvastatin compared with simvastatin-based therapies in the management of severe familial hyperlipidaemias. QJM. 1999;92:387–94. doi: 10.1093/qjmed/92.7.387. [DOI] [PubMed] [Google Scholar]
- 6.Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581–5. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- 7.Sinzinger H, Schmid P, O’Grady J. Two different types of exercise-induced muscle pain without myopathy and CK-elevation during HMG-Co-enzyme-A-reductase inhibitor treatment. Atherosclerosis. 1999;143:459–60. doi: 10.1016/s0021-9150(98)00310-4. [DOI] [PubMed] [Google Scholar]
- 8.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Does more aggressive statin therapy increase muscle and liver risk?. Presented at the 55th annual meeting of the American College of Cardiology; Atlanta, GA. March 11–14, 2006. [Google Scholar]
- 9.Dobkin BH. Underappreciated statin-induced myopathic weakness causes disability. Neurorehabil Neural Repair. 2005;19:259–63. doi: 10.1177/1545968305277167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.England JD, Walsh JC, Stewart P, Boyd I, Rohan A, Halmagyi GM. Mitochondrial myopathy developing on treatment with the HMG CoA reductase inhibitors—simvastatin and pravastatin. Aust N Z J Med. 1995;25:374–5. doi: 10.1111/j.1445-5994.1995.tb01912.x. [DOI] [PubMed] [Google Scholar]
- 11.England JD, Viles A, Walsh JC, Stewart PM. Muscle side effects associated with simvastatin therapy. Med J Aust. 1990;153:562–3. doi: 10.5694/j.1326-5377.1990.tb126217.x. [DOI] [PubMed] [Google Scholar]
- 12.Sinzinger H. Does vitamin E beneficially affect muscle pains during HMG-Co-enzyme-A-reductase inhibitors without CK-elevation [letter]? Atherosclerosis. 2000;149:225. doi: 10.1016/s0021-9150(99)00422-0. [DOI] [PubMed] [Google Scholar]
- 13.Silva M, Matthews ML, Jarvis C, et al. Meta-analysis of drug-induced adverse events associated with intensive-dose statin therapy. Clin Ther. 2007;29:253–60. doi: 10.1016/j.clinthera.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Antons KA, Williams CD, Baker SK, Phillips PS. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119:400–9. doi: 10.1016/j.amjmed.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Cziraky MJ, Willey VJ, McKenney JM. Statin safety: an assessment using an administrative claims database. Am J Cardiol. 2006;97:C61–8. doi: 10.1016/j.amjcard.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy: a genomewide study. N Engl J Med. 2008;359:789–99. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 17.Sinzinger H, Chehne F, Lupattelli G. Oxidation injury in patients receiving HMG-CoA reductase inhibitors: occurrence in patients without enzyme elevation or myopathy. Drug Saf. 2002;25:877–83. doi: 10.2165/00002018-200225120-00005. [DOI] [PubMed] [Google Scholar]
- 18.Sinzinger H, Lupattelli G, Chehne F, Oguogho A, Furberg CD. Isoprostane 8-epi-PGF2alpha is frequently increased in patients with muscle pain and/or CK-elevation after HMG-Co-enzyme-A-reductase inhibitor therapy. J Clin Pharm Ther. 2001;26:303–10. doi: 10.1046/j.1365-2710.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- 19.Sinzinger H, O’Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol. 2004;57:525–8. doi: 10.1111/j.1365-2125.2004.02044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinzinger H, Wolfram R, Peskar BA. Muscular side effects of statins. J Cardiovasc Pharmacol. 2002;40:163–71. doi: 10.1097/00005344-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Vladutiu GD, Simmons Z, Isackson PJ, et al. Genetic risk factors associated with lipid-lowering drug–induced myopathies. Muscle Nerve. 2006;34:153–62. doi: 10.1002/mus.20567. [DOI] [PubMed] [Google Scholar]
- 22.Phillips PS, Haas RH. Observations from a statin myopathy clinic. Arch Intern Med. 2006;166:1232–3. doi: 10.1001/archinte.166.11.1232-b. [DOI] [PubMed] [Google Scholar]
- 23.Fisher S, Bryant SG, Kent TA, Davis JE. Patient drug attributions and postmarketing surveillance. Pharmacotherapy. 1994;14:202–9. [PubMed] [Google Scholar]
- 24.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients: the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–14. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 25.Golomb BA, McGraw JJ, Evans MA, Dimsdale JE. Physician response to patient reports of adverse drug effects: implications for patient-targeted adverse effect surveillance. Drug Saf. 2007;30:669–75. doi: 10.2165/00002018-200730080-00003. [DOI] [PubMed] [Google Scholar]
- 26.Griffin GC, Parkinson RW, Woolley BH. Report every adverse drug reaction! We’re all in this together. Postgrad Med. 1997;101:13–16. doi: 10.3810/pgm.1997.04.192. [DOI] [PubMed] [Google Scholar]
- 27.Naranjo CC, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 28.Food and Drug Administration. [Accessed March 2, 2005];FDA public health advisory on Crestor (rosuvastatin) Available from http://wwwfdagov/cder/drug/advisory/crestor_3_2005htm.
