Abstract
Background
Characteristics of pretransplant antibodies directed at donor HLA (DSA) associated with adverse outcomes in kidney transplant recipients are being elucidated but uncertainties exist.
Methods
Prospectively screening of pretransplant sera from 543 kidney recipients using single antigen bead assays identified 154 recipients with DSA and 389 without. We investigated the association of DSA features to acute rejection (AR) and graft failure.
Results
One-year AR incidence was higher in DSA positive group (P<0.001), primarily due to antibody mediated rejection (AMR, 13% vs. 1.8%, P<0.001) and not T-cell mediated rejection (ACR, 5% vs.6%, P=0.65). Risk of AMR increased progressively with a rise in DSA MFI-Sum (P<0.0001). Both DSA MFI-Sum ≥6000 (OR=18; 95%CI, 7.0 to 47; P<0.001) and DSA specificity, presence of DSA against both HLA class I and II (OR=39; 95%CI, 14 to 106; P<0.0001), predicted one-year AMR, independent of other covariates. In a combined model, DSA specificity predicted AMR, independent of DSA MFI-Sum. In multivariable Cox proportional hazards models, the covariate-adjusted hazard ratio for graft failure was 2.03 (95%CI, 1.05 to 3.92; P=0.04) for DSA MFI-Sum≥6000 and 2.23 (95% CI, 1.04 to 4.80; P=0.04) for class I and II DSA. Prediction of graft loss was not independent of AMR.
Conclusions
Our study supports the hypothesis that characterization of pretransplant DSA, specifically presence of DSA against both HLA class I and II and the strength, as quantified by DSA MFI-Sum, is useful to estimate AMR and graft failure risk in kidney graft recipients. Elevated risk of graft failure is attributable to increased risk of AMR.
Keywords: donor specific antibodies, acute rejection, graft loss, kidney transplant
Introduction
Preformed donor specific antibodies, detected using the CDC crossmatch (CDC XM), have been associated with a very high rate of hyperacute rejection and graft loss (1). To avoid this complication, kidney transplants are currently performed following a negative donor T-cell CDC XM. Antibody mediated injury however remains a major cause of kidney allograft failure (1, 2).
Several sensitive techniques (solid phase assays using flow cytometer, ELISA and Luminex fluoroanalyzer) have been developed to detect HLA antibodies (3–7). The clinical utility of detecting circulating antibodies directed at donor HLA (DSA) using these sensitive techniques for organ allocation, risk stratification and treatment decisions remains to be fully defined (6, 8, 9).
The most sensitive and specific assay for DSA detection is the single antigen bead (SAB) assay in which beads coated with single recombinant HLA are used as the target and the bound antibody labeled with a fluorescent signal is detected using the Luminex fluoroanalyzer (10). Refinement of this assay identifies anti-HLA antibodies that can bind complement fraction C1q, a critical step in the activation of the classic complement cascade (4).
Existing literature both support (11–15) and refute (16–21) the increased risk of antibody-mediated rejection (AMR) and/or graft loss associated with DSA. Impact of DSA strength, reflected by mean fluorescence intensity (MFI), and type of DSA (class I vs. II) on outcomes is not fully resolved (11, 13–15). Furthermore, guidelines on how to evaluate the clinical significance of multiple DSAs associated with different MFI values are lacking (9, 22).
Current study addresses whether the DSA strength as quantified by the sum of MFI of DSAs against HLA-A/B/Cw/DR/DQ (DSA MFI-Sum) and DSA specificity (that is DSA directed at class I, class II or both class I and II HLA) are associated with acute rejection (AR) and kidney graft failure. Our single-center prospective study of 543 kidney graft recipients correlated allograft outcomes with DSA MFI-Sum and DSA specificity identified in the pre-transplant serum using SAB assay.
RESULTS
Baseline Characteristics
Among the 543 kidney graft recipients, 154 (28%) had circulating DSA (DSA positive group) detected in pre-transplant sera (collected 10 ± 9 days prior). Table 1 summarizes recipient and donor characteristics stratified by the presence or absence of DSA. Recipient age, gender and ethnicity as well as cause of end stage renal disease (ESRD), donor age and type of donor were significantly different between the two groups. Variables associated with increased risk of AR – specifically, history of a prior failed transplant (P<0.001), CPRA (P<0.001), and number of HLA-A/B/DR/DQ (P<0.001) – were also different by bivariate analysis. Within the DSA positive group, 35% of the patients had class I DSA only, 42% had class II DSA only and 23% had both class I and II DSA.
TABLE 1.
Baseline Characteristics of the 543 kidney graft recipients, stratified by the presence or absence of DSAa
| Variable | DSA Negative Group (N=389) | DSA Positive Groupa (N=154) | P Valueb |
|---|---|---|---|
| Recipient | |||
| Age, (mean±SD, yr) | 54 ± 14 | 50 ± 13 | 0.004 |
| Female, N (%) | 128 (33) | 73 (47) | 0.002 |
| African-American, N (%) | 76 (20) | 56 (36) | <0.001 |
| Cause of ESRD | 0.08 | ||
| Diabetes Mellitus, N (%) | 118 (30) | 30 (19) | 0.01 |
| Hypertension, N (%) | 66 (17) | 36 (23) | 0.09 |
| Systemic Lupus, N (%) | 16 (4) | 11 (7) | 0.19 |
| Glomerulonephritis, N (%) | 69 (18) | 26 (17) | 0.90 |
| Polycystic Kidney Disease, N (%) | 45 (12) | 18 (12) | 0.99 |
| Other, N (%) | 75 (19) | 33(21) | 0.63 |
| Donor | |||
| Age, (mean±SD, yr) | 47 ± 16 | 44 ± 16 | 0.01 |
| Female, N (%) | 218 (56) | 75 (49) | 0.13 |
| African-American, N(%) | 45(12) | 27 (18) | 0.07 |
| Deceased donor, N (%) | 154 (40) | 79 (51) | 0.02 |
| SCD, N (%)c | 82 (53) | 46 (58) | 0.49 |
| ECD, N (%)c | 60 (39) | 26 (33) | 0.39 |
| DCD, N (%)c | 12 (8) | 7 (9) | 0.80 |
| Cold ischemia time, (mean±SD, hr)c | 23.5 ± 9.8 | 24.2 ± 8.5 | 0.78 |
| Transplant related variables | |||
| Prior transplant recipient, N (%) | 28 (7) | 43 (28) | <0.001 |
| CPRA | 1.9 ±10.6 | 20.5 ± 33.8 | <0.001 |
| HLA mismatches | |||
| HLA A/B/DR/DQ mismatch (mean ± SD) | 4.5 ± 2.3 | 5.3 ± 1.8 | <0.001 |
| Type of anti-HLA DSA in pre-transplant serum, N=154 | |||
| Class I DSA only, N (%) | — | 54 (35) | n/a |
| Class II DSA only, N(%) | — | 64 (42) | n/a |
| Class I and II DSA, N(%) | — | 36 (23) | n/a |
| Donor Specific Crossmatch (XM), N=543 | |||
| Complement Dependent Cytotoxicity (CDC)d | |||
| T-cell positive, N (%) | 0 (0) | 0 (0) | 1.0 |
| B-cell positive, N (%) | 4 (1) | 4 (3) | 0.17 |
| Flow Cytometry Crossmatchd, N=210 | 120 (31) | 90 (59) | <0.001 |
| T and/or B-cell positive, N (%) | 6 (1.3) | 41 (27) | <0.001 |
| Donor T-cell XM median channel shift | 0.33±14 | 22±40 | <0.001 |
| Donor B-cell XM median channel shift | 11±54 | 73±78 | <0.001 |
The presence of circulating donor-specific anti-HLA-A, -B,-Cw, -DR, -DQ and -DP antibodies was determined using single antigen flow bead (SAB) assays on a Luminex platform (LABScreenR, One Lambda). DSA positive status was defined by the presence of at least one DSA directed at donor HLA-A, -B, -Cw, -DR or -DQ with MFI value >1000.
