Abstract
Human adenocarcinomas overexpress a hypoglycosylated, tumor-associated form of the mucin-like glycoprotein MUC1 containing abnormal mono- and disaccharide antigens, such as Tn, sialyl-Tn, and TF, as well as stretches of unglycosylated protein backbone in the variable number of tandem repeats (VNTR) region. Both peptide and glycopeptide epitopes generated from the VNTR are candidates for cancer vaccines and we performed experiments to evaluate their relative potential to elicit tumor-MUC1-specific immunity. We show here that immunization with the 100 amino acid-long VNTR peptide (MUC1p) elicits weaker responses in MUC1 transgenic mice compared to wild type mice suggesting self-tolerance. In contrast, when glycosylated with tumor-associated Tn antigen (GalNAc-O-S/T), TnMUC1 induces glycopeptide-specific T cell and antibody responses in both strains of mice and helps enhance responses to MUC1p in MUC1 transgenic mice. Using newly derived MUC1-specific mouse T cell hybridomas we show that the only antigen-presenting cells able to cross-present TnMUC1 glycopeptide are dendritic cells (DCs). This is likely due to their exclusive expression of receptors capable of binding TnMUC1. We conclude that MUC1 glycopeptides induce stronger immunity in MUC1-Tg mice because they are recognized as ‘foreign’ rather than ‘self’ and because they are cross-presented preferentially by DCs.
Keywords: cancer, glycoprotein, Tn antigen, tumor antigen, vaccine
Introduction
Transformed cells can aberrantly express many self-proteins that elicit immune responses. Because they are not mutated and because they are also expressed, albeit differently, on normal cells, they are generally termed tumor-associated antigens (Ryan et al., 2007). Several new mouse models that express human tumor antigens as transgenes are beginning to reveal that in spite of the ability of many of these antigens to induce antibodies and T cells in cancer patients, they may nevertheless be subject to a certain level of self-tolerance mediated by different mechanisms (Tempero et al., 1998; Reilly et al., 2000; Nichols et al., 2007; Bos et al., 2008). This may in part explain why patients with detectable immune responses against tumor-associated peptides still succumb to the disease, and why attempts to boost these responses using peptide vaccines have been met with only a limited success. Many of these antigens are potential sources of epitopes that are very different when derived from the tumor form of the antigen versus the normal form found on normal cells, and it is of interest to explore if self-tolerance can be avoided by choosing epitopes that are more tumor-specific.
Most human adenocarcinomas overexpress an abnormal form of the transmembrane glycoprotein mucin 1 (MUC1), characterized by exposure of the bare VNTR protein backbone in its extracellular domain due to its marked hypoglycosylation. MUC1-specific CD8+ cytotoxic T cells (CTL) recognizing variable number of tandem repeat (VNTR) peptides are found in patients with MUC1+ tumors indicating that an immune response can be generated against them (Jerome et al., 1991, 1993). However, clinical trials using MUC1 peptide based vaccines to boost this immunity have resulted in little change in CTL activity and ineffective anti-MUC1 antibody class switching beyond the IgM isotype (Goydos et al., 1996; Ramanathan et al., 2005; Lepisto et al., 2008). Studies in the MUC1-transgenic (MUC1-Tg) mouse model, which mimics the self-tolerant environment for MUC1 peptide-specific immune responses of humans, have shown that low antibody and CTL responses to MUC1 VNTR peptides are due in part to very low level of CD4 T cell responses compared to responses in transgene-negative litter mates (Gerloni et al., 2005; Turner et al., 2007; Ding et al., 2008). This is of concern since CD4 T helper cells are critical components of effective anti-tumor immunity and required for effective B cell priming and antibody isotype switching, CD8 CTL expansion, and CD8 memory responses (Kennedy and Celis, 2008).
As an alternative to tumor-derived peptides that induce low responses, tumor-associated glycoprotein antigens, such as MUC1, could be targeted via their tumor-associated glycopeptides (Vlad and Finn, 2004). Some tumor-associated glycoproteins carry the Tn (GalNAc-O-S/T) and T (Gal-GalNAc-O-S/T) glycan antigens. These carbohydrate antigens are not normally exposed on healthy cells or tissues with benign disease. Conversely, approximately 90% of carcinomas (many being adenocarcinomas) express these tumor-associated carbohydrates (Springer, 1997). Tumor-associated glycopeptides derived from tumor MUC1 have been shown to be viable targets for T cells and anti-tumor immunity (Vlad et al., 2002; Xu et al., 2004; Ninkovic et al., 2009). It has not yet been determined, however, whether tumor-derived glycopeptides would face self-tolerance similar to that of peptides. Using two different vaccination methods we show for the first time that incorporation of tumor-associated Tn antigens on MUC1 VNTR peptides not only induces glycopeptide-specific responses but can also boost previously suppressed MUC1 peptide-specific T cell and antibody responses in the MUC1-Tg mouse. We generated MUC1 glycopeptide-specific CD4 T cell hybridomas and could show that dendritic cells and B cells differ in their ability to present epitopes derived from the MUC1 glycopeptide, but are equally efficient in presenting the MUC1 peptide antigen.