- 29.Roberts WC. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am J Cardiol. 1997;80:106–7. [PubMed] [Google Scholar]
- 30.Miller AE, Hansen LB, Saseen JJ. Switching statin therapy using a pharmacist-managed therapeutic conversion program versus usual care conversion among indigent patients. Pharmacotherapy. 2008;28:553–61. doi: 10.1592/phco.28.5.553. [DOI] [PubMed] [Google Scholar]
- 31.Golomb BA, Criqui MH, White HL, Dimsdale JE. The UCSD statin study: a randomized controlled trial assessing the impact of statins on selected noncardiac outcomes. Control Clin Trials. 2004;25:178–202. doi: 10.1016/j.cct.2003.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark P, Lavielle P, Martinez H. Learning from pain scales: patient perspective. J Rheumatol. 2003;30:1584–8. [PubMed] [Google Scholar]
- 33.Rosier EM, Iadarola MJ, Coghill RC. Reproducibility of pain measurement and pain perception. Pain. 2002;98:205–16. doi: 10.1016/s0304-3959(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 34.Gill TM, Feinstein AR. A critical appraisal of the quality of quality-of-life measurements. JAMA. 1994;272:619–26. [PubMed] [Google Scholar]
- 35.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 36.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 37.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 38.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas coronary atherosclerosis prevention study. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 39.Criqui MH, Golomb BA. Low and lowered cholesterol and total mortality. J Am Coll Cardiol. 2004;44:1009–10. doi: 10.1016/j.jacc.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 40.Kaan A. Serious adverse event analysis: lipid-lowering therapy revisited. Therapeutics Letter. 2001;42(Aug–Oct):1–2. [PubMed] [Google Scholar]
- 41.Golomb BA, Parrish J, Broadwin JA. Statins and mortality. On the Risk. 2009;25:66–71. [Google Scholar]
- 42.IMS Health. [Accessed April 17, 2009];Commonly requested therapeutic class and product Information. (update February 2006). Available from http://www1imshealthcom/ims/portal/front/articleC/0,2777,6599_18731_77056778,00html.
- 43.Mazzu AL, Lasseter KC, Shamblen EC, Agarwal V, Lettieri J, Sundaresen P. Itraconazole alters the pharmacokinetics of atorvastatin to a greater extent than either cerivastatin or pravastatin. Clin Pharmacol Ther. 2000;68:391–400. doi: 10.1067/mcp.2000.110537. [DOI] [PubMed] [Google Scholar]
- 44.Ose L, Luurila O, Eriksson J, Olsson A, Lithell H, Widgren B for the Cerivastatin Study Group. Efficacy and safety of cerivastatin, 0.2 mg and 0. 4 mg, in patients with primary hypercholesterolaemia: a multinational, randomised, double-blind study. Curr Med Res Opin. 1999;15:228–40. doi: 10.1185/03007999909114095. [DOI] [PubMed] [Google Scholar]
- 45.Farnier M, Davignon J. Current and future treatment of hyperlipidemia: the role of statins. Am J Cardiol. 1998;82:J3–10. doi: 10.1016/s0002-9149(98)00423-8. [DOI] [PubMed] [Google Scholar]
- 46.Lalani SR, Vladutiu GD, Plunkett K, Lotze TE, Adesina AM, Scaglia F. Isolated mitochondrial myopathy associated with muscle coenzyme Q10 deficiency. Arch Neurol. 2005;62:317–20. doi: 10.1001/archneur.62.2.317. [DOI] [PubMed] [Google Scholar]
- 47.Golomb BA, Evans MA. Statin adverse effects: a review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs. 2008;8:373–418. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mortensen SA, Leth A, Agner E, Rohde M. Dose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitors. Mol Aspects Med. 1997;18(suppl):S137–44. doi: 10.1016/s0098-2997(97)00014-9. [DOI] [PubMed] [Google Scholar]
- 49.Hill CF, Schwartz LM, Thompson PD, Clarkson PM. Effects of statin treatment and supplemental CoQ10 on C2C12 myotubes: 2436. Med Sci Sports Exerc. 2005;37(5 suppl):S466–7. [Google Scholar]
- 50.Rossignol R, Faustin B, Rocher C, Malgat M, Mazat JP, Letellier T. Mitochondrial threshold effects. Biochem J. 2003;370(pt 3):751–62. doi: 10.1042/BJ20021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 52.Matthews CE, Jurj AL, Shu XO, et al. Influence of exercise, walking, cycling, and overall nonexercise physical activity on mortality in Chinese women. Am J Epidemiol. 2007;165:1343–50. doi: 10.1093/aje/kwm088. [DOI] [PubMed] [Google Scholar]