P values were calculated using Fischer’s Exact test for categorical variables, including those with more than two categories, and Mann-Whitney test for continuous variables.
Data from recipients of a deceased donor graft (N=154, DSA negative group and N=79, DSA positive group).
Method details provided in SDC Methods.
DSA, donor specific antibody; ESRD, end stage renal disease; SCD, standard criteria donor; ECD, expanded criteria donor; DCD, donation after cardiac death.
All 543 patients had a negative donor T-cell CDC XM but 3% in the DSA positive group and 1% in the DSA negative group had a positive donor B-cell CDC XM (P=0.17). Flow cytometry crossmatch (FCXM), performed in 210 patients, was positive in 27% of the DSA positive group and in 1% of the DSA negative group. As expected the median channel shift for T and B-cell FCXM were higher in the DSA positive group (Table 1). Within the DSA positive group, the median channel shifts for donor T-cell FCXM correlated positively with HLA class I DSA MFI-Sum and shifts for B-cell FCXM correlated positively with combined class I and II DSA MFI-Sum (P<0.0001, SDC, Fig. S1A and S1B). Proportion of patients testing positive for FCXM increased significantly with higher levels of DSA MFI-Sum (SDC, Fig. S1C and S1D).
Breadth of sensitization (23) was characterized for each patient by determining the calculated PRA (CPRA) value using our current criteria for listing unacceptable HLA on UNetSM, recipient HLA antibodies associated with MFI value >10,000. Mean (±SD) CPRA was significantly higher in patients with a DSA (Table 1). However, analysis restricted to patients with DSA showed that the CPRA is not significantly different between those patients who developed AMR compared to patients without AMR (33.2±41.5 vs. 18.5±32.2, P=0.14).
Characteristics of Pretransplant DSA and Incidence of AR
Incidence of biopsy confirmed AR in the first year of transplantation among the 543 patients was 9.8% and significantly higher in the DSA positive vs. DSA negative group (18% vs. 6%, P<0.001, Fisher’s exact test).
Figure 1A–D portray the incidence and risk of AMR (1A and 1C) or ACR (1B and 1D) for different levels of DSA MFI-Sum. The incidence of AMR (Fig. 1A, P<0.001), but not the incidence of ACR (Fig. 1B, P=0.49), differed significantly by DSA MFI-Sum. The odds ratio (OR) for AMR is significantly higher in patients with DSA MFI-Sum =3000–5999 (Fig. 1C, P=0.02), and in those with DSA MFI-Sum ≥6000 (P<0.0001) compared to patients without DSA or with DSA MFI-Sum=1000–2999. In a model that contained DSA MFI-Sum for class I only, class II only, and combined class I and II, DSA MFI-Sum≥6000 for combined class I and class II DSA (OR=13.4; 95%CI, 2.9 to 62.2; P=0.001) predicted AMR better than DSA MFI-Sum≥6000 for class I DSA only (OR=1.4; 95%CI, 0.3 to 6.0; P=0.65) or class II DSA only (OR=0.7; 95%CI, 0.17 to 3.1; P=0.65).
Figure 1. Incidence and Risk of Acute Rejection in the First Year of Kidney Transplantation in the DSA Positive Cohort Stratified by DSA MFI-Sum and HLA Class in Pretransplant Serum.
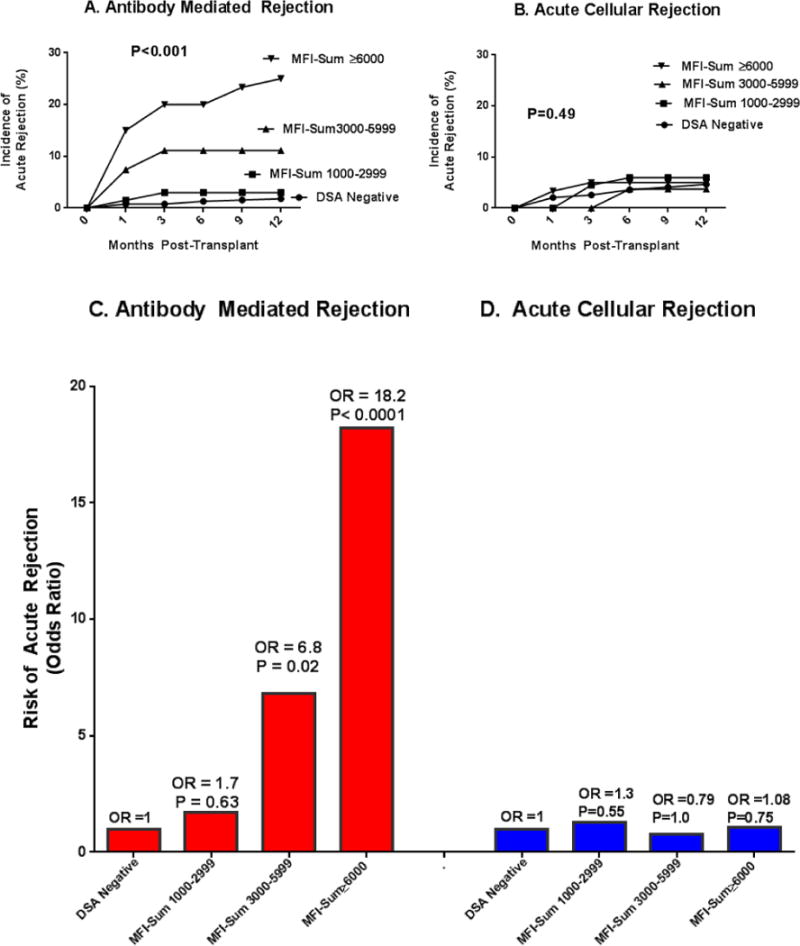
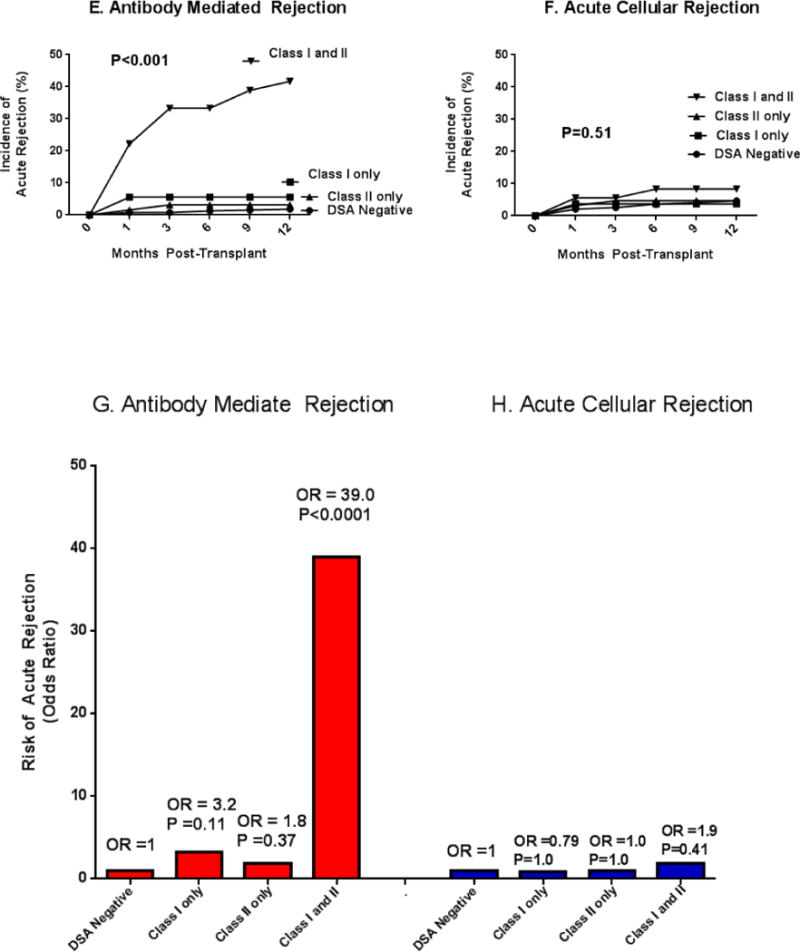
Figure 1 portrays the incidence and risk of biopsy confirmed acute antibody mediated rejection (AMR, n=27 biopsies from 27 patients, 21 of 27 biopsies showing acute AMR only and 6 showing both acute AMR and ACR) and biopsy confirmed T-cell mediated acute cellular rejection (ACR, n=26 biopsies from 26 patients) during the first year of transplantation, stratified according to DSA MFI-Sum (Fig. 1A to 1D) and DSA HLA class (Fig. 1E and H). In Panels A and B, the study cohort of 543 patients was divided into four groups based on DSA MFI-Sum in the pretransplant serum – DSA negative group, patients without a single DSA with MFI>1000 (n=389); patients with DSA MFI-Sum between 1000 and 2999 (n=67); patients with DSA MFI-Sum between 3000 and 5999 (n=27); and patients with DSA MFI-Sum≥6000 (n=60). The incidence of AMR (Panel A) was significantly higher in those with DSA MFI-Sum≥3000 (P<0.001) while the incidence of ACR was not significantly different among the four groups (P=0.9, Panel B). In Panel C, the incidence of AMR in DSA negative group was used as the reference and the relative increase in the Odds of AMR in the first year of transplantation was calculated for those with DSA MFI-Sum of 1000–2999 (OR=1.7; 95%CI: 0.34, 8.26), DSA MFI-Sum of 3000–5999 (OR=6.8; 95%CI: 1.7, 28.1) and DSA MFI-Sum≥6000 (OR=18.2; 95%CI: 7.0, 47.0). Panel D displays the Odds of ACR associated with different levels of DSA MFI-Sum (P>0.05). P-values based on Fisher’s exact test.