Results
MUC1 peptide (MUC1p) and MUC1 glycopeptide (TnMUC1) are handled differently by the immune systems of wild type (WT) versus MUC1-Tg mice
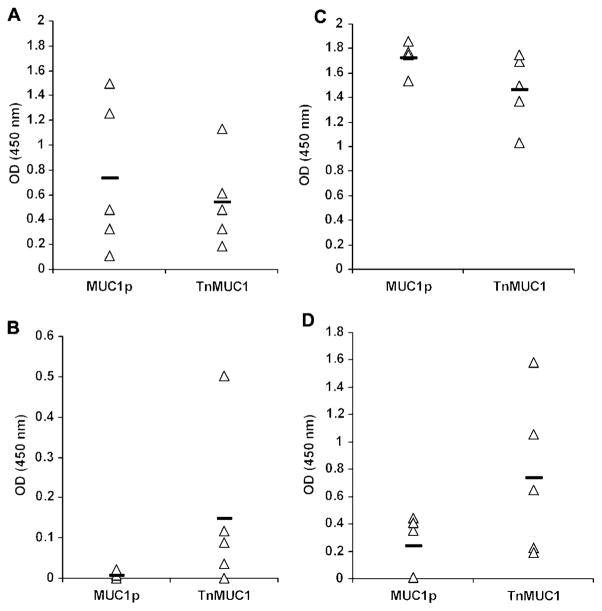
To address the effect of tumor-associated carbohydrates on anti-MUC1 peptide immune responses in MUC1-Tg mice, where the peptides may be seen as self, we compared them to responses in transgene-negative (WT) mice where the human MUC1 peptide sequence, which is not homologous to mouse Muc1, might be seen as foreign. The MUC1 vaccine was composed of a soluble adjuvant E6020, a synthetic Lipid A mimetic (Przetak et al., 2003), mixed with either a 100 amino acid peptide from the MUC1 VNTR region (MUC1p) or the same MUC1p glycosylated in vitro to contain multiple tumor-associated Tn (GalNAc-O-S/T) moieties (TnMUC1). As we expected, E6020-MUC1p and E6020-TnMUC1 induced strong anti-MUC1 IgG antibody responses in WT mice (Figure 1A) indicating an effective T cell help for T cell dependent isotype switch from IgM. MUC1-Tg mice were hyporesponsive to the E6020-MUC1p vaccine, producing no detectable MUC1-specific IgG antibody (Figure 1B) suggesting a lack of helper T cell activation. This result is consistent with the hypothesis that these transgenic mice may see MUC1p as a self-antigen. In contrast, MUC1-specific IgG responses were observed in the majority (80%) of MUC1-Tg mice after two doses of the E6020-TnMUC1 vaccine (Figure 1B). These responses were further boosted by giving a third vaccine dose (Figure 1D), although antibody titers were 10-fold lower than in WT mice (Figure 1C).
Figure 1. TnMUC1 vaccine induces anti-MUC1 IgG in MUC1-Tg mice, while MUC1p vaccine does not.
WT and MUC1-Tg mice were vaccinated subcutaneously with E6020 (3 μg) adjuvant plus equimolar amounts of MUC1p (80 μg) or TnMUC1 (104 μg). Anti-MUC1 IgG was measured by ELISA at 4 d after the second vaccine dose in WT (A) and MUC1-Tg (B) mice. Antibody measurements were performed again 6 d following the final vaccine dose in WT (C) and MUC1-Tg (D) mice. Individual groups are labeled by the vaccine received. Data points represent optical density (OD) values (post-vaccine OD subtracted from pre-vaccine OD) at 1:1000 serum dilution (A, C) and 1:100 serum dilution (B, D). The average OD value of each individual group (n=5) is indicated by a solid bar.
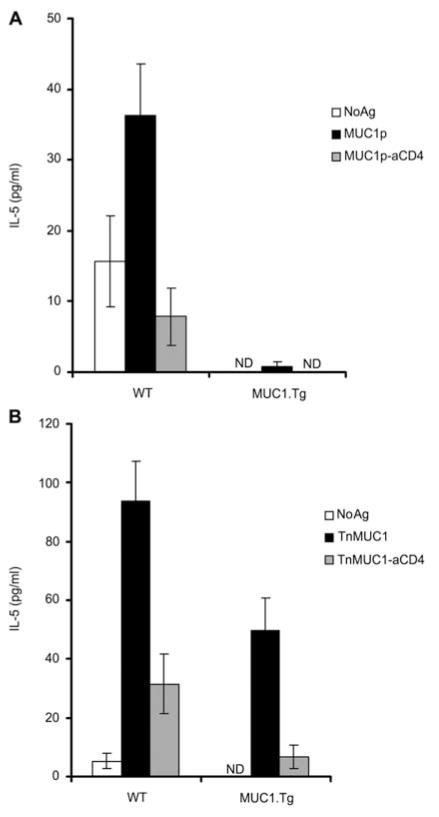
Consistent with the weak MUC1-specific IgG antibody response to the E6020-MUC1p vaccine, in MUC1-Tg mice we could not detect MUC1-specific T cells. Strong MUC1p-specific antibody responses in WT mice were accompanied by MUC1-specific CD4 T cell activation (Figure 2A). The E6020-TnMUC1 vaccine induced MUC1-specific CD4 T cell responses in both MUC1-Tg mice and WT mice (Figure 2B), as measured by specific production of interleukin 5 (IL-5). We measured IL-5 instead of interferon γ (IFN-γ) because it is a T cell specific cytokine and this way we avoid measuring IFN-γ produced by other cells in culture, such as natural killer or natural killer T cells, which may have been stimulated by the adjuvant.
Figure 2. TnMUC1 plus adjuvant vaccine elicits CD4 T cells in MUC1-Tg mice.
WT mice and MUC1-Tg mice were vaccinated three times with E6020 (3 μg) plus equimolar amounts of (A) MUC1p (2 μg) or (B) TnMUC1 (2.6 μg). Seven days following the final vaccine mice were sacrificed and lymphocytes were collected. Cells isolated from each mouse were stimulated once in vitro using DCs pulsed with the same form of MUC1 used in the vaccine, followed by a second in vitro stimulation 7 d later. To detect MUC1-specific responses, cells from each mouse were restimulated with either DC alone (NoAg, white bar), DCs pulsed with MUC1 (black bar), or DCs pulsed with MUC1 in the presence of anti-CD4 blocking antibody (gray bar). Then, 48 h following the second stimulation cell supernatants were collected and IL-5 was detected by ELISA. Responses from representative mice per vaccine group are shown.