- 53.Treat-Jacobson D, Walsh ME. Treating patients with peripheral arterial disease and claudication. J Vasc Nurs. 2003;21:5–14. doi: 10.1067/mvn.2003.2. [DOI] [PubMed] [Google Scholar]
- 54.Khan S, Cleanthis M, Smout J, Flather M, Stansby G. Lifestyle modification in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2005;29:2–9. doi: 10.1016/j.ejvs.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 55.Okumiya K, Matsubayashi K, Wada T, Kimura S, Doi Y, Ozawa T. Effects of exercise on neurobehavioral function in community-dwelling older people more than 75 years of age. J Am Geriatr Soc. 1996;44:569–72. doi: 10.1111/j.1532-5415.1996.tb01444.x. [DOI] [PubMed] [Google Scholar]
- 56.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Ernst E. Exercise for female osteoporosis: a systematic review of randomised clinical trials. Sports Med. 1998;25:359–68. doi: 10.2165/00007256-199825060-00002. [DOI] [PubMed] [Google Scholar]
- 58.Gaesser GA. Exercise for prevention and treatment of cardiovascular disease, type 2 diabetes, and metabolic syndrome. Curr Diab Rep. 2007;7:14–19. doi: 10.1007/s11892-007-0004-8. [DOI] [PubMed] [Google Scholar]
- 59.Church TS, Cheng YJ, Earnest CP, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care. 2004;27:83–8. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- 60.Bernstein L, Patel AV, Ursin G, et al. Lifetime recreational exercise activity and breast cancer risk among black women and white women. J Natl Cancer Inst. 2005;97:1671–9. doi: 10.1093/jnci/dji374. [DOI] [PubMed] [Google Scholar]
- 61.Hirose K, Hamajima N, Takezaki T, Miura S, Tajima K. Physical exercise reduces risk of breast cancer in Japanese women. Cancer Sci. 2003;94:193–9. doi: 10.1111/j.1349-7006.2003.tb01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schnohr P, Parner J, Lange P. Joggers live longer: the Osterbro study [in Danish] Ugeskr Laeger. 2001;163:2633–5. [PubMed] [Google Scholar]
- 63.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–90. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 64.De Vivo DC, DiMauro S. Mitochondrial defects of brain and muscle. Biol Neonate. 1990;58(suppl 1):54–69. doi: 10.1159/000243300. [DOI] [PubMed] [Google Scholar]
- 65.Scranton RE, Cantillon C, Gagnon D, Fiore L, Gaziano JM. Occurrences of rhabdomyolysis or myositis among statin users in a Veteran Affairs population [abstract] Circulation. 2004;109:P154. [Google Scholar]
- 66.Golomb B, Yang E, Denenberg J, Criqui M. Statin-associated muscle adverse effects [abstract] Circulation. 2003;107:e7028–9. [Google Scholar]
- 67.Danish Medicines Agency. [Accessed January 15, 2005];Changes of product information for Crestor (rosuvastatin) [updated 2004]. Available from http://www.dkma.dk/1024/visUKLSArtikel.asp?artikelID=3546.
- 68.Hosein S. Warnings issued in Canada and the European Union about lipid drug. [Accessed January 15, 2005];CATIE News. 2004 Jun 28; Available from http://www.aegis.com/news/catie/2004/CATE-N20040602.html.
- 69.Davidson MH. Rosuvastatin safety: lessons from the FDA review and post-approval surveillance. Expert Opin Drug Saf. 2004;3:547–57. doi: 10.1517/14740338.3.6.547. [DOI] [PubMed] [Google Scholar]
- 70.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Arterioscler Thromb Vasc Biol. 2004;24:e149–61. doi: 10.1161/01.ATV.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 71.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 72.Fisher S, Bryant SG, Kluge RM. New approaches to postmarketing surveillance. Psychopharmacology (Berl) 1986;90:347–50. doi: 10.1007/BF00179189. [DOI] [PubMed] [Google Scholar]
- 73.Fisher S, Bryant SG. Postmarketing surveillance: accuracy of patient drug attribution judgments. Clin Pharmacol Ther. 1990;48:102–7. doi: 10.1038/clpt.1990.123. [DOI] [PubMed] [Google Scholar]
- 74.Fisher S, Bryant SG. Postmarketing surveillance of adverse drug reactions: patient self-monitoring. J Am Board Fam Pract. 1992;5:17–25. [PubMed] [Google Scholar]
- 75.Jarernsiripornkul N, Krska J, Richards RM, Capps PA. Patient reporting of adverse drug reactions: useful information for pain management? Eur J Pain. 2003;7:219–24. doi: 10.1016/S1090-3801(02)00114-3. [DOI] [PubMed] [Google Scholar]