In Panels E to H, the study cohort was divided into four groups according to HLA class of the DSA present in the pretransplant serum – DSA negative group (n=389); patients with DSA against HLA class I only (n=54); patients with DSA against HLA class II only (n=64); and patients with DSA against HLA class I and II (n=36). The incidence of AMR (Panel E) was significantly higher in those with DSA directed against both HLA class I and II (P<0.001) whereas the incidence of ACR was not significantly different among the four groups (P=0.5, Panel F). The frequency of AMR was not different when the data were analyzed according to the HLA locus (A, B, Cw, DR or DQ) that was targeted by the DSA (P=0.62, Chi-Square test). In Panel G, the DSA negative group was used as the reference and the relative increase in the Odds of AMR in the first year of transplantation was calculated for DSA against HLA class I only (OR=3.2; 95%CI: 0.8, 12.8), DSA against HLA class II only (OR=1.8; 95%CI: 0.36, 8.7) and DSA against HLA class I and II (OR=39; 95%CI: 14, 106); Panel H displays the Odds of ACR associated with HLA class of DSA (P>0.05). P-values based on Fisher’s exact test.
Figures 1E to 1H display the incidence and risk of AMR (Fig. 1E and 1G) or ACR (Fig. 1F and 1H) for DSA directed at HLA class I, class II or both class I and II. The incidence of AMR, but not the incidence of ACR, was associated with the presence of DSA class I and II (P<0.001). Odds of AMR in those with both class I and class II DSA was increased (OR=39, 95%CI, 14 to 105; P<0.0001).
DSA Status and For-Cause Biopsies in the First Post-Transplant Year
During the first year of transplantation, 157 patients (60-DSA positive and 97-DSA negative) underwent clinically indicated (for-cause) biopsies for graft dysfunction and 52 patients underwent a follow-up biopsy within the first year of transplantation. Table 2 lists the index biopsy diagnosis, pretransplant DSA status, DSA status at the time of for-cause biopsy and the number of follow-up biopsies with chronic AMR (CAMR) and transplant glomerulopathy (TG). Nine (17%) follow-up biopsies were classified as CAMR and 7 of these biopsies showed features of TG. CAMR was only found in patients who had a prior episode of AMR or ACR.
Table 2.
Kidney Allograft Biopsy Diagnosis, DSA Status and the Incidence of Chronic Antibody Mediated (CAMR)/Transplant Glomerulopathy (TG) Within One-Year Post-Transplantation.
| Index Biopsy Diagnosisa and Number of Patients (n=157) | Time of Biopsy (post-transplant month, mean ±SD) | Pre- Transplant DSA Statusb Negative/Positive |
Number of Patients tested for DSA at the time of index biopsy (n=135)/number of patients with DSAb (n=52) | Follow-up Biopsy within first year post-transplantc Number of biopsies (n=52)/number with CAMR (n=9)/number with TG (n=7) |
|---|---|---|---|---|
| Acute AMRd (n=27) | 1.7±2.5 | Negative (n=6) | 6/6 | 4/3/3 |
| Positive (n=21) | 18/18 | 12/4/2 | ||
| ACR (n=26) | 2.3 ±2.6 | Negative (n=18) | 18/1 | 7/1/1 |
| Positive (n=8) | 8/7 | 4/1/1 | ||
| ATN/Toxicity (n=44) | 2.1± 2.5 | Negative (n=30) | 25/1 | 7/0/0 |
| Positive (n=14) | 8/3 | 5/0/0 | ||
| Other Diagnosis (n=31) | 5.4 ±3.7 | Negative (n=23) | 20/4 | 6/0/0 |
| Positive (n=8) | 8/6 | 2/0/0 | ||
| No Specific Abnormality (n=29) | 3.6 ±3.6 | Negative (n=19) | 16/1 | 3/0/0 |
| Positive (n=10) | 8/6 | 2/0/0 |
Biopsies performed at the time of graft dysfunction were classified, using the Banff ‘09 schema. Patients were grouped according to the index biopsy diagnosis and each patient contributed only once to the index biopsy diagnosis. Five of the 27 acute AMR biopsies were classified as type I, 20 as type II and the remaining 2 as type III.
Positive DSA status was defined by the presence of at least one HLA-A, -B, -Cw, -DR or -DQ DSA with MFI value >1000.
Only biopsies performed within the first year post-transplantation were included.
In the AMR cohort (n=27), three patients experienced AMR within the first week and did not have repeat DSA testing at the time of for-cause biopsy.