MUC1-Tg mice have improved responses to TnMUC1-versus MUC1p-pulsed dendritic cell (DC) vaccine
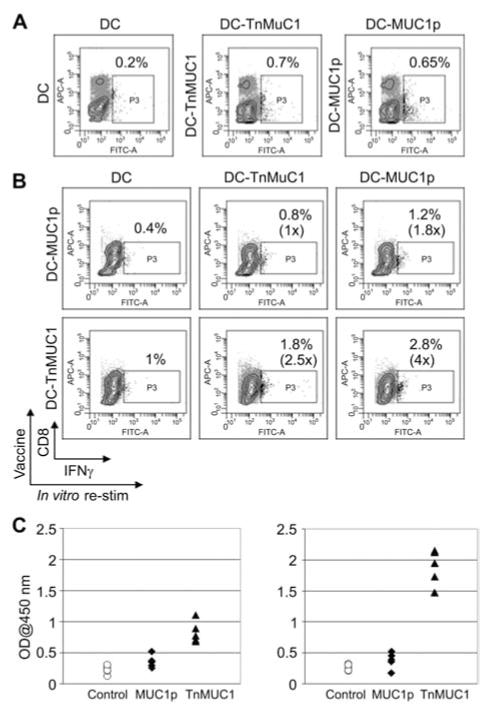
In addition to MUC1 plus adjuvant vaccines, we also tested anti-MUC1 peptide and glycopeptide responses when triggered by antigen-pulsed dendritic cells (DC-MUC1 vaccines). The DC-MUC1p vaccine has been used in both preclinical (Soares et al., 2001) and clinical (Lepisto et al., 2008) studies, but it has not been compared side-by-side with the DC-TnMUC1 vaccine. We vaccinated MUC1-Tg mice with either DC-MUC1p or DC-TnMUC1 and following the final boost we examined the T cell response to the respective forms of MUC1 used to vaccinate. Both DC-MUC1p and DC-TnMUC1 vaccines elicited similar low percentages of MUC1-specific CD4 T cells when examined directly ex vivo (Figure 3A). However, expansion of MUC1-specific IFN-γ producing CD4 T cells after in vitro stimulation was much greater from mice vaccinated and restimulated with TnMUC1 (Figure 3B). Here, we measured antigen-specific IFN-γ production because unlike soluble adjuvants that can stimulate IFN-γ production by other cell types, DC vaccines induce primarily IFN-γ producing T cells. Paralleling the cellular response, we found that MUC1-Tg mice receiving DC-MUC1p vaccine produced much lower IgG antibody responses compared to MUC1-Tg mice vaccinated with DC-TnMUC1 (Figure 3C). Not only did the TnMUC1 vaccine induce antibody against Tn-MUC1 but it also induced higher IgG titers and CD4 T cell responses against MUC1p epitopes. DCs pulsed with TnMUC1 can present both peptide and glycopeptide epitopes to CD4 T cells (Figure 4) (Vlad et al., 2002) and this result shows that T cells reacting to the glycopeptide epitope help the peptide-specific T cells rather than being inhibited by the mechanisms that suppress their function in the absence of the Tn antigen.
Figure 3. TnMUC1-pulsed DC vaccine induces antibody and cellular responses in MUC1-Tg mice and improves MUC1 peptide-specific immunity.
(A) Ex vivo staining for intracellular IFN-γ in spleen cells from mice vaccinated with either control (non-pulsed) DC, MUC1 peptide-pulsed DC (DC-MUC1p) or MUC1 glycopeptide-pulsed DC (DC-TnMUC1). Staining was performed using a pooled fraction of spleen cells from three mice in each group. The cells were stimulated for 4 h with their respective vaccine in the presence of Brefeldin A. Numbers shown represent percentage of T cells secreting IFN-γ. (B) Intracellular staining for IFN-γ after two in vitro restimulations. Spleen cells from DC-MUC1p (upper row) or DC-TnMUC1 (lower row) vaccinated mice were restimulated twice with either control DC (left), DC-TnMUC1 (middle), or DC-MUC1p (right). (C) MUC1-specific serum IgG1 antibodies detected by ELISA. The plates were coated with either MUC1p (left) or TnMUC1 (right). The serum was diluted 1:150.
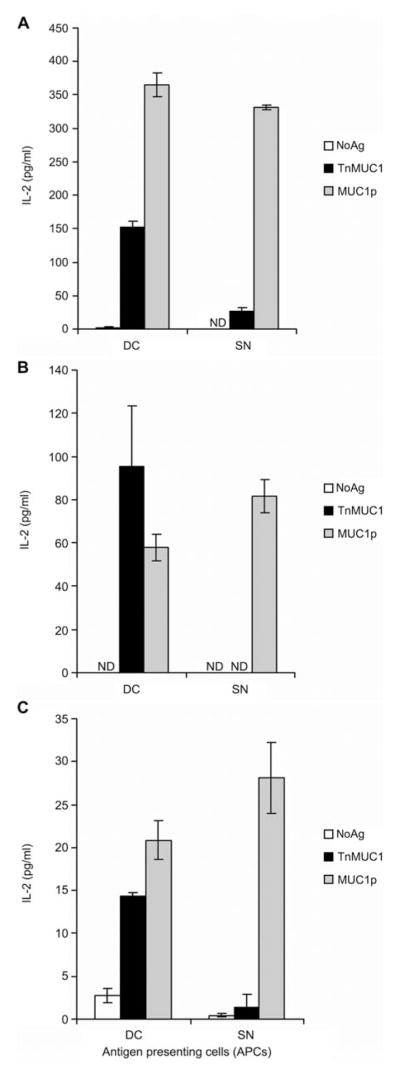
Figure 4. Dendritic cells (DCs) and splenocytes differ in Tn-MUC1 antigen presentation.