Figure 2 exhibits the post-transplant DSA MFI-Sums measured in 135 of the157 (52-DSA positive and 83-DSA negative) patients undergoing for-cause biopsies, grouped by index biopsy diagnosis. DSA MFI-Sum was significantly increased at the time of index biopsy in the AMR group only (Fig. 2A, P=0.003) and in the remaining groups, majority of the patients had DSA MFI-Sum below 6000 at the time of index biopsy (Fig. 2B to 2E).
Figure 2. DSA MFI-Sums Pretransplant and at the Time of For-Cause Biopsy.
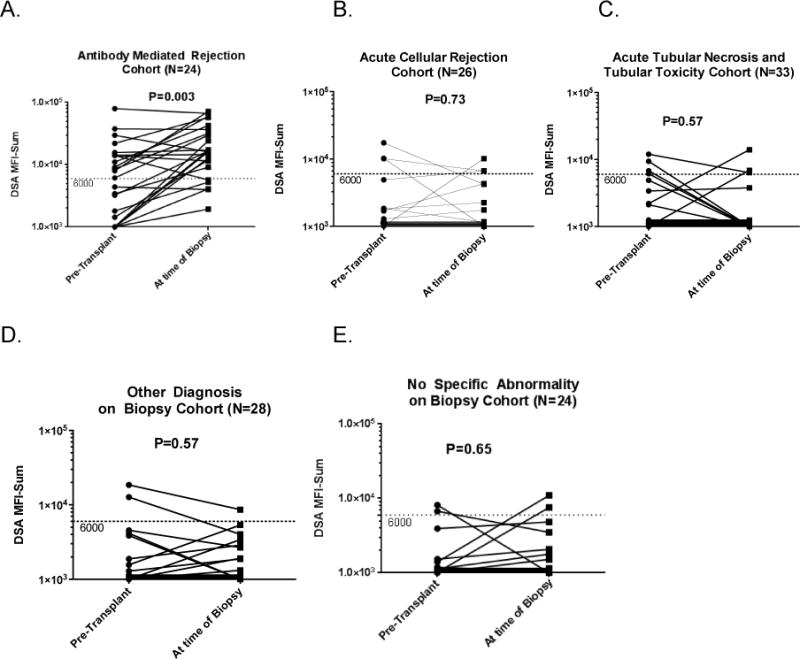
Patients undergoing for-cause biopsy (n=157) were divided into groups according to the diagnosis of the index for-cause biopsy obtained within the first year post-transplantation. Each patient contributed only once and was assigned to one group only. In the AMR cohort (n=27), three patients experience AMR within the first week and did not have repeat DSA testing at the time of for-cause biopsy. The DSA MFI-Sum pre-transplant and at the time of for-cause biopsy are shown for the remaining 24 AMR patients. In the remaining groups, 26 patients with ACR, 33 with acute tubular necrosis/tubular toxicity, 28 with other diagnosis and 24 with no specific abnormality were screened for DSA at the time of for-cause biopsy. DSA MFI-Sum levels obtained pretransplant were compared to levels obtained at the time of for-cause biopsy using Wilcoxon test.
Independent Predictors of AMR
Since DSA MFI-Sum and DSA class were highly correlated (P<0.0001, SDC Table S1), we examined their relationships to 1-year incident AMR separately, while controlling for other risk factors associated with AMR at P<0.05 by bivariate analysis (SDC Table S2). In a multivariable logistic regression analysis both DSA MFI-Sum= 3000–5999 and DSA MFI-Sum ≥6000 significantly predicted an increased risk for AMR, independent of the other factors (Table 3A). Presence of class I and II DSA also significantly and independently predicted risk for AMR (Table 3B). In both models, CPRA was not a risk factor for AMR but the degree of HLA mismatch was a risk factor for AMR while older age was protective.
Table 3.
Multivariable analysis of risk factors for antibody mediated rejection (N=543)a
| (A)|Model with DSA MFI-Suma | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| DSA MFI-Sum ≥6000 | 13.4 (4.25, 42.3) | <0.001 |
| DSA MFI-Sum 3000–5999 | 4.42 (1.05 18.7) | 0.04 |
| Age (per 10 years) | 0.60 (0.42, 0.85) | 0.004 |
| HLA mismatch (A, B, DR, DQ) | 1.29 (1.01, 1.64) | 0.04 |
| Prior transplant | 1.03 (0.33, 3.21) | 0.96 |
| CPRA | 1.00 (0.98, 1.01) | 0.72 |
| Cause of ESRD = Lupus | 2.47 (0.67, 9.16) | 0.18 |
| (B) Model with Class I and II DSA c | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| DSA Class I and II | 23.0 (7.25, 73.0) | <0.001 |
| Age (per 10 years) | 0.57 (0.39, 0.84) | 0.004 |
| HLA mismatch (A,B, DR, DQ) | 1.30 (1.0, 1.69) | 0.05 |
| Prior transplant | 0.92 (0.27, 3.14) | 0.90 |
| CPRA | 1.0 (0.98, 1.01) | 0.77 |
| Cause of ESRD = Lupus | 2.59 (0.66, 10.1) | 0.66 |
Multivariable logistic regression analysis was performed using DSA MFI-Sum as the predictor along with other variables that were found to be associated with AMR with P<0.05 (SDC Table S2). Class of DSA was not included in the model due to co-linearity with DSA MFI-Sum. Steroid maintenance therapy was not included since, to a substantial extent, it was a response to learning that a patient was DSA Positive (SDC Table S2).
Multivariable logistic regression analysis was performed using DSA Class I&II as the predictor instead of DSA MFI-Sum.
We analyzed DSA MFI-Sum (≥6000) and class I and II DSA together in a model, controlling for the other covariates shown in Table 3, and found that class I and II DSA continued to independently predict AMR (OR=18.6, 95% CI 3.2 to 107.8; P=0.001) whereas DSA MFI-Sum did not (P=0.9). Furthermore, there was no evidence of an interaction (e.g., synergistic) effect of DSA MFI-Sum and DSA class (P=0.51).
FCXM was performed in 210 patients and of these 19 developed AMR (120-DSA negative and 90-DSA positive) and of the 90 DSA positive patients, 41 were scored as T-cell or B-cell FCXM positive (Table 1). One-year incidence of acute AMR was 29% in the 41 patients with both DSA and a FCXM positive result, and 12 % in the 49 patients with DSA and FCXM negative result (P=0.04, Fisher’s exact test). Although the association was significant by univariate analysis, in the multivariable analysis restricted to patients with FCXM results (n=210 patients), a positive FCXM result was not an independent risk factor for acute AMR (Table S4). However, in the 210 patients with FCXM results, DSA MFI-Sum=3000 to 5999 (OR=13.9, 95% CI, 1.6 to 118; P=0.02) and ≥6000 (OR=19.2, 95% CI, 2.9 to 125.7; P=0.002) (SDC Table S4A) and presence of class I and II DSA (OR=48; 95%CI 7.5 to 304; P<0.001, SDC Table S4B) were independent predictors of AMR in their respective models. As in the entire study cohort, class I and II DSA was a significant predictor of AMR (P=0.001) while DSA MFI-Sum was not when both variables were included in the same model.
Since non-adherence can contribute to AR and graft loss (24), we systematically evaluated patient charts and levels of immunosuppressive drugs at the time of for-cause biopsy and identified only 5 patients whose rejection episodes were associated with insufficient immunosuppression (2 in DSA positive group secondary to patient non-adherence and 3 in DSA negative group secondary to BKV infection; SDC Methods). SDC Figure S2 demonstrates that tacrolimus trough levels and MMF dose at the time of for-cause biopsies were not statistically different between those with and without AR.