Antigen-specific responses by CD4 T cell hybridomas were measured by IL-2 detection by ELISA after 48 h stimulation. Hybridoma population RF78 (A), RF141 (B), or VF5 hybridoma clone (C) were stimulated with either DCs or spleen cells (SN) loaded with nothing (white bar), TnMUC1 (black bar), or MUC1p (gray bar). Data are represented as the average and standard deviation of triplicate wells.
Dendritic cells and B cells differ in MUC1 antigen presentation
Due to the different outcomes we saw in immune responsiveness to MUC1p versus TnMUC1 with both the adjuvant-based and DC-based vaccines, we postulated that there may be inherent differences in how the immune system encounters the two forms (Hiltbold et al., 2000). The soluble antigen delivered with the adjuvant might be presented in vivo by multiple antigen-presenting cell (APC) populations, not only DCs, and different APCs might differ in their ability to take up and present the two different forms, MUC1p and TnMUC1. To test this in vitro we needed MUC1-specific T cell lines; however, these are difficult to establish and maintain long-term. As an alternative, we generated T cell hybridomas with specificity for TnMUC1-derived epitopes and used them to explore differences in antigen presentation.
Our previous work had shown that MUC1 VNTR peptides either unglycosylated (MUC1p) or carrying the tumor-associated Tn antigens (TnMUC1) are taken up by DCs where both forms are processed into peptides presented in MHC class II for peptide-specific CD4 T cell recognition (Vlad et al., 2002). To better understand if TnMUC1 can also be a source of glycopeptides able to activate glycopeptide-specific CD4 T cells, we generated a panel of CD4 T cell hybridomas using T cells from WT mice vaccinated with DC-TnMUC1. We first tested polyclonal hybridoma populations for the presence of glycopeptide specificity. We found that these cell populations were specific for the immunogen TnMUC1, but responded differently when the antigen was presented on bone marrow derived DCs (BMDCs) versus splenocytes. Furthermore, we found that TnMUC1-pulsed DCs can present both glycopeptide and peptide epitopes. For example, when BMDCs were used as APCs, hybridoma population RF78 responded strongly to both TnMUC1 and MUC1p, as monitored by their level of IL-2 secretion (Figure 4A). However, when splenocytes, consisting mainly of B cells (70%), were used as APCs, the response to TnMUC1 by the same RF78 population decreased, while responses to MUC1p remained high (Figure 4A). This was seen with another hybridoma population, RF141, which also responded strongly to Tn-MUC1 when BMDCs were used as APCs, but not when splenocytes were used to present the antigen (Figure 4B). Experiments were repeated using lipopolysaccharide activated B cells as APCs and the same results were obtained as with total spleen cells (data not shown), suggesting that B cells can present MUC1 peptides but not glycopeptides. We confirmed that peptide epitopes are derived from TnMUC1 and that DCs are the only APCs capable of presenting TnMUC1-derived epitopes by using the single-cell cloned VF5 hybridoma (Figure 4C), previously characterized for specificity to a peptide epitope derived from MUC1p (Vlad et al., 2002).
DCs process and present epitopes from TnMUC1 that are recognized by T cells only if glycosylated
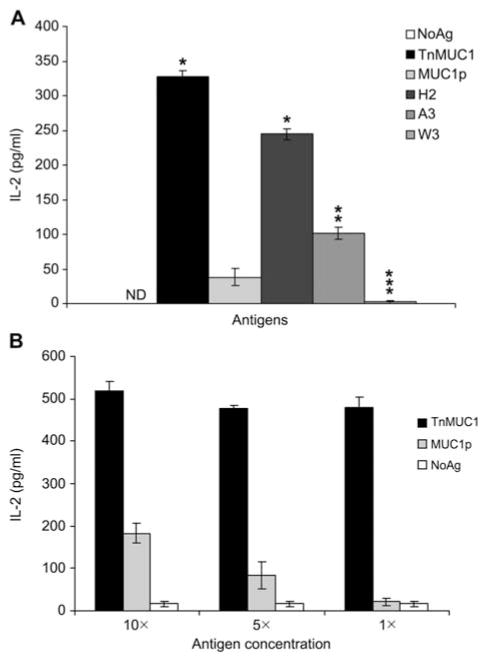
To identify T cell hybridoma clones responding exclusively to glycoepitopes, we subcloned the RF141 population described above and isolated the RF6 clone that responds to DCs pulsed with TnMUC1 and not MUC1p, suggesting that only glycopeptides and not peptides are recognized by its T cell receptor (TCR). We determined that RF6 responds to glycopeptides carrying the Tn antigen on the GS(GalNAc-O-T)A motif (Figure 5A). In addition, RF6 responsiveness decreased when the number of carbohydrate residues O-linked to the threonines in the GSTA motif increased from 1 (optimal) to 3 (Figure 5A). At high antigen concentrations, we observed some cross-reactivity with unglycosylated epitopes derived from MUC1p that decreased precipitously at lower antigen concentration, showing that inclusion of the carbohydrate increases the binding affinity the RF6 TCR and that its preferred epitope is the glycopeptide (Figure 5B). Further characterization of the RF6 hybridoma showed that its TCR is MHC class II restricted (data not shown).
Figure 5. RF6 hybridoma response to TnMUC1-derived epitopes is glycosylation-dependent.
(A) RF6 was stimulated with BMDCs alone (NoAg, white bar) or with different MUC1 peptides: TnMUC1, MUC1p, H2 [Gal-NAc(α1-O)], A3 [Gal(β1-3)-GalNAc(α1-O)], and W3 [Glc(α1-4)-Glc(β1-4)-GalNAc(α1-O)]. (B) Cross-reactivity with peptide epitopes at high antigen concentrations. RF6 were stimulated with the same number of DCs loaded with 26, 13, 2.6 μg/ml TnMUC1 or 20, 10, 2 μg/ml MUC1p (10×, 5×, 1×, respectively). Data are represented as the average and standard deviation of triplicate cell culture wells.
Discussion
Past and present studies in the MUC1-Tg mouse model have indicated that in the context of a ‘self’ environment immune responses to unglycosylated MUC1 VNTR peptides face self-tolerance (Tempero et al., 1998; Soares et al., 2001; Gerloni et al., 2005; Turner et al., 2007; Ding et al., 2008). As a strategy to increase the potency of MUC1-peptide vaccines, we altered the peptide immunogen by incorporating tumor-associated glycans within its structure (i.e., Tn antigen), thus more closely resembling epitopes that would be displayed on MUC1+ tumors and on APCs that cross-present MUC1+ tumors to T cells in patients.
In the present study, we show that incorporation of tumor-associated Tn antigens on MUC1 VNTR peptides (TnMUC1) can boost MUC1 peptide-specific CD4 T cells and promote effective antibody responses that are otherwise suppressed in the MUC1-Tg mouse. In vitro studies indicate that DCs are the main APCs capable of cross-presenting TnMUC1 for CD4 T cell stimulation. Ensuring that vaccines will target TnMUC1 to DCs in vivo would be beneficial not only because they are the professional APCs responsible for driving strong adaptive immune responses, but also because the optimal MUC1 antigen is not presented by other APCs. Based on our observations, it could be extrapolated that intradermal delivery of the vaccines would be optimal and adjuvants that are administered via this route and able to stimulate dermal DCs would be expected to be more efficient than those administered via an intramuscular route.
An efficient humoral response has been elicited previously in the MUC1-Tg mouse with a vaccine composed of MUC1 VNTR peptides carrying Tn antigens linked to keyhole limpet hemocyanin (KLH) (Sorensen et al., 2006). In this formulation, KLH served as a strong inducer of helper T cell responses. However, since this help is not related to MUC1, these types of vaccines providing heterologous help would not be expected to induce effective anti-tumor memory T cells. MUC1 glycopeptide-specific T cells, on the other hand, can provide MUC1-specific help at the time of priming and also at any later time when, as memory cells, they are challenged with the same antigen expressed on tumor cells.
By directly comparing antibody responses induced by MUC1p and TnMUC1 vaccines in WT versus MUC1-Tg mice, we noticed that even though TnMUC1 can elicit responses in MUC1-Tg mice, those responses were not as strong as the response in WT mice. We postulate that peptide epitopes, processed from TnMUC1 simultaneously with the glycopeptide epitopes, may induce T regulatory cells that may have a suppressive effect on the glycopeptide-specific T cells as well, albeit not to the same extent. We have recently shown that TnMUC1 glycopeptide epitopes are seen as foreign in WT and MUC1-Tg mice (Ryan et al., submitted), thus we believe that a similar TnMUC1-specific CD4 T cell repertoire would be present in both environments. This T cell repertoire would likely recognize Tn-glycosylation in concert with the underling peptide (Deck et al., 1999). However, further studies are needed to confirm this and define the fine specificity of those T cells responding to glycopeptide epitopes derived from TnMUC1. Ideally, we would choose those epitopes that have only a stimulatory effect and delete those that stimulate regulatory T cells. The CD4 T cell hybridomas we described will be useful reagents for such studies. We note that the T cell receptor of the RF6 hybridoma that we described above as being dependent on the glycosylation of the threonine in the GSTA motif of the VNTR region has the same specificity as antibodies generated using TnMUC1 coupled to KLH (Sorensen et al., 2006; Tarp et al., 2007). This epitope is thus recognized both in its native form on the whole antigen as well as a processed peptide in MHC class II.
Studies have shown that different forms of MUC1, depending on glycosylation, are endocytosed by DCs though different means (Hiltbold et al., 2000; Napoletano et al., 2007), yet DCs are not the only APCs that can stimulate CD4 T cell response to a vaccine containing soluble antigen. We show a difference in the ability of BMDCs and splenocytes to present TnMUC1 derived epitopes. While BMDC preparations are routinely 80%–90% CD11c+, the majority of APCs found in bulk splenocytes are B cells (ca. 70%) and splenic DCs make up only a minor percentage. We postulate that the difference in TnMUC1 presentation is due to the absence on B cells of the receptor for TnMUC1, namely the macrophage galactose-type lectin (MGL). The MGL receptor is expressed on immature DCs and macrophages but not on B cells in both mouse (Tsuiji et al., 2002) and human (Valladeau et al., 2001). In addition, the MGL receptor has been shown to specifically bind the Tn antigen (GalNAc) of glycosylated MUC1 and facilitate its internalization into DCs (Napoletano et al., 2007; Saeland et al., 2007). By nature, immature DCs that express the MGL receptor are highly endocytic but are weak stimulators. It might be possible for circulating glycosylated proteins, selectively processed and sub-optimally presented by immature DCs, to induce peripheral deletion of glycopeptide-specific T cells or induction of T regulatory cells (Hawiger et al., 2001; Bruder et al., 2005; Kretschmer et al., 2005). Thus, soluble TnMUC1 should be administered with a potent adjuvant that will properly mature endogenously antigen-loaded DCs (Saeland et al., 2007).
Owing to its antigenicity and escape from tolerance in MUC1-Tg mice, TnMUC1 should be evaluated in clinical trials. Similar to many others, we have evaluated MUC1 VNTR peptides, completing a trial of a DC-MUC1p vaccine (Lepisto et al., 2008) and two trials of MUC1p vaccine using two different adjuvants (Goydos et al., 1996; Ramanathan et al., 2005). Taken together with the studies in the preclinical MUC1-Tg mouse model, the low level responses in patients to the peptide vaccine suggest that improvements are needed. Choosing MUC1 epitopes that are more tumor-specific and less self-like should lead to an improved response. This strategy applies to other tumor-associated antigens that are uniquely modified on tumors, such as those with altered glycosylation, phosphorylation (Zarling et al., 2006), and other post-translational modifications. Focusing the vaccines on altered rather than self-epitopes should improve both efficacy and safety of cancer vaccines.