Patient and Graft Outcomes
Overall 60 of 543 patients (11%) died during the observed follow-up (mean, 41 months; range, 0.5 to 73 months) and among these patients, 46 died with a functioning graft. Twenty-five percent of our study population was over the age of 65 and the major causes of death were cardiovascular and infectious complications. Patient survival was not different between the DSA positive and DSA negative groups (estimated survival at 48 months was 93% vs. 95%, respectively; P=0.61 by log-rank test).
The incidence of delayed graft function (DGF, defined as the need for dialysis during the first week following transplantation) was 23% in the DSA positive group and 16% in the negative group (P=0.05). Serum creatinine level, at one year post-transplantation, was numerically higher in the DSA positive group (1.8±1.6 vs. 1.5±1.1, P=0.23).
As of May 2014, 52 kidney grafts failed and the 3-year survival rates were 89% and 94% in the DSA positive and DSA negative group respectively (P=0.02, Fig. 3A). Kaplan-Meier estimates of graft survival were compared among the four groups stratified by DSA MFI-Sum (P=0.06, Fig. 3B). Graft survival at 3-years was 84% in patients with DSA MFI-Sum≥6000, 93% in patients with DSA MFI-Sum =3000–5999, and 92% in patients with DSA MFI-Sum=1000–2999 and 94% in patients without DSA. We also compared graft survival stratified by the class of DSA present. Graft survival at 3-years was 85% in those with class I and II DSA compared to 93% in those with class I DSA only, 88% in those with class II DSA only and 94% in those without DSA (P=0.02, Fig. 3C).
Figure 3. Kaplan-Meier Curves for Kidney Graft Survival Stratified by DSA MFI-Sum and HLA Class in Pretransplant Serum.
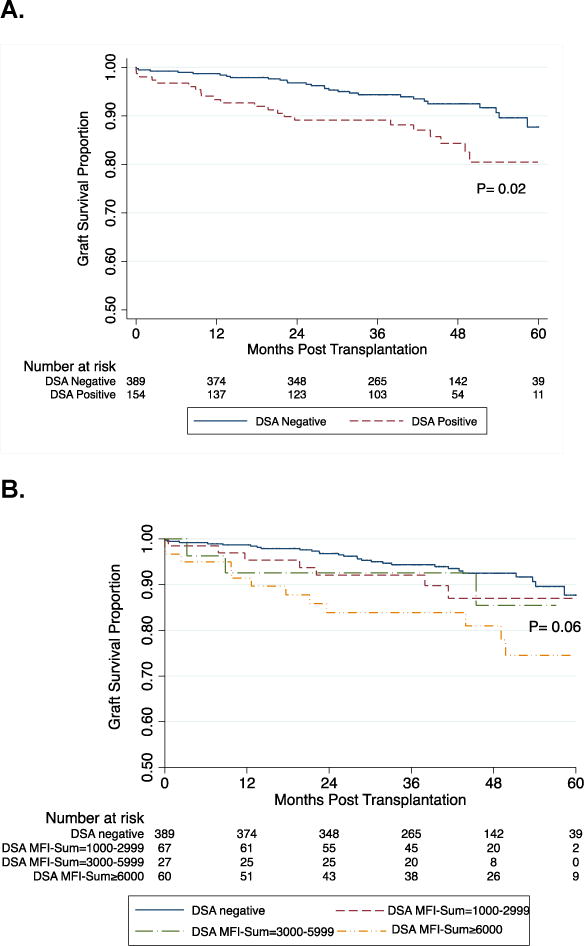
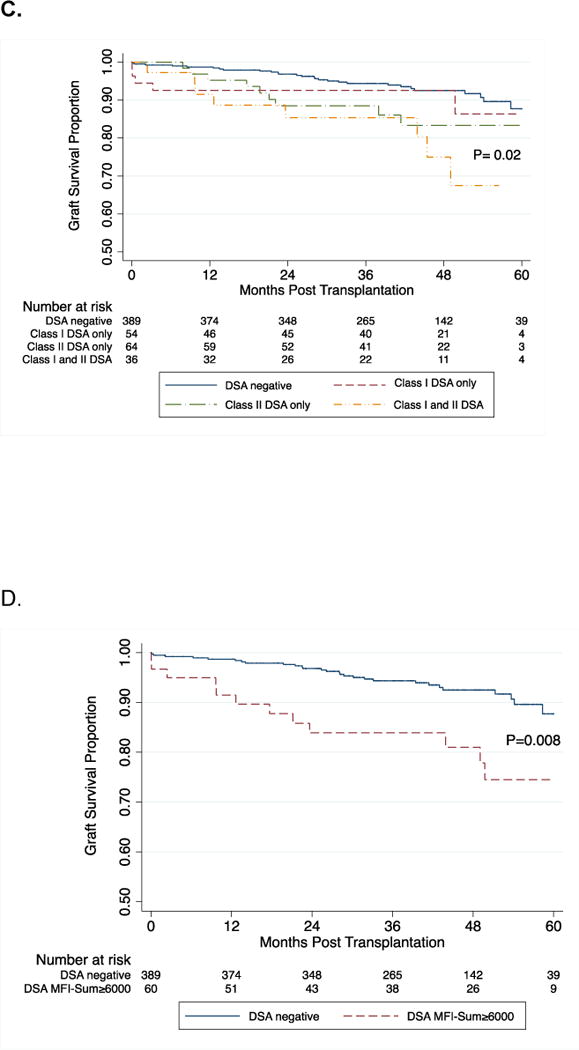
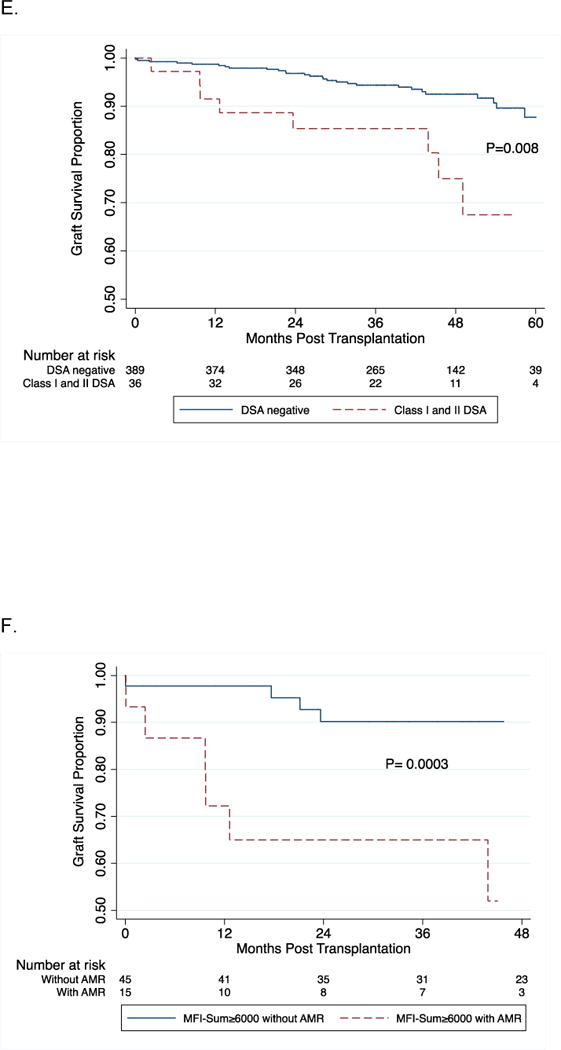
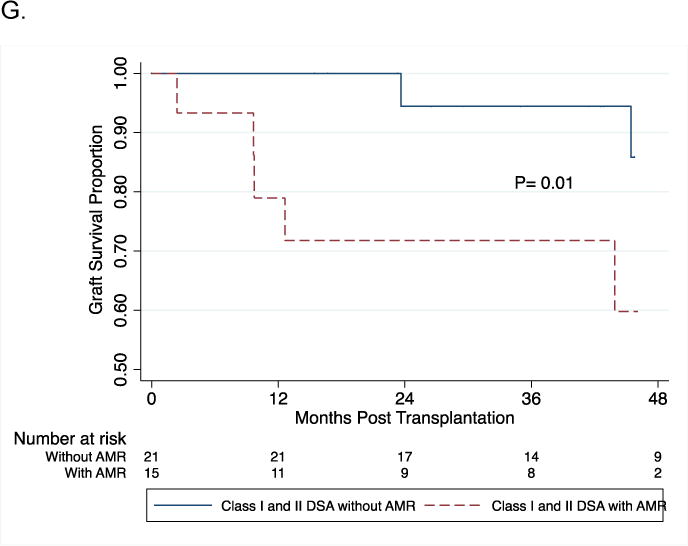
Log-rank test was used to compare kidney graft survival curves in patients with and without DSA (Panel A). Panel B demonstrates graft survival in the four groups stratified by the DSA MFI-Sum in the pretransplant serum. Graft survival at 3-years post-transplantation was 84% in the patients with DSA MFI-Sum≥6000, 93% in patients with DSA MFI-Sum= 3000–5999, and 92% in patients with DSA MFI-Sum= 1000–2999 and 94% in patients without DSA (P=0.06). Graft survival in the group with DSA MFI-Sum≥6000 was significantly lower compared to all three other groups combined (DSA negative + DSA MFI-Sum =1000–2999 + DSA MFI-Sum = 3000–5999) (P=0.003). In Panel C, log-rank test was used to compare kidney graft survival curves in the four groups stratified by the DSA HLA class in the pretransplant serum. Graft survival at 3-years post-transplantation was 85% in the patients with DSA HLA class I and II, 93% in patients with DSA HLA class I only, 88% in patients with DSA HLA class II only and 94% in those without DSA (P=0.02). Graft survival in the group with DSA against both HLA class I and II was significantly lower compared to all three other groups combined (DSA negative + class I DSA only + and class II DSA only) (P=0.02). Panels 3D and 3E demonstrate a significant difference in graft survival in those at high risk for AMR – those with DSA MFI-Sum ≥ 6000 vs. no DSA (Fig 3D, P=0.008) and those with DSA Class I and II vs. no DSA (Fig. 3E, P=0.008). Within the group with DSA MFI-Sum ≥6000, the occurrence of an episode of AMR during the first year of transplantation resulted in a significantly lower graft survival (53% vs. 89%; P=0.0003, Fig. 3F). Within the group with DSA directed at HLA- class I and II, the occurrence of an episode of AMR during the first year of transplantation resulted in lower graft survival (60% vs. 90%; P=0.01, Fig. 3G).
Figure 3D and 3E demonstrate a significant difference in graft survival in those at high risk for AMR (DSA MFI-Sum ≥6000 vs. no DSA [P=0.008, Fig. 3D] and DSA Class I and II vs. no DSA [P=0.008, Fig. 3E]). Within the group with DSA MFI-Sum ≥6000, AMR episode during the first year of transplantation resulted in a significantly lower graft survival (53% vs. 89%; P=0.0003, Fig. 3F). Within the group with DSA directed at class I and II HLA, AMR episode during the first year of transplantation also resulted in lower graft survival (60% vs. 90%; P=0.01, Fig. 3G). Five of the 27 acute AMR biopsies were classified as type I, 20 as type II and the remaining 2 as type III, and the graft failure rates at 2 years were 20%, 25% and 100%, respectively.