Materials and methods
Mice and cell lines
All mice were maintained in a pathogen-free environment at the University of Pittsburgh. C57BL/6 mice were originally purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and MUC1-Tg mice (Rowse et al., 1998) from Dr. S. Gendler (Mayo Clinic, Scottsdale, AZ, USA). MUC1-transgene positive and transgene negative (WT) mice from MUC1-Tg by C57BL/6 mouse breeding were identified by PCR analysis. T cell hybrid-omas were generated using a similar method as previously described (Vlad et al., 2002). Briefly, C57BL/6 mice were vaccinated three times using a DC-TnMUC1 vaccine (Soares et al., 2001; Vlad et al., 2002). Seven days after the final vaccination lymphocytes were collected and stimulated once in vitro with TnMUC1-pulsed DCs. Following in vitro stimulation, cells were collected and then fused with the HAT-sensitive BW5147α-β-thymoma line (White et al., 1989) using polyethylene glycol 1500 (Roche, Mannheim, Germany). The resulting T cell hybridomas were screened for CD3 and CD4 expression by FACS analysis before further selection based on IL-2 production in response to TnMUC1 or MUC1p. IL-2 was measured using a BD OptEIA ELI-SA kit following the manufacturer’s protocol (BD Biosciences, San José, CA, USA). The RF6 hybridoma was cloned using single cell limiting dilution.
MUC1 peptides and glycopeptides
The 100mer peptide (MUC1p) represents 5 repeats of the 20 amino acid sequence HGVTSAPDTR PAPGSTAPPA from the MUC1 VNTR region and was synthesized as described previously (Turner et al., 2007). The GalNAc-100mer (Tn100mer/TnMUC1) was prepared by enzymatic addition of GalNAc to the synthetic peptide substrate using a recombinant human UDP-GalNAc:polypeptide N-acetyl-galactosaminyltransferase rGalNAc-T1 as previously described elsewhere (Brokx et al., 2003). The final reaction product contained a heterogeneous mixture of 9–15 GalNAc residues per 100mer peptide molecule that were incorporated within threonine in VTSA region and adjoining serine and threonine within GSTA region as described previously (Brokx et al., 2003). Both MUC1p and TnMUC1 were synthesized at the University of Pittsburgh Genomics and Proteomics Core Laboratories. Glycopeptides H2 [GalNAc(α1-O)], A3 [Gal(β1-3)-GalNAc(α1-O)] and W3 [Glc(α1-4)-Glc(β1-4)-GalNAc(α1-O)] were synthesized as described previously (Vlad et al., 2002).
Generation of bone marrow-derived DCs (BMDCs) and vaccination protocols
BMDCs were generated as previously described (Vlad et al., 2002) with a few modifications. Briefly, bone marrow (C57Bl/6 mice) was collected and subjected to RBC lysis before being plated at 1×106 cells/ml serum-free AIM-V media (Invitrogen, Carlsbad, CA, USA) containing sodium pyruvate, non-essential amino acids, and 2-ME; supplemented with 10 ng/ml each of GM-CSF and IL-4 (a generous gift from Immunex, Seattle, WA, USA). Cells were fed on day 3 by replacing one-half of the culture volume with fresh AIM-V plus 10 ng/ml GM-CSF and IL-4. DCs were purified on day 6 of culture using a Nycoprep 1.068 (Accurate Chemical, Westbury, NY, USA) gradient.
For the DC vaccine, peptides and glycopeptides were first conjugated to BioPORTER (Sigma, St. Louis, MO, USA) for 5 min at room temperature, according to the manufacturer’s instructions, before being added to BMDCs. BMDCs were pulsed with TnMUC1-BioPorter, MUC1p-BioPorter or PBS-BioPORTER for 4 h at 37°C. The pulsed BMDCs were washed with PBS, counted, and 3×105 viable cells/100 μl sterile, endotoxin-free PBS were injected subcutaneously in the hip area of MUC1-Tg mice. The mice were boosted twice, 3 weeks apart.
For vaccines involving the use of the adjuvant E6020 (a kind gift from Eisai, Boston, MA, USA), 3 μg of E6020 was mixed with either TnMUC1 (2.6 μg or 104 μg) or MUC1p (2 μg or 80 μg) and injected in a final volume of 100 μl (s.c.) or 200 μl (i.p.) prepared in endotoxin-free PBS.
In vitro functional assays for cellular responses
One to two weeks after the third vaccination, spleens and lymph nodes (inguinal, mesenteric) were collected. Single cell suspensions were prepared by mechanical disruption and RBC lysis before being cultured in 24-well plates at a density of 1–1.5×106 cells/ml. The culture medium used was cDMEM-10 (10% FBS, penicillin and streptomycin, L-glutamine, sodium pyruvate, non-essential amino acids, and 2-ME), supplemented with 20 U/ml IL-2.
Cells were tested at two time points for intracellular cytokine detection by FACS, either after an ex vivo stimulation or after two rounds of in vitro restimulation. The restimulation was carried out 10 d apart, with 1×105 antigen pulsed or non-pulsed DCs for each 1×106 spleen cells in culture. The first or final stimulation, respectively, was carried out for 6 h. Brefeldin A was added during the last 4 h. Cells were stained with anti-mouse CD3 (PerCP), CD4 (APC), and IFN-γ (FITC) antibodies. Isotype control antibodies were used to set up the gates. All antibodies were from BD Biosciences and staining was carried out according to the manufacturer’s protocol. Stained cells were analyzed on a LSR II Flow Cytometer using the FACSDiva data analysis software (BD Biosciences). Testing for soluble cytokine production was carried out following a similar in vitro restimulation protocol as described above. A mouse anti-CD4 blocking antibody (clone H129.19; BD Biosciences) was used during the final re-stimulation at 2.5 μg/ml. Cell culture supernatants were collected 48 h following the final restimulation and IL-5 concentrations determined by ELISA using a BD OptEIA ELISA kit following the manufacturer’s protocol (BD Biosciences).