Multivariable Cox proportional hazards models were used to investigate, the relationship of DSA MFI-Sum and DSA class to graft loss (SDC, Table S5). In separate models, after controlling for ethnicity (African-American) and donor source (deceased), DSA MFI-Sum≥6000 (HR=2.03, 95% CI, 1.05 to 3.92; P=0.04) and presence of class I and II DSA, predicted graft loss (HR=2.23, 95% CI, 1.04 to 4.80; P=0.04). In both models, donor source and ethnicity contributed to increased risk of graft loss (P<0.05). When we statistically control for those who develop AMR, DSA Class and DSA MFI-Sum no longer predict graft loss (P>0.50 for each), suggesting that the primary mechanism by which DSA increases the risk of graft loss is through its effect on AMR risk.
DISCUSSION
Our single center prospective study of 543 kidney allograft recipients demonstrates that the incidence of AR in the first year of transplantation is higher in DSA positive group, primarily due to a higher incidence of AMR while the incidence of ACR is not affected. We demonstrated that the risk of AMR increases progressively with a rise in DSA MFI-Sum and both DSA MFI-Sum and the presence of DSA against HLA-class I and II are predictors of one-year AMR, independent of other covariates.
We developed a method to calculate the cumulative strength of multiple DSAs by setting a pre-specified criterion for a positive reaction (MFI>1000) and by calculating the DSA MFI-Sum by adding the MFI values of antibodies directed against HLA-A, B, Cw, DR, and DQ antigens. Although the use of MFI>1000 as the threshold to score a positive reaction is somewhat arbitrary, we used this cutpoint since we have found that anti-HLA antibody specificities identified using this threshold in American Society of Histocompatibility and Immunogentics (ASHI) proficiency testing program demonstrates good agreement across histocompatibility laboratories. Our strategy identified a progressive increase in the risk for AMR associated with the rise in the DSA MFI-Sum. Others have found that a sequential rise in MFI value of the highest ranked DSA in recipients’ sera correlates with increased risk of rejection and graft loss (11, 12, 15). However, these studies did not address the role of multiple DSAs. It is noteworthy that we report in this manuscript that DSA MFI-Sum > 3000 in the pretransplant serum is an independent risk factor for acute AMR in the first year of transplantation, and very recently Heilman et al. found in a prospective study of 245 kidney graft recipients that post-transplant development of de novo DSA with MFI>3000 in the first year of transplantation is associated with an increased risk of AMR (25).
Presence of DSA against both class I and II in the pre-transplant serum is also an independent predictor of AMR, even in the presence of a positive FCXM. Additionally, we identified for the first time that the presence of class I and II DSA was a stronger predictor of AMR compared to DSA MFI-Sum by including both variables in a single model. We did not find a synergistic (or modifier) effect between DSA class and DSA MFI-Sum. Our observations are consistent with the report of Fidler and colleagues that the risk of AMR is highest in those with DSA directed against class I and II combined (HR=10, P<0.001) (15).
We found that the association of DSA MFI-Sum or class I and II DSA with graft loss is not independent of an episode of AMR within the first year. This suggests that the mechanism by which DSA confers an increased risk of graft loss is through its effect on AMR risk. These data are consistent with other published data in which DSA-positive patients who developed AMR are at risk for graft loss while those who did not develop AMR had similar graft survival to the DSA negative group(3, 14, 15, 26).
An important goal of screening patients for DSA is to identify apriori patients at risk for AR and initiate preemptive or adjunctive therapy to reduce the risk. At the current time, we use the presence of anti-HLA antibody with >10,000 MFI as the threshold to list that HLA as an unacceptable antigen on the UNetSM wait list. In addition, patients with DSA and a positive flow cytometry crossmatch against their potential living donor are encouraged to enter the Donor Swap Program and are also eligible to undergo a desensitization treatment protocol comprised of a combination of rituximab, mycophenolate mofetil, tacrolimus, IVIG and apheresis. Indeed, 91 patients received, in addition to maintenance steroid therapy, adjunctive IVIG and/or rituximab therapy. Disappointingly, these additional therapies did not seem to influence the development of AMR in those with DSA Sum-MFI ≥6000 and in those with class I and II DSA (SDC Table S6).
A potential limitation of our study is that we did not investigate the C1q-binding ability of DSA found in the pretransplant serum. The C1q assay, developed by Tyan and colleagues, helps to identify the subset of IgG antibodies that are able to fix complement and in some but not all studies C1Qq-fixing antibodies have been associated with acute rejection and/or an inferior graft survival rate (27–29). In a large multicenter study, the development of C1q-binding DSA was the most important predictor of renal allograft outcomes (30). It is of interest that 65% of patients positive for the C1q-binding DSA, had DSA MFI≥6000 while only 10% of patients with non-C1q-binding DSA had DSA MFI≥6000 (Figure S2, Loupy et al. NEJM 2013).
Recent data also suggests that de novo DSA (26, 31, 32) and persistent DSA (33, 34) are an important predictor of AMR and graft survival. Indeed we noted a rise in DSA MFI-Sum at the time of for-cause biopsy in the group with AMR. However, our ability to study de novo and persistent DSA in this cohort is limited by the absence of serial monitoring of DSA post-transplantation.
In the light of lack of validated guidelines for scoring a DSA reaction as clinically significant, our single center study of 543 kidney graft recipients evaluated systematically for pretransplant antibodies directed at HLA enables classification of DSA positive patients in to those at risk for AMR and graft failure and those not at risk. Our algorithm for calculating DSA offers a new, simple and testable approach to manage sensitized patients and may stimulate new studies to investigate effectiveness of new treatment plans (e.g., boretezmib) in patients identified in our investigation to be at high risk for AMR and graft failure.
Materials and Methods
Study population
Between January 2008 and December 2010, 644 patients underwent kidney transplantation at NYPH-Weill Cornell Medical Center and 543 met the inclusion/exclusion criteria (SDC Methods). Of the 543 patients, 157 patients underwent at least one clinically indicated (for-cause) biopsy, and 52 of 157 underwent a follow-up for-cause biopsy during the first 12 months of transplantation and were classified using the Banff ’09 schema (SDC Methods). Table 2 lists the index biopsy diagnosis, pretransplant DSA status, DSA status at the time of index biopsy, and follow up biopsy diagnosis.
HLA typing and detection of DSA
Recipients and donors were typed for class I and class II HLA antigens using standard molecular techniques. Recipients’ sera were screened for circulating DSA, using SAB assays, prior to transplantation and at the time of for-cause biopsy. A mean baseline-normalized MFI value of greater that 1000 directed against donor’s HLA antigen was classified as a positive result and DSA MFI-Sum was calculated by adding all MFI values of DSA>1000 using the MFI value of the highest reactive bead for a given antigen (SDC, Methods).
Statistical Analysis
Details of data analyses are summarized in SDC, Methods. All of the analyses were performed using the SAS version 9.3 and STATA version 12 statistical software packages, and all reported P-values are two-sided with statistical significance evaluated at the 0.05 alpha-level.
Supplementary Material
Acknowledgments
MS is a recipient of NIH MERIT award (R37 AI051652) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. TM is a recipient of K08 DK087824 from the National Institute of Diabetes and Digestive and Kidney Diseases. JL is a recipient of KL2 TR000458 post-doctoral scholar award from the Clinical and Translational Science Center at Weill Cornell Medical College. Additional statistical support was provided by Paul Christos who is supported by the UL1 RR024996 of The Clinical and Translational Science Center from the National Center for Advancing Translational Sciences, National Institutes of Health. The authors would like acknowledge the contribution of Mr. Hans Cabrera who assisted in obtaining the current graft and patient status information and the entire kidney transplant team involved in the care of our patients.
Footnotes
Author Contributions
Dinesh Kannabhiran - Participated in research design, performance of the research, data analysis, and in the writing of the paper. No conflict of interest.
John Lee - Participated in the performance of the research and data analysis. No conflict of interest.
Joseph E. Schwartz - Participated in the performance of data analysis and in the writing of the paper. No conflict of interest.
Rex Friedlander - Participated in the performance of the research and in the review of the paper. No conflict of interest.
Meredith Aull - Participated in the performance of data analysis and in the review of paper. No conflict of interest.
Thangamani Muthukumar - Participated in data analysis and in the writing of the paper. No conflict of interest.
Sean Campbell – Participated in the performance of the research. No conflict of interest.
David Epstein - Participated in performance of the research. No conflict of interest.
Surya V. Seshan - Participated in the performance of the research and review of the paper. No conflict of interest.
Sandip Kapur - Participated performance of the research and in the review of the paper. No conflict of interest.
Vijay K. Sharma - Participated in performance of the research and in the review of the paper. No conflict of interest.
Manikkam Suthanthiran - Participated in the research design, performance of data analysis, and in the writing of the paper. No conflict of interest.
Darshana Dadhania - Participated in the research design, performance of the research, performance of data analysis, and in the writing of the paper. No conflict of interest.