For T cell hybridoma functional responses, 1×105 hybridomas were co-cultured in cDMEM-10 with 1×104 BMDCs per well (96-well plate) in the presence or absence of the indicated MUC1 antigen. In some cases, BMDCs were replaced with 3×104 splenocytes (C57BL/6) per well. The final culture volume was 200 μl/well and each condition was done in triplicate. After 48 h, cell culture supernatant was collected for IL-2 cytokine analysis by ELISA using a BD OptEIA ELISA kit (BD Biosciences) following the manufacturer’s protocol. Data for all cytokine ELISAs are represented as the average and standard deviation of triplicate cell culture wells.
Anti-MUC1 antibody ELISA
At specified time points, blood was collected and serum isolated after coagulation. The serum was stored at −80°C until ready to use. Serum from individual mice was tested for the presence of MUC1-specific antibodies. 96-well Immulon 4HBX plates (Fisher Scientific, Pittsburgh, PA, USA) were coated overnight with 10 μg/ml of MUC1p peptide or 15 μg/ml TnMUC1 glycopeptide or 2.5% BSA to serve as negative control. Plates were then washed with PBS and blocked with 2.5% BSA. Serial dilutions of sera were carried out in 2.5% BSA and added to the plates in triplicate. Plates were washed three times with PBS/0.1% Tween 20, then HRP-conjugated goat anti-mouse IgG1 or pan-IgG secondary antibody (Sigma) was added (diluted 1:500 in 2.5% BSA). The plates were washed three times with PBS/0.1% Tween 20 and then incubated with tetramethylbenzidine substrate (BD Biosciences). The reaction was stopped with 2 N sulfuric acid, and the absorbance was measured at 450 nm. Data were represented using the average and standard deviation of triplicate wells, after subtracting the background readings of control wells.
Acknowledgments
This work was supported by National Institute of Health grants T32CA82084 (S.R.), RO1CA56103, and PO1CA73743 (O.F.) and a grant from the Canadian Breast Cancer Research Alliance in association with the Canadian Cancer Society (J.G.).
References
- Bos R, van Duikeren S, Morreau H, Franken K, Schumacher TNM, Haanen JB, van der Burg SH, Melief CJM, Offringa R. Balancing between antitumor efficacy and autoimmune pathology in T-cell-mediated targeting of carcinoembryonic antigen. Cancer Res. 2008;68:8446–8455. doi: 10.1158/0008-5472.CAN-08-1864. [DOI] [PubMed] [Google Scholar]
- Brokx RD, Revers L, Zhang Q, Yang S, Mal TK, Ikura M, Gariepy J. Nuclear magnetic resonance-based dissection of a glycosyltransferase specificity for the mucin MUC1 tandem repeat. Biochemistry. 2003;42:13817–13825. doi: 10.1021/bi0353070. [DOI] [PubMed] [Google Scholar]
- Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian Y, von Boehmer H, Mahnke K, Buer J. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes. 2005;54:3395–3401. doi: 10.2337/diabetes.54.12.3395. [DOI] [PubMed] [Google Scholar]
- Deck MB, Sjolin P, Unanue ER, Kihlberg J. MHC-restricted, glycopeptide-specific T cells show specificity for both carbohydrate and peptide residues. J Immunol. 1999;162:4740–4744. [PubMed] [Google Scholar]
- Ding C, Wang L, Marroquin J, Yan J. Targeting of antigens to B cells augments antigen-specific T-cell responses and breaks immune tolerance to tumor-associated antigen MUC1. Blood. 2008;112:2817–2825. doi: 10.1182/blood-2008-05-157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloni M, Castiglioni P, Zanetti M. The cooperation between two CD4 T cells induces tumor protective immunity in MUC.1 transgenic mice. J Immunol. 2005;175:6551–6559. doi: 10.4049/jimmunol.175.10.6551. [DOI] [PubMed] [Google Scholar]
- Goydos JS, Elder E, Whiteside TL, Finn OJ, Lotze MT. A Phase I trial of a synthetic mucin peptide vaccine: induction of specific immune reactivity in patients with adenocarcinoma. J Surg Res. 1996;63:298–304. doi: 10.1006/jsre.1996.0264. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltbold EM, Vlad AM, Ciborowski P, Watkins SC, Finn OJ. The mechanism of unresponsiveness to circulating tumor antigen MUC1 is a block in intracellular sorting and processing by dendritic cells. J Immunol. 2000;165:3730–3741. doi: 10.4049/jimmunol.165.7.3730. [DOI] [PubMed] [Google Scholar]
- Jerome KR, Barnd DL, Bendt KM, Boyer CM, Taylor-Papadimitriou J, McKenzie IFC, Bast RC, Jr, Finn OJ. Cytotoxic T-lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells. Cancer Res. 1991;51:2908–2916. [PubMed] [Google Scholar]
- Jerome KR, Domenech N, Finn OJ. Tumor-specific cytotoxic T cell clones from patients with breast and pancreatic adenocarcinoma recognize EBV-immortalized B cells transfected with polymorphic epithelial mucin complementary DNA. J Immunol. 1993;151:1654–1662. [PubMed] [Google Scholar]
- Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–144. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, Mc-Kolanis JR, Geller BA, Schmotzer A, Potter DP, Whiteside T, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6:955–964. [PMC free article] [PubMed] [Google Scholar]
- Napoletano C, Rughetti A, Agervig Tarp MP, Coleman J, Bennett EP, Picco G, Sale P, Denda-Nagai K, Irimura T, Mandel U, et al. Tumor-associated Tn-MUC1 glycoform is internalized through the macrophage galactose-type C-type lectin and delivered to the HLA class I and II compartments in dendritic cells. Cancer Res. 2007;67:8358–8367. doi: 10.1158/0008-5472.CAN-07-1035. [DOI] [PubMed] [Google Scholar]
- Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- Ninkovic T, Kinarsky L, Engelmann K, Pisarev V, Sherman S, Finn OJ, Hanisch F-G. Identification of O-glycosylated decapeptides within the MUC1 repeat domain as potential MHC class I (A2) binding epitopes. Mol Immunol. 2009 doi: 10.1016/j.molimm.2008.09.032. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przetak M, Chow J, Cheng H, Rose J, Hawkins LD, Ishizaka ST. Novel synthetic LPS receptor agonists boost systemic and mucosal antibody responses in mice. Vaccine. 2003;21:961–970. doi: 10.1016/s0264-410x(02)00737-5. [DOI] [PubMed] [Google Scholar]
- Ramanathan RK, Lee KM, McKolanis J, Hitbold E, Schraut W, Moser AJ, Warnick E, Whiteside T, Osborne J, Kim H, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254–264. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly RT, Gottlieb MBC, Ercolini AM, Machiels JPH, Kane CE, Okoye FI, Muller WJ, Dixon KH, Jaffee EM. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 2000;60:3569–3576. [PubMed] [Google Scholar]
- Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–321. [PubMed] [Google Scholar]
- Ryan SO, Gantt KR, Finn OJ. Tumor antigen-based immunotherapy and immunoprevention of cancer. Int Arch Allergy Immunol. 2007;142:179–189. doi: 10.1159/000097020. [DOI] [PubMed] [Google Scholar]
- Saeland E, van Vliet S, Bäckström M, van den Berg V, Geijtenbeek T, Meijer G, van Kooyk Y. The C-type lectin MGL expressed by dendritic cells detects glycan changes on MUC1 in colon carcinoma. Cancer Immunol Immunother. 2007;56:1225–1236. doi: 10.1007/s00262-006-0274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MM, Mehta V, Finn OJ. Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection. J Immunol. 2001;166:6555–6563. doi: 10.4049/jimmunol.166.11.6555. [DOI] [PubMed] [Google Scholar]
- Sorensen AL, Reis CA, Tarp MA, Mandel U, Ramachandran K, Sankaranarayanan V, Schwientek T, Graham R, Taylor-Papadimitriou J, Hollingsworth MA, et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16:96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
- Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- Tarp MA, Sorensen AL, Mandel U, Paulsen H, Burchell J, Taylor-Papadimitriou J, Clausen H. Identification of a novel cancer-specific immunodominant glycopeptide epitope in the MUC1 tandem repeat. Glycobiology. 2007;17:197–209. doi: 10.1093/glycob/cwl061. [DOI] [PubMed] [Google Scholar]
- Tempero RM, VanLith ML, Morikane K, Rowse GJ, Gendler SJ, Hollingsworth MA. CD4+ lymphocytes provide MUC1-specific tumor immunity in vivo that is undetectable in vitro and is absent in MUC1 transgenic mice. J Immunol. 1998;161:5500–5506. [PubMed] [Google Scholar]
- Tsuiji M, Fujimori M, Ohashi Y, Higashi N, Onami TM, Hedrick SM, Irimura T. Molecular cloning and characterization of a novel mouse macrophage C-type lectin, mMGL2, which has a distinct carbohydrate specificity from mMGL1. J Biol Chem. 2002;277:28892–28901. doi: 10.1074/jbc.M203774200. [DOI] [PubMed] [Google Scholar]
- Turner MS, Cohen PA, Finn OJ. Lack of effective MUC1 tumor antigen-specific immunity in MUC1-transgenic mice results from a Th/T regulatory cell imbalance that can be corrected by adoptive transfer of wild-type Th cells. J Immunol. 2007;178:2787–2793. doi: 10.4049/jimmunol.178.5.2787. [DOI] [PubMed] [Google Scholar]
- Valladeau J, Duvert-Frances V, Pin JJ, Kleijmeer MJ, Ait-Yahia S, Ravel O, Vincent C, Vega F, Jr, Helms A, Gorman D, et al. Immature human dendritic cells express asialoglycoprotein receptor isoforms for efficient receptor-mediated endocytosis. J Immunol. 2001;167:5767–5774. doi: 10.4049/jimmunol.167.10.5767. [DOI] [PubMed] [Google Scholar]
- Vlad AM, Finn OJ. Glycoprotein tumor antigens for immunotherapy of breast cancer. Breast Dis. 2004;20:73–79. doi: 10.3233/bd-2004-20109. [DOI] [PubMed] [Google Scholar]
- Vlad AM, Muller S, Cudic M, Paulsen H, Otvos L, Jr, Hanisch FG, Finn OJ. Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J Exp Med. 2002;196:1435–1446. doi: 10.1084/jem.20020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Blackman M, Bill J, Kappler J, Marrack P, Gold D, Born W. Two better cell lines for making hybridomas expressing specific T cell receptors. J Immunol. 1989;143:1822–1825. [PubMed] [Google Scholar]
- Xu Y, Gendler SJ, Franco A. Designer glycopeptides for cytotoxic T cell-based elimination of carcinomas. J Exp Med. 2004;199:707–716. doi: 10.1084/jem.20031865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling AL, Polefrone JM, Evans AM, Mikesh LM, Shabanowitz J, Lewis ST, Engelhard VH, Hunt DF. Identification of class I MHC-associated phosphopeptides as targets for cancer immunotherapy. Proc Natl Acad Sci USA. 2006;103:14889–14894. doi: 10.1073/pnas.0604045103. [DOI] [PMC free article] [PubMed] [Google Scholar]