References
- 1.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. The New England journal of medicine. 1969;280(14):735–9. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 2.Ozawa M, Terasaki PI, Castro R, Alberu J, Morales-Buenrostro L, Alvarez I, et al. 14th International HLA and Immunogenetics Workshop Prospective Chronic Rejection Project: a three-year follow-up analysis. Clinical transplants. 2007:255–60. [PubMed] [Google Scholar]
- 3.Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(2):324–31. doi: 10.1111/j.1600-6143.2007.02072.x. [DOI] [PubMed] [Google Scholar]
- 4.Gibney EM, Cagle LR, Freed B, Warnell SE, Chan L, Wiseman AC. Detection of donor-specific antibodies using HLA-coated microspheres: another tool for kidney transplant risk stratification. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21(9):2625–9. doi: 10.1093/ndt/gfl202. [DOI] [PubMed] [Google Scholar]
- 5.Pei R, Lee J, Chen T, Rojo S, Terasaki PI. Flow cytometric detection of HLA antibodies using a spectrum of microbeads. Human immunology. 1999;60(12):1293–302. doi: 10.1016/s0198-8859(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 6.Buscaroli A, De Sanctis LB, Iannelli S, Stipo L, Bertuzzi V, Raimondi C, et al. Application of Prastat ELISA in the determination of anti-HLA specificity for immunized patients awaiting kidney transplant: five years’ experience. Transplant international: official journal of the European Society for Organ Transplantation. 2000;13(Suppl 1):S99–105. doi: 10.1007/s001470050289. [DOI] [PubMed] [Google Scholar]
- 7.Chin C, Chen G, Sequeria F, Berry G, Siehr S, Bernstein D, et al. Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2011;30(2):158–63. doi: 10.1016/j.healun.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Zachary AA, Sholander JT, Houp JA, Leffell MS. Using real data for a virtual crossmatch. Human immunology. 2009;70(8):574–9. doi: 10.1016/j.humimm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Nikaein A, Cherikh W, Nelson K, Baker T, Leffell S, Bow L, et al. Organ procurement and transplantation network/united network for organ sharing histocompatibility committee collaborative study to evaluate prediction of crossmatch results in highly sensitized patients. Transplantation. 2009;87(4):557–62. doi: 10.1097/TP.0b013e3181943c76. [DOI] [PubMed] [Google Scholar]
- 10.Lee PC, Ozawa M, Hung CJ, Lin YJ, Chang SS, Chou TC. Reappraisal of HLA antibody analysis and crossmatching in kidney transplantation. Transplantation proceedings. 2009;41(1):95–8. doi: 10.1016/j.transproceed.2008.10.074. [DOI] [PubMed] [Google Scholar]
- 11.Singh N, Djamali A, Lorentzen D, Pirsch JD, Leverson G, Neidlinger N, et al. Pretransplant donor-specific antibodies detected by single-antigen bead flow cytometry are associated with inferior kidney transplant outcomes. Transplantation. 2010;90(10):1079–84. doi: 10.1097/TP.0b013e3181f6a07b. [DOI] [PubMed] [Google Scholar]
- 12.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. Journal of the American Society of Nephrology: JASN. 2010;21(8):1398–406. doi: 10.1681/ASN.2009101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caro-Oleas JL, Gonzalez-Escribano MF, Gonzalez-Roncero FM, Acevedo-Calado MJ, Cabello-Chaves V, Gentil-Govantes MA, et al. Clinical relevance of HLA donor-specific antibodies detected by single antigen assay in kidney transplantation. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27(3):1231–8. doi: 10.1093/ndt/gfr429. [DOI] [PubMed] [Google Scholar]
- 14.Amico P, Honger G, Mayr M, Steiger J, Hopfer H, Schaub S. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009;87(11):1681–8. doi: 10.1097/TP.0b013e3181a5e034. [DOI] [PubMed] [Google Scholar]
- 15.Fidler SJ, Irish AB, Lim W, Ferrari P, Witt CS, Christiansen FT. Pre-transplant donor specific anti-HLA antibody is associated with antibody-mediated rejection, progressive graft dysfunction and patient death. Transplant immunology. 2013;28(4):148–53. doi: 10.1016/j.trim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Yoo PS, Bonnel A, Kamoun M, Levine MH. Clinical outcomes among renal transplant recipients with pre-transplant weakly reactive donor-specific antibodies. Clinical transplantation. 2014;28(1):127–33. doi: 10.1111/ctr.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Susal C, Ovens J, Mahmoud K, Dohler B, Scherer S, Ruhenstroth A, et al. No association of kidney graft loss with human leukocyte antigen antibodies detected exclusively by sensitive Luminex single-antigen testing: a Collaborative Transplant Study report. Transplantation. 2011;91(8):883–7. doi: 10.1097/TP.0b013e3182100f77. [DOI] [PubMed] [Google Scholar]
- 18.Sicard A, Amrouche L, Suberbielle C, Carmagnat M, Candon S, Thervet E, et al. Outcome of kidney transplantations performed with preformed donor-specific antibodies of unknown etiology. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(1):193–201. doi: 10.1111/ajt.12512. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A, Iveson V, Varagunam M, Bodger S, Sinnott P, Thuraisingham RC. Pretransplant donor-specific antibodies in cytotoxic negative crossmatch kidney transplants: are they relevant? Transplantation. 2008;85(8):1200–4. doi: 10.1097/TP.0b013e31816b1c37. [DOI] [PubMed] [Google Scholar]
- 20.van den Berg-Loonen EM, Billen EV, Voorter CE, van Heurn LW, Claas FH, van Hooff JP, et al. Clinical relevance of pretransplant donor-directed antibodies detected by single antigen beads in highly sensitized renal transplant patients. Transplantation. 2008;85(8):1086–90. doi: 10.1097/TP.0b013e31816b3ed1. [DOI] [PubMed] [Google Scholar]
- 21.Marfo K, Ajaimy M, Colovai A, Kayler L, Greenstein S, Lubetzky M, et al. Pretransplant Immunologic Risk Assessment of Kidney Transplant Recipients With Donor-Specific Anti-Human Leukocyte Antigen Antibodies. Transplantation. 2014 doi: 10.1097/TP.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 22.Tait BD, Susal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95(1):19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 23.Tambur AR, Leventhal JR, Walsh RC, Zitzner JR, Friedewald JJ. HLA-DQ barrier: effects on cPRA calculations. Transplantation. 2013;96(12):1065–72. doi: 10.1097/TP.0b013e3182a452a5. [DOI] [PubMed] [Google Scholar]
- 24.Gaynor JJ, Ciancio G, Guerra G, Sageshima J, Hanson L, Roth D, et al. Graft failure due to noncompliance among 628 kidney transplant recipients with long-term follow-up: a single-center observational study. Transplantation. 2014;97(9):925–33. doi: 10.1097/01.TP.0000438199.76531.4a. [DOI] [PubMed] [Google Scholar]
- 25.Heilman RL, Nijim A, Desmarteau YM, Khamash H, Pando MJ, Smith ML, et al. De Novo Donor-Specific Human Leukocyte Antigen Antibodies Early After Kidney Transplantation. Transplantation. 2014 doi: 10.1097/TP.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 26.Devos JM, Gaber AO, Teeter LD, Graviss EA, Patel SJ, Land GA, et al. Intermediate-term graft loss after renal transplantation is associated with both donor-specific antibody and acute rejection. Transplantation. 2014;97(5):534–40. doi: 10.1097/01.TP.0000438196.30790.66. [DOI] [PubMed] [Google Scholar]
- 27.Otten HG, Verhaar MC, Borst HP, Hene RJ, van Zuilen AD. Pretransplant donor-specific HLA class-I and -II antibodies are associated with an increased risk for kidney graft failure. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(6):1618–23. doi: 10.1111/j.1600-6143.2011.03985.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen G, Sequeira F, Tyan DB. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Human immunology. 2011;72(10):849–58. doi: 10.1016/j.humimm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Yabu JM, Higgins JP, Chen G, Sequeira F, Busque S, Tyan DB. C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation. 2011;91(3):342–7. doi: 10.1097/TP.0b013e318203fd26. [DOI] [PubMed] [Google Scholar]
- 30.Loupy A, Lefaucheur C, Vernerey D, Prugger C, van Huyen JP, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. The New England journal of medicine. 2013;369(13):1215–26. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 31.Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013;95(3):410–7. doi: 10.1097/TP.0b013e31827d62e3. [DOI] [PubMed] [Google Scholar]
- 32.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(5):1157–67. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 33.Wu P, Jin J, Everly MJ, Lin C, Terasaki PI, Chen J. Impact of alloantibody strength in crossmatch negative DSA positive kidney transplantation. Clinical biochemistry. 2013;46(15):1389–93. doi: 10.1016/j.clinbiochem.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 34.Burns JM, Cornell LD, Perry DK, Pollinger HS, Gloor JM, Kremers WK, et al. Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(12):2684–94. doi: 10.1111/j.1600-6143.2008.02441.x. [DOI] [PubMed] [Google Scholar]
- 35.Friedlander R, Putheti P, Diaz E, Menon A, Ponce B, Muthukumar T, et al. On the detection of anti-HLA antibodies using single antigen bead Luminex assay: lot-to-lot variations in MFI. Transplantation. 2013;96(4):e24–6. doi: 10.1097/TP.0b013e31829c2481